The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
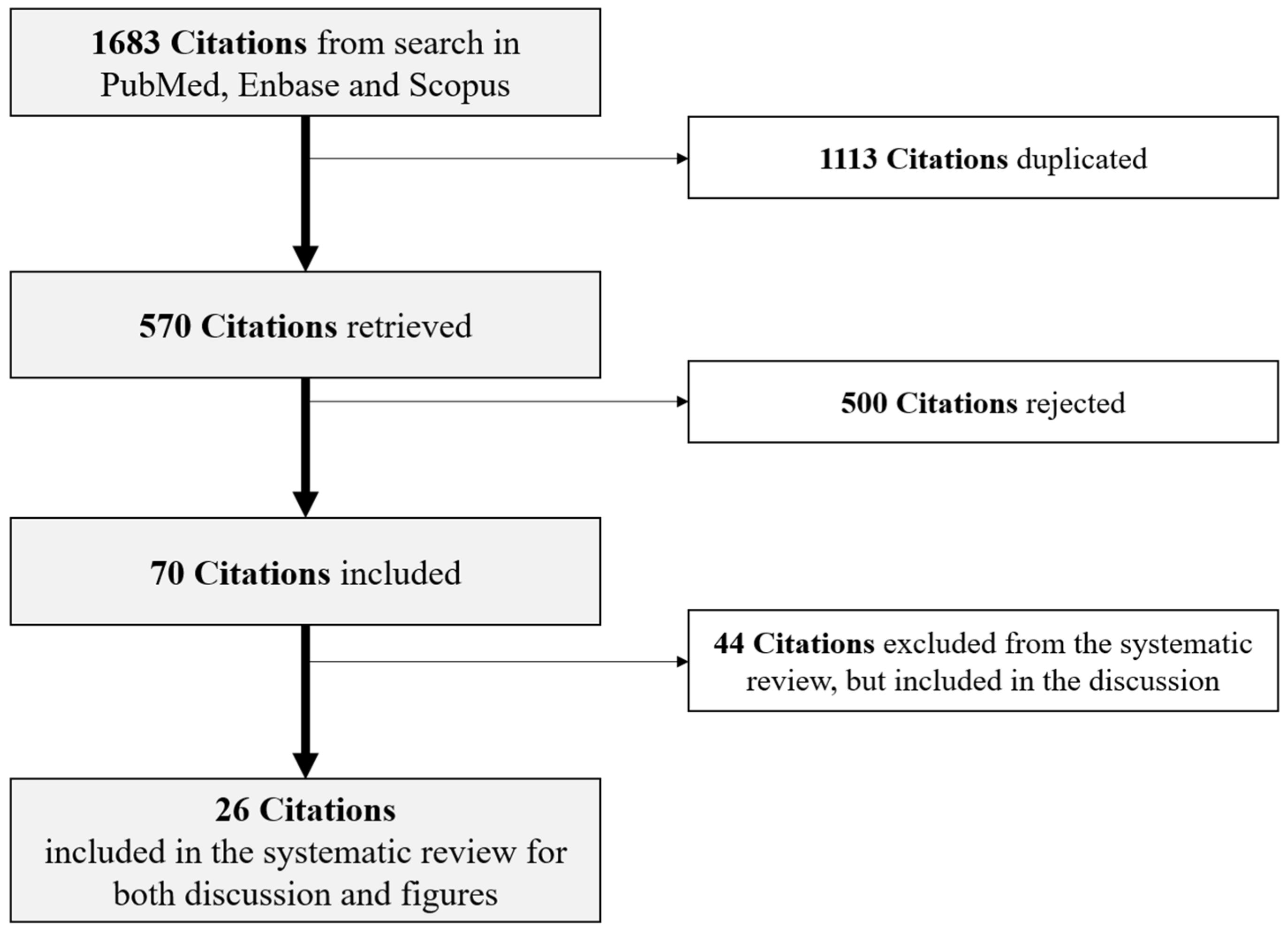
2. Materials and Methods
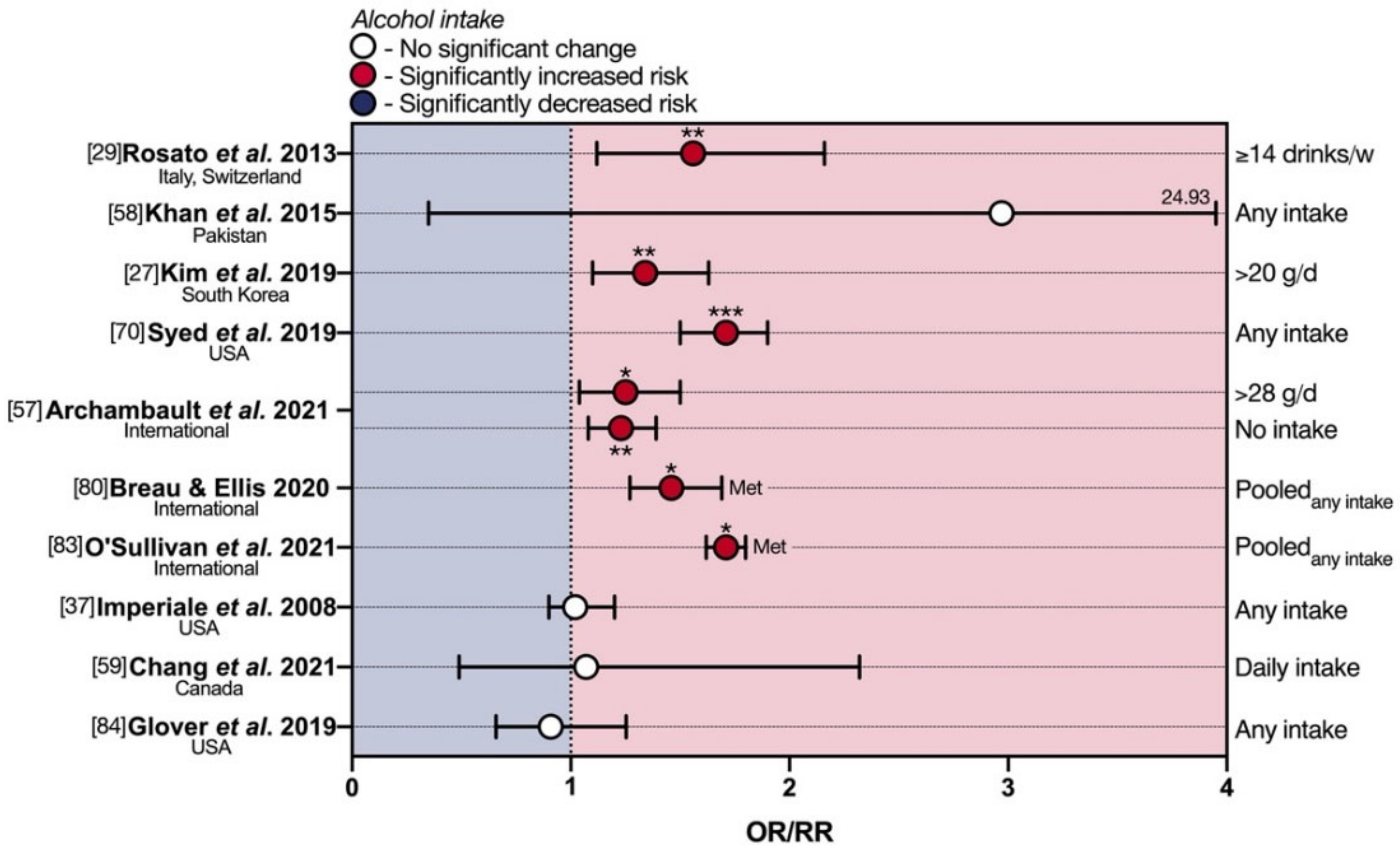
3. Diet and eoCRC
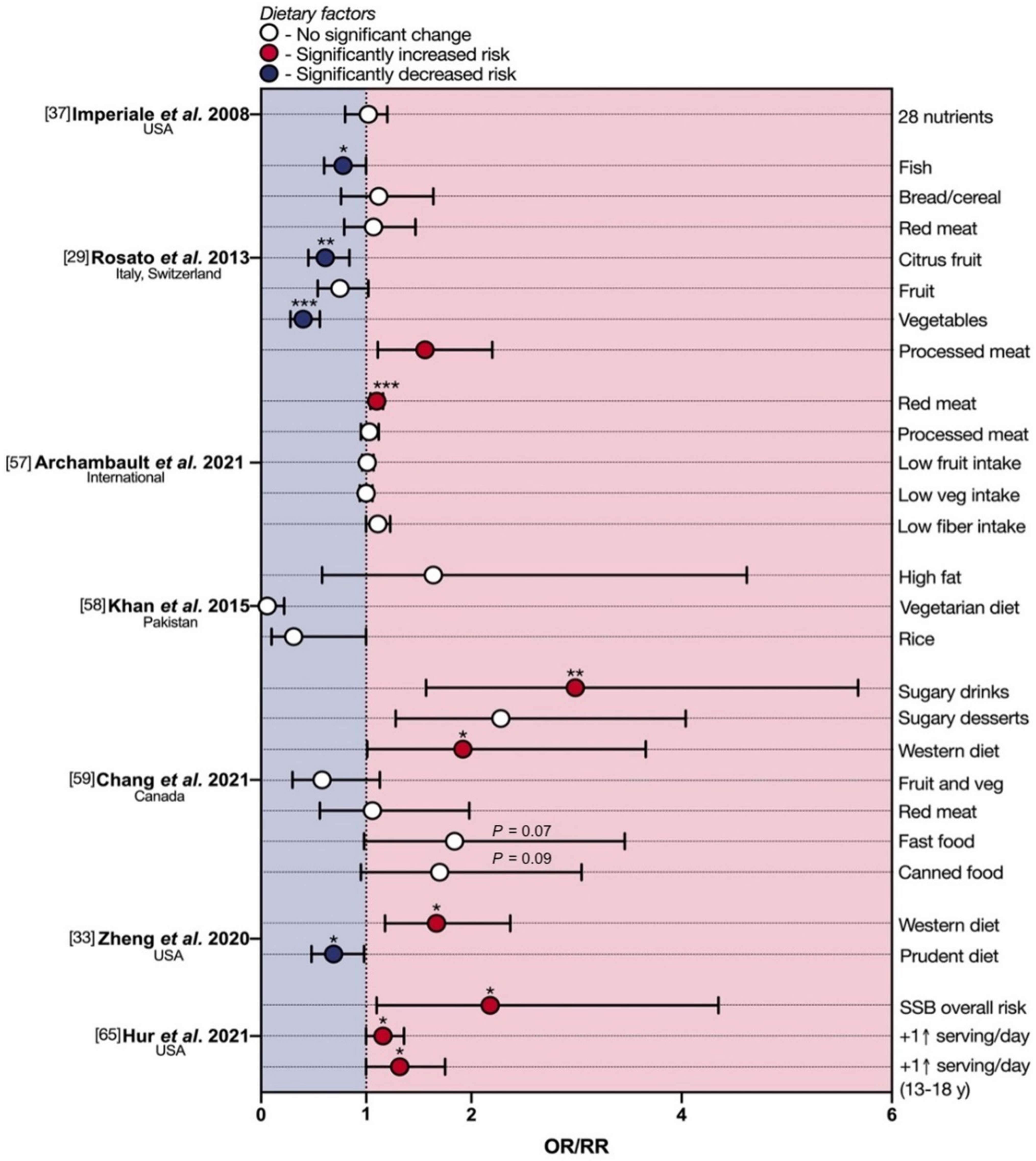
3.1. Foods and eoCRC
3.2. Drinks and eoCRC
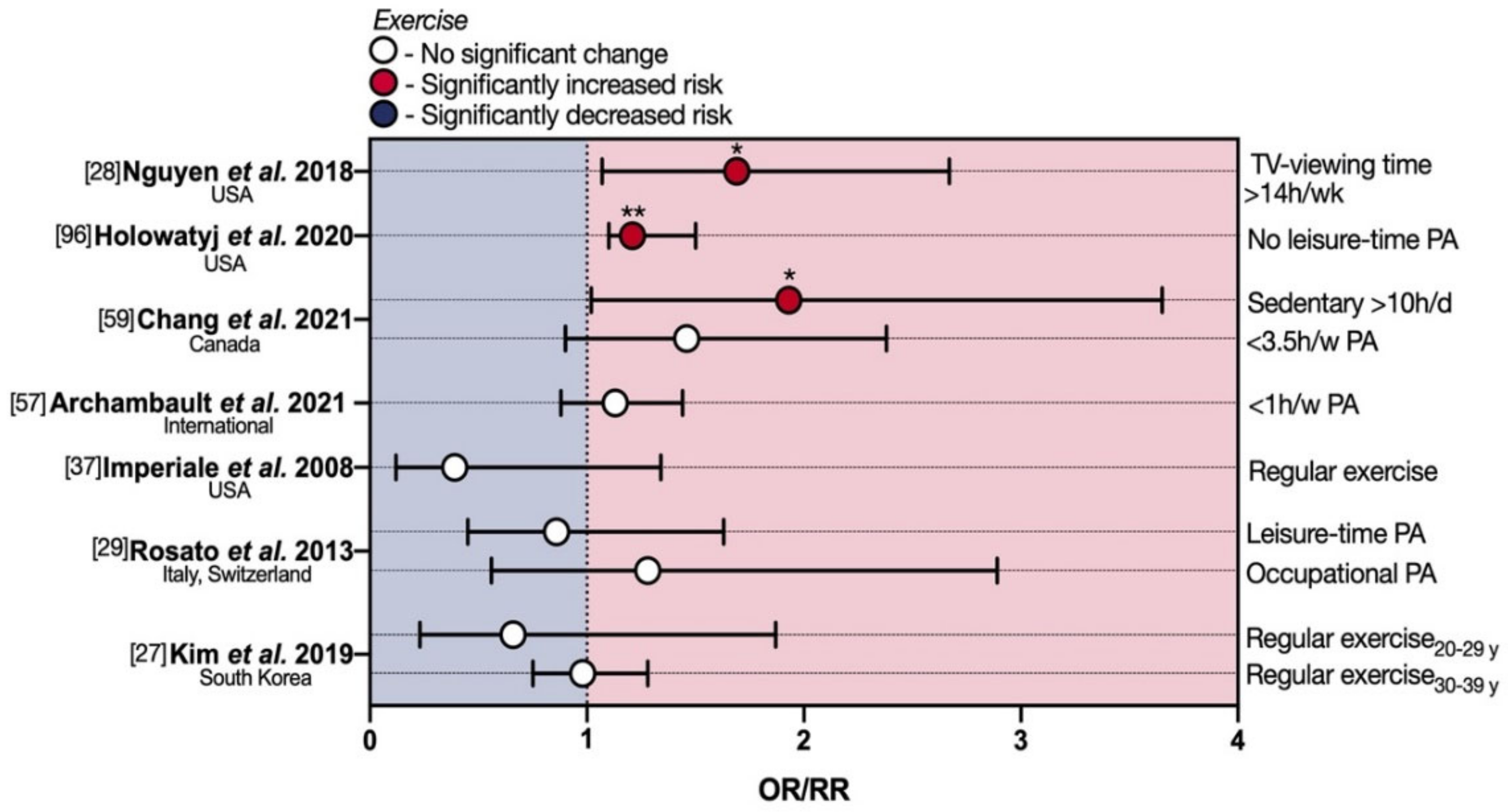
4. Physical Activity and eoCRC
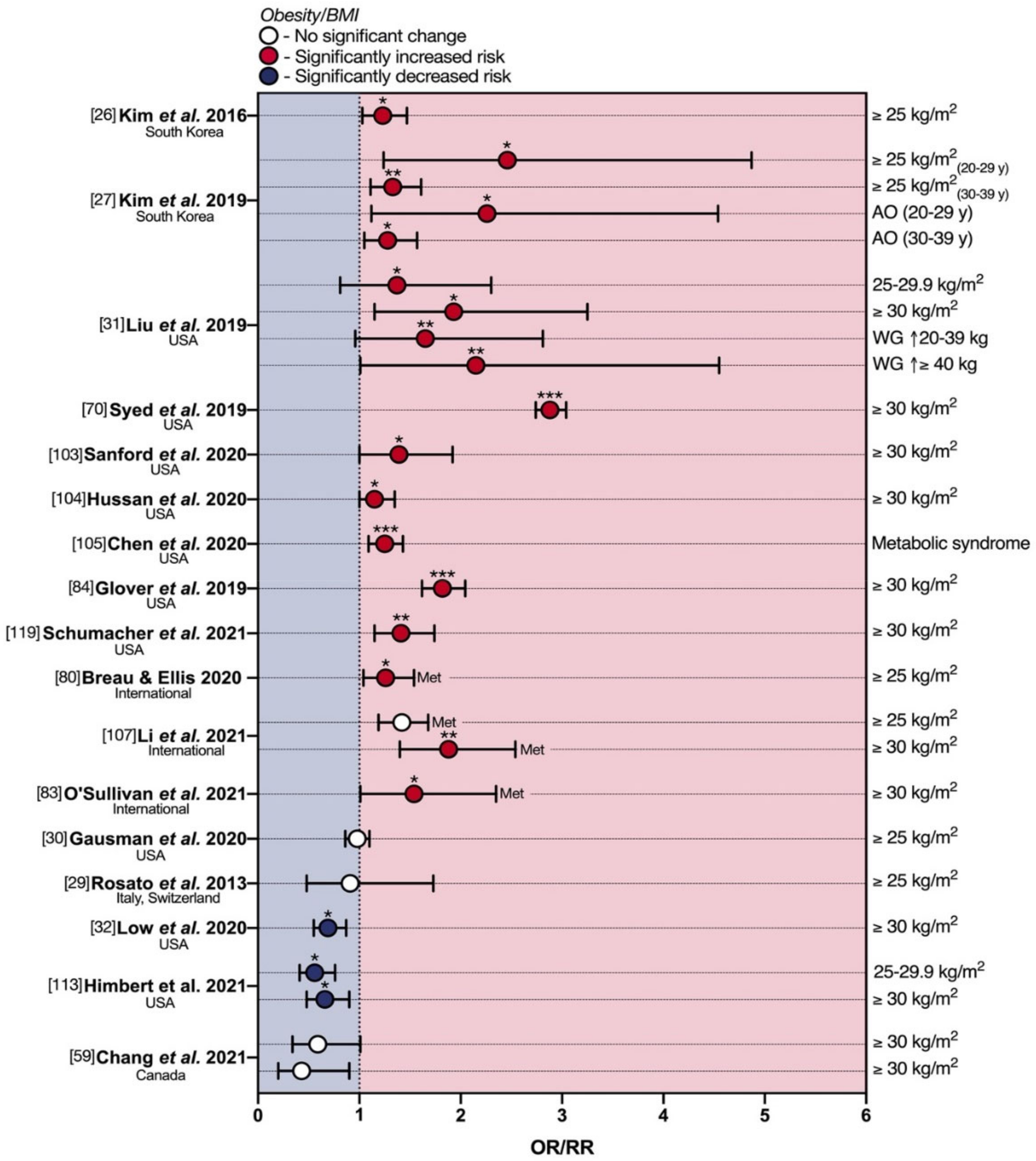
5. Obesity and eoCRC
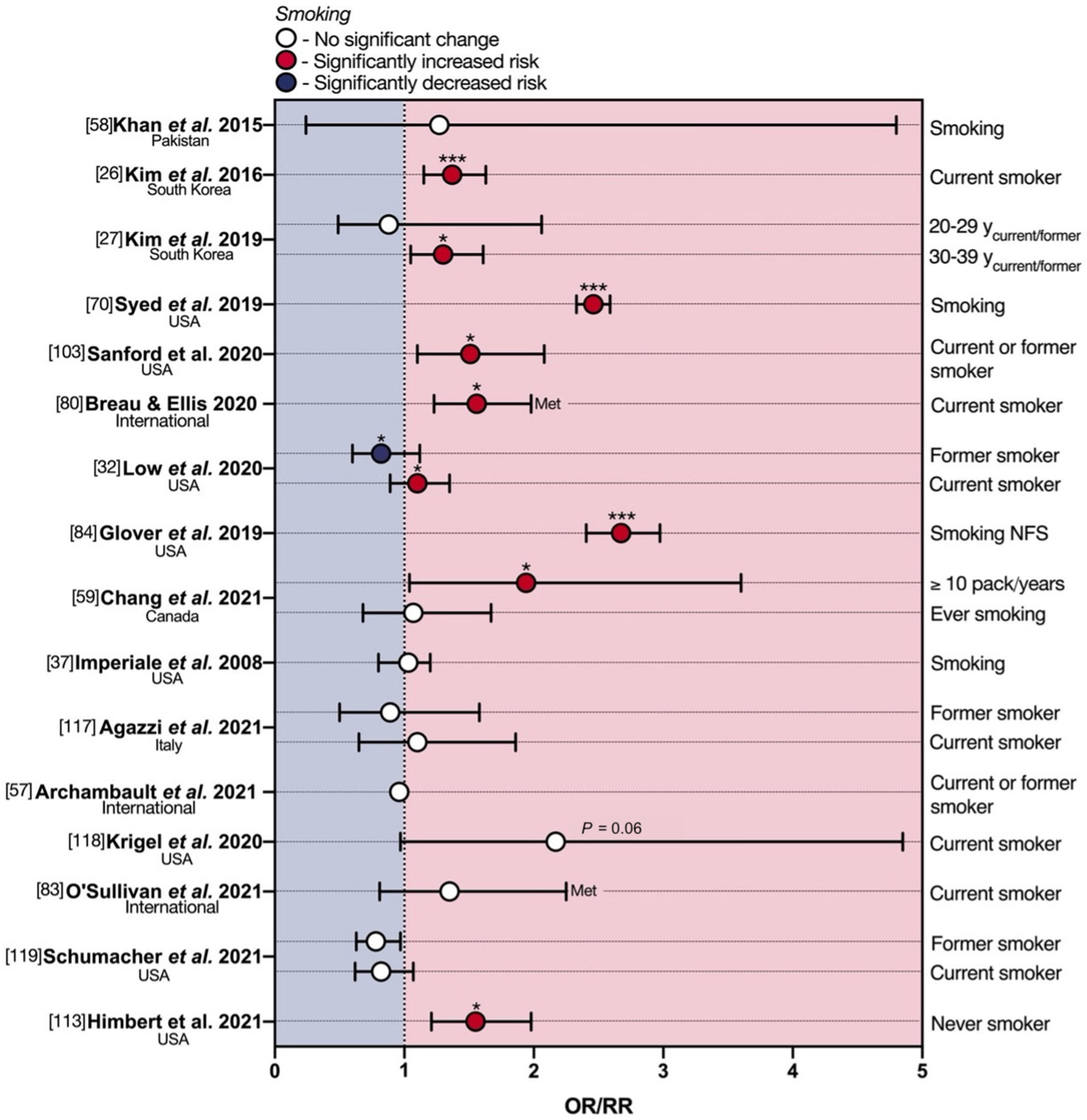
6. Smoking and eoCRC
7. The Relationship of Other Risk Factors for eoCRC with Diet and Lifestyle
7.1. Epigenetics and eoCRC
7.2. Microbiota, Early Exposure to Risk Factors, and eoCRC
7.3. Other Non-Modifiable Risk Factors and eoCRC
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahnen, D.J.; Wade, S.W.; Jones, W.F.; Sifri, R.; Mendoza Silveiras, J.; Greenamyer, J.; Guiffre, S.; Axilbund, J.; Spiegel, A.; You, Y.N. The increasing incidence of young-onset colorectal cancer: A call to action. Mayo Clin. Proc. 2014, 89, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.T.; Pai, R.K.; Rybicki, L.A.; Dimaio, M.A.; Limaye, M.; Jayachandran, P.; Koong, A.C.; Kunz, P.A.; Fisher, G.A.; Ford, J.M.; et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol. 2012, 25, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Araghi, M.; Soerjomataram, I.; Bardot, A.; Ferlay, J.; Cabasag, C.J.; Morrison, D.S.; De, P.; Tervonen, H.; Walsh, P.M.; Bucher, O.; et al. Changes in colorectal cancer incidence in seven high-income countries: A population-based study. Lancet Gastroenterol. Hepatol. 2019, 4, 511–518. [Google Scholar] [CrossRef]
- Russo, A.; Andreano, A.; Sartore-Bianchi, A.; Mauri, G.; Decarli, A.; Siena, S. Increased incidence of colon cancer among individuals younger than 50 years: A 17 years analysis from the cancer registry of the municipality of Milan, Italy. Cancer Epidemiol. 2019, 60, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [Green Version]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellise, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Zorzi, M.; Dal Maso, L.; Francisci, S.; Buzzoni, C.; Rugge, M.; Guzzinati, S.; Group, A.W. Trends of colorectal cancer incidence and mortality rates from 2003 to 2014 in Italy. Tumori 2019, 105, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Cavestro, G.M.; Guzzinati, S.; Dal Maso, L.; Rugge, M.; Group, A.W. Decline in the incidence of colorectal cancer and the associated mortality in young Italian adults. Gut 2020, 69, 1902–1903. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef]
- Yeo, H.; Betel, D.; Abelson, J.S.; Zheng, X.E.; Yantiss, R.; Shah, M.A. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin. Colorectal Cancer 2017, 16, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, M.; Zuppardo, R.A.; Puzzono, M.; Ditonno, I.; Mannucci, A.; Antoci, G.; Russo Raucci, A.; Patricelli, M.G.; Elmore, U.; Tamburini, A.M.; et al. Risk factors and clinical characteristics of early-onset colorectal cancer vs. late-onset colorectal cancer: A case-case study. Eur. J. Gastroenterol. Hepatol. 2020, 33, 1153–1160. [Google Scholar] [CrossRef]
- Yeo, S.; Chew, M.; Koh, P.; Tang, C. Young colorectal carcinoma patients do not have a poorer prognosis: A comparative review of 2426 cases. Tech. Coloproctol. 2013, 17, 653–661. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Mannucci, A.; Zuppardo, R.A.; Di Leo, M.; Stoffel, E.; Tonon, G. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig. Liver Dis. 2018, 50, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline Genetic Features of Young Individuals with Colorectal Cancer. Gastroenterology 2018, 154, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Lau, R.; Chan, D.S.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011, 141, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.L.; Shu, L.; Zheng, P.F.; Zhang, X.Y.; Si, C.J.; Yu, X.L.; Gao, W.; Zhang, L. Dietary patterns and colorectal cancer risk: A meta-analysis. Eur. J. Cancer Prev. 2017, 26, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Barrubés, L.; Babio, N.; Becerra-Tomás, N.; Rosique-Esteban, N.; Salas-Salvadó, J. Association between dairy product consumption and colorectal cancer risk in adults: A systematic review and meta-analysis of epidemiologic studies. Adv. Nutr. 2019, 10, S190–S211. [Google Scholar] [CrossRef]
- Castelló, A.; Amiano, P.; de Larrea, N.F.; Martín, V.; Alonso, M.H.; Castaño-Vinyals, G.; Pérez-Gómez, B.; Olmedo-Requena, R.; Guevara, M.; Fernandez-Tardon, G. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur. J. Nutr. 2019, 58, 1495–1505. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Petimar, J.; Smith-Warner, S.A.; Fung, T.T.; Rosner, B.; Chan, A.T.; Hu, F.B.; Giovannucci, E.L.; Tabung, F.K. Recommendation-based dietary indexes and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Am. J. Clin. Nutr. 2018, 108, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef] [Green Version]
- Chapelle, N.; Martel, M.; Toes-Zoutendijk, E.; Barkun, A.N.; Bardou, M. Recent advances in clinical practice: Colorectal cancer chemoprevention in the average-risk population. Gut 2020, 69, 2244–2255. [Google Scholar] [CrossRef]
