The Release of Inflammatory Mediators from Acid-Stimulated Mesenchymal Stromal Cells Favours Tumour Invasiveness and Metastasis in Osteosarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Observational Clinical Pilot Study
2.2. Circulating Markers and ELISA of IL6 and IL8
2.3. 2D Cell Cultures
2.4. Spheroids
2.5. Immunofluorescence
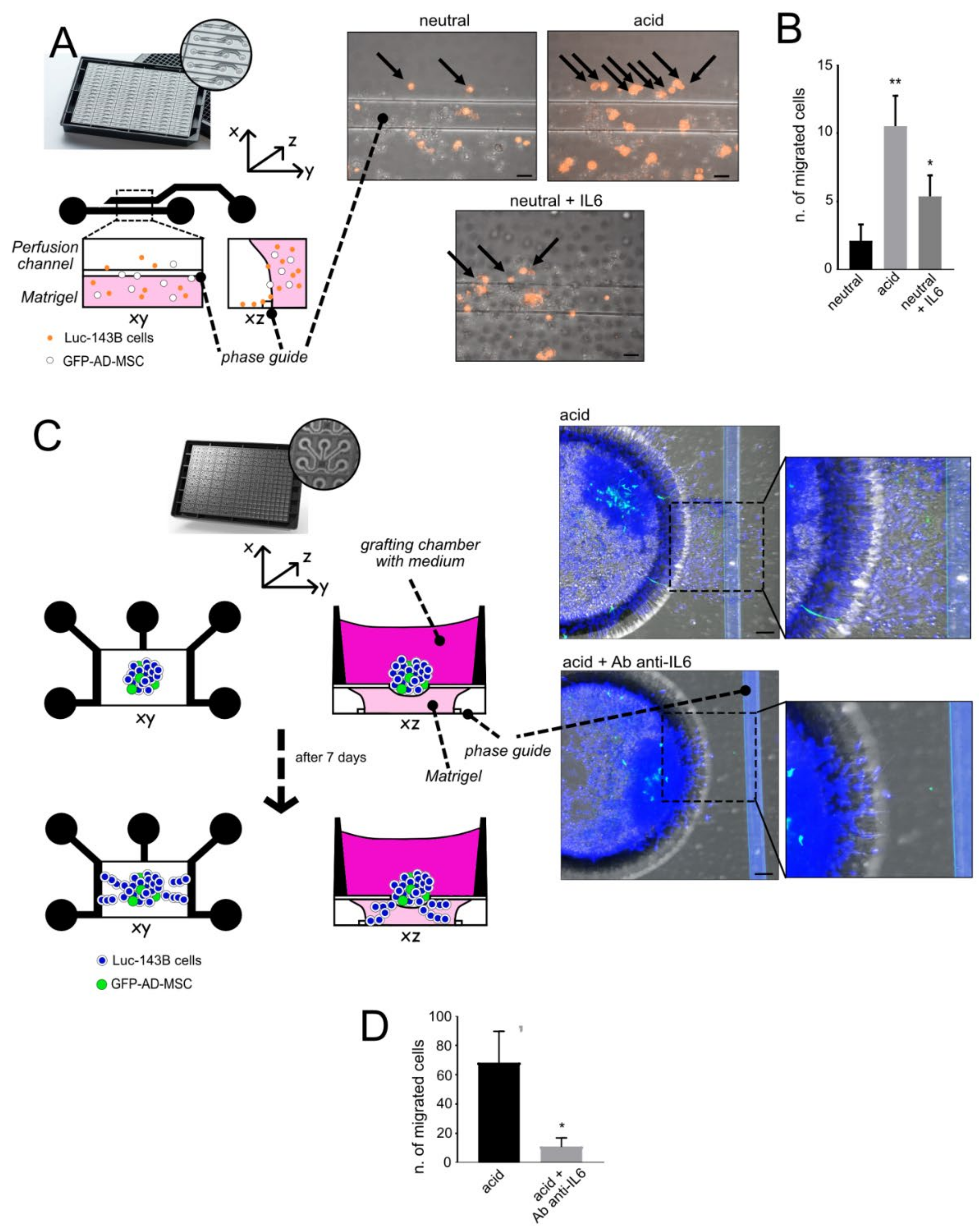
2.6. 3D Cell Cultures: Microfluidic Models
- (a)
- Single-cell invasion ability: A single-cell suspension of luc-143B cells (1 × 103 cells/channel) was obtained from the 70% confluent flask, detached in trypsin and counted. Cells were stained with Vibrant DIL Cell-Label (Life Technologies, Carlsbad, CA, USA) for 20 min at 37 °C following the manufacturer’s procedure. Cells were then mixed in a 1:3 ratio with GFP-AD-MSC and resuspended in 50% Matrigel®, 25% neutralised Rat Tail Collagen I (Cultrex, RD Systems), and 25% cell culture medium supplemented with 0.1% FCS. Cells embedded in the gel were added in the lower channel of a microfluidic 2-lane OrganoPlate® (Mimetas, Oegstgeest, The Netherlands) and placed for 15 min a 37 °C to allow for gel polymerisation. The channel was then filled with culture medium at neutral (7.4) or acidic (6.0) pH. As a stimulus for cell migration, the upper perfusion channel was added with 10% FCS medium, and Recombinant Human IL6 (Peprotech, 50 µg/mL) was used as a positive control. To simulate physiological perfusion flow, the OrganoPlate® was placed on a rocking plate: the rocker was set to change the 7° tilting angle every 8 min. Cell migration was visualised with confocal microscopy after 24 h. Only DIL-positive cells migrated in the perfusion channel were counted. Images were acquired with an objective 20x air, numerical aperture 0.75, Galvano scanning, zoom at 1, line average of 4, A1R MP confocal microscope, Nikon, scale bar 50 μM. The total acquired Z-stack was 161 µm, with a Z-step of 1.67 µm: 2 images/channel on 5 different channels.
