Relationship between Treatment Plan Dosimetry, Toxicity, and Survival following Intensity-Modulated Radiotherapy, with or without Chemotherapy, for Stage III Inoperable Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, R.; Schramel, F.; van Dartel, M.; Hilarius, D.; de Boer, A.; Wouters, M.; Smit, H. The Dutch Lung Cancer Audit: Nationwide quality of care evaluation of lung cancer patients. Lung Cancer 2020, 149, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Nederlandse Kankerregistratie. Available online: www.cijfersoverkanker.nl (accessed on 6 April 2021).
- Popat, S.; Navani, N.; Kerr, K.M.; Smit, E.F.; Batchelor, T.J.P.; Paul, V.S.P.; McDonald, F. Navigating Diagnostic and Treatment Decisions in Non-Small Cell Lung Cancer: Expert Commentary on the Multidisciplinary Team Approach. Oncologist 2021, 26, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Vansteenkiste, J.; Lee, K.H.; Pentheroudakis, G.; Zhou, C.; Prabhash, K.; Seto, T.; Voon, P.; Tan, D.; Yang, J.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann. Oncol. 2020, 31, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickhoff, C.; Dahele, M.; De Langen, A.J.; Paul, M.A.; Smit, E.F.; Senan, S.; Hartemink, K.J.; Damhuis, R.A. Population-Based Patterns of Surgical Care for Stage IIIA NSCLC in the Netherlands between 2010 and 2013. J. Thorac. Oncol. 2016, 11, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Dickhoff, C.; Dahele, M.; Smit, E.; Paul, M.; Senan, S.; Hartemink, K.; Damhuis, R. Patterns of care and outcomes for stage IIIB non-small cell lung cancer in the TNM-7 era: Results from the Netherlands Cancer Registry. Lung Cancer 2017, 110, 14–18. [Google Scholar] [CrossRef]
- Ronden, M.I.; Bahce, I.; Hashemi, S.M.; Dickhoff, C.; de Haan, P.F.; Becker, A.; Spoelstra, F.O.; Dahele, M.R.; Ali, R.; Tiemessen, M.A.; et al. Factors influencing multi-disciplinary tumor board recommendations in stage III non-small cell lung cancer. Lung Cancer 2020, 152, 149–156. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC—an Update From the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Adizie, J.; Khakwani, A.; Beckett, P.; Navani, N.; West, D.; Woolhouse, I.; Harden, S.V. Stage III Non-small Cell Lung Cancer Management in England. Clin. Oncol. 2019, 31, 688–696. [Google Scholar] [CrossRef]