- Wang, L.; Lo, C.H.; He, X.; Hang, D.; Wang, M.; Wu, K.; Chan, A.T.; Ogino, S.; Giovannucci, E.L.; Song, M. Risk Factor Profiles Differ for Cancers of Different Regions of the Colorectum. Gastroenterology 2020, 159, 241–256. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, Y.; Li, Q.; Wang, F.; Ge, X.; Zhou, G.; Miao, L. Association between Mediterranean diet adherence and colorectal cancer: A dose-response meta-analysis. Am. J. Clin. Nutr. 2020, 111, 1214–1225. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, Y.S.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Choi, K.Y.; Park, D.I. Different risk factors for advanced colorectal neoplasm in young adults. World J. Gastroenterol. 2016, 22, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jung, Y.S.; Yang, H.J.; Park, S.K.; Park, J.H.; Park, D.I.; Sohn, C.I. Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20–39 Years Old). Clin. Gastroenterol. Hepatol. 2019, 17, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.H.; Liu, P.H.; Zheng, X.; Keum, N.; Zong, X.; Li, X.; Wu, K.; Fuchs, C.S.; Ogino, S.; Ng, K.; et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018, 2, pky073. [Google Scholar] [CrossRef] [Green Version]
- Rosato, V.; Bosetti, C.; Levi, F.; Polesel, J.; Zucchetto, A.; Negri, E.; La Vecchia, C. Risk factors for young-onset colorectal cancer. Cancer Causes Control 2013, 24, 335–341. [Google Scholar] [CrossRef]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759. [Google Scholar] [CrossRef]
- Liu, P.-H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.H.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019, 5, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Low, E.E.; Demb, J.; Liu, L.; Earles, A.; Bustamante, R.; Williams, C.D.; Provenzale, D.; Kaltenbach, T.; Gawron, A.J.; Martinez, M.E.; et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020, 159, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hur, J.; Nguyen, L.H.; Liu, J.; Song, M.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Willett, W.C.; Chan, A.T.; et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J. Natl. Cancer Inst. 2020, 113, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.; Cotterchio, M.; Kreiger, N.; Nadalin, V.; Block, T.; Block, G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006, 9, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Decarli, A.; Franceschi, S.; Ferraroni, M.; Gnagnarella, P.; Parpinel, M.T.; La Vecchia, C.; Negri, E.; Salvini, S.; Falcini, F.; Giacosa, A. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Ann. Epidemiol. 1996, 6, 110–118. [Google Scholar] [CrossRef]
- Huxley, R.R.; Ansary-Moghaddam, A.; Clifton, P.; Czernichow, S.; Parr, C.L.; Woodward, M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: A quantitative overview of the epidemiological evidence. Int. J. Cancer 2009, 125, 171–180. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Kahi, C.J.; Stuart, J.S.; Qi, R.; Born, L.J.; Glowinski, E.A.; Rex, D.K. Risk factors for advanced sporadic colorectal neoplasia in persons younger than age 50. Cancer Detect. Prev. 2008, 32, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Trock, B.; Lanza, E.; Greenwald, P. Dietary fiber, vegetables, and colon cancer: Critical review and meta-analyses of the epidemiologic evidence. J. Natl. Cancer Inst. 1990, 82, 650–661. [Google Scholar] [CrossRef]
- Tse, G.; Eslick, G.D. Cruciferous vegetables and risk of colorectal neoplasms: A systematic review and meta-analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Woo, H.D.; Park, S.; Oh, K.; Kim, H.J.; Shin, H.R.; Moon, H.K.; Kim, J. Diet and cancer risk in the Korean population: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8509–8519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous vegetables intake and the risk of colorectal cancer: A meta-analysis of observational studies. Ann. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sun, Y.; Qi, L.; Zhong, R.; Miao, X. Dietary legume consumption reduces risk of colorectal cancer: Evidence from a meta-analysis of cohort studies. Sci. Rep. 2015, 5, 8797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashino, I.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Nagata, C.; Inoue, M.; Tsugane, S. Vegetable consumption and colorectal cancer risk: An evaluation based on a systematic review and meta-analysis among the Japanese population. Jpn. J. Clin. Oncol. 2015, 45, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Geelen, A.; Schouten, J.M.; Kamphuis, C.; Stam, B.E.; Burema, J.; Renkema, J.M.; Bakker, E.J.; van’t Veer, P.; Kampman, E. Fish consumption, n-3 fatty acids, and colorectal cancer: A meta-analysis of prospective cohort studies. Am. J. Epidemiol. 2007, 166, 1116–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, N.M.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Nagata, C.; Inoue, M.; Tsugane, S. Fish consumption and colorectal cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2013, 43, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.J.; Zhou, J.D.; Dong, J.Y.; Ding, W.Q.; Wu, J.C. Dietary intake of n-3 fatty acids and colorectal cancer risk: A meta-analysis of data from 489 000 individuals. Br. J. Nutr. 2012, 108, 1550–1556. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Feng, B.; Li, K.; Zhu, X.; Liang, S.; Liu, X.; Han, S.; Wang, B.; Wu, K.; Miao, D.; et al. Fish consumption and colorectal cancer risk in humans: A systematic review and meta-analysis. Am. J. Med. 2012, 125, 551–559. [Google Scholar] [CrossRef]
- Yu, X.F.; Zou, J.; Dong, J. Fish consumption and risk of gastrointestinal cancers: A meta-analysis of cohort studies. World J. Gastroenterol. 2014, 20, 15398–15412. [Google Scholar] [CrossRef]
