Prognostic Significance of Estrogen Receptor Alpha in Oral Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Patients
2.3. Immunohistochemistry
2.4. Statistical Analysis
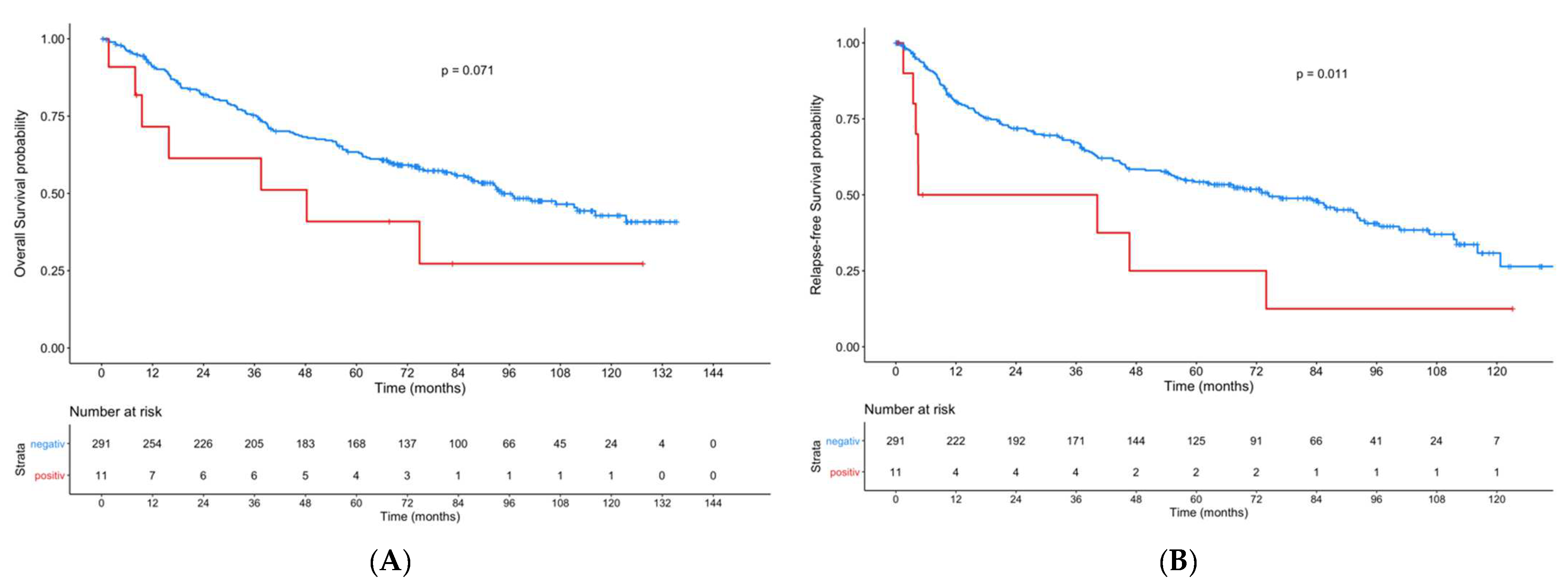
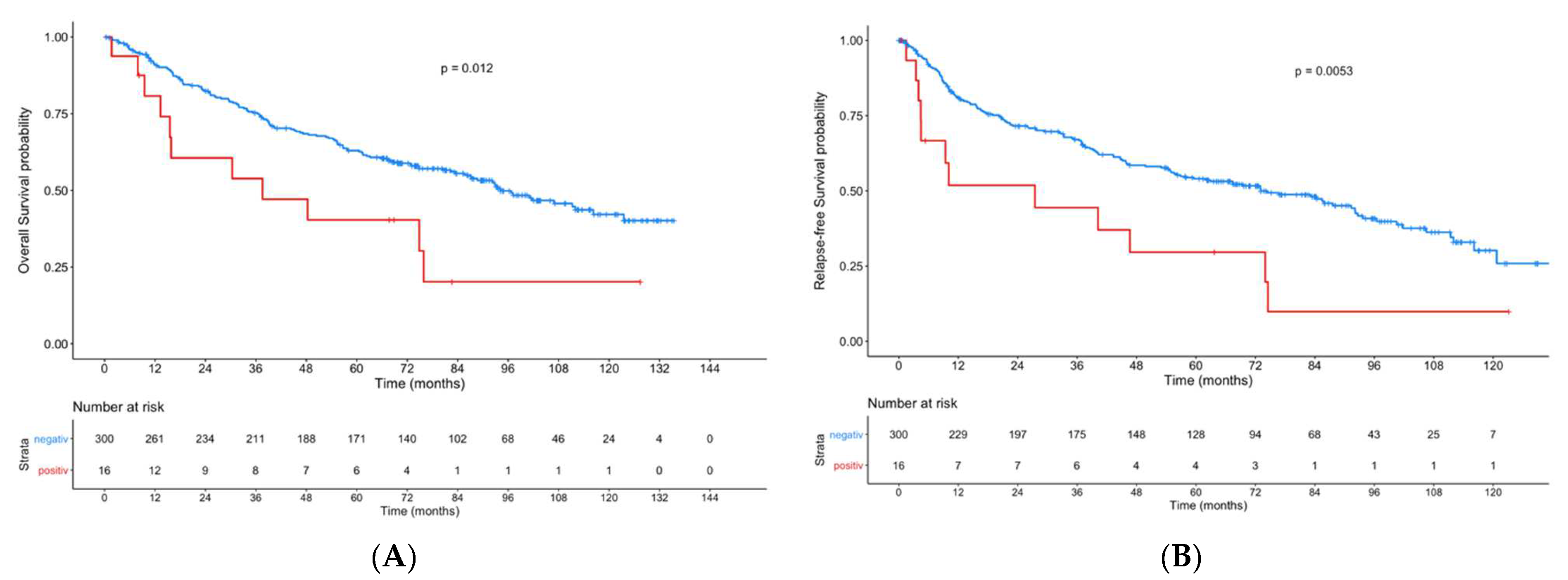
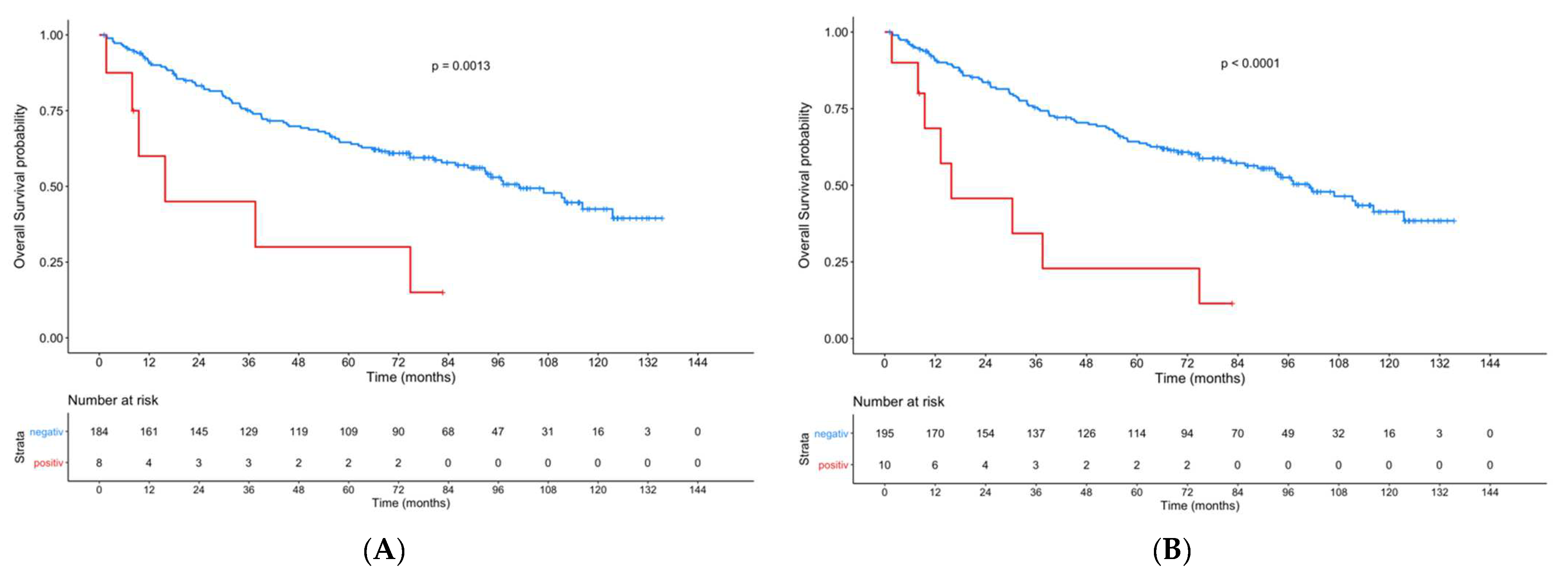
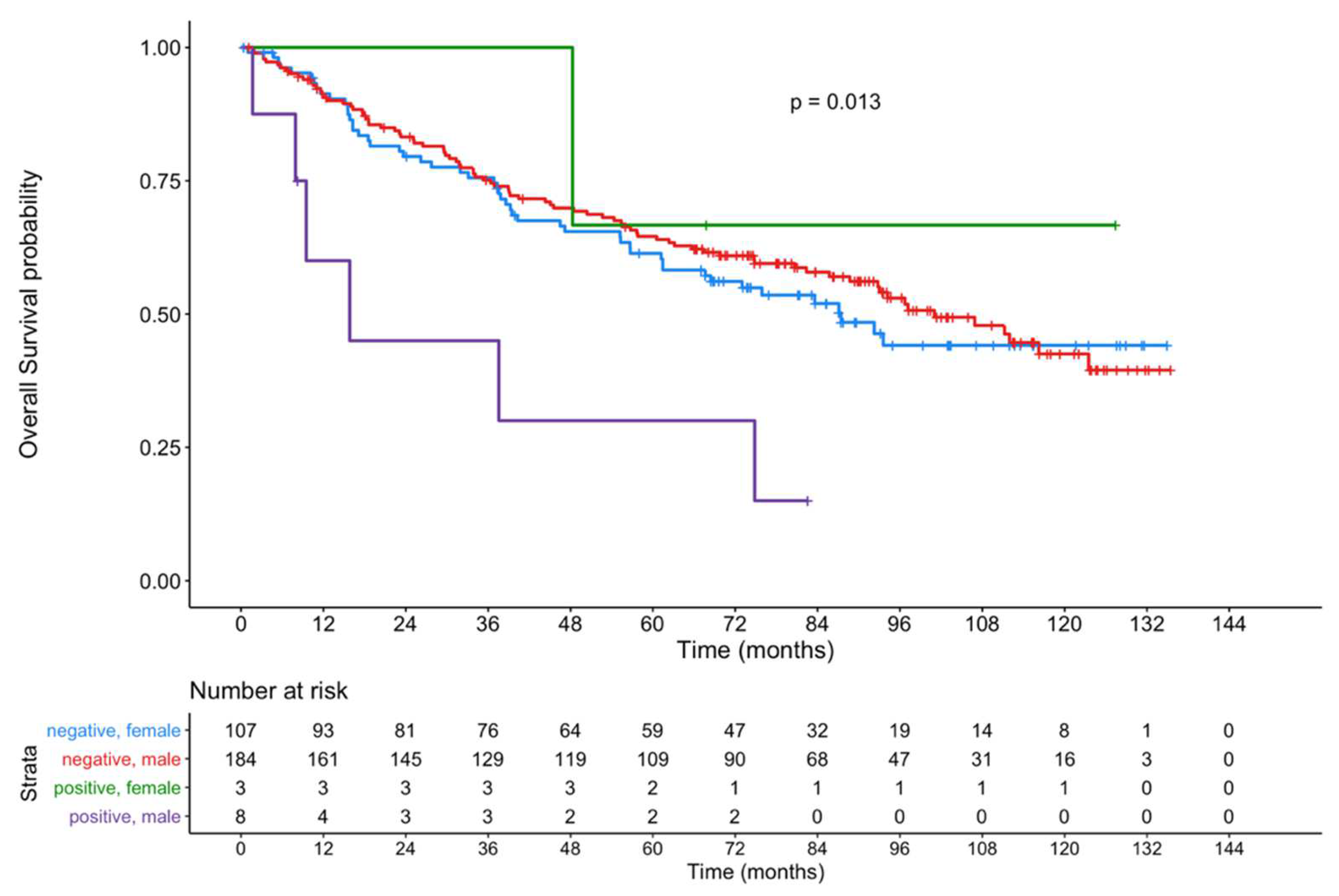
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Amit, M.; Yen, T.C.; Liao, C.T.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Ebrahimi, A.; Clark, J.R.; Kreppel, M.; Zöller, J.; et al. Improvement in survival of patients with oral cavity squamous cell carcinoma: An international collaborative study. Cancer 2013, 119, 4242–4248. [Google Scholar] [CrossRef]
- Neckel, N.; Michael, M.; Troeltzsch, D.; Wüster, J.; Koerdt, S.; Doll, C.; Jöhrens, K.; Neumann, K.; Heiland, M.; Raguse, J.-D. Rediscussing the Role of Traditional Risk Factors in Young Adults With Oral Squamous Cell Carcinoma. Anticancer Res. 2020, 40, 6987–6995. [Google Scholar] [CrossRef]
- Gotz, C.; Drecoll, E.; Straub, M.; Bissinger, O.; Wolff, K.D.; Kolk, A. Impact of HPV infection on oral squamous cell carcinoma. Oncotarget 2016, 7, 76704–76712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gustafsson, J.A. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Brunello, A.; Maruzzo, M.; Basso, U.; Basso, S.M. Treatment of estrogen receptor-positive breast cancer. Curr. Med. Chem. 2013, 20, 596–604. [Google Scholar] [CrossRef]