- Miller, E.D.; Fisher, J.L.; E Haglund, K.; Grecula, J.C.; Xu-Welliver, M.; Bertino, E.M.; He, K.; Shields, P.G.; Carbone, D.P.; Williams, T.M.; et al. Identifying patterns of care for elderly patients with non-surgically treated stage III non-small cell lung cancer: An analysis of the national cancer database. Radiat. Oncol. 2018, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Or, M.; Liu, B.; Lam, J.; Vinod, S.; Xuan, W.; Yeghiaian-Alvandi, R.; Hau, E. A systematic review and meta-analysis of treatment-related toxicities of curative and palliative radiation therapy in non-small cell lung cancer. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Verbakel, W.F.A.R.; van Reij, E.; Ladenius-Lischer, I.; Cuijpers, J.; Slotman, B.J.; Senan, S. Clinical application of a novel hybrid intensity-modulated radiotherapy technique for stage III lung cancer and dosimetric comparison with four other techniques. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Blom, G.J.; Verbakel, W.F.A.R.; Dahele, M.; Hoffmans, D.; Slotman, B.J.; Senan, S. Improving radiotherapy planning for large volume lung cancer: A dosimetric comparison between hybrid-IMRT and RapidArc. Acta Oncol. 2014, 54, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Z.X.; Komaki, R.R.; Thames, H.D., Jr.; Liu, H.H.; Tucker, S.L.; Mohan, R.; Martel, M.K.; Wei, X.; Yang, K.; Kim, E.S.; et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small cell lung cancer receiving concomitant chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Koshy, M.; Malik, R.; Spiotto, M.; Mahmood, U.; Rusthoven, C.G.; Sher, D.J. Association between intensity modulated radiotherapy and survival in patients with stage III non-small cell lung cancer treated with chemoradiotherapy. Lung Cancer 2017, 108, 222–227. [Google Scholar] [CrossRef]