- Phillips, D.H.; Grover, P.L. Polycyclic hydrocarbon activation: Bay regions and beyond. Drug Metab. Rev. 1994, 26, 443–467. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T. Environmental Factors, Gut Microbiota, and Colorectal Cancer Prevention. Clin. Gastroenterol. Hepatol. 2019, 17, 275–289. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The association of diet, gut microbiota and colorectal cancer: What we eat may imply what we get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, A.J.; Ferrucci, L.M.; Risch, A.; Graubard, B.I.; Ward, M.H.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R. A large prospective study of meat consumption and colorectal cancer risk: An investigation of potential mechanisms underlying this association. Cancer Res. 2010, 70, 2406–2414. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.M.; Sinha, R.; Huang, W.Y.; Berndt, S.I.; Katki, H.A.; Schoen, R.E.; Hayes, R.B.; Cross, A.J. Meat consumption and the risk of incident distal colon and rectal adenoma. Br. J. Cancer 2012, 106, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohoe, D.R.; Holley, D.; Collins, L.B.; Montgomery, S.A.; Whitmore, A.C.; Hillhouse, A.; Curry, K.P.; Renner, S.W.; Greenwalt, A.; Ryan, E.P. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota-and butyrate-dependent manner. Cancer Discov. 2014, 4, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Archambault, A.N.; Lin, Y.; Jeon, J.; Harrison, T.A.; Bishop, D.T.; Brenner, H.; Casey, G.; Chan, A.T.; Chang-Claude, J.; Figueiredo, J.C.; et al. Nongenetic Determinants of Risk for Early-Onset Colorectal Cancer. JNCI Cancer Spectr. 2021, 5, pkab029. [Google Scholar] [CrossRef]
- Khan, N.A.; Hussain, M.; ur Rahman, A.; Farooqui, W.A.; Rasheed, A.; Memon, A.S. Dietary Practices, Addictive Behavior and Bowel Habits and Risk of Early Onset Colorectal Cancer: A Case Control Study. Asian Pac. J. Cancer Prev. 2015, 16, 7967–7973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, V.C.; Cotterchio, M.; De, P.; Tinmouth, J. Risk factors for early-onset colorectal cancer: A population-based case-control study in Ontario, Canada. Cancer Causes Control. 2021, 32, 1063–1083. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Brown, L.S.; Fung, T.T. Dietary Patterns and Colorectal Cancer Risk: A Review of 17 Years of Evidence (2000–2016). Curr. Colorectal Cancer Rep. 2017, 13, 440–454. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Morton, V.; Norat, T.; Moreira, A.; Potts, J.F.; Reeves, T.; Bakolis, I. Dietary patterns derived from principal component analysis (PCA) and risk of colorectal cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2019, 73, 366–386. [Google Scholar] [CrossRef]
- Nimptsch, K.; Malik, V.S.; Fung, T.T.; Pischon, T.; Hu, F.B.; Willett, W.C.; Fuchs, C.S.; Ogino, S.; Chan, A.T.; Giovannucci, E.; et al. Dietary patterns during high school and risk of colorectal adenoma in a cohort of middle-aged women. Int. J. Cancer 2014, 134, 2458–2467. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.; Cade, J.E.; Evans, C.E.L.; Hancock, N.; Greenwood, D.C. The Mediterranean diet and risk of colorectal cancer in the UK Women’s Cohort Study. Int. J. Epidemiol. 2017, 46, 1786–1796. [Google Scholar] [CrossRef] [Green Version]
- Agnoli, C.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Giurdanella, M.C.; Pala, V.; et al. Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int. J. Cancer 2013, 132, 1404–1411. [Google Scholar] [CrossRef]
- Hur, J.; Otegbeye, E.; Joh, H.K.; Nimptsch, K.; Ng, K.; Ogino, S.; Meyerhardt, J.A.; Chan, A.T.; Willett, W.C.; Wu, K.; et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021, 70, 2330–2336. [Google Scholar] [CrossRef]
- Aune, D.; Lau, R.; Chan, D.S.M.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dairy products and colorectal cancer risk: A systematic review and meta-analysis of cohort studies. Ann. Oncol. 2012, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ralston, R.A.; Truby, H.; Palermo, C.E.; Walker, K.Z. Colorectal cancer and nonfermented milk, solid cheese, and fermented milk consumption: A systematic review and meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2014, 54, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Aune, D.; Greenwood, D.C.; Ju, W.; Giovannucci, E.L. Calcium intake and colorectal cancer risk: Dose-response meta-analysis of prospective observational studies. Int. J. Cancer 2014, 135, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, S.A.; Lipkin, M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann. N. Y. Acad. Sci. 2001, 952, 73–87. [Google Scholar] [CrossRef]
- Syed, A.R.; Thakkar, P.; Horne, Z.D.; Abdul-Baki, H.; Kochhar, G.; Farah, K.; Thakkar, S. Old vs new: Risk factors predicting early onset colorectal cancer. World J. Gastrointest. Oncol. 2019, 11, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Blangiardo, M.; La Vecchia, C.; Corrao, G. A meta-analysis of alcohol drinking and cancer risk. Br. J. Cancer 2001, 85, 1700–1705. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Light alcohol drinking and cancer: A meta-analysis. Ann. Oncol. 2013, 24, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Corrao, G.; Bagnardi, V.; Zambon, A.; Arico, S. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: A meta-analysis. Addiction 1999, 94, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C. Alcohol drinking and colorectal cancer risk: An overall and dose–response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Orza, M.J.; Adams, M.E.; Vioque, J.; Chalmers, T.C. A meta-analysis of alcoholic beverage consumption in relation to risk of colorectal cancer. Cancer Causes Control. 1990, 1, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Moskal, A.; Norat, T.; Ferrari, P.; Riboli, E. Alcohol intake and colorectal cancer risk: A dose-response meta-analysis of published cohort studies. Int. J. Cancer 2007, 120, 664–671. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, H.; Yang, H.; Lin, J. A pooled analysis of alcohol intake and colorectal cancer. Int. J. Clin. Exp. Med. 2015, 8, 6878–6889. [Google Scholar]
- Zhang, C.; Zhong, M. Consumption of beer and colorectal cancer incidence: A meta-analysis of observational studies. Cancer Causes Control. 2015, 26, 549–560. [Google Scholar] [CrossRef]
