Safety of COVID-19 mRNA Vaccines in Patients with Cancer Enrolled in Early-Phase Clinical Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Data Collection
2.2. Ethics Statement
2.3. Endpoint and Outcome
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Overall Population
3.2. Type of COVID-19 Vaccine
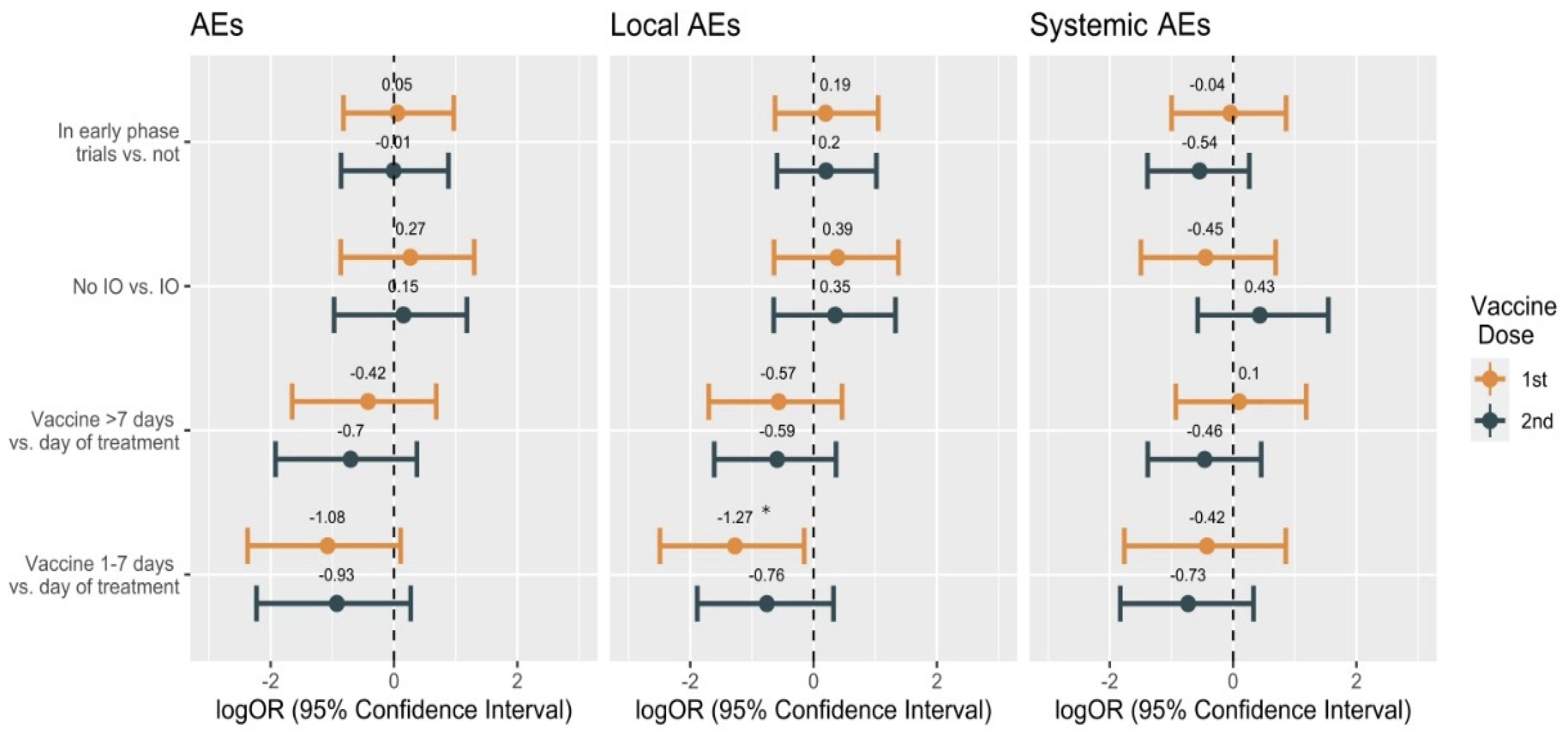
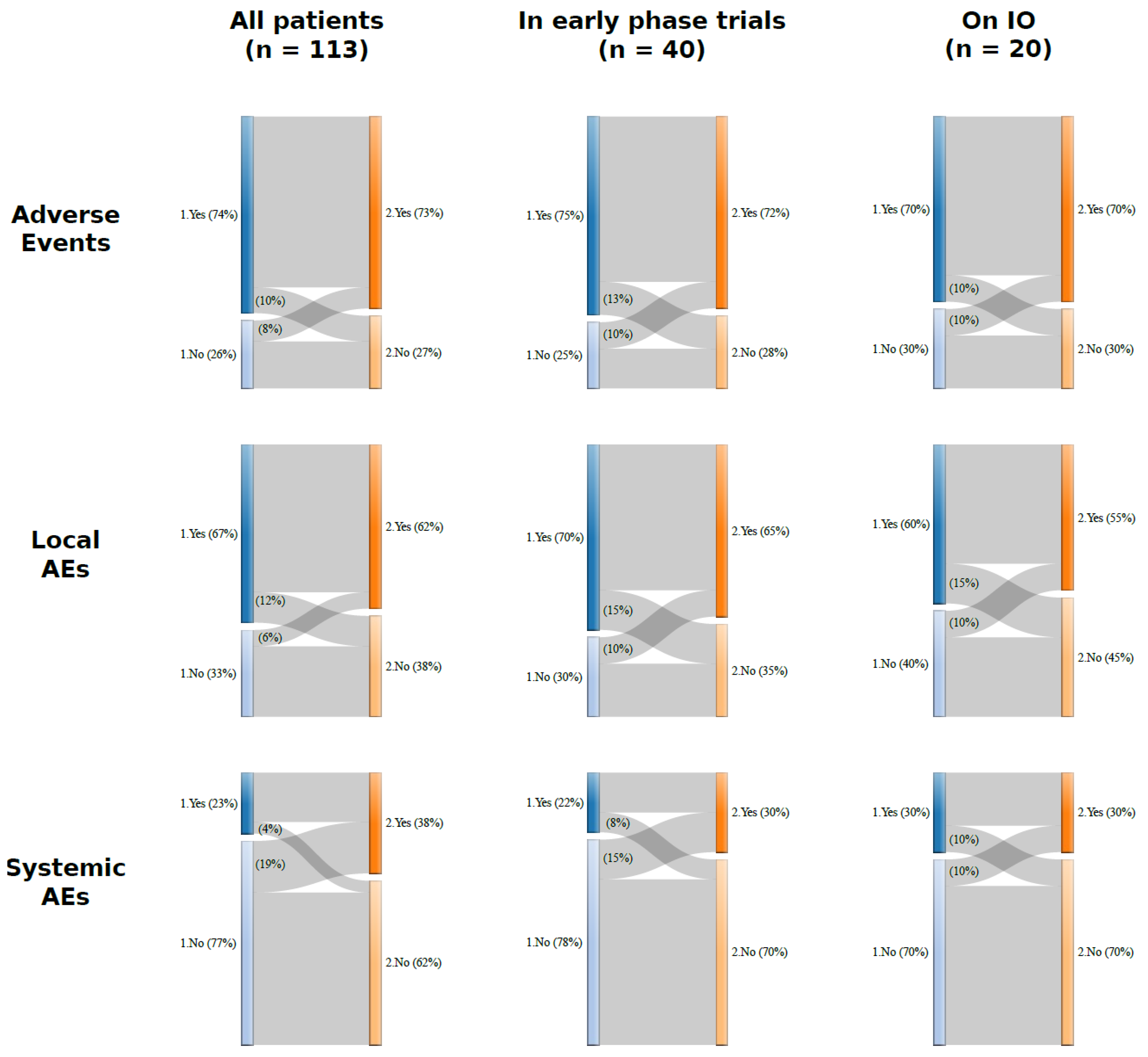
3.3. Safety
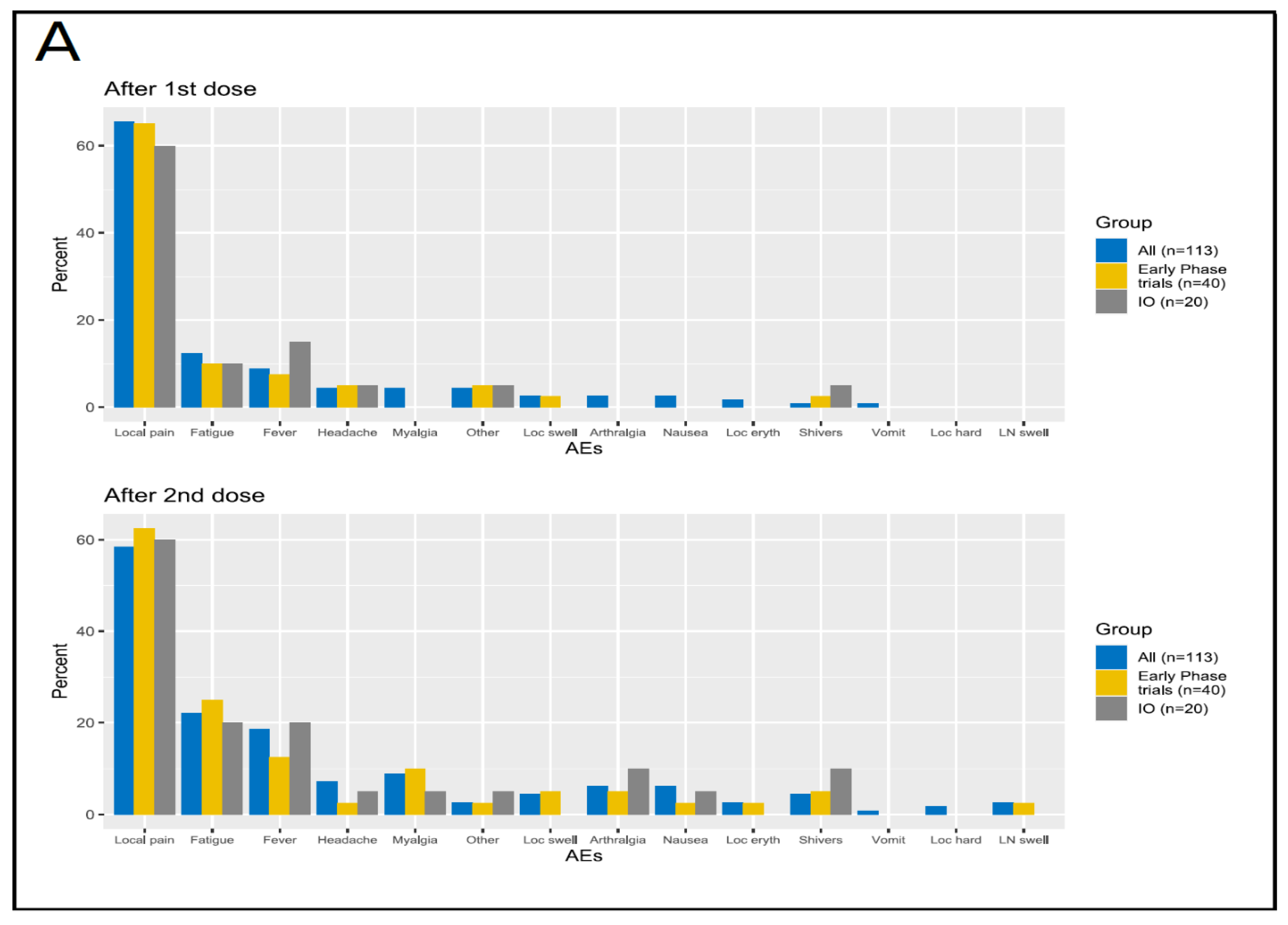
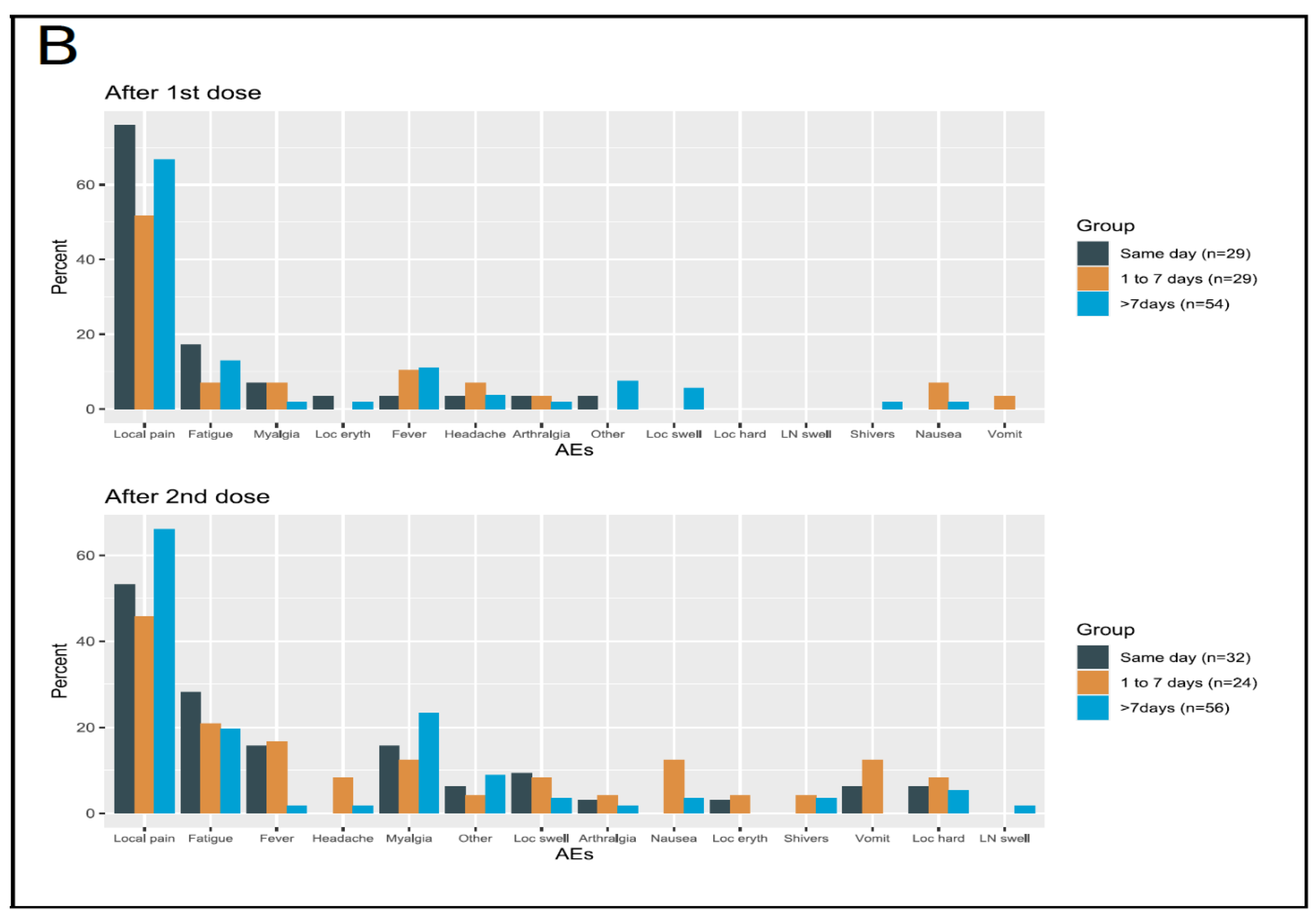
3.3.1. Safety in the Overall Population
3.3.2. Safety in the Cohort of Patients Enrolled in Early-Phase Clinical Trials
3.3.3. Safety in the Group Receiving Experimental Immunotherapy Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrière, J.; Gal, J.; Hoch, B.; Cassuto, O.; Leysalle, A.; Chamorey, E.; Borchiellini, D. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: A cross-sectional survey. Ann. Oncol. 2021, 32, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez, M.G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; Lopes, G.D.L.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Desai, A.; Gupta, R.; Advani, S.; Ouellette, L.; Kuderer, N.M.; Lyman, G.H.; Li, A. Mortality in hospitalized patients with cancer and coronavirus disease 2019: A systematic review and meta-analysis of cohort studies. Cancer 2020, 127, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Tagliamento, M.; Agostinetto, E.; Bruzzone, M.; Ceppi, M.; Saini, K.S.; de Azambuja, E.; Punie, K.; Westphalen, C.B.; Morgan, G.; Pronzato, P.; et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: A systematic review and meta-analysis. Crit. Rev. Oncol. 2021, 163, 103365. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Crimini, E.; Tarantino, P.; Pravettoni, G.; Eggermont, A.M.; Delaloge, S.; Curigliano, G. SARS-CoV-2 vaccines for cancer patients: A call to action. Eur. J. Cancer 2021, 148, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Trapani, D.; Curigliano, G. COVID-19 vaccines in patients with cancer. Lancet Oncol. 2021, 22, 738–739. [Google Scholar] [CrossRef]
- Spiera, R.; Jinich, S.; Jannat-Khah, D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021, 80, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, D.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021. [Google Scholar] [CrossRef]
- Au, L.; Fendler, A.; Shepherd, S.T.C.; Rzeniewicz, K.; Cerrone, M.; Byrne, F.; Carlyle, E.; Edmonds, K.; Del Rosario, L.; Shon, J.; et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat. Med. 2021, 27, 1362–1366. [Google Scholar] [CrossRef]
