AMP-Activated Protein Kinase Contributes to Apoptosis Induced by the Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
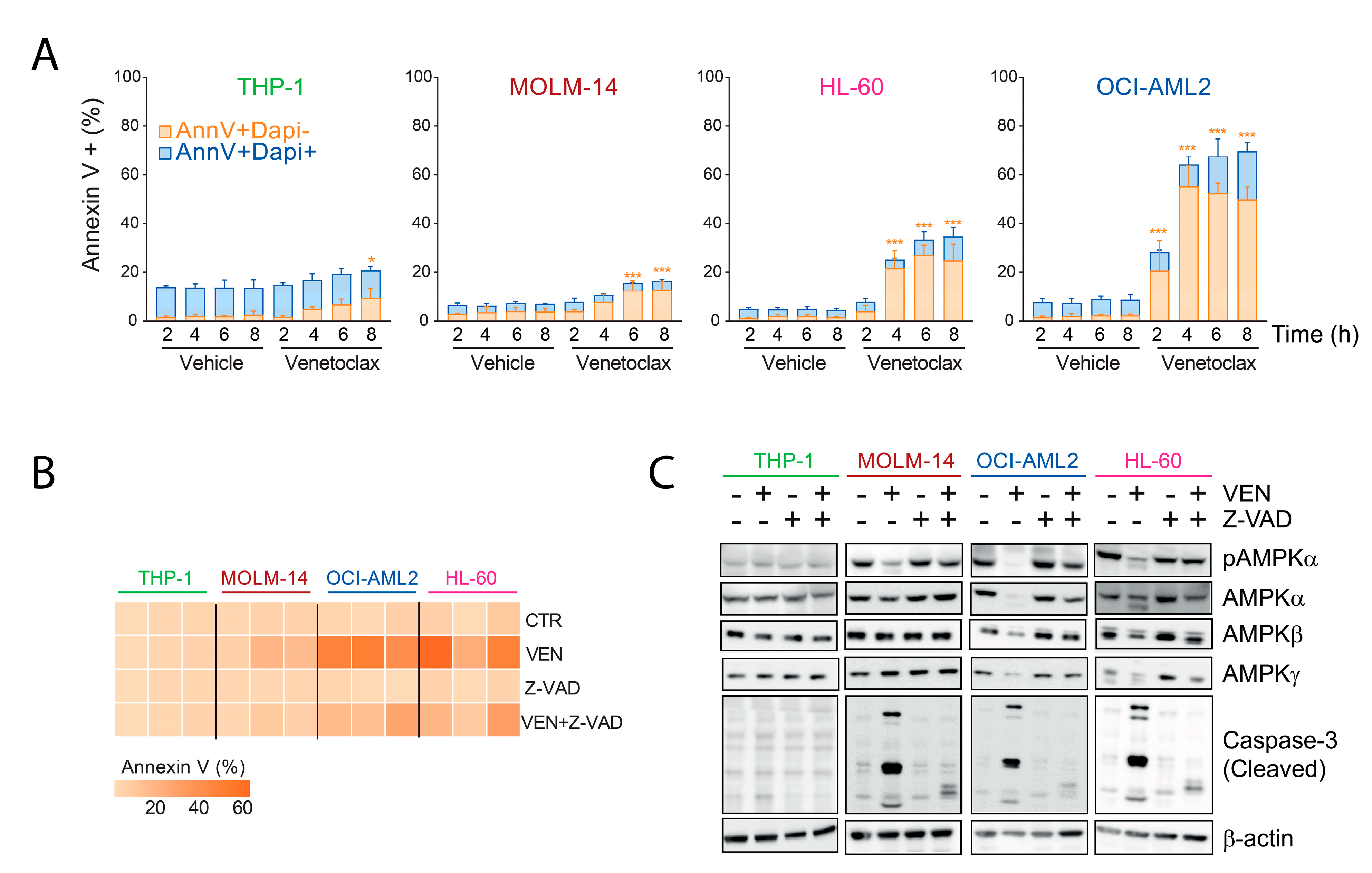
2.1. Inhibition of AMPK Activity by Venetoclax in AML Cells
2.2. Decreased Amount of AMPK Subunits by Venetoclax in AML
2.3. AMPK Degradation Is Due to On-Target Caspase Activation by Venetoclax
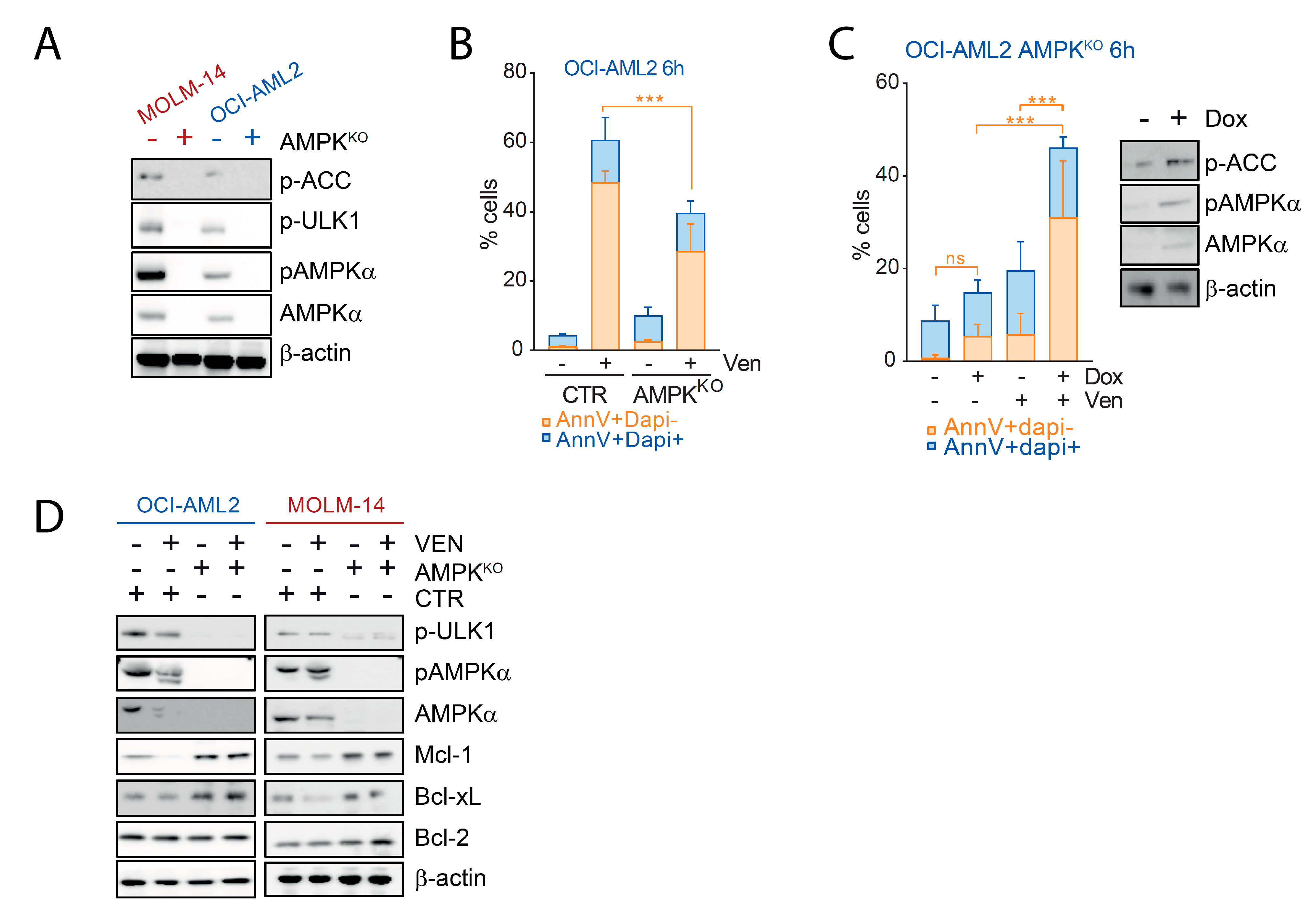
2.4. AMPK Contributes to the Pro-Apoptotic Activity of Venetoclax in AML
3. Discussion
4. Methods
4.1. Cell Lines and Reagents
4.2. AMPK Expression Vector
4.3. Cell Viability Assay
4.4. ATP Quantification
4.5. Western Blots
4.6. In Vitro Kinase Assays
4.7. Flow Cytometry
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [Green Version]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing Platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Pan, R.; Hogdal, L.J.; Benito, J.M.; Bucci, D.; Han, L.; Borthakur, G.; Cortes, J.; DeAngelo, D.J.; Debose, L.; Mu, H.; et al. Selective BCL-2 Inhibition by ABT-199 Causes On Target Cell Death in Acute Myeloid Leukemia. Cancer Discov. 2014, 4, 362–375. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.H.; Strickland, S.A.; Hou, J.-Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients with Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, G.; Ohanian, M.; Alvarado, Y.; et al. 10-Day Decitabine with Venetoclax for Newly Diagnosed Intensive Chemotherapy Ineligible, and Relapsed or Refractory Acute Myeloid Leukaemia: A Single-Centre, Phase 2 Trial. Lancet Haematol. 2020, 7, e724–e736. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Sharon, D.; Cathelin, S.; Mirali, S.; Di Trani, J.M.; Yanofsky, D.J.; Keon, K.A.; Rubinstein, J.L.; Schimmer, A.D.; Ketela, T.; Chan, S.M. Inhibition of Mitochondrial Translation Overcomes Venetoclax Resistance in AML through Activation of the Integrated Stress Response. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; et al. Venetoclax with Azacitidine Disrupts Energy Metabolism and Targets Leukemia Stem Cells in Patients with Acute Myeloid Leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Hardie, D.G. AMPK: Sensing Glucose as Well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Adam, K.; Lambert, M.; Lestang, E.; Champenois, G.; Dusanter-Fourt, I.; Tamburini, J.; Bouscary, D.; Lacombe, C.; Zermati, Y.; Mayeux, P. Control of Pim2 Kinase Stability and Expression in Transformed Human Haematopoietic Cells. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadokoro, D.; Takahama, S.; Shimizu, K.; Hayashi, S.; Endo, Y.; Sawasaki, T. Characterization of a Caspase-3-Substrate Kinome Using an N- and C-Terminally Tagged Protein Kinase Library Produced by a Cell-Free System. Cell Death Dis. 2010, 1, e89. [Google Scholar] [CrossRef]
- Grenier, A.; Sujobert, P.; Olivier, S.; Guermouche, H.; Mondésir, J.; Kosmider, O.; Viollet, B.; Tamburini, J. Knockdown of Human AMPK Using the CRISPR/Cas9 Genome-Editing System. Methods Mol. Biol. 2018, 1732, 171–194. [Google Scholar] [CrossRef] [Green Version]
- Schiffer, C.A. Promoting Apoptosis with Venetoclax—A Benefit for Older Patients with AML. N. Engl. J. Med. 2020, 383, 677–679. [Google Scholar] [CrossRef]
- Sujobert, P.; Poulain, L.; Paubelle, E.; Zylbersztejn, F.; Grenier, A.; Lambert, M.; Townsend, E.C.; Brusq, J.-M.; Nicodeme, E.; Decrooqc, J.; et al. Co-Activation of AMPK and MTORC1 Induces Cytotoxicity in Acute Myeloid Leukemia. Cell Rep. 2015, 11, 1446–1457. [Google Scholar] [CrossRef]
- Tamburini, J.; Green, A.S.; Chapuis, N.; Bardet, V.; Lacombe, C.; Mayeux, P.; Bouscary, D. Targeting Translation in Acute Myeloid Leukemia: A New Paradigm for Therapy? Cell Cycle 2009, 8, 3893–3899. [Google Scholar] [CrossRef]
- Mills, J.R.; Hippo, Y.; Robert, F.; Chen, S.M.H.; Malina, A.; Lin, C.-J.; Trojahn, U.; Wendel, H.-G.; Charest, A.; Bronson, R.T.; et al. MTORC1 Promotes Survival through Translational Control of Mcl-1. Proc. Natl. Acad. Sci. USA 2008, 105, 10853–10858. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, H.E.; Fischer, M.A.; Lee, T.; Gorska, A.E.; Arrate, M.P.; Fuller, L.; Boyd, K.L.; Strickland, S.A.; Sensintaffar, J.; Hogdal, L.J.; et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018, 8, 1566–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guièze, R.; Liu, V.M.; Rosebrock, D.; Jourdain, A.A.; Hernández-Sánchez, M.; Martinez Zurita, A.; Sun, J.; Ten Hacken, E.; Baranowski, K.; Thompson, P.A.; et al. Mitochondrial Reprogramming Underlies Resistance to BCL-2 Inhibition in Lymphoid Malignancies. Cancer Cell 2019, 36, 369–384.e13. [Google Scholar] [CrossRef]
- Stahl, M.; Menghrajani, K.; Derkach, A.; Chan, A.; Xiao, W.; Glass, J.; King, A.C.; Daniyan, A.F.; Famulare, C.; Cuello, B.M.; et al. Clinical and Molecular Predictors of Response and Survival Following Venetoclax Therapy in Relapsed/Refractory AML. Blood Adv. 2021, 5, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular Patterns of Response and Treatment Failure after Frontline Venetoclax Combinations in Older Patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Hardie, D.G. Molecular Pathways: Is AMPK a Friend or a Foe in Cancer? Clin. Cancer Res. 2015, 21, 3836–3840. [Google Scholar] [CrossRef] [Green Version]
- Vara-Ciruelos, D.; Russell, F.M.; Hardie, D.G. The Strange Case of AMPK and Cancer: Dr Jekyll or Mr Hyde? Open Biol. 2019, 9, 190099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Chapple, R.H.; Lin, A.; Kitano, A.; Nakada, D. AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell Stem Cell 2015, 17, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Pei, S.; Minhajuddin, M.; Adane, B.; Khan, N.; Stevens, B.M.; Mack, S.C.; Lai, S.; Rich, J.N.; Inguva, A.; Shannon, K.M.; et al. AMPK/FIS1-Mediated Mitophagy Is Required for Self-Renewal of Human AML Stem Cells. Cell Stem Cell 2018, 23, 86–100.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, S.; Minhajuddin, M.; Jang, J.E.; Eom, J.-I.; Jeung, H.-K.; Cheong, J.-W.; Lee, J.Y.; Kim, J.S.; Min, Y.H. Targeting AMPK-ULK1-mediated autophagy for combating BET inhibitor resistance in acute myeloid leukemia stem cells. Autophagy 2017, 13, 761–762. [Google Scholar] [CrossRef] [Green Version]


| Cell Lines | AMPKα | AMPKβ | AMPKγ |
|---|---|---|---|
| OCI-AML2 | 34 h | 18.9 h | 16.2 h |
| OCI-AML2 VEN | 4.8 h | 4.8 h | 4 h |
| MOLM-14 | 7.7 h | 40.7 h | 9 h |