- Jegadeesh, N.; Liu, Y.; Gillespie, T.; Fernandes, F.; Ramalingam, S.; Mikell, J.; Lipscomb, J.; Curran, W.J.; Higgins, K.A. Evaluating intensity/modulate radiation therapy in locally advanced non-small-cell lung cancer: Results from the national cancer data base. Clin. Lung Cancer 2016, 17, 398–405. [Google Scholar] [CrossRef]
- Khalil, A.A.; Hoffmann, L.; Moeller, D.S.; Farr, K.P.; Knap, M.M. New dose constraint reduces radiation-induced fatal pneumonitis in locally advanced non-small cell lung cancer patients treated with intensity-modulated radiotherapy. Acta Oncol. 2015, 54, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Verbakel, W.F.A.R.; Doornaert, P.A.H.; Raaijmakers, C.P.J.; Bos, L.J.; Essers, M.; Kamer van de, B.J.; Kaanders, J.H. Targeted intervention to improve the quality of head and neck radiation therapy treatment planning in the Netherlands: Short and long-term impact. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 514–524. [Google Scholar] [CrossRef]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Martinez, A.M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schildc, S.E.; Bradley, J.D. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef]
- Brade, A.M.; Wenz, F.; Koppe, F.; Livens, Y.; San Antonio, B.; Iscoe, N.A.; Senan, S. Radiation therapy quality assurance (RTQA) of concurrent chemoradiation therapy for locally advanced non-small cell lung cancer in the PROCLAIM phase 3 trail. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 927–934. [Google Scholar] [CrossRef]
- Evers, J.; de Jaeger, K.; Hendriks, L.E.L.; van der Sangen, M.; Terhaard, C.; Siesling, S.; Aarts, M.J. Trends and variations in treatment of stage I-III non-small cell lung cancer from 2008 to 2018: A nationwide population-based study from the NetherlandsLung. Cancer 2021, 155, 103–113. [Google Scholar]
- Vansteenkiste, J.; De Ruysscher, D.; Eberhardt, W.E.E.; Lim, E.; Senan, S.; Felip, E.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi89–vi98. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chanski, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Yokoi, K. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Nestle, U.; Ruysscher de, D.; Ricardi, U.; Geets, X.; Belderbos, J.; Pootgen, C.; Van Houtte, P. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. J. Rad. Onc. 2018, 127, 1–5. [Google Scholar] [CrossRef]