- (b)
- Cellular escape from spheroids: We obtained heterotypic spheroids (luc-143B/GFP-AD-MSC) with the hanging-drop method after 96 h of culture in RPMI pH 7.4, as already described. The spheroids were placed in the open grafting chamber of a microfluidic OrganoPlate® Graft (Mimetas,), previously filled with 50% Matrigel®, 25% neutralised Rat Tail Collagen I (Cultrex, RD Systems), and 25% cell culture medium supplemented with 0.1% FCS. The chamber was then covered by 0.1% FCS medium at neutral (7.4) or acidic (6.0) pH. The lateral channels were filled with 10% medium to provide a stimulus for cell invasion. Cells were allowed to invade the gel for 7 days, with or without the anti-IL6 antibody (Tocilizumab, 100 µg/mL, Roche) that was added daily to the grafting chamber. At the endpoint, cells were fixed with 3.7% paraformaldehyde and nuclei were stained with Hoechst 33342 (Sigma). Images were acquired with an objective 20x air, numerical aperture 0.75, Resonant scanning, zoom at 1, line average of 4 (A1R MP confocal microscope, Nikon, scale bar 50 μM). The total acquired Z-stack was 200 µm, with a Z-step of 5 µm: 4 images/chamber were acquired and only cells visualised inside the gel were counted. The experiment was performed with three replicates.
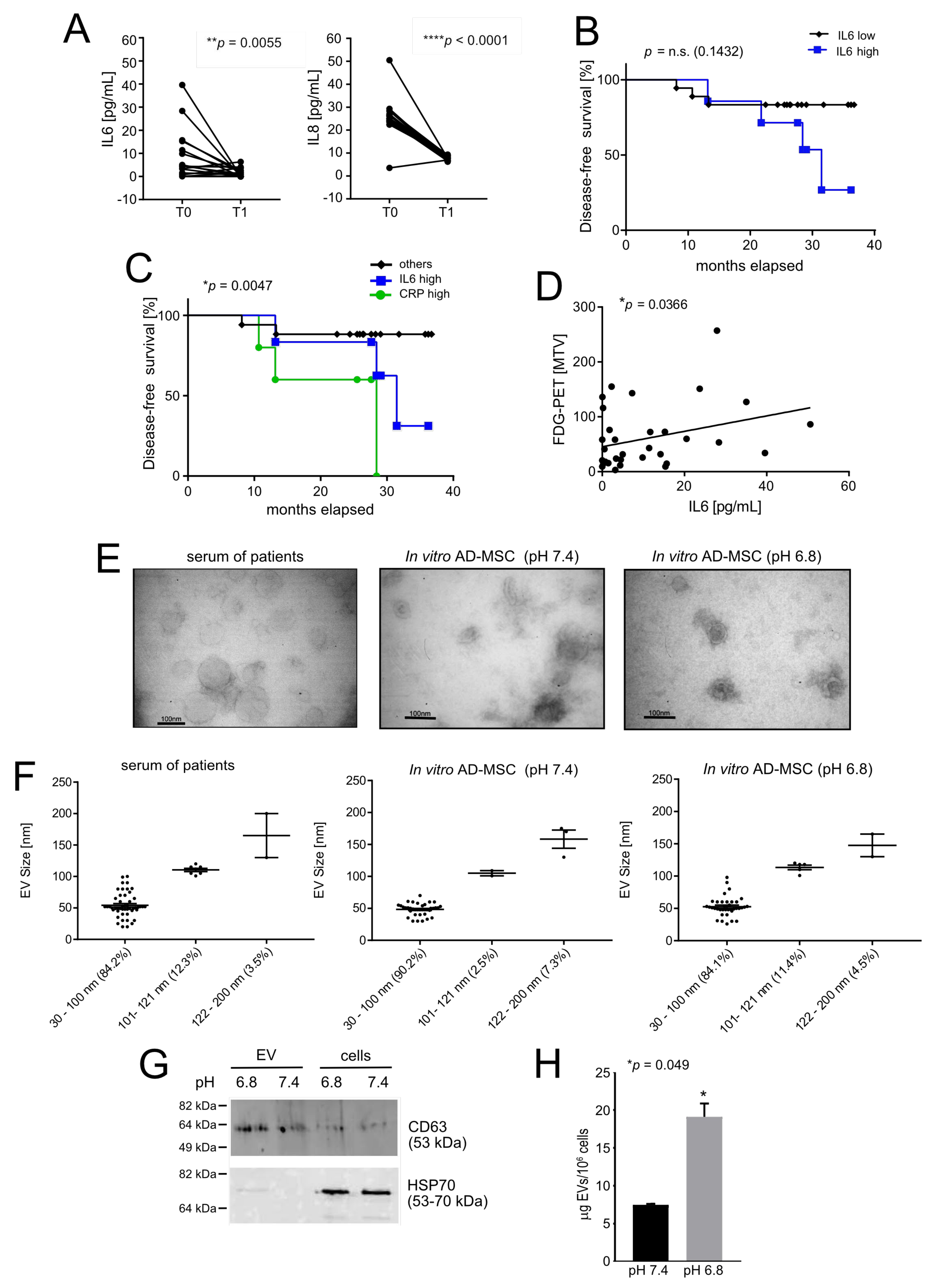
2.7. Extracellular Nanovesicle Isolation
- (a)
- Isolation of extracellular nanovesicles (EV) from AD-MSC: We assessed cell density and viability of AD-MSC at different pH (6.8 or 7.4) by the erythrosine B (Sigma-Aldrich) dye exclusion method. AD-MSC were grown in complete medium until 70–80% confluence. Cells were washed with Phosphate-Buffered Saline (PBS) and then incubated for 48 h with serum-free α-MEM at pH 6.8 or 7.4 (Sigma-Aldrich). The supernatant was collected after two consecutive periods (18 h and an additional 18 h) from AD-MSC grown on 20 Petri dish (diameter 150 mm, 18 mL/Petri). The EV were purified by differential centrifugation: 500× g for 10 min (twice), 2000× g for 15 min (twice), and 10,000× g for 30 min (twice) at 4 °C to remove floating cells and cellular debris. The supernatant was then ultracentrifuged at 110,000× g for 1 h at 4 °C. The EV pellet was resuspended in PBS and centrifuged at 110,000× g for 1 h at 4 °C (Beckman Coulter, Milan, Italy). The EV pellet was resuspended in PBS and stored at −80 °C until use. EV quantity was determined by the Bradford method (Bio-Rad, Milan, Italy).