- Hsu, L.H.; Chu, N.M.; Kao, S.H. Estrogen, Estrogen Receptor and Lung Cancer. Int. J. Mol. Sci. 2017, 18, 1713. [Google Scholar] [CrossRef]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Estrogen receptors and their implications in colorectal carcinogenesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef]
- Lan, Y.L.; Zou, S.; Wang, X.; Lou, J.C.; Xing, J.S.; Yu, M.; Zhang, B. Update on the therapeutic significance of estrogen receptor beta in malignant gliomas. Oncotarget 2017, 8, 81686–81696. [Google Scholar] [CrossRef][Green Version]
- Vucicevic Boras, V.; Fucic, A.; Virag, M.; Gabric, D.; Blivajs, I.; Tomasovic-Loncaric, C.; Rakusic, Z.; Bisof, V.; Novere, N.L.; Velimir Vrdoljak, D. Significance of stroma in biology of oral squamous cell carcinoma. Tumori 2018, 104, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Gender-related hormonal risk factors for oral cancer. Pathol. Oncol. Res. 2007, 13, 195–202. [Google Scholar] [CrossRef]
- Satgunaseelan, L.; Allanson, B.M.; Asher, R.; Reddy, R.; Low, H.T.H.; Veness, M.; Gopal Iyer, N.; Smee, R.I.; Palme, C.E.; Gupta, R.; et al. The incidence of squamous cell carcinoma of the oral tongue is rising in young non-smoking women: An international multi-institutional analysis. Oral. Oncol. 2020, 110, 104875. [Google Scholar] [CrossRef] [PubMed]