- Palma, D.A.; Verbakel, W.F.; Otto, K.; Senan, S. New developments in arc radiation therapy: A review. Cancer Treat. Rev. 2010, 36, 393–399. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, N.; Roque, I.; Figuls, M.; Bernado, N.F.; Macbeth, F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst. Rev. 2010, 6, CD002140. [Google Scholar] [CrossRef] [PubMed]
- van Reij, A.J.F.; Dahele, M.; van de Ven, P.M.; de Haan, P.F.; Verbakel, W.F.A.R.; Smit, F.E.; Slotman, B.J.; Senan, S. Cahnges in non-surgical management of stage III non-small cell lung cancer at a single institution between 2003 and 2010. Acta Oncol. 2014, 53, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Palma, D.A.; Senan, S.; Tsujino, K.; Barriger, R.B.; Rengan, R.; Moreno, J.D.B.; Hyun, K.T.; Ramella, S.; Marks, L.B.; de Petris, S.L.; et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, D.A.; Senan, S.; Oberije, C.; Belderbos, J.; de Rodrigues Dios, N.; Bradley, J.D.; Barriger, R.B.; Moreno-Jimenez, M.; Hyun Kim, T.; Ramella, S.; et al. Predicting esophagitis after chemoradiation therapy for non-small cell lung cancer: An individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 690–696. [Google Scholar] [CrossRef]
- van Diessen, J.N.; Kwint, M.; Sonke, J.J.; Walraven, I.; Stam, B.; de Langen, A.J.; Belderbos, J.S. Safety and efficacy of reduced dose and marginst to involved lymph node metastasis in locally advances NSCLC patients. Radiother. Oncol. 2020, 143, 66–72. [Google Scholar] [CrossRef]
- Speirs, C.K.; DeWees, T.A.; Rehman, S.; Moltievschi, A.; Velez, M.A.; Mullen, D.; Fergus, S.; Trovo, M.; Bradely, J.D.; Robinson, C.G. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J. Thorac. Oncol. 2016, 12, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lambam, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac radiation dose, cardiac disease and mortality in patients with lung cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef]