- Breau, G.; Ellis, U. Risk Factors Associated with Young-Onset Colorectal Adenomas and Cancer: A Systematic Review and Meta-Analysis of Observational Research. Cancer Control 2020, 27, 1073274820976670. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, S.; Park, T.; Kim, S.K.; Jung, Y.S.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; et al. Development and validation of a scoring system for advanced colorectal neoplasm in young Korean subjects less than age 50 years. Intest. Res. 2019, 17, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Jo, H.B.; Kwack, W.G.; Jeong, Y.J.; Yoon, Y.J.; Kang, H.W. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J. Gastroenterol. 2016, 22, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, S1542-3565(21)00087-2. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.; Mansoor, E.; Panhwar, M.; Parasa, S.; Cooper, G.S. Epidemiology of Colorectal Cancer in Average Risk Adults 20–39 Years of Age: A Population-Based National Study. Dig. Dis. Sci. 2019, 64, 3602–3609. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef]
- Tsuruya, A.; Kuwahara, A.; Saito, Y.; Yamaguchi, H.; Tsubo, T.; Suga, S.; Inai, M.; Aoki, Y.; Takahashi, S.; Tsutsumi, E.; et al. Ecophysiological consequences of alcoholism on human gut microbiota: Implications for ethanol-related pathogenesis of colon cancer. Sci. Rep. 2016, 6, 27923. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Starkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruya, A.; Kuwahara, A.; Saito, Y.; Yamaguchi, H.; Tenma, N.; Inai, M.; Takahashi, S.; Tsutsumi, E.; Suwa, Y.; Totsuka, Y.; et al. Major Anaerobic Bacteria Responsible for the Production of Carcinogenic Acetaldehyde from Ethanol in the Colon and Rectum. Alcohol Alcohol. 2016, 51, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Robsahm, T.E.; Aagnes, B.; Hjartaker, A.; Langseth, H.; Bray, F.I.; Larsen, I.K. Body mass index, physical activity, and colorectal cancer by anatomical subsites: A systematic review and meta-analysis of cohort studies. Eur. J. Cancer Prev. 2013, 22, 492–505. [Google Scholar] [CrossRef]
- Slattery, M.L. Physical activity and colorectal cancer. Sports Med. 2004, 34, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [Green Version]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Holowatyj, A.N.; Langston, M.E.; Han, Y.; Viskochil, R.; Perea, J.; Cao, Y.; Rogers, C.R.; Lieu, C.H.; Moore, J.X. Community health behaviors and geographic variation in early-onset colorectal cancer survival among women. Clin. Transl. Gastroenterol. 2020, 11, e00266. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Capuano, A.; Bellastella, G.; Maiorino, M.I.; Rafaniello, C.; Panagiotakos, D.B.; Giugliano, D. Colorectal cancer association with metabolic syndrome and its components: A systematic review with meta-analysis. Endocrine 2013, 44, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.T.; Nieuwdorp, M.; Backhed, F. Microbial modulation of insulin sensitivity. Cell Metab. 2014, 20, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Grimm, S.A.; Chrysovergis, K.; Kosak, J.; Wang, X.; Du, Y.; Burkholder, A.; Janardhan, K.; Mav, D.; Shah, R. Obesity, rather than diet, drives epigenomic alterations in colonic epithelium resembling cancer progression. Cell Metab. 2014, 19, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Grimm, S.A.; Mav, D.; Gu, H.; Djukovic, D.; Shah, R.; Merrick, B.A.; Raftery, D.; Wade, P.A. Transcriptome and DNA Methylome Analysis in a Mouse Model of Diet-Induced Obesity Predicts Increased Risk of Colorectal Cancer. Cell Rep. 2018, 22, 624–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanford, N.N.; Giovannucci, E.L.; Ahn, C.; Dee, E.C.; Mahal, B.A. Obesity and younger versus older onset colorectal cancer in the United States, 1998–2017. J. Gastrointest. Oncol. 2020, 11, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hussan, H.; Patel, A.; Le Roux, M.; Cruz-Monserrate, Z.; Porter, K.; Clinton, S.K.; Carethers, J.M.; Courneya, K.S. Rising Incidence of Colorectal Cancer in Young Adults Corresponds with Increasing Surgical Resections in Obese Patients. Clin. Transl. Gastroenterol. 2020, 11, e00160. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Zong, X.; Li, Z.; Li, N.; Hur, J.; Fritz, C.D.; Chapman, W., Jr.; Nickel, K.B.; Tipping, A.; et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2020, 70, 1147–1154. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, C.H.; Kim, N.H.; Lee, M.Y.; Park, D.I. Impact of Age on the Risk of Advanced Colorectal Neoplasia in a Young Population: An Analysis Using the Predicted Probability Model. Dig. Dis. Sci. 2017, 62, 2518–2525. [Google Scholar] [CrossRef]
- Li, H.; Boakye, D.; Chen, X.; Hoffmeister, M.; Brenner, H. Association of Body Mass Index with Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2021, 116, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Dash, C.; Yu, J.; Nomura, S.; Lu, J.; Rosenberg, L.; Palmer, J.R.; Adams-Campbell, L.L. Obesity is an initiator of colon adenomas but not a promoter of colorectal cancer in the Black Women’s Health Study. Cancer Causes Control. 2020, 31, 291–302. [Google Scholar] [CrossRef]
- Elangovan, A.; Skeans, J.; Landsman, M.; Ali, S.M.J.; Elangovan, A.G.; Kaelber, D.C.; Sandhu, D.S.; Cooper, G.S. Colorectal Cancer, Age, and Obesity-Related Comorbidities: A Large Database Study. Dig. Dis. Sci. 2021, 66, 3156–3163. [Google Scholar] [CrossRef]