- Garassino, M.C.; Vyas, M.; de Vries, E.; Kanesvaran, R.; Giuliani, R.; Peters, S. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann. Oncol. 2021, 32, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Gainor, J.F.; Hegde, A.; Schram, A.M.; Curigliano, G.; Pal, S.; Liu, S.V.; Halmos, B.; Groisberg, R.; Grande, E.; et al. Author Correction: COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat. Rev. Clin. Oncol. 2021, 18, 320. [Google Scholar] [CrossRef] [PubMed]
- A Yap, T.; Siu, L.L.; Calvo, E.; Lolkema, M.P.; LoRusso, P.M.; Soria, J.-C.; Plummer, R.; de Bono, J.S.; Tabernero, J.; Banerji, U. SARS-CoV-2 vaccination and phase 1 cancer clinical trials. Lancet Oncol. 2021, 22, 298–301. [Google Scholar] [CrossRef]
- National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health & Human Services: Washington, DC, USA, 2017.
- ASCO. Inclusion of Individuals with Cancer on COVID-19 Vaccine Trials; A Joint Position Statement from the American Society of Clinical Oncology and Friends of Cancer Research Approved by the ASCO Board of Directors 20 May 2021; ASCO: Alexandria, VA, USA, 2021. [Google Scholar]
- Patel, M.K.; Bergeri, I.; Bresee, J.S.; Cowling, B.J.; Crowcroft, N.S.; Fahmy, K.; Hirve, S.; Kang, G.; Katz, M.A.; Lanata, C.F.; et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: Summary of interim guidance of the World Health Organization. Vaccine 2021, 39, 4013–4024. [Google Scholar] [CrossRef]
- WHO. COVID-19 and Mandatory Vaccination: Ethical Considerations and Caveats. Policy Br. 2021, pp. 13–17. Available online: https://apps.who.int/iris/rest/bitstreams/1342697/retrieve (accessed on 8 July 2021).