- (b)
- Isolation of EV from serum (see Table 1): EV were precipitated from the serum (600 µL) by using the miRCURY Exosome Isolation Kit—Serum and plasma (QIAGEN, Hilden, Germany)
2.8. RNA Isolation and qPCR
- (a)
- Extracellular nanovesicles: miRNA isolation from EV isolated from serum and their analysis were conducted by QIAGEN Genomic Services. Total RNA was isolated from serum-derived EV by using the exoRNeasy Serum/Plasma Kit (QIAGEN) according to the manufacturer’s instructions. Total RNA (7 µL) was reverse transcribed in 35 µL reactions using the miRCURY LNA RT Kit (QIAGEN). cDNA was diluted 50× and assayed in 10 µL PCR reactions following the protocol for miRCURY LNA miRNA PCR; each miRNA was assayed once by qPCR on the miRNA Ready-to-Use PCR, Serum/Plasma Focus panel (192 miRNA assay) by using the miRCURY LNA SYBR Green master mix. Negative controls excluding the template from the reverse transcription reaction were performed and profiled like the samples. The amplification was performed in a LightCycler® 480 Real-Time PCR System (Roche) in 384-well plates. The amplification curves were analysed using Roche LC software, both for determination of Cq (by the 2nd derivative method) and for melting curve analysis. All assays were inspected for distinct melting curves and the Tm was checked to be within known specifications for the assay. Furthermore, assays must be detected with 5 Cq less than the negative control, and with Cq < 37 to be included in the data analysis. Data that did not pass these criteria were omitted from any further analysis. Cq was calculated as the 2nd derivative. All data were normalised to the average of assays detected in all samples [32]. Total RNA was isolated also from AD-MSC-derived EV by using miRCURY RNA Isolation Kits (QIAGEN) and reverse transcribed by using the miRCURY LNA RT Kit (QIAGEN). cDNA was diluted 20× and assayed in 10 µL PCR reactions according to the protocol for miRCURY LNA miRNA PCR, by using the hsa-miR-136-5p miRCURY LNA miRNA PCR Assay and miRCURY LNA SYBR Green master mix (QIAGEN). RNA spike-in templates (UniSp2, UniSp4 and UniSp5) and synthetic transcript UniSp6 (QIAGEN) were used as controls. The amplification was performed in a Bio-Rad CFX96 Touch System (Bio-rad, Hercules, CA, USA) in 96-well plates.
- (b)
- From tumour tissues and cell cultures: Total RNA was collected from frozen xenografts that were powdered by using a Mikro-Dismembrator (B. Braun Biotech International, Melsungen, Germany) with TRIzol reagent (ThermoFisher Scientific, Waltham, MA, USA). Total RNA was reverse transcribed into cDNA using RNase inhibitor and MuLV Reverse Transcriptase (Applied Biosystems, Foster City, CA). First-strand cDNA was synthesised by RT-qPCR using random hexamers. Real-time Polymerisation Chain Reaction (Real-time PCR) was performed by amplifying 500 ng of cDNA using the Light Cycler instrument and the Universal Probe Library system (Light Cycler instrument, Roche Applied Science, Monza, Italy). Probes and primers were selected by using a web-based assay design software (ProbeFinder, https://www.roche-applied-science.com, accessed on 18 October 2021). All primers were validated by BLAST (National Centre for Biotechnology Information) and Oligo Primer Analysis Software (Oligo, Colorado Springs, CO, USA); for primer information, see Table S1. The amplification protocol was 95 °C for 10 min, 45 cycles (95 °C for 10 s, 60 °C for 30 s, and 72 °C for 1 s); 40 °C for 30 s. Relative gene expression was obtained with the ratio with the geometric mean of the different housekeeping genes (HKG) (Gusb, YWHZ, 18S RNA, GAPDH) according to the ΔΔCt model, as previously described [24]. These housekeeping genes were considered suitable for the evaluation of mRNA expression under acidic conditions, as previously demonstrated [33]. All the experiments were replicated three times.
2.9. Electron Microscopy
- (a)
- Analysis of EV: Transmission electron microscopy was used to evaluate the morphology and size of EV, isolated from both human serum and cell cultures. Briefly, EV were resuspended in 2% paraformaldehyde and deposited onto formvar-carbon-coated grids. Then, EV were fixed in 1% glutaraldehyde, washed, counterstained with a solution of uranyl oxalate, pH 7.0, and embedded in a mixture of 4% uranyl acetate and 2% methylcellulose before observation with a Zeiss-EM 109 transmission electron microscope (Zeiss, Jena, Germany). Minor modification of this protocol was applied for electron microscopy analysis of serum-derived EV, as previously described [34]; the diameter of EVs was measured, and the percentage of size distribution was calculated.
- (b)
- Analysis of spheroids: Spheroids, obtained as described above, were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer pH 7.6 for 1 h at room temperature, post-fixed with 1% OsO4 in cacodylate buffer for 1 h, dehydrated in an ethanol series and embedded in Epon resin. Ultrathin sections stained with uranyl acetate and lead citrate were observed with a Jem-1011 transmission electron microscope (Jeol Inc., Peabody, MA, USA).