- Howell, R.E.; Wright, B.A.; Dewar, R. Trends in the incidence of oral cancer in Nova Scotia from 1983 to 1997. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 205–212. [Google Scholar] [CrossRef]
- Ng, J.H.; Iyer, N.G.; Tan, M.H.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2017, 39, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Muscat, J.E.; Richie, J.P., Jr.; Thompson, S.; Wynder, E.L. Gender differences in smoking and risk for oral cancer. Cancer Res. 1996, 56, 5192–5197. [Google Scholar]
- Chang, Y.L.; Hsu, Y.K.; Wu, T.F.; Huang, C.M.; Liou, L.Y.; Chiu, Y.W.; Hsiao, Y.H.; Luo, F.J.; Yuan, T.C. Regulation of estrogen receptor alpha function in oral squamous cell carcinoma cells by FAK signaling. Endocr. Relat. Cancer 2014, 21, 555–565. [Google Scholar] [CrossRef]
- Colella, G.; Izzo, G.; Carinci, F.; Campisi, G.; Lo Muzio, L.; D’Amato, S.; Mazzotta, M.; Cannavale, R.; Ferrara, D.; Minucci, S. Expression of sexual hormones receptors in oral squamous cell carcinoma. Int. J. Immunopathol. Pharm. 2011, 24, 129–132. [Google Scholar] [CrossRef]
- Doll, C.; Arsenic, R.; Lage, H.; Johrens, K.; Hartwig, S.; Nelson, K.; Raguse, J.D. Expression of Estrogen Receptors in OSCC in Relation to Histopathological Grade. Anticancer. Res. 2015, 35, 5867–5872. [Google Scholar]
- Egloff, A.M.; Rothstein, M.E.; Seethala, R.; Siegfried, J.M.; Grandis, J.R.; Stabile, L.P. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6529–6540. [Google Scholar] [CrossRef]