- Centers for Disease Control and Prevention (CDC). Different COVID-19 Vaccines; CDC: Atlanta, GA, USA. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html (accessed on 8 July 2021).
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef]
- Mazzarella, L.; Duso, B.A.; Trapani, D.; Belli, C.; D’Amico, P.; Ferraro, E.; Viale, G.; Curigliano, G. The evolving landscape of ‘next-generation’ immune checkpoint inhibitors: A review. Eur. J. Cancer 2019, 117, 14–31. [Google Scholar] [CrossRef]
- Hosseini, I.; Gadkar, K.; Stefanich, E.; Li, C.-C.; Sun, L.L.; Chu, Y.-W.; Ramanujan, S. Mitigating the risk of cytokine release syndrome in a Phase I trial of CD20/CD3 bispecific antibody mosunetuzumab in NHL: Impact of translational system modeling. npj Syst. Biol. Appl. 2020, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, L.; Giugliano, S.; D’Amico, P.; Belli, C.; Duso, B.A.; Rescigno, M.; Curigliano, G. Evidence for interleukin 17 involvement in severe immune-related neuroendocrine toxicity. Eur. J. Cancer 2020, 141, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ceschi, A.; Noseda, R.; Palin, K.; Verhamme, K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front. Pharmacol. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.-F.; et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef]
| All Patients | In Early-Phase Clinical Trials | Experimental IO e | |
|---|---|---|---|
| N | 113 (100%) | 40 (35.4%) | 20 (17.7%) |
| Sex | |||
| Male | 17 (15.0%) | 16 (40%) | 11 (55%) |
| Female | 96 (85.0%) | 24 (60%) | 9 (45%) |
| Age, median (IQR) | 60 (53, 69) | 59 (49, 67) | 58 (50, 66.5) |
| Smoking status | |||
| Never smoker | 86 (76.1%) | 29 (73%) | 12 (60%) |
| Smoker | 19 (16.8%) | 8 (20%) | 5 (25%) |
| Former smoker | 8 (7.1%) | 3 (8%) | 3 (15%) |
| History of previous COVID-19 | |||
| Yes a | 10 (8.8%) | 7 (17.5%) | 3 (15%) |
| No | 103 (91.2%) | 33 (82.5%) | 17 (85%) |
| Comorbidities | |||
| No | 21 (18.6%) | 8 (20%) | 4 (20%) |
| Yes | 92 (81.4%) | 32 (80%) | 16 (80%) |
| Obesity | |||
| No | 102 (90.3%) | 37 (93%) | 18 (90%) |
| Yes | 11 (9.7%) | 3 (8%) | 2 (10%) |
| Tumor type | |||
| Breast | 86 (76.1%) | 14 (35%) | 5 (25%) |
| Lung | 7 (6.2%) | 7 (18%) | 1 (5%) |
| Melanoma | 6 (5.3%) | 6 (15%) | 6 (30%) |
| Other b | 14 (12.4%) | 13 (33%) | 8 (40%) |
| TNM Stage | |||
| I-III | 3 (2.6%) | 0 (0%) | 0 (0%) |
| IV | 110 (97.4%) | 40 (100%) | 20 (100%) |
| Number of previous lines of treatment | |||
| 0 | 29 (25.7%) | 11 (28%) | 5 (25%) |
| 1 | 30 (26.5%) | 13 (33%) | 9 (45%) |
| 2 | 19 (16.8%) | 6 (15%) | 2 (10%) |
| 3 | 13 (11.5%) | 2 (5%) | 1 (5%) |
| >3 | 22 (19.5%) | 8 (20%) | 3 (15%) |
| Ongoing treatment | |||