2.10. Western Blotting
2.11. In Vivo Models
- (a)
- Subcutaneous model of OS: NOD/SCID animals were housed and maintained in a pathogen-free environment. The 5-week-old male NOD/SCID mice (Charles River Laboratories International, Wilmington, MA, USA) were randomly split into two groups for the subcutaneous injection in the flank of homotypic or heterotypic cell populations, mixed with reduced growth factor Matrigel® (BD Life Sciences, Biosciences, Franklin Lakes, NJ, USA). For the number and type of cells, see Table 1 (experiment 1 and 2). For group #2, an isotonic solution containing GFP-AD-MSC was injected in the tail vein, on day 7 after surgery, once a week. Weights were taken daily during treatment. Mice were euthanised when tumour volume exceeded 2500 mm3.
- (b)
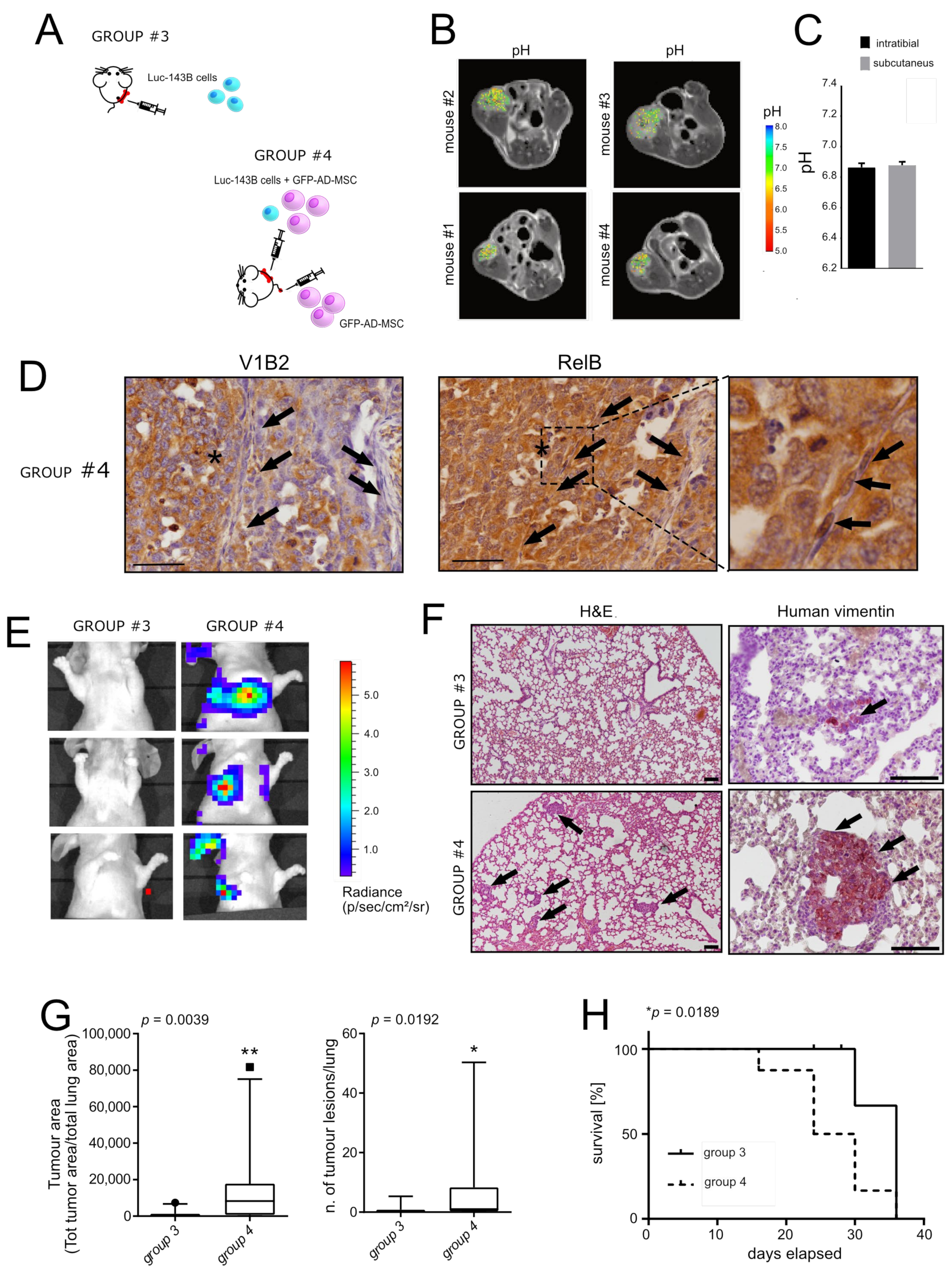
- Orthotopic model of OS: NOD/SCID animals were housed and maintained in a pathogen-free environment. The 5-week-old male NOD/SCID mice (Charles River Laboratories International) were randomly split into two groups and injected with luc-143B cells, w/o GFP-AD-MSC (see Exp. 4 in Table 1), in the left tibia. Cells were suspended in an isotonic saline solution. As previously described [22], the skin at the tibial plateau of the left calf was cut and a sterile incision was carefully made to expose the tibia. Next, 10 µL of cell suspension was slowly injected into the medullary cavity of the left tibia. The micro-syringe was then removed, and bone wax was used to seal the hole. Finally, the aseptic incision was sutured with vicryl polyglactin. Mice were sacrificed at different time points (n = 1/group at 8 days only for group #4; n = 4/group at days 12 days, n = 4/group at 15 days, n = 4/group at 18 days). In any case, mice were euthanised if the tumour volume exceeded 2500 mm3 or when mice showed signs of lung metastases development (respiratory distress, weakness, weight loss, dorsal kyphosis) or were positive for the luciferase signal, as described in the following section. The tibia and the lungs were extracted for histological and macroscopic analysis.