- Ishida, H.; Wada, K.; Masuda, T.; Okura, M.; Kohama, K.; Sano, Y.; Nakajima, A.; Kogo, M.; Kamisaki, Y. Critical role of estrogen receptor on anoikis and invasion of squamous cell carcinoma. Cancer Sci. 2007, 98, 636–643. [Google Scholar] [CrossRef]
- Nelson, K.; Helmstaedter, V.; Lage, H. The influence of tamoxifen on growth behavior and cell-cell adhesion in OSCC in vitro. Oral Oncol. 2007, 43, 720–727. [Google Scholar] [CrossRef]
- Nelson, K.; Helmstaedter, V.; Moreau, C.; Lage, H. Estradiol, tamoxifen and ICI 182,780 alter alpha3 and beta1 integrin expression and laminin-1 adhesion in oral squamous cell carcinoma cell cultures. Oral Oncol. 2008, 44, 94–99. [Google Scholar] [CrossRef]
- Marocchio, L.S.; Giudice, F.; Correa, L.; Pinto Junior Ddos, S.; de Sousa, S.O. Oestrogens and androgen receptors in oral squamous cell carcinoma. Acta Odontol. Scand. 2013, 71, 1513–1519. [Google Scholar] [CrossRef]
- Kwon, S.; Ahn, S.H.; Jeong, W.J.; Jung, Y.H.; Bae, Y.J.; Paik, J.H.; Chung, J.H.; Kim, H. Estrogen receptor alpha as a predictive biomarker for survival in human papillomavirus-positive oropharyngeal squamous cell carcinoma. J. Transl. Med. 2020, 18, 240. [Google Scholar] [CrossRef]
- Grimm, M.; Biegner, T.; Teriete, P.; Hoefert, S.; Krimmel, M.; Munz, A.; Reinert, S. Estrogen and Progesterone hormone receptor expression in oral cavity cancer. Med. Oral Patol. Oral Y Cir. Bucal. 2016, 21, e554–e558. [Google Scholar] [CrossRef][Green Version]