- Kantor, E.D.; Udumyan, R.; Signorello, L.B.; Giovannucci, E.L.; Montgomery, S.; Fall, K. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut 2016, 65, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Pasche, C.; La Vecchia, C.; Lucchini, F.; Franceschi, S. Food groups and colorectal cancer risk. Br. J. Cancer 1999, 79, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.L.; Bradlee, M.L.; Singer, M.R.; Splansky, G.L.; Proctor, M.H.; Ellison, R.C.; Kreger, B.E. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himbert, C.; Figueiredo, J.C.; Shibata, D.; Ose, J.; Lin, T.; Huang, L.C.; Peoples, A.R.; Scaife, C.L.; Pickron, B.; Lambert, L.; et al. Clinical Characteristics and Outcomes of Colorectal Cancer in the ColoCare Study: Differences by Age of Onset. Cancers 2021, 13, 3817. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and colorectal cancer: A meta-analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.S.; Chen, T.Y.; Giovannucci, E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2406–2415. [Google Scholar] [CrossRef]
- Agazzi, S.; Lenti, M.V.; Klersy, C.; Strada, E.; Pozzi, L.; Rovedatti, L.; Bardone, M.; Mauro, A.; Costetti, M.; Costa, S.; et al. Incidence and risk factors for preneoplastic and neoplastic lesions of the colon and rectum in patients under 50 referred for colonoscopy. Eur. J. Intern. Med. 2021, 87, 36–43. [Google Scholar] [CrossRef]
- Krigel, A.; Zhou, M.; Terry, M.B.; Kastrinos, F.; Lebwohl, B. Symptoms and demographic factors associated with early-onset colorectal neoplasia among individuals undergoing diagnostic colonoscopy. Eur. J. Gastroenterol. Hepatol. 2020, 32, 821–826. [Google Scholar] [CrossRef]
- Schumacher, A.J.; Chen, Q.; Attaluri, V.; McLemore, E.C.; Chao, C.R. Metabolic Risk Factors Associated with Early-Onset Colorectal Adenocarcinoma: A Case-Control Study at Kaiser Permanente Southern California. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1792–1798. [Google Scholar] [CrossRef]
- Baars, J.; Kuipers, E.; Van Haastert, M.; Nicolaï, J.; Poen, A.; Van der Woude, C. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: A nationwide, long-term survey. J. Gastroenterol. 2012, 47, 1308–1322. [Google Scholar] [CrossRef] [Green Version]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef] [PubMed]
- Seppala, T.T.; Latchford, A.; Negoi, I.; Sampaio Soares, A.; Jimenez-Rodriguez, R.; Sanchez-Guillen, L.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; et al. European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender. Br. J. Surg. 2021, 108, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Balaguer, F.; Dekker, E.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Ricciardiello, L.; Rupinska, M.; et al. Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 1082–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Dekker, E.; Jover, R.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Pellise, M.; Saurin, J.C.; et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 877–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A.; Zandanell, S.; Kiesslich, T.; Neureiter, D.; Klieser, E.; Holzinger, J.; Berr, F. Systematic Review on Optical Diagnosis of Early Gastrointestinal Neoplasia. J. Clin. Med. 2021, 10, 2794. [Google Scholar] [CrossRef]
- Wijnands, A.M.; Mahmoud, R.; Lutgens, M.; Oldenburg, B. Surveillance and management of colorectal dysplasia and cancer in inflammatory bowel disease: Current practice and future perspectives. Eur. J. Intern. Med. 2021, 93, 35–41. [Google Scholar] [CrossRef]
- Yang, J.; Gurudu, S.R.; Koptiuch, C.; Agrawal, D.; Buxbaum, J.L.; Abbas Fehmi, S.M.; Fishman, D.S.; Khashab, M.A.; Jamil, L.H.; Jue, T.L.; et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest. Endosc. 2020, 91, 963–982. [Google Scholar] [CrossRef]
- Nimptsch, K.; Wu, K. Is timing important? The role of diet and lifestyle during early life on colorectal neoplasia. Curr. Colorectal Cancer Rep. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Wolff, E.M.; Byun, H.M.; Han, H.F.; Sharma, S.; Nichols, P.W.; Siegmund, K.D.; Yang, A.S.; Jones, P.A.; Liang, G. Hypomethylation of a LINE-1 promoter activates an alternate transcript of the MET oncogene in bladders with cancer. PLoS Genet. 2010, 6, e1000917. [Google Scholar] [CrossRef]
- Goelz, S.E.; Vogelstein, B.; Hamilton, S.R.; Feinberg, A.P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science 1985, 228, 187–190. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas, S.; Lafuente, M.J.; Crescenti, A.; Trias, M.; Ballesta, A.; Molina, R.; Zheng, S.; Wiencke, J.K.; Lafuente, A. Lower specific micronutrient intake in colorectal cancer patients with tumors presenting promoter hypermethylation in p16INK4a, p14ARF and hMLH1. Anticancer Res. 2007, 27, 1151–1156. [Google Scholar] [PubMed]
- Van Engeland, M.; Weijenberg, M.P.; Roemen, G.M.; Brink, M.; de Bruïne, A.P.; Goldbohm, R.A.; van den Brandt, P.A.; Baylin, S.B.; de Goeij, A.F.; Herman, J.G. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: The Netherlands cohort study on diet and cancer. Cancer Res. 2003, 63, 3133–3137. [Google Scholar] [PubMed]
- Schernhammer, E.S.; Giovannucci, E.; Kawasaki, T.; Rosner, B.; Fuchs, C.S.; Ogino, S. Dietary folate, alcohol and B vitamins in relation to LINE-1 hypomethylation in colon cancer. Gut 2010, 59, 794–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogna, P.; O’Sullivan, D.E.; King, W.D. The effect of inflammation-related lifestyle exposures and interactions with gene variants on long interspersed nuclear element-1 DNA methylation. Epigenomics 2018, 10, 785–796. [Google Scholar] [CrossRef]
- Martin, E.M.; Fry, R.C. Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations. Annu. Rev. Public Health 2018, 39, 309–333. [Google Scholar] [CrossRef] [Green Version]