2.12. In Vivo MRI-CEST Intratumoural pH Imaging
2.13. H&E and Immunostaining
2.14. Quantitation of Tumour Growth and Metastasis
- (a)
- Tumour growth at the site of injection: Tumour growth was assessed at 8 days, using the bioluminescence generated by the injected luciferase-expressing luc-143B cells in mice (n = 6) as the read-out. At the endpoint, mice were injected intraperitoneally (150 μg/g of body weight) with a D-luciferin (Promega, Madison, WI, USA) solution in PBS, anaesthetised with isofluorane and imaged using an IVIS in vivo imaging system (PerkinElmer) with Living Image software (version 4.3.1, Perkin Elmer). Images were taken within the first 12 min after D-luciferin administration.
- (b)
- Metastatic formation: We used both luciferase assay in live animals, as described above, and vimentin immunostaining analysis to enlighten the development of tumour clones in the lung (using tissue sectioning followed by standard light microscopy techniques) after the mice’s sacrifice. Results that could not be confirmed with both immunohistochemistry and bioluminescence were excluded from the analysis. As parameters for the evaluation of metastasis, we used the tumour area (as measured by the quantification of the area of the lung that was positive to vimentin signal with respect to the total lung area), the number of tumour lesions (as measured by the quantification of the number of spots associated with the vimentin signal), and survival. Tumour area and the number of tumour lesions were analysed from three distinguished lung areas (top, middle and bottom). For survival analysis, uncensored corresponded to mice that were positive for both luciferase signal and vimentin (n = 8 mice for group #3 and n = 9 mice for group #4). The remaining mice were excluded.
2.15. Statistics
3. Results
3.1. Acid-Related Systemic Inflammatory Mediators in Osteosarcoma Patients
3.2. Intratumoural Acidosis Induces IL6 and IL8 Expression in Tumour-Associated Mesenchymal Stroma in a Subcutaneous Model of OS
3.3. Reactive MSC in the TME Promote Lung Metastasis in an Orthotopic Model of OS
3.4. Acid-Stimulated MSC Are Crucial for the First Step of the Metastatic Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef] [PubMed]
- Cortini, M.; Avnet, S.; Baldini, N. Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett. 2017, 405, 90–99. [Google Scholar] [CrossRef]
- Pouyssegur, J.; Dayan, F.; Mazure, N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006, 441, 437–443. [Google Scholar] [CrossRef]
- Kolosenko, I.; Avnet, S.; Baldini, N.; Viklund, J.; De Milito, A. Therapeutic implications of tumor interstitial acidification. Semin. Cancer Biol. 2017, 43, 119–133. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Sonveaux, P.; Stock, C.; Perez-Sayans, M.; De Milito, A.; Avnet, S.; Garcia, A.G.; Harguindey, S.; Fais, S. Proton channels and exchangers in cancer. Biochim. Biophys. Acta 2015, 1848, 2715–2726. [Google Scholar] [CrossRef]
- Longo, D.L.; Bartoli, A.; Consolino, L.; Bardini, P.; Arena, F.; Schwaiger, M.; Aime, S. In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res. 2016, 76, 6463–6470. [Google Scholar] [CrossRef]
- Avnet, S.; Di Pompo, G.; Lemma, S.; Salerno, M.; Perut, F.; Bonuccelli, G.; Granchi, D.; Zini, N.; Baldini, N. V-ATPase is a candidate therapeutic target for Ewing sarcoma. Biochim. Biophys. Acta 2013, 1832, 1105–1116. [Google Scholar] [CrossRef][Green Version]
- Perut, F.; Avnet, S.; Fotia, C.; Baglio, S.R.; Salerno, M.; Hosogi, S.; Kusuzaki, K.; Baldini, N. V-ATPase as an effective therapeutic target for sarcomas. Exp. Cell Res. 2014, 320, 21–32. [Google Scholar] [CrossRef]
- Cortini, M.; Armirotti, A.; Columbaro, M.; Longo, D.L.; Di Pompo, G.; Cannas, E.; Maresca, A.; Errani, C.; Longhi, A.; Righi, A.; et al. Exploring Metabolic Adaptations to the Acidic Microenvironment of Osteosarcoma Cells Unveils Sphingosine 1-Phosphate as a Valuable Therapeutic Target. Cancers 2021, 13, 311. [Google Scholar] [CrossRef]
- Di Pompo, G.; Cortini, M.; Baldini, N.; Avnet, S. Acid Microenvironment in Bone Sarcomas. Cancers 2021, 13, 3848. [Google Scholar] [CrossRef]
- Avnet, S.; Longhi, A.; Salerno, M.; Halleen, J.M.; Perut, F.; Granchi, D.; Ferrari, S.; Bertoni, F.; Giunti, A.; Baldini, N. Increased osteoclast activity is associated with aggressiveness of osteosarcoma. Int. J. Oncol. 2008, 33, 1231–1238. [Google Scholar] [CrossRef]
- Barcellos-de-Souza, P.; Gori, V.; Bambi, F.; Chiarugi, P. Tumor microenvironment: Bone marrow-mesenchymal stem cells as key players. Biochim. Biophys. Acta 2013, 1836, 321–335. [Google Scholar] [CrossRef]
- Hass, R. Role of MSC in the Tumor Microenvironment. Cancers 2020, 12, 2107. [Google Scholar] [CrossRef]
- Atiya, H.; Frisbie, L.; Pressimone, C.; Coffman, L. Mesenchymal Stem Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 31–42. [Google Scholar] [CrossRef]
- Truffi, M.; Sorrentino, L.; Corsi, F. Fibroblasts in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 15–29. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Donnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Xu, W.T.; Bian, Z.Y.; Fan, Q.M.; Li, G.; Tang, T.T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009, 281, 32–41. [Google Scholar] [CrossRef]