- Chang, J.Y.; Zang, X.; Wang, X.; Kang, Y.; Riley, B.; Bilton, S.; Cox, J.D. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I of stage II non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Pan, X.; Li, X.; Mohan, R.; Komaki, R.; Chang, J.Y. Intensity-Modulated Proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage III non-small-cell lung cancer: A virtual clinical study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 357–366. [Google Scholar] [PubMed] [Green Version]
- Nichols, R.C.; Huh, S.; Henderson, R.; Mendenhall, N.P.; Flampouri, S.; Li, Z.; Hoppe, B.S. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalation radiation therapy for unresectable stage III non-small-cell lung cancer: A dosimetric study. Clin. Lung Cancer 2011, 12, 252–255. [Google Scholar] [CrossRef] [PubMed]
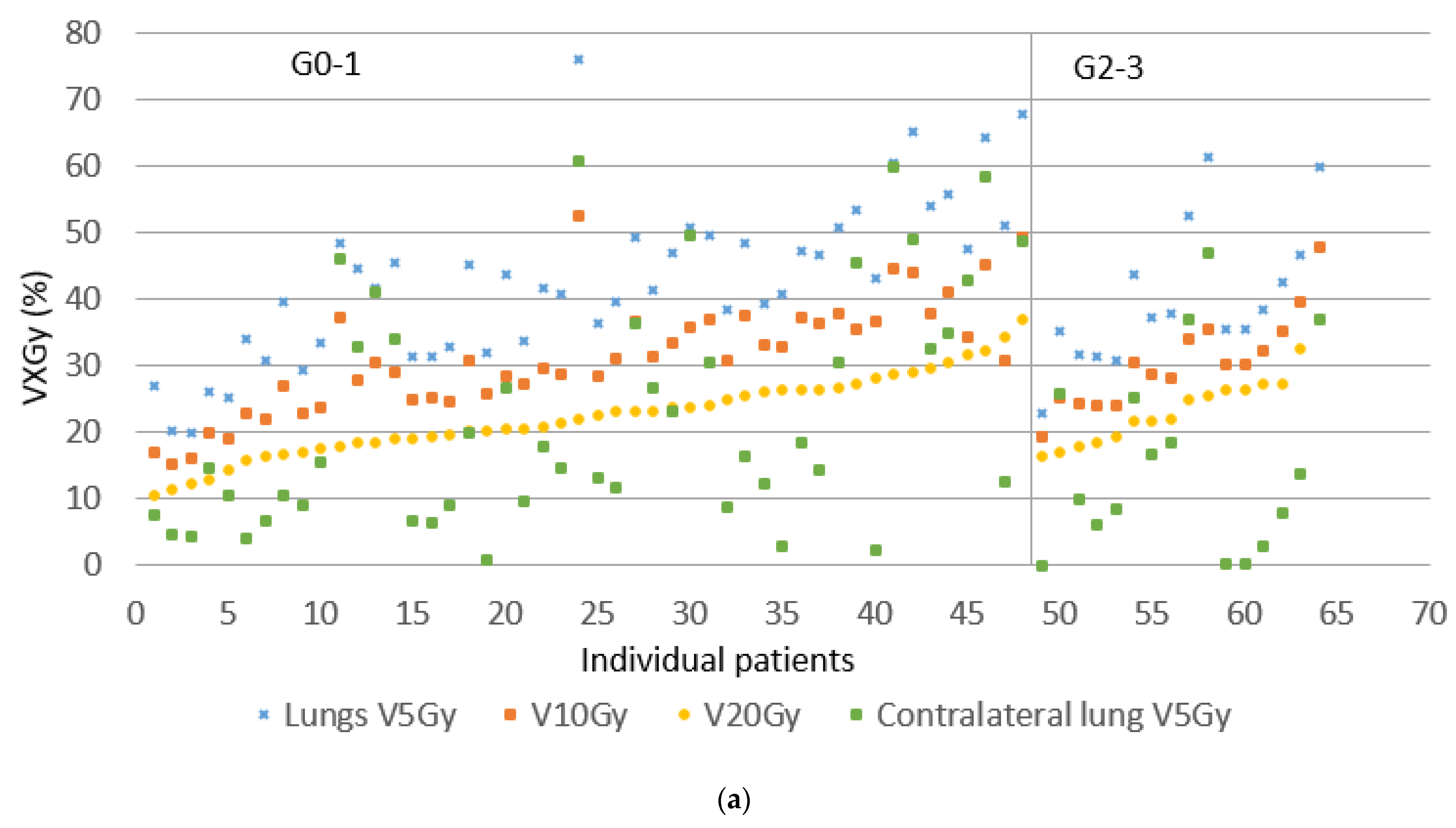
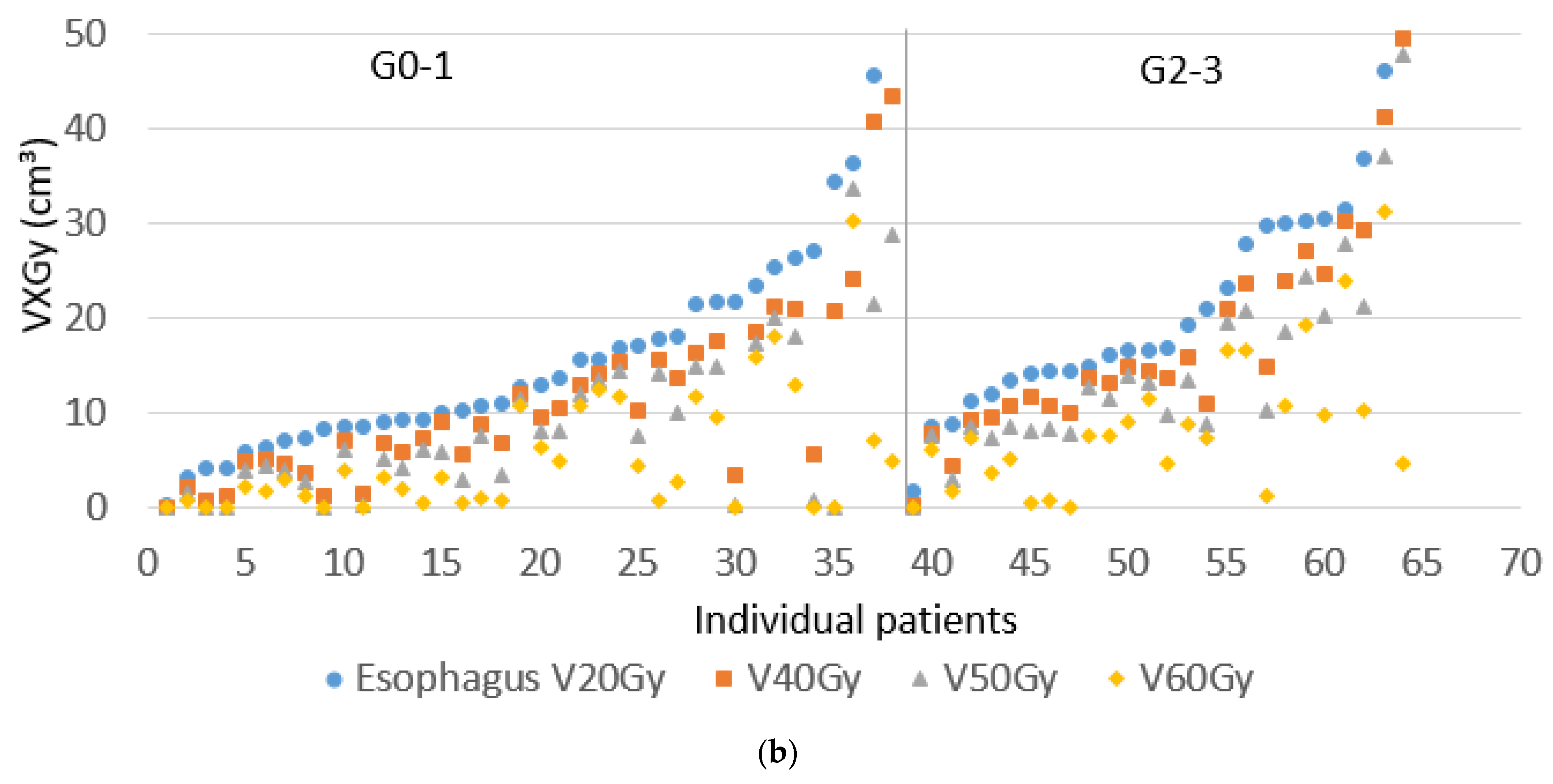

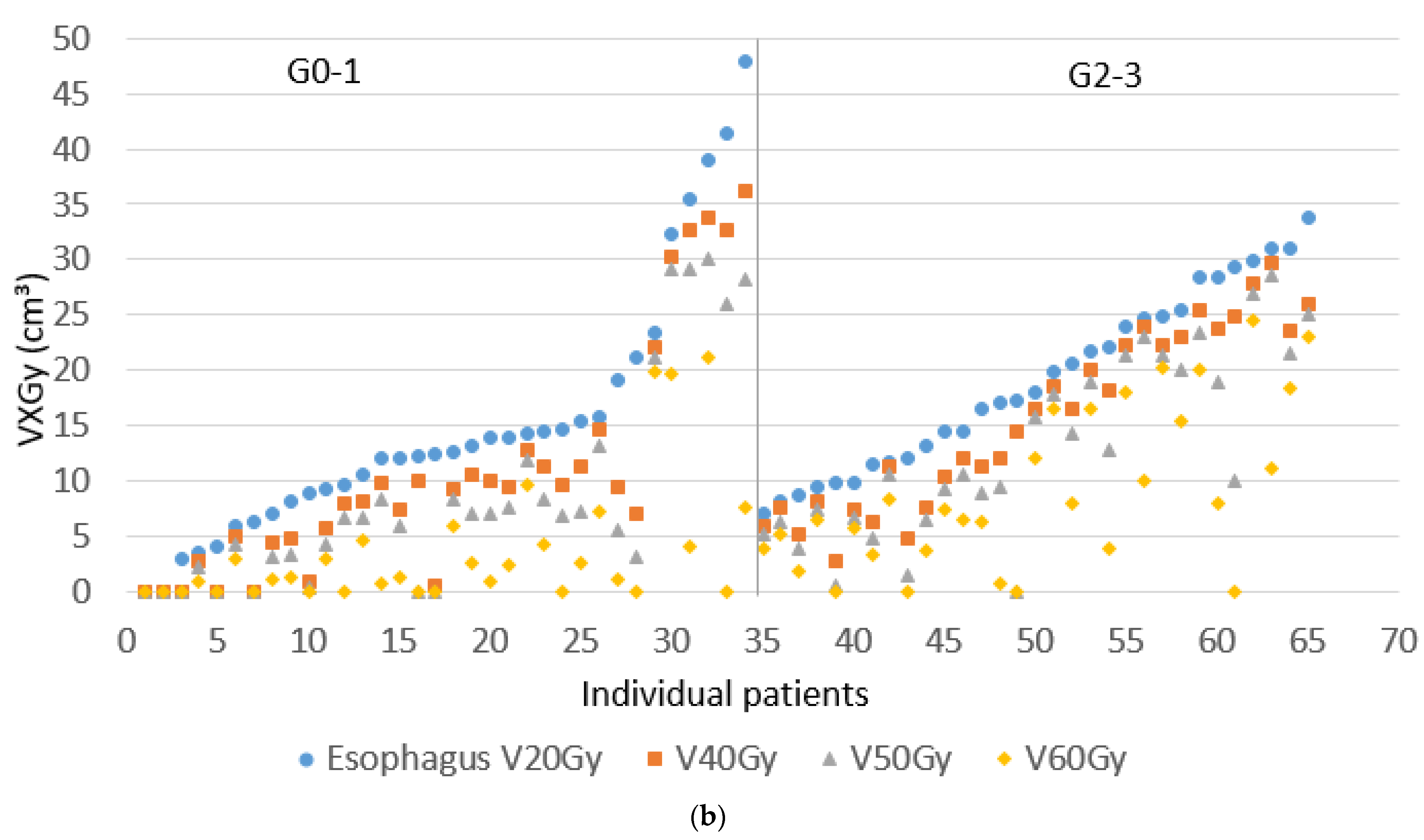
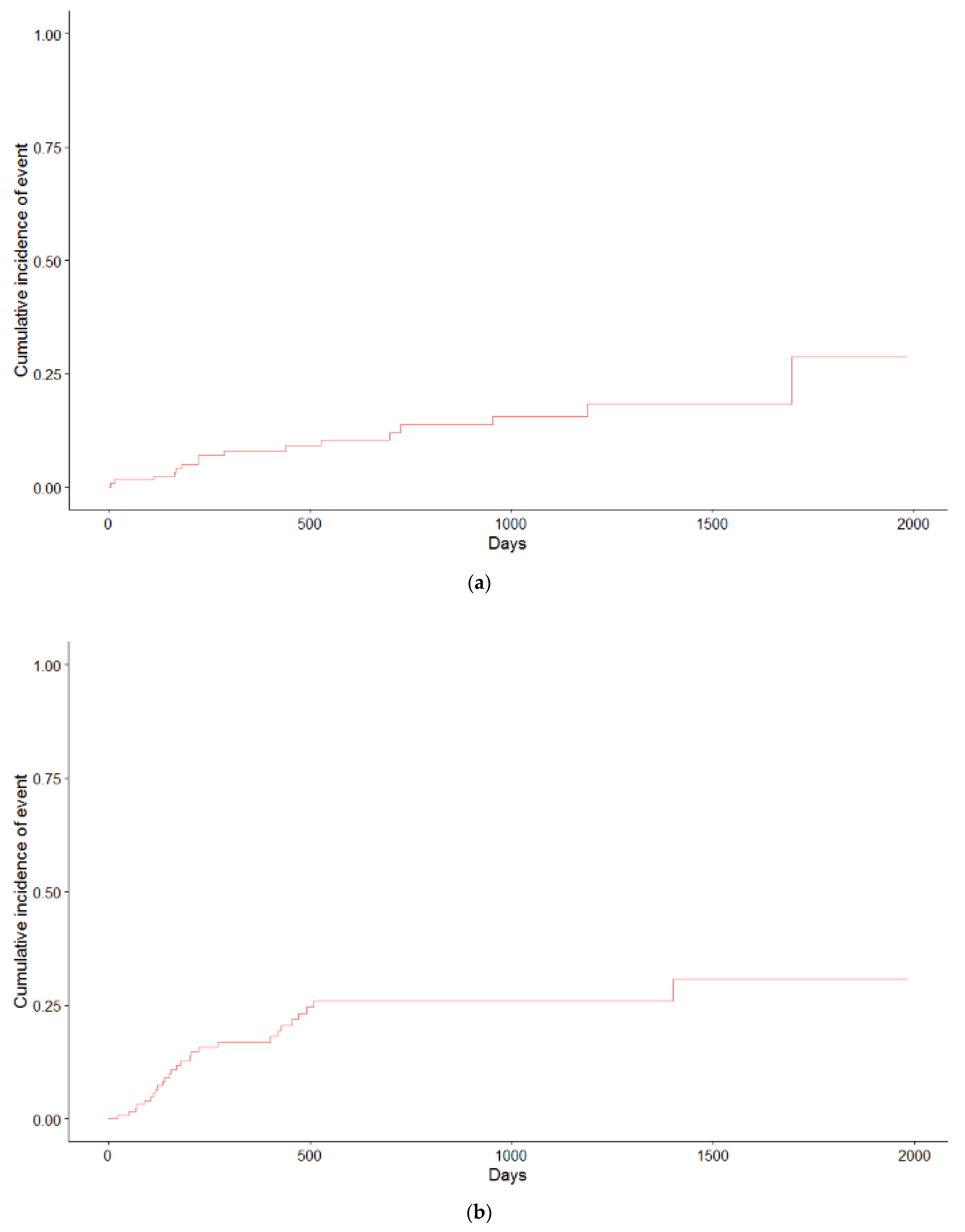
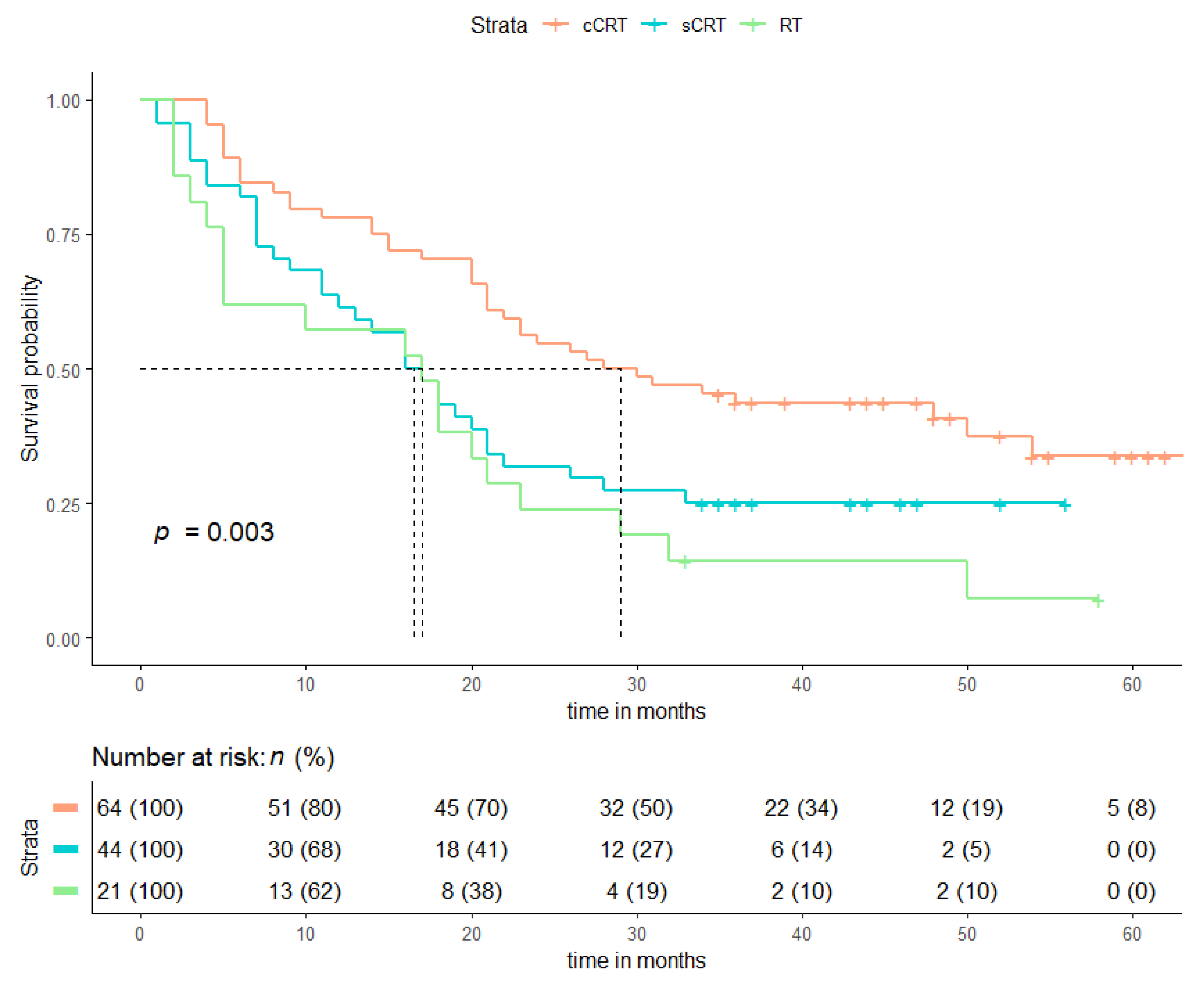
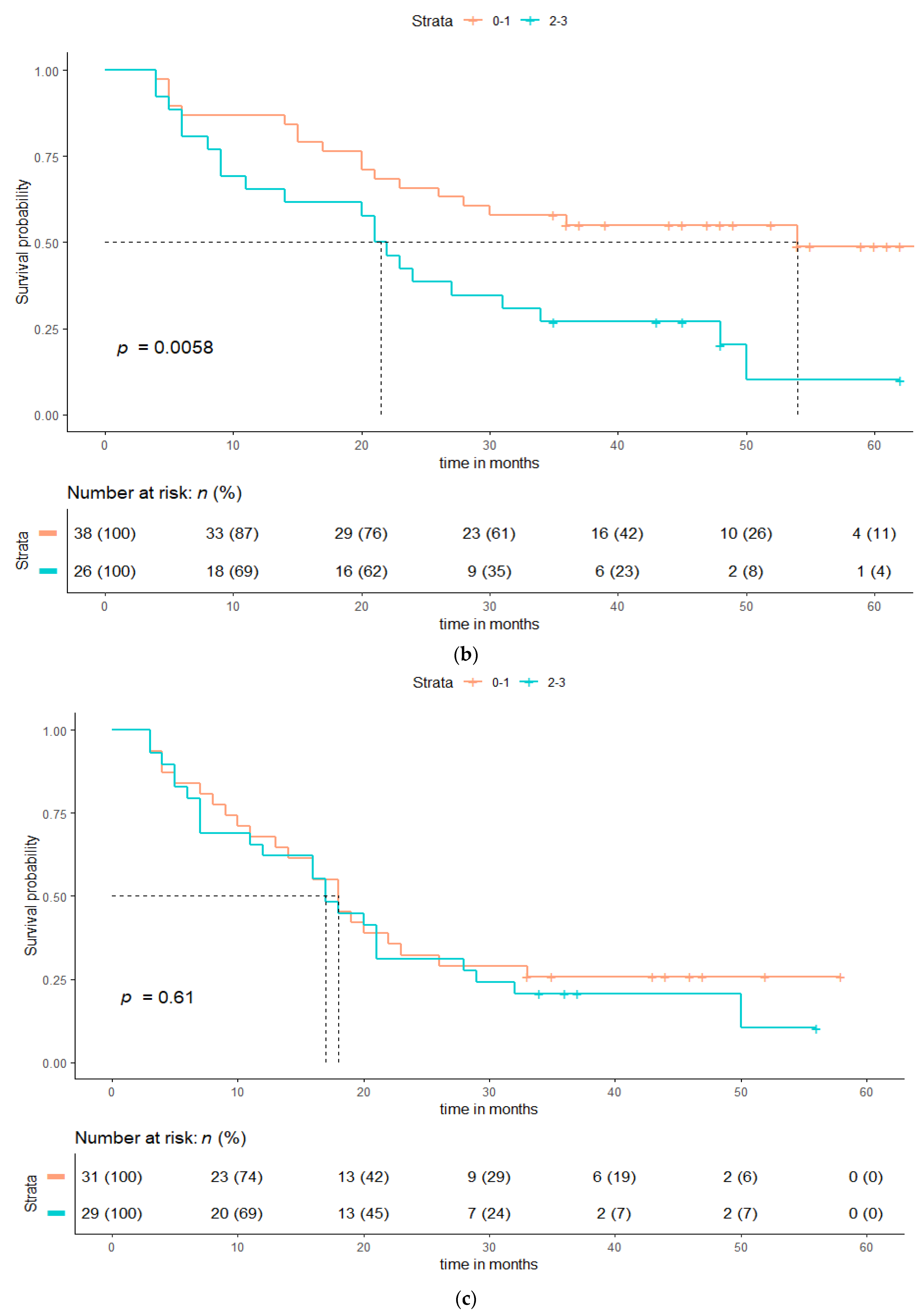
| Patient Characteristics | cCRT (n = 64), n (%) | sCRT + RT (n = 65), n (%) | p-Value |
|---|---|---|---|
| Age, median (IQR) | 67 (50–89) | 67 (33–90) | 0.60 |
| Male | 32 (50) | 36 (55) | 0.54 |
| Smoking history | 61 (95) | 64 (98) | 0.31 |
| WHO performance score | <0.01 | ||
| 0 | 38 (59) | 22 (34) | |
| 1 | 26 (41) | 31(48) | |
| 2 | - | 9 (14) | |
| 3 | - | 1 (2) | |
| 4 | - | - | |
| Missing | - | 2 (3) | |
| AJCC stage | 0.58 | ||
| IIIA | 30 (47) | 29 (45) | |
| IIIB | 27 (42) | 25 (38) | |
| IIIC | 7 (11) | 11 (17) | |
| T-stage | 0.08 | ||
| T1 | 16 (25) | 7 (11) | |
| T2 | 15 (23) | 15 (23) | |
| T3 | 13 (20) | 20 (31) | |
| T4 | 19 (30) | 19 (29) | |
| Tx | 1 (2) | 4 (6) | |
| N-stage | 0.85 | ||
| N0 | 5 (8) | 6 (9) | |
| N1 | 3 (5) | 6 () | |
| N2 | 39 (61) | 34 (52) | |
| N3 | 17 (27) | 19 (29) | |
| Prescribed total dose | <0.01 | ||
| 66 Gy (33 × 2 Gy) | 53 (83) | - | |
| 65 Gy (25 × 2.6 Gy) | - | 47 (72) | |
| 60 Gy (30 × 2 Gy) | 11 (17) | 5 (8) | |
| 60 Gy (20 × 3 Gy) | - | 3 (5) | |
| 59.8 Gy (23 × 2.6 Gy) | - | 8 (12) | |
| 57.5 Gy (23 × 2.5 Gy) | - | 1 (2) | |