- Antelo, M.; Balaguer, F.; Shia, J.; Shen, Y.; Hur, K.; Moreira, L.; Cuatrecasas, M.; Bujanda, L.; Giraldez, M.D.; Takahashi, M.; et al. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS ONE 2012, 7, e45357. [Google Scholar] [CrossRef]
- Akimoto, N.; Zhao, M.; Ugai, T.; Zhong, R.; Lau, M.C.; Fujiyoshi, K.; Kishikawa, J.; Haruki, K.; Arima, K.; Twombly, T.S.; et al. Tumor Long Interspersed Nucleotide Element-1 (LINE-1) Hypomethylation in Relation to Age of Colorectal Cancer Diagnosis and Prognosis. Cancers 2021, 13, 2016. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [Green Version]
- Daly, K.; Shirazi-Beechey, S.P. Microarray analysis of butyrate regulated genes in colonic epithelial cells. DNA Cell Biol. 2006, 25, 49–62. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, S2485–S2493. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dong, T.S.; Dalal, S.R.; Wu, F.; Bissonnette, M.; Kwon, J.H.; Chang, E.B. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS ONE 2011, 6, e16221. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Orang, A.; Marri, S.; McKinnon, R.A.; Meech, R.; Michael, M.Z. Integrative Transcriptomic Network Analysis of Butyrate Treated Colorectal Cancer Cells. Cancers 2021, 13, 636. [Google Scholar] [CrossRef]
- Humphreys, K.J.; Cobiac, L.; Le Leu, R.K.; Van der Hoek, M.B.; Michael, M.Z. Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol. Carcinog. 2013, 52, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, R.; Bagchi, A. Prediction of the differentially expressed circRNAs to decipher their roles in the onset of human colorectal cancers. Gene 2020, 762, 145035. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Lan, Z.; Xiong, X.; Ao, H.; Feng, Y.; Gu, H.; Yu, M.; Cui, Q. The Dual Role of MicroRNAs in Colorectal Cancer Progression. Int. J. Mol. Sci. 2018, 19, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafaee, F.; Diakos, C.; Kirschner, M.B.; Reid, G.; Michael, M.Z.; Horvath, L.G.; Alinejad-Rokny, H.; Cheng, Z.J.; Kuncic, Z.; Clarke, S. A data-driven, knowledge-based approach to biomarker discovery: Application to circulating microRNA markers of colorectal cancer prognosis. NPJ Syst. Biol. Appl. 2018, 4, 20. [Google Scholar] [CrossRef]
- Xiong, G.; Zhang, J.; Zhang, Y.; Pang, X.; Wang, B.; Zhang, Y. Circular RNA_0074027 participates in cell proliferation, apoptosis and metastasis of colorectal cancer cells through regulation of miR5253p. Mol. Med. Rep. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Angius, A.; Pira, G.; Scanu, A.M.; Uva, P.; Sotgiu, G.; Saderi, L.; Manca, A.; Serra, C.; Uleri, E.; Piu, C.; et al. MicroRNA-425-5p Expression Affects BRAF/RAS/MAPK Pathways in Colorectal Cancers. Int. J. Med. Sci. 2019, 16, 1480–1491. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Cao, T.; Xie, H.; Li, T.; Sun, L.; Liu, H.; Guo, H.; Wang, X.; Liu, Q.; Kim, T.; et al. KRAS Mutation-Responsive miR-139-5p inhibits Colorectal Cancer Progression and is repressed by Wnt Signaling. Theranostics 2020, 10, 7335–7350. [Google Scholar] [CrossRef]
- Senaldi, L.; Smith-Raska, M. Evidence for germline non-genetic inheritance of human phenotypes and diseases. Clin. Epigenet. 2020, 12, 136. [Google Scholar] [CrossRef]
- Legoff, L.; D’Cruz, S.C.; Tevosian, S.; Primig, M.; Smagulova, F. Transgenerational Inheritance of Environmentally Induced Epigenetic Alterations during Mammalian Development. Cells 2019, 8, 1559. [Google Scholar] [CrossRef] [Green Version]
- Skinner, M.K.; Nilsson, E.E. Role of environmentally induced epigenetic transgenerational inheritance in evolutionary biology: Unified Evolution Theory. Environ. Epigenet. 2021, 7, dvab012. [Google Scholar] [CrossRef] [PubMed]
- Bollati, V.; Baccarelli, A. Environmental epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, L.E.; Kee, F.; Collins, B.J.; Patterson, C.C.; Houlston, R.S. Colorectal cancer mortality in first-degree relatives of early-onset colorectal cancer cases. Dis. Colon. Rectum. 2002, 45, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.; de la Chapelle, A.; Hampel, H. Mutation Frequencies in Patients with Early-Onset Colorectal Cancer-Reply. JAMA Oncol. 2017, 3, 1587. [Google Scholar] [CrossRef] [PubMed]
- Stanich, P.P.; Pelstring, K.R.; Hampel, H.; Pearlman, R. A High Percentage of Early-age Onset Colorectal Cancer Is Potentially Preventable. Gastroenterology 2021, 160, 1850–1852. [Google Scholar] [CrossRef]
- Strum, W.B.; Boland, C.R. Clinical and Genetic Characteristics of Colorectal Cancer in Persons under 50 Years of Age: A Review. Dig. Dis. Sci. 2019, 64, 3059–3065. [Google Scholar] [CrossRef]
- Zhang, Q.; Berger, F.G.; Love, B.; Banister, C.E.; Murphy, E.A.; Hofseth, L.J. Maternal stress and early-onset colorectal cancer. Med. Hypotheses 2018, 121, 152–159. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef] [Green Version]
- Hughes, L.A.; van den Brandt, P.A.; Goldbohm, R.A.; de Goeij, A.F.; de Bruïne, A.P.; van Engeland, M.; Weijenberg, M.P. Childhood and adolescent energy restriction and subsequent colorectal cancer risk: Results from the Netherlands Cohort Study. Int. J. Epidemiol. 2010, 39, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Keinan-Boker, L.; Vin-Raviv, N.; Liphshitz, I.; Linn, S.; Barchana, M. Cancer incidence in Israeli Jewish survivors of World War II. J. Natl. Cancer Inst. 2009, 101, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.C.; Singal, A.G.; Baron, J.A.; Sandler, R.S. Decrease in Incidence of Young-Onset Colorectal Cancer Before Recent Increase. Gastroenterology 2018, 155, 1716–1719. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.H.; Patel, N.; Du, M.; Liang, P.S. Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database. Clin. Gastroenterol. Hepatol. 2021, S1542-3565(21)00817-X. [Google Scholar] [CrossRef]