- Perrot, P.; Rousseau, J.; Bouffaut, A.L.; Redini, F.; Cassagnau, E.; Deschaseaux, F.; Heymann, M.F.; Heymann, D.; Duteille, F.; Trichet, V.; et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS ONE 2010, 5, e10999. [Google Scholar] [CrossRef]
- Baglio, S.R.; Lagerweij, T.; Perez-Lanzon, M.; Ho, X.D.; Leveille, N.; Melo, S.A.; Cleton-Jansen, A.M.; Jordanova, E.S.; Roncuzzi, L.; Greco, M.; et al. Blocking Tumor-Educated MSC Paracrine Activity Halts Osteosarcoma Progression. Clin. Cancer Res. 2017, 23, 3721–3733. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Avnet, S.; Grisendi, G.; Salerno, M.; Granchi, D.; Dominici, M.; Kusuzaki, K.; Baldini, N. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget 2014, 5, 7575–7588. [Google Scholar] [CrossRef]
- Avnet, S.; Di Pompo, G.; Chano, T.; Errani, C.; Ibrahim-Hashim, A.; Gillies, R.J.; Donati, D.M.; Baldini, N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-kappaB activation. Int. J. Cancer 2017, 140, 1331–1345. [Google Scholar] [CrossRef]
- Cummings, N.A.; Nordby, G.L. Measurement of synovial fluid pH in normal and arthritic knees. Arthritis Rheum. 1966, 9, 47–56. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Antimicrobial Mechanisms of Macrophages and the Immune Evasion Strategies of Staphylococcus aureus. Pathogens 2015, 4, 826–868. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Di Pompo, G.; Lemma, S.; Canti, L.; Rucci, N.; Ponzetti, M.; Errani, C.; Donati, D.M.; Russell, S.; Gillies, R.; Chano, T.; et al. Intratumoral acidosis fosters cancer-induced bone pain through the activation of the mesenchymal tumor-associated stroma in bone metastasis from breast carcinoma. Oncotarget 2017, 8, 54478–54496. [Google Scholar] [CrossRef]
- Grisendi, G.; Bussolari, R.; Cafarelli, L.; Petak, I.; Rasini, V.; Veronesi, E.; De Santis, G.; Spano, C.; Tagliazzucchi, M.; Barti-Juhasz, H.; et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010, 70, 3718–3729. [Google Scholar] [CrossRef] [PubMed]
- Marx, J.C.; Allay, J.A.; Persons, D.A.; Nooner, S.A.; Hargrove, P.W.; Kelly, P.F.; Vanin, E.F.; Horwitz, E.M. High-efficiency transduction and long-term gene expression with a murine stem cell retroviral vector encoding the green fluorescent protein in human marrow stromal cells. Hum. Gene Ther. 1999, 10, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, P.; Van Vlierberghe, P.; De Weer, A.; Muth, D.; Westermann, F.; Speleman, F.; Vandesompele, J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009, 10, R64. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S.; Avnet, S.; Meade, M.J.; Chano, T.; Baldini, N. Validation of Suitable Housekeeping Genes for the Normalization of mRNA Expression for Studying Tumor Acidosis. Int. J. Mol. Sci. 2018, 19, 2930. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and Non-Exosomal Transport of Extra-Cellular microRNAs in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef]
- Longo, D.L.; Dastru, W.; Digilio, G.; Keupp, J.; Langereis, S.; Lanzardo, S.; Prestigio, S.; Steinbach, O.; Terreno, E.; Uggeri, F.; et al. Iopamidol as a responsive MRI-chemical exchange saturation transfer contrast agent for pH mapping of kidneys: In vivo studies in mice at 7 T. Magn. Reason. Med. 2011, 65, 202–211. [Google Scholar] [CrossRef]
- Anemone, A.; Consolino, L.; Conti, L.; Reineri, F.; Cavallo, F.; Aime, S.; Longo, D.L. In vivo evaluation of tumour acidosis for assessing the early metabolic response and onset of resistance to dichloroacetate by using magnetic resonance pH imaging. Int. J. Oncol. 2017, 51, 498–506. [Google Scholar] [CrossRef]
- Rutkowski, P.; Kaminska, J.; Kowalska, M.; Ruka, W.; Steffen, J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: Correlations with local tumor extent and prognosis. J. Surg. Oncol. 2003, 84, 151–159. [Google Scholar] [CrossRef]
- Errani, C.; Cosentino, M.; Ciani, G.; Ferra, L.; Alfaro, P.A.; Bordini, B.; Donati, D.M. C-reactive protein and tumour diagnosis predict survival in patients treated surgically for long bone metastases. Int. Orthop. 2021, 45, 1337–1346. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.L.; Garcia-Posadas, L.; Diebold, Y. Extracellular Vesicles from Human Adipose-Derived Mesenchymal Stem Cells: A Review of Common Cargos. Stem Cell Rev. Rep. 2021. [Google Scholar] [CrossRef]
- Deng, G.; Gao, Y.; Cen, Z.; He, J.; Cao, B.; Zeng, G.; Zong, S. miR-136-5p Regulates the Inflammatory Response by Targeting the IKKbeta/NF-kappaB/A20 Pathway After Spinal Cord Injury. Cell. Physiol. Biochem. 2018, 50, 512–524. [Google Scholar] [CrossRef]
- He, J.; Zhao, J.; Peng, X.; Shi, X.; Zong, S.; Zeng, G. Molecular Mechanism of MiR-136-5p Targeting NF-kappaB/A20 in the IL-17-Mediated Inflammatory Response after Spinal Cord Injury. Cell. Physiol. Biochem. 2017, 44, 1224–1241. [Google Scholar] [CrossRef]
- Avnet, S.; Lemma, S.; Cortini, M.; Di Pompo, G.; Perut, F.; Lipreri, M.V.; Roncuzzi, L.; Columbaro, M.; Errani, C.; Longhi, A.; et al. Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy. Personal observation. 2021.