| 55 Gy (20 × 2.75 Gy) | - | 1 (2) | |
| Radiotherapy technique | 0.36 | ||
| hIMRT | 2 (3) | 2 (3) | |
| hVMAT | 46 (72) | 42 (65) | |
| FullVMAT | 16 (25) | 21 (32) | |
| PTV cm³, median (range) | 607 (203–2053) | 577 (48–1659) | |
| Radiotherapy characteristics | Median (range) | ||
| PTV mean dose (Gy) | 66.6 (48.4–70.2) | 65.3 (10.6–68.7) | <0.01 |
| Mean lung dose (Gy) | 13.8 (6.5–22.0) | 13.5 (1.2–20.2) | 0.60 |
| Mean lung dose ipsilateral (Gy) | 30.6 (0.5–48.0) | 28.3 (4.2–47.9) | 0.27 |
| Mean lung dose contralateral (Gy) | 3.5 (0.4–13.8) | 3.7 (0.2–16.8) | 0.93 |
| V5 Gy lung (%) | 41 (10–76) | 41 (14–78) | 0.93 |
| V10 Gy lung (%) | 31 (15–52) | 31 (11–53) | 0.92 |
| V15 Gy lung (%) | 26 (12–42) | 26 (9–44) | 0.86 |
| V20 Gy lung (%) | 22 (11–37) | 22 (5–37) | 0.74 |
| V25 Gy lung (%) | 19 (9–34) | 20 (0–32) | 0.89 |
| V5 Gy contralateral lung (%) | 15 (0–61) | 17 (0- 70) | 0.73 |
| V20 Gy esophagus (cm3) | 15.5 (0–51.2) | 14.4 (0–48.0) | 0.79 |
| V40 Gy esophagus (cm3) | 11.1 (0–50.0) | 10.4 (0–36.1) | 0.87 |
| V50 Gy esophagus (cm3) | 8.4 ( 0–47.7) | 7.6 ( 0–30.0) | 0.84 |
| V60 Gy esophagus (cm3) | 4.6 (0–31.2) | 3.9 (0–21.2) | 0.70 |
| V65 Gy esophagus (cm3) | 0.6 (0–19.0) | 0.1 (0–13.5) | 0.07 |
| Mean heart dose (Gy) | 11.1 (0.8–42.2) | 12.9 (0.5–44.2) | 0.62 |
| V40 Gy heart (%) | 10 (0–58) | 12 (0–60) | 0.83 |
| Toxicity | cCRT (n = 64), n (%) | sCRT/RT (n = 65), n(%) | |
|---|---|---|---|
| Pneumonitis grade | p-value | ||
| 0–1 | 48 (75%) | 54 (83%) | 0.18 |
| 2 | 15 (23%) | 9 (14%) | |
| 3 | 0 (0%) | 2 (3%) | |
| 4 | 1 (2%) | 0 (0%) | |
| Esophagitis grade | |||
| 0–1 | 38 (59%) | 34 (52%) | 0.20 |
| 2 | 15 (23%) | 24 (37%) | |
| 3 | 11 (17%) | 7 (11%) | |
| 4 | 0 (0%) | 0 (0%) | |
| Cardiac event | |||
| No cardiac event | 59 (92%) | 54 (83%) | |
| Any cardiac event | 5 (8%) | 11 (17%) | 0.12 |
| cCRT | sCRT/RT | |||||
|---|---|---|---|---|---|---|
| Univariate Variables | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| WHO performance score ≥ 2 | - | - | - | 2.61 | 0.72–10.78 | 0.15 |
| PTV volume (cc) | 1.30 | 1.07–1.64 | <0.01 | 1.08 | 0.92–1.28 | 0.33 |