- Lukits, J.; Remenar, E.; Raso, E.; Ladanyi, A.; Kasler, M.; Timar, J. Molecular identification, expression and prognostic role of estrogen- and progesterone receptors in head and neck cancer. Int. J. Oncol. 2007, 30, 155–160. [Google Scholar] [CrossRef][Green Version]
- Akyu, R.; Tomihara, K.; Yamazaki, M.; Moniruzzaman, R.; Heshiki, W.; Sekido, K.; Tachinami, H.; Sakurai, K.; Yonesi, A.; Imaue, S.; et al. Protumor role of estrogen receptor expression in oral squamous cell carcinoma cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B. AJCC Cancer Staging Manual, 7th ed.; Stephen, B.E., Ed.; Springer: New York, NY, USA; London, UK, 2010. [Google Scholar]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der. Pathol. 1987, 8, 138–140. [Google Scholar]
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sanger, J.; Baum, R.P.; Prasad, V.; Hommann, M. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 2012, 5, 187–194. [Google Scholar] [PubMed]
- Naipal, K.A.; Raams, A.; Bruens, S.T.; Brandsma, I.; Verkaik, N.S.; Jaspers, N.G.; Hoeijmakers, J.H.; van Leenders, G.J.; Pothof, J.; Kanaar, R.; et al. Attenuated XPC expression is not associated with impaired DNA repair in bladder cancer. PLoS ONE 2015, 10, e0126029. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 13 April 2021).