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Salerno, M.; Avnet, S.; Bonuccelli, G.; Hosogi, S.; Granchi, D.; Baldini, N. Impairment of lysosomal activity as a therapeutic modality targeting cancer stem cells of embryonal rhabdomyosarcoma cell line RD. PLoS ONE 2014, 9, e110340. [Google Scholar] [CrossRef]
- Karin, M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a000141. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, L.; Luo, G.; Son, H.; Prectoni, J.H.; Zheng, W. Effect of the cytokine levels in serum on osteosarcoma. Tumour Biol. 2014, 35, 1023–1028. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Tu, B.; Du, L.; Fan, Q.M.; Tang, Z.; Tang, T.T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012, 325, 80–88. [Google Scholar] [CrossRef]
- Riemann, A.; Wussling, H.; Loppnow, H.; Fu, H.; Reime, S.; Thews, O. Acidosis differently modulates the inflammatory program in monocytes and macrophages. Biochim. Biophys. Acta 2016, 1862, 72–81. [Google Scholar] [CrossRef]
- Sheen, H.; Kim, W.; Byun, B.H.; Kong, C.B.; Song, W.S.; Cho, W.H.; Lim, I.; Lim, S.M.; Woo, S.K. Metastasis risk prediction model in osteosarcoma using metabolic imaging phenotypes: A multivariable radiomics model. PLoS ONE 2019, 14, e0225242. [Google Scholar] [CrossRef]
- O'Neill, C.P.; Gilligan, K.E.; Dwyer, R.M. Role of Extracellular Vesicles (EVs) in Cell Stress Response and Resistance to Cancer Therapy. Cancers 2019, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Tang, H.; Zhang, Y.; Zhang, Z.; Huang, P.; Zhu, J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J. Cell. Physiol. 2020, 235, 4734–4745. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.; Leeper, D.B.; Cater, J.R.; Thistlethwaite, A.J.; Tupchong, L.; McFarlane, J.D. Extracellular pH distribution in human tumours. Int. J. Hyperth. 1995, 11, 211–216. [Google Scholar] [CrossRef]
- Martano, M.; Morello, E.; Avnet, S.; Costa, F.; Sammartano, F.; Kusuzaki, K.; Baldini, N. Photodynamic Surgery for Feline Injection-Site Sarcoma. Biomed. Res. Int. 2019, 2019, 8275935. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Vazquez-Arreguin, K.; Kwak, C.; Sugimoto, H.; Zheng, X.; Li, B.; Kirtley, M.L.; LeBleu, V.S.; Kalluri, R. alphaSMA(+) fibroblasts suppress Lgr5(+) cancer stem cells and restrain colorectal cancer progression. Oncogene 2021, 40, 4440–4452. [Google Scholar] [CrossRef]
- Avnet, S.; Lemma, S.; Cortini, M.; Pellegrini, P.; Perut, F.; Zini, N.; Kusuzaki, K.; Chano, T.; Grisendi, G.; Dominici, M.; et al. Altered pH gradient at the plasma membrane of osteosarcoma cells is a key mechanism of drug resistance. Oncotarget 2016, 7, 63408–63423. [Google Scholar] [CrossRef]
- Shi, Y.; He, R.; Zhuang, Z.; Ren, J.; Wang, Z.; Liu, Y.; Wu, J.; Jiang, S.; Wang, K. A risk signature-based on metastasis-associated genes to predict survival of patients with osteosarcoma. J. Cell. Biochem. 2020, 121, 3479–3490. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, H.; Pan, B.; Lu, L.; Wei, Z.; Shi, L.; Yao, X.; Kang, Y.; Feng, S. Exploring the key genes and pathways of osteosarcoma with pulmonary metastasis using a gene expression microarray. Mol. Med. Rep. 2017, 16, 7423–7431. [Google Scholar] [CrossRef]
- Itoh, H.; Kadomatsu, T.; Tanoue, H.; Yugami, M.; Miyata, K.; Endo, M.; Morinaga, J.; Kobayashi, E.; Miyamoto, T.; Kurahashi, R.; et al. TET2-dependent IL-6 induction mediated by the tumor microenvironment promotes tumor metastasis in osteosarcoma. Oncogene 2018, 37, 2903–2920. [Google Scholar] [CrossRef]
- Salerno, M.; Avnet, S.; Bonuccelli, G.; Eramo, A.; De Maria, R.; Gambarotti, M.; Gamberi, G.; Baldini, N. Sphere-forming cell subsets with cancer stem cell properties in human musculoskeletal sarcomas. Int. J. Oncol. 2013, 43, 95–102. [Google Scholar] [CrossRef][Green Version]
- Gillies, R.J. Cancer heterogeneity and metastasis: Life at the edge. Clin. Exp. Metastasis 2021. [Google Scholar] [CrossRef]


| Variable | Total Series (n = 40) | Subseries Screened for miRNA Content in Circulating EV (n = 10) |
|---|---|---|
| Age (y) | ||
| ≤14 >14 | 21 19 | 4 6 |
| Sex | ||
| Male Female | 24 16 | 5 5 |
| Anatomic site | ||