- Murphy, C.C.; Wallace, K.; Sandler, R.S.; Baron, J.A. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2019, 156, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, S.G. Young-onset rectal cancer patients: In need of answers. Future Oncol. 2019, 15, 1053–1055. [Google Scholar] [CrossRef] [Green Version]
- Boursi, B.; Haynes, K.; Mamtani, R.; Yang, Y.X. Impact of antibiotic exposure on the risk of colorectal cancer. Pharm. Drug Saf. 2015, 24, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Dik, V.K.; van Oijen, M.G.; Smeets, H.M.; Siersema, P.D. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Dig. Dis. Sci. 2016, 61, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, Y.; Gao, K.; Peng, Y.; Mu, C.L.; Zhu, W.Y. Antibiotic-induced alterations of the gut microbiota and microbial fermentation in protein parallel the changes in host nitrogen metabolism of growing pigs. Animal 2019, 13, 262–272. [Google Scholar] [CrossRef]
- Gerber, J.S.; Prasad, P.A.; Localio, A.R.; Fiks, A.G.; Grundmeier, R.W.; Bell, L.M.; Wasserman, R.C.; Rubin, D.M.; Keren, R.; Zaoutis, T.E. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics 2013, 131, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, M.K.; Johnson, T.J.; Chamberlain, J.M.; Casper, T.C.; Simmons, T.; Alessandrini, E.A.; Bajaj, L.; Grundmeier, R.W.; Gerber, J.S.; Lorch, S.A.; et al. Racial and Ethnic Differences in Antibiotic Use for Viral Illness in Emergency Departments. Pediatrics 2017, 140, e20170203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Du, G.; Guo, J.; Zhang, Y. Bugs, drugs, and cancer: Can the microbiome be a potential therapeutic target for cancer management? Drug Discov. Today 2019, 24, 1000–1009. [Google Scholar] [CrossRef]
- Scott, A.J.; Alexander, J.L.; Merrifield, C.A.; Cunningham, D.; Jobin, C.; Brown, R.; Alverdy, J.; O’Keefe, S.J.; Gaskins, H.R.; Teare, J.; et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019, 68, 1624–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Yang, T.; Owen, J.L.; Lightfoot, Y.L.; Kladde, M.P.; Mohamadzadeh, M. Microbiota impact on the epigenetic regulation of colorectal cancer. Trends Mol. Med. 2013, 19, 714–725. [Google Scholar] [CrossRef] [Green Version]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef] [Green Version]
- Flemer, B.; Lynch, D.B.; Brown, J.M.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kosumi, K.; Hamada, T.; Koh, H.; Borowsky, J.; Bullman, S.; Twombly, T.S.; Nevo, D.; Masugi, Y.; Liu, L.; da Silva, A.; et al. The Amount of Bifidobacterium Genus in Colorectal Carcinoma Tissue in Relation to Tumor Characteristics and Clinical Outcome. Am. J. Pathol. 2018, 188, 2839–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mima, K.; Cao, Y.; Chan, A.T.; Qian, Z.R.; Nowak, J.A.; Masugi, Y.; Shi, Y.; Song, M.; da Silva, A.; Gu, M.; et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin. Transl. Gastroenterol. 2016, 7, e200. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [Green Version]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K.; et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015, 1, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Hwang, I.; Park, Y.J.; Kim, Y.R.; Kim, Y.N.; Ka, S.; Lee, H.Y.; Seong, J.K.; Seok, Y.J.; Kim, J.B. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015, 29, 2397–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, A.; Farver, M.; Smilowitz, J.T. The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants. Nutr. Metab. Insights 2015, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.S.; Das, M.; Jeffery, I.B.; O’Toole, P.W. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. eLife 2020, 9, e50240. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Wang, H.; Geng, X.; Shokralla, S.; Bakhshi, E.; Chaldekas, K.; Harris, B.T.; Clagett, D.; Marshall, J. A comparison study of the intratumoral microbiome in younger verses older-onset colorectal cancer (COSMO CRC). J. Clin. Oncol. 2020, 38, 241. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J. Clin. Oncol. 2020, 38, 1000. [Google Scholar] [CrossRef]
- Ramasamy, S.; Singh, S.; Taniere, P.; Langman, M.J.; Eggo, M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G288–G296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.H.; Ma, W.; Wang, D.D.; Cao, Y.; Mallick, H.; Gerbaba, T.K.; Lloyd-Price, J.; Abu-Ali, G.; Hall, A.B.; Sikavi, D.; et al. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology 2020, 158, 1313–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.H.; Cao, Y.; Hur, J.; Mehta, R.S.; Sikavi, D.R.; Wang, Y.; Ma, W.; Wu, K.; Song, M.; Giovannucci, E.L.; et al. The Sulfur Microbial Diet Is Associated with Increased Risk of Early-Onset Colorectal Cancer Precursors. Gastroenterology 2021, 161, 1423–1432. [Google Scholar] [CrossRef]
- Hogan, N.M.; Hanley, M.; Hogan, A.M.; Sheehan, M.; McAnena, O.J.; Regan, M.P.; Kerin, M.J.; Joyce, M.R. Awareness and uptake of family screening in patients diagnosed with colorectal cancer at a young age. Gastroenterol. Res. Pr. 2015, 2015, 194931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manne, S.; Markowitz, A.; Winawer, S.; Meropol, N.J.; Haller, D.; Rakowski, W.; Babb, J.; Jandorf, L. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psychol. 2002, 21, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Xirasagar, S.; Li, Y.J.; de Groen, P.C. Colonoscopy screening among US adults aged 40 or older with a family history of colorectal cancer. Prev. Chronic. Dis. 2015, 12, e80. [Google Scholar] [CrossRef] [Green Version]
- Boland, C.R.; Goel, A.; Patel, S.G. The genetic and epigenetic landscape of early-onset colorectal cancer. Colorectal Cancer 2020, 9, CRC23. [Google Scholar] [CrossRef]