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERalpha) and beta (ERbeta): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjanen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef]
- Valimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Makela, S.; Kujari, H.; Gustafsson, J.A.; Laine, M. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef]
- Nebel, D.; Bratthall, G.; Ekblad, E.; Norderyd, O.; Nilsson, B.O. Estrogen regulates DNA synthesis in human gingival epithelial cells displaying strong estrogen receptor beta immunoreactivity. J. Periodontal Res. 2011, 46, 622–628. [Google Scholar] [CrossRef]
- Gourdy, P.; Guillaume, M.; Fontaine, C.; Adlanmerini, M.; Montagner, A.; Laurell, H.; Lenfant, F.; Arnal, J.F. Estrogen receptor subcellular localization and cardiometabolism. Mol. Metab. 2018, 15, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, J.M.; Stabile, L.P. Estrongenic steroid hormones in lung cancer. Semin. Oncol. 2014, 41, 5–16. [Google Scholar] [CrossRef]

| Variable | Primary Tumor (n = 302) | Primary Lymph Node Metastasis (n = 52) | ||||
|---|---|---|---|---|---|---|
| ERα− (n = 291) | ERα+ (n = 11) | p | ERα− (n = 47) | ERα+ (n = 5) | p | |
| Sex | ||||||
| Female | 107 (97.3%) | 3 (2.7%) | 0.521 | 10 (76.9%) | 3 (23.1%) | 0.057 |
| Male | 184 (95.8%) | 8 (4.2%) | 37 (94.9%) | 2 (5.1%) | ||
| Age group | 0.504 | 0.121 | ||||
| ≤49 | 45 (93.8%) | 3 (6.3%) | 10 (100%) | 0 (0%) | ||
| 50–59 | 79 (98.8%) | 1 (1.3%) | 13 (81.3%) | 3 (18.8%) | ||
| 60–69 | 100 (96.2%) | 4 (3.8%) | 18 (94.7%) | 1 (5.3%) | ||
| 70–79 | 52 (94.5%) | 3 (5.5%) | 5 (100%) | 0 (0%) | ||
| 80+ | 15 (100%) | 0 (0%) | 1 (50%) | 1 (50%) | ||
| Tumor localization | 0.006 | 0.336 | ||||
| Floor of mouth | 65 (98.5%) | 1 (1.5%) | 14 (82.4%) | 3 (17.6%) | ||
| Maxilla | 13 (100%) | 0 (0%) | - | - | ||
| Mandible | 27 (84.4%) | 5 (15.6%) | 4 (80%) | 1 (20%) | ||
| Cheek | 13 (100%) | 0 (0%) | - | - | ||
| Tongue | 80 (98.8%) | 1 (1.2%) | 8 (100%) | 0 (0%) | ||
| Multiple localizations | 93 (95.9%) | 4 (4.1%) | 21 (95.5%) | 1 (4.5%) | ||
| History of Smoking | 0.216 | 0.459 | ||||
| Yes | 195 (95.1%) | 10 (4.9%) | 39 (90.7%) | 4 (9.3%) | ||
| No | 67 (98.5%) | 1 (1.5%) | 4 (80.0%) | 1 (20.0%) | ||
| History of Alcohol | 0.681 | 0.851 | ||||
| Yes | 202 (96.2%) | 8 (3.8%) | 35 (89.7%) | 4 (10.3%) | ||