| MOLM-14 VEN | 4.35 h | 5.5 h | 9 h |
| HL-60 | 4.8 h | 10.5 h | 11.6 h |
| HL-60 VEN | 4.5 h | 7.5 h | 7.8 h |
| THP-1 | 14 h | N/E | N/E |
| THP-1 VEN | 3.3 h | N/E | 8 h |
| Antibody | Manufacturer | Reference |
|---|---|---|
| β-actin | Sigma-Aldrich | A-74 |
| AMPKα | Cell signaling | 2532 |
| AMPKα1 | Cell signaling | 2795 |
| AMPKβ | Cell signaling | 12,063 |
| AMPKγ | Cell signaling | 4187 |
| Phospho-ACC (S79) | Cell signaling | 3661 |
| Phospho-ULK-1 (S555) | Cell signaling | 5869 |
| Phospho-AMPK (T172) | Cell signaling | 4188 |
| Bcl-2 | Cell signaling | 4223 |
| Bcl-xL | Cell signaling | 2762 |
| Mcl-1 | Cell signaling | 94,296 |
| Caspase 3 | Cell signaling | 9662 |
| PARP | Cell signaling | 9542 |
| rabbit HRP-linked IgG | Cell signaling | 7074 |
| mouse HRP-linked IgG | Cell signaling | 7076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legrand, N.; Pradier, A.; Poulain, L.; Mouche, S.; Birsen, R.; Larrue, C.; Simonetta, F.; Tamburini, J. AMP-Activated Protein Kinase Contributes to Apoptosis Induced by the Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia. Cancers 2021, 13, 5966. https://doi.org/10.3390/cancers13235966
Legrand N, Pradier A, Poulain L, Mouche S, Birsen R, Larrue C, Simonetta F, Tamburini J. AMP-Activated Protein Kinase Contributes to Apoptosis Induced by the Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia. Cancers. 2021; 13(23):5966. https://doi.org/10.3390/cancers13235966
Chicago/Turabian StyleLegrand, Noémie, Amandine Pradier, Laury Poulain, Sarah Mouche, Rudy Birsen, Clément Larrue, Federico Simonetta, and Jerome Tamburini. 2021. "AMP-Activated Protein Kinase Contributes to Apoptosis Induced by the Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia" Cancers 13, no. 23: 5966. https://doi.org/10.3390/cancers13235966
APA StyleLegrand, N., Pradier, A., Poulain, L., Mouche, S., Birsen, R., Larrue, C., Simonetta, F., & Tamburini, J. (2021). AMP-Activated Protein Kinase Contributes to Apoptosis Induced by the Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia. Cancers, 13(23), 5966. https://doi.org/10.3390/cancers13235966