| Femur Tibia Humerus Pelvis Fibula Radius | 24 10 2 2 1 1 | 6 3 0 1 0 0 |
| Histological subtype | ||
| Osteoblastic Chondroblastic Fibroblastic Osteoblastic/Chondroblastic Osteoblastic/Fibroblastic Osteoblastic/Telangiectatic | 24 1 1 5 4 5 | 5 0 0 2 1 2 |
| Animal Trial n. | Representative Scheme of the Experiment | Site of Injection and Cells (n) | N. of Groups (n. Mice/Group), Code Group | Endpoints (Days From Injection) | Type of Assay |
|---|---|---|---|---|---|
| 1 | Figure 2A | Subcutaneous, one-off (luc-143B 75,000) | 1 (4) Group #1 | 21 | MRI-CEST |
| 2 | Figure 2B | Subcutaneous, one-off (luc-143B 75,000/GFP-AD-MSC 225,000) + tail vain once/week (GFP-AD-MSC 225,000) | 2(6) Group #1 and #2 | 22 | RNA lysis from frozen tissues; Luc. Assay to the site of injection; E&E from paraffin-embedded tissues |
| 3 | Figure 3A | Intratibial, one-off (luc-143B 75,000/ GFP-ADMSC 225,000) + tail vain once/week (GFP-AD-MSC 135,000) | 1 (4) Group #3 | 21 | MRI-CEST (bone) |
| 4 | Figure 3A | Intratibial, one-off (luc-143B 75,000/GFP-AD-MSC 225,000) + tail vain once/week (GFP-AD-MSC 135,000) | 2 (12 for group #3 and 13 for group #4) Group #3 and #4 | 8 (bone) 8-12-15-18 (lung) | Luc Assay to the site of injection and to the lung; H&E to the site of injection and to the lung; Immunohistochemistry for V1B2 and RelB at the site of injection, for vimentin at the lung |
| miRNA Name | Standard Deviation (SD) | Fold Change | p-Value (t-Test) | p-Value (BH adj) |
|---|---|---|---|---|
| Decreased miRNA (from T0 to T1) | ||||
| hsa-miR-376a-3p | 0.68 | −2.4 | 0.00022 | 0.035 |
| hsa-miR-154-5p | 0.55 | −2.8 | 0.00041 | 0.035 |
| hsa-miR-136-5p | 0.63 | −2.0 | 0.00061 | 0.035 |
| hsa-miR-376c-3p | 0.81 | −2.3 | 0.0010 | 0.043 |
| hsa-miR-495-3p | 1.2 | −3.0 | 0.0047 | 0.14 |
| hsa-miR-320c | 0.27 | −1.2 | 0.0048 | 0.14 |
| hsa-miR-99b-5p | 0.65 | −1.6 | 0.0080 | 0.16 |
| hsa-miR-409-3p | 0.95 | −2.3 | 0.0095 | 0.16 |
| hsa-miR-485-3p | 0.61 | −2.1 | 0.016 | 0.21 |
| hsa-miR-199a-3p | 0.34 | −1.2 | 0.031 | 0.30 |
| hsa-miR-186-5p | 0.52 | −1.3 | 0.031 | 0.30 |
| Increased miRNA (from T0 to T1) | ||||
| hsa-miR-144-5p | 0.94 | 1.9 | 0.015 | 0.21 |
| hsa-miR-30a-5p | 0.51 | 1.4 | 0.010 | 0.16 |
| hsa-miR-22-3p | 0.28 | 1.2 | 0.017 | 0.21 |
| hsa-miR-374a-5p | 0.23 | 1.1 | 0.020 | 0.23 |
| hsa-miR-20b-5p | 1.3 | 2.3 | 0.027 | 0.29 |
| hsa-miR-139-5p | 0.50 | 1.4 | 0.0096 | 0.16 |
| hsa-miR-18b-5p | 0.41 | 1.2 | 0.035 | 0.31 |
| hsa-miR-126-5p | 0.31 | 1.2 | 0.036 | 0.31 |
| hsa-miR-424-5p | 0.46 | 1.4 | 0.0064 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avnet, S.; Lemma, S.; Cortini, M.; Di Pompo, G.; Perut, F.; Lipreri, M.V.; Roncuzzi, L.; Columbaro, M.; Errani, C.; Longhi, A.; et al. The Release of Inflammatory Mediators from Acid-Stimulated Mesenchymal Stromal Cells Favours Tumour Invasiveness and Metastasis in Osteosarcoma. Cancers 2021, 13, 5855. https://doi.org/10.3390/cancers13225855
Avnet S, Lemma S, Cortini M, Di Pompo G, Perut F, Lipreri MV, Roncuzzi L, Columbaro M, Errani C, Longhi A, et al. The Release of Inflammatory Mediators from Acid-Stimulated Mesenchymal Stromal Cells Favours Tumour Invasiveness and Metastasis in Osteosarcoma. Cancers. 2021; 13(22):5855. https://doi.org/10.3390/cancers13225855
Chicago/Turabian StyleAvnet, Sofia, Silvia Lemma, Margherita Cortini, Gemma Di Pompo, Francesca Perut, Maria Veronica Lipreri, Laura Roncuzzi, Marta Columbaro, Costantino Errani, Alessandra Longhi, and et al. 2021. "The Release of Inflammatory Mediators from Acid-Stimulated Mesenchymal Stromal Cells Favours Tumour Invasiveness and Metastasis in Osteosarcoma" Cancers 13, no. 22: 5855. https://doi.org/10.3390/cancers13225855
APA StyleAvnet, S., Lemma, S., Cortini, M., Di Pompo, G., Perut, F., Lipreri, M. V., Roncuzzi, L., Columbaro, M., Errani, C., Longhi, A., Zini, N., Heymann, D., Dominici, M., Grisendi, G., Golinelli, G., Consolino, L., Longo, D. L., Nanni, C., Righi, A., & Baldini, N. (2021). The Release of Inflammatory Mediators from Acid-Stimulated Mesenchymal Stromal Cells Favours Tumour Invasiveness and Metastasis in Osteosarcoma. Cancers, 13(22), 5855. https://doi.org/10.3390/cancers13225855