| Packyears smoking | 1.02 | 0.99–1.05 | 0.12 | 0.99 | 0.98–1.01 | 0.82 |
| Mean lung dose (cGy) | 1.20 | 1.02–1.44 | 0.03 | 1.16 | 1.04–1.30 | <0.01 |
| V20 Gy lung (%) | 2.05 | 0.86–5.27 | 0.12 | 2.09 | 1.16–4.00 | 0.02 |
| V5 Gy contralateral lung (%) | 1.66 | 1.20–2.40 | <0.01 | 1.36 | 1.02–1.87 | 0.05 |
| V20 Gy esophagus (cm3) | 1.01 | 1.00–1.02 | 0.07 | 1.01 | 0.99–1.02 | 0.11 |
| V40 Gy esophagus (cm3) | 1.01 | 1.00–1.02 | 0.02 | 1.01 | 1.00–1.03 | 0.04 |
| V50 Gy esophagus (cm3) | 1.02 | 1.00–1.03 | <0.01 | 1.02 | 1.00–1.03 | <0.01 |
| V60 Gy esophagus (cm3) | 1.02 | 1.00–1.03 | 0.06 | 1.03 | 1.01–1.04 | <0.01 |
| N-stage 2 | 0.93 | 0.20–5.10 | 0.93 | 1.78 | 0.47–7.72 | 0.38 |
| N-stage 3 | 1.88 | 0.34–11.66 | 0.47 | 2.75 | 0.63–13.51 | 0.19 |
| Radiotherapy technique full VMAT | 1.105 | 0.34–3.49 | 0.86 | 0.68 | 0.23–1.95 | 0.48 |
| Age | 0.97 | 0.92–1.03 | 0.34 | 0.98 | 0.94–1.03 | 0.51 |
| cCRT | sCRT/RT | |||||
|---|---|---|---|---|---|---|
| Univariate Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| V25 Gy heart (%) | 1.11 | 0.90–1.34 | 0.34 | 0.94 | 0.80–1.11 | 0.45 |
| V40 Gy heart (%) | 1.13 | 0.99–1.42 | 0.25 | 0.94 | 0.76–1.15 | 0.53 |
| V60 Gy heart (%) | 1.29 | 0.92–1.81 | 0.14 | 1.01 | 0.72–1.38 | 0.96 |
| Mean heart dose (cGy) | 1.00 | 0.91–1.11 | 0.27 | 0.99 | 0.96–1.00 | 0.54 |
| V20 Gy lungs (%) | 1.14 | 0.67–1.93 | 0.62 | 0.95 | 0.71–1.28 | 0.73 |
| V25 Gy lungs (%) | 1.11 | 0.63–1.95 | 0.72 | 0.97 | 0.70–1.33 | 0.83 |
| V5 Gy contralateral lung (%) | 1.21 | 1.01–1.45 | 0.04 | 0.91 | 0.78–1.06 | 0.24 |
| Mean lung dose (cGy) | 1.00 | 0.93–1.11 | 0.60 | 1.00 | 0.91–1.11 | 0.91 |
| V20 Gy esophagus (cm3) | 1.02 | 0.99–1.05 | 0.12 | 1.01 | 0.98–1.04 | 0.57 |
| V40 Gy esophagus (cm3) | 1.03 | 1.00–1.05 | 0.07 | 1.00 | 0.98–1.03 | 0.80 |
| V50 Gy esophagus (cm3) | 1.04 | 1.01–1.07 | 0.02 | 0.99 | 0.97–1.03 | 0.92 |
| V60 Gy esophagus (cm3) | 1.03 | 0.99–1.08 | 0.13 | 1.01 | 0.97–1.05 | 0.61 |
| N-stage 2 | 0.51 | 0.17–1.48 | 0.21 | 1.93 | 0.58–6.38 | 0.28 |
| N-stage 3 | 0.57 | 0.18–1.81 | 0.34 | 1.34 | 0.39–4.82 | 0.62 |