- Zhunussova, G.; Afonin, G.; Abdikerim, S.; Jumanov, A.; Perfilyeva, A.; Kaidarova, D.; Djansugurova, L. Mutation Spectrum of Cancer-Associated Genes in Patients with Early Onset of Colorectal Cancer. Front. Oncol. 2019, 9, 673. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.W. GH Hardy (1908) and Hardy-Weinberg equilibrium. Genetics 2008, 179, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Coletta, A.M.; Peterson, S.K.; Gatus, L.A.; Krause, K.J.; Schembre, S.M.; Gilchrist, S.C.; Pande, M.; Vilar, E.; You, Y.N.; Rodriguez-Bigas, M.A.; et al. Energy balance related lifestyle factors and risk of endometrial and colorectal cancer among individuals with lynch syndrome: A systematic review. Fam Cancer 2019, 18, 399–420. [Google Scholar] [CrossRef]
- Miguchi, M.; Hinoi, T.; Tanakaya, K.; Yamaguchi, T.; Furukawa, Y.; Yoshida, T.; Tamura, K.; Sugano, K.; Ishioka, C.; Matsubara, N.; et al. Alcohol consumption and early-onset risk of colorectal cancer in Japanese patients with Lynch syndrome: A cross-sectional study conducted by the Japanese Society for Cancer of the Colon and Rectum. Surg. Today 2018, 48, 810–814. [Google Scholar] [CrossRef]
- Van Duijnhoven, F.J.; Botma, A.; Winkels, R.; Nagengast, F.M.; Vasen, H.F.; Kampman, E. Do lifestyle factors influence colorectal cancer risk in Lynch syndrome? Fam Cancer 2013, 12, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Botma, A.; Vasen, H.F.; van Duijnhoven, F.J.; Kleibeuker, J.H.; Nagengast, F.M.; Kampman, E. Dietary patterns and colorectal adenomas in Lynch syndrome: The GEOLynch cohort study. Cancer 2013, 119, 512–521. [Google Scholar] [CrossRef]
- Brouwer, J.G.; Makama, M.; van Woudenbergh, G.J.; Vasen, H.F.; Nagengast, F.M.; Kleibeuker, J.H.; Kampman, E.; van Duijnhoven, F.J. Inflammatory potential of the diet and colorectal tumor risk in persons with Lynch syndrome. Am. J. Clin. Nutr. 2017, 106, 1287–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eijkelboom, A.H.; Brouwer, J.G.M.; Vasen, H.F.A.; Bisseling, T.M.; Koornstra, J.J.; Kampman, E.; van Duijnhoven, F.J.B. Diet quality and colorectal tumor risk in persons with Lynch syndrome. Cancer Epidemiol. 2020, 69, 101809. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Gareth Evans, D.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.L.; Keita, A.V.; Duncan, S.H.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn’s disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.L.; Rushworth, S.L.; Richman, E.; Rhodes, J.M. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J. Crohn’s Colitis 2013, 7, 338–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm. Bowel. Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Chassaing, B. First victim, later aggressor: How the intestinal microbiota drives the pro-inflammatory effects of dietary emulsifiers? Gut Microbes 2018, 9, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Viennois, E.; Merlin, D.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifier-Induced Low-Grade Inflammation Promotes Colon Carcinogenesis. Cancer Res. 2017, 77, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Viennois, E.; Chassaing, B. Consumption of Select Dietary Emulsifiers Exacerbates the Development of Spontaneous Intestinal Adenoma. Int. J. Mol. Sci. 2021, 22, 2602. [Google Scholar] [CrossRef]
- Cougnoux, A.; Dalmasso, G.; Martinez, R.; Buc, E.; Delmas, J.; Gibold, L.; Sauvanet, P.; Darcha, C.; Dechelotte, P.; Bonnet, M.; et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 2014, 63, 1932–1942. [Google Scholar] [CrossRef]
- Sears, C.L.; Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Dziubanska-Kusibab, P.J.; Berger, H.; Battistini, F.; Bouwman, B.A.M.; Iftekhar, A.; Katainen, R.; Cajuso, T.; Crosetto, N.; Orozco, M.; Aaltonen, L.A.; et al. Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nat. Med. 2020, 26, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Secher, T.; Boury, M.; Brehin, C.; Menard, S.; Salvador-Cartier, C.; Cuevas-Ramos, G.; Watrin, C.; Marcq, I.; Nougayrede, J.P.; et al. Maternally acquired genotoxic Escherichia coli alters offspring’s intestinal homeostasis. Gut Microbes 2014, 5, 313–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrarese, R.; Zuppardo, R.A.; Puzzono, M.; Mannucci, A.; Amato, V.; Ditonno, I.; Patricelli, M.G.; Raucci, A.R.; Clementi, M.; Elmore, U.; et al. Oral and Fecal Microbiota in Lynch Syndrome. J. Clin. Med. 2020, 9, 2735. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Drew, D.A.; Markowitz, A.; Lloyd-Price, J.; Abu-Ali, G.; Nguyen, L.H.; Tran, C.; Chung, D.C.; Gilpin, K.K.; Meixell, D.; et al. Structure of the Mucosal and Stool Microbiome in Lynch Syndrome. Cell Host Microbe 2020, 27, 585–600. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puzzono, M.; Mannucci, A.; Grannò, S.; Zuppardo, R.A.; Galli, A.; Danese, S.; Cavestro, G.M. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 5933. https://doi.org/10.3390/cancers13235933
Puzzono M, Mannucci A, Grannò S, Zuppardo RA, Galli A, Danese S, Cavestro GM. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers. 2021; 13(23):5933. https://doi.org/10.3390/cancers13235933
Chicago/Turabian StylePuzzono, Marta, Alessandro Mannucci, Simone Grannò, Raffaella Alessia Zuppardo, Andrea Galli, Silvio Danese, and Giulia Martina Cavestro. 2021. "The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review" Cancers 13, no. 23: 5933. https://doi.org/10.3390/cancers13235933
APA StylePuzzono, M., Mannucci, A., Grannò, S., Zuppardo, R. A., Galli, A., Danese, S., & Cavestro, G. M. (2021). The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers, 13(23), 5933. https://doi.org/10.3390/cancers13235933