| Chemotherapy | 25 (22.1%) | 2 (5%) | 0 (0%) |
| Chemotherapy + Targeted therapy | 2 (1.8%) | 1 (3%) | 0 (0%) |
| Endocrine Treatment alone | 8 (7.1%) | 0 (0%) | 0 (0%) |
| Endocrine Treatment + Targeted Therapy c | 31 (27.4%) | 0 (0%) | 0 (0%) |
| IO | 18 (15.9%) | 18 (45%) | 18 (90%) |
| IO + Targeted Therapy | 2 (1.8%) | 2 (5%) | 2 (10%) |
| Targeted Therapy | 27 (23.9%) | 17 (43%) d | 0 (0%) |
| Performance Status (ECOG) | |||
| 0 | 94 (83.2%) | 32 (80%) | 16 (80%) |
| 1 | 17 (15.0%) | 8 (20%) | 4 (20%) |
| 2 | 2 (1.8%) | 0 (0%) | 0 (0%) |
| Steroids use | |||
| No | 106 (93.8%) | 37 (92.5%) | 18 (90%) |
| Yes | 7 (6.2%) | 3 (7.5%) | 2 (10%) |
| Type of Vaccine | |||
| AstraZeneca | 2 (1.8%) | 0 (0%) | 0 (0%) |
| Moderna | 59 (52.2%) | 18 (45%) | 8 (40%) |
| Pfizer | 52 (46.0%) | 22 (55%) | 12 (60%) |
| After first dose of vaccine | |||
| AEs | 84 (74.3%) | 30 (75%) | 14 (70%) |
| Local AEs | 76 (67.3%) | 28 (70%) | 12 (60%) |
| Systemic AEs | 26 (23.0%) | 9 (23%) | 6 (30%) |
| After second dose of vaccine | |||
| AEs | 82 (72.6%) | 29 (73%) | 14 (70%) |
| Local AEs | 70 (61.9%) | 26 (65%) | 11 (55%) |
| Systemic AEs | 43 (38.1%) | 12 (30%) | 6 (30%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trillo Aliaga, P.; Trapani, D.; Sandoval, J.L.; Crimini, E.; Antonarelli, G.; Vivanet, G.; Morganti, S.; Corti, C.; Tarantino, P.; Friedlaender, A.; et al. Safety of COVID-19 mRNA Vaccines in Patients with Cancer Enrolled in Early-Phase Clinical Trials. Cancers 2021, 13, 5829. https://doi.org/10.3390/cancers13225829
Trillo Aliaga P, Trapani D, Sandoval JL, Crimini E, Antonarelli G, Vivanet G, Morganti S, Corti C, Tarantino P, Friedlaender A, et al. Safety of COVID-19 mRNA Vaccines in Patients with Cancer Enrolled in Early-Phase Clinical Trials. Cancers. 2021; 13(22):5829. https://doi.org/10.3390/cancers13225829
Chicago/Turabian StyleTrillo Aliaga, Pamela, Dario Trapani, José Luis Sandoval, Edoardo Crimini, Gabriele Antonarelli, Grazia Vivanet, Stefania Morganti, Chiara Corti, Paolo Tarantino, Alex Friedlaender, and et al. 2021. "Safety of COVID-19 mRNA Vaccines in Patients with Cancer Enrolled in Early-Phase Clinical Trials" Cancers 13, no. 22: 5829. https://doi.org/10.3390/cancers13225829
APA StyleTrillo Aliaga, P., Trapani, D., Sandoval, J. L., Crimini, E., Antonarelli, G., Vivanet, G., Morganti, S., Corti, C., Tarantino, P., Friedlaender, A., Belli, C., Minchella, I., Locatelli, M., Esposito, A., Criscitiello, C., & Curigliano, G. (2021). Safety of COVID-19 mRNA Vaccines in Patients with Cancer Enrolled in Early-Phase Clinical Trials. Cancers, 13(22), 5829. https://doi.org/10.3390/cancers13225829