| Technique full VMAT | 1.05 | 0.51–2.15 | 0.90 | 0.65 | 0.35–1.21 | 0.18 |
| Age | 1.03 | 0.99–1.07 | 0.16 | 1.02 | 0.99–1.04 | 0.17 |
| Smoking history | 1.01 | 0.99–1.02 | 0.14 | 0.99 | 0.99–1.01 | 0.54 |
| PTV volume (cc) | 1.22 | 1.11–1.35 | <0.01 | 1.11 | 0.82–1.22 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remmerts de Vries, I.F.; Ronden, M.I.; Bahce, I.; Spoelstra, F.O.B.; De Haan, P.F.; Haasbeek, C.J.A.; Lissenberg-Witte, B.I.; Slotman, B.J.; Dahele, M.; Verbakel, W.F.A.R. Relationship between Treatment Plan Dosimetry, Toxicity, and Survival following Intensity-Modulated Radiotherapy, with or without Chemotherapy, for Stage III Inoperable Non-Small Cell Lung Cancer. Cancers 2021, 13, 5923. https://doi.org/10.3390/cancers13235923
Remmerts de Vries IF, Ronden MI, Bahce I, Spoelstra FOB, De Haan PF, Haasbeek CJA, Lissenberg-Witte BI, Slotman BJ, Dahele M, Verbakel WFAR. Relationship between Treatment Plan Dosimetry, Toxicity, and Survival following Intensity-Modulated Radiotherapy, with or without Chemotherapy, for Stage III Inoperable Non-Small Cell Lung Cancer. Cancers. 2021; 13(23):5923. https://doi.org/10.3390/cancers13235923
Chicago/Turabian StyleRemmerts de Vries, Isabel F., Merle I. Ronden, Idris Bahce, Femke O. B. Spoelstra, Patricia F. De Haan, Cornelis J. A. Haasbeek, Birgit I. Lissenberg-Witte, Ben J. Slotman, Max Dahele, and Wilko F. A. R. Verbakel. 2021. "Relationship between Treatment Plan Dosimetry, Toxicity, and Survival following Intensity-Modulated Radiotherapy, with or without Chemotherapy, for Stage III Inoperable Non-Small Cell Lung Cancer" Cancers 13, no. 23: 5923. https://doi.org/10.3390/cancers13235923
APA StyleRemmerts de Vries, I. F., Ronden, M. I., Bahce, I., Spoelstra, F. O. B., De Haan, P. F., Haasbeek, C. J. A., Lissenberg-Witte, B. I., Slotman, B. J., Dahele, M., & Verbakel, W. F. A. R. (2021). Relationship between Treatment Plan Dosimetry, Toxicity, and Survival following Intensity-Modulated Radiotherapy, with or without Chemotherapy, for Stage III Inoperable Non-Small Cell Lung Cancer. Cancers, 13(23), 5923. https://doi.org/10.3390/cancers13235923