| No | 57 (95.0%) | 3 (5.0%) | 7 (87.5%) | 1 (12.5%) | ||
| G-Stage | 0.950 | 0.891 | ||||
| 1 | 19 (95.0%) | 1 (5.0%) | 2 (100%) | 0 (0%) | ||
| 2 | 215 (96.4%) | 8 (3.6%) | 28 (90.3%) | 3 (9.7%) | ||
| 3 | 53 (96.4%) | 2 (3.6%) | 17 (89.5%) | 2 (10.5%) | ||
| pT | 0.619 | 0.362 | ||||
| 1 | 124 (97.6%) | 3 (2.4%) | 12 (80.0%) | 3 (20.0%) | ||
| 2 | 109 (95.6%) | 5 (4.4%) | 16 (94.1%) | 1 (5.9%) | ||
| 3 | 33 (97.1%) | 1 (2.9%) | 10 (100%) | 0 (0%) | ||
| 4a | 21 (91.3%) | 2 (8.7%) | 9 (90%) | 1 (10%) | ||
| x | 4 (100%) | 0 (0%) | ||||
| pN | 0.092 | - | ||||
| N0 | 187 (97.4%) | 5 (2.6%) | - | - | ||
| N1/N2 | 82 (93.2%) | 6 (6.8%) | 47 (90.4%) | 5 (9.6%) | ||
| UICC | 0.026 | 0.198 | ||||
| I | 97 (100%) | 0 (0%) | - | - | ||
| II | 75 (96.2%) | 3 (3.8%) | - | - | ||
| III | 49 (96.1%) | 2 (3.9%) | 12 (100%) | 0 (0%) | ||
| IV | 58 (90.6%) | 6 (9.4%) | 35 (87.5%) | 5 (12.5%) | ||
| Extracapsular spread (ECS) | 0.204 | 0.704 | ||||
| N− | 187 (97.4%) | 5 (2.6%) | - | - | ||
| N+ ECS− | 47 (92.2%) | 4 (7.8%) | 23 (92.0%) | 2 (8.0%) | ||
| N+ ECS+ | 35 (94.6%) | 2 (5.4%) | 24 (88.9%) | 3 (11.1%) | ||
| Bony infiltration | 0.049 | 0.913 | ||||
| no | 231 (97.5%) | 6 (2.5%) | 27 (90.0%) | 3 (10.0%) | ||
| yes | 60 (92.3%) | 5 (7.7%) | 20 (90.9%) | 2 (9.1%) | ||
| No | Gender | Age | Localization | TNM | Grading | R-Status | ERα IRS | Smoking | Alcohol | Adjuvant Therapy | Survival (Months) | Time to Recurrence (Months) | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male | 66 | Lower jaw | pT1pN1 cM0 | G2 | R0 | 2.00 | + | + | − | 75 | None | |
| 2 | male | 64 | Floor of mouth | pT2pN1 cM0 | G2 | R0 cm * | 2.00 | + | − | + | 8 (alive) | None | Lost to follow-up after 8 months. |
| 3 | male | 49 | Floor of mouth, tongue | pT2pN0 cM0 | G2 | R0 | 2.00 | + | + | − | 83 (alive) | Patient lost to clinical follow-up | Micrometastasis in one lymph node. The patient did not show up for regular clinical follow-up. However, the patient was still alive after 83 months. |
| 4 | male | 39 | Lower jaw | pT1pN2b cM0 | G3 | R0 * | 6.00 | + | + | (+) | 8 | 3 | Patient initially refused recommendation for adj. therapy. It was carried out 3 months after surgical therapy due to recurrence. |
| 5 | male | 42 | Tongue, floor of mouth | pT2pN2b cM0 | G2 | R0 | 2.00 | + | + | + | 9 | 4 | |
| 6 | female | 63 | Lower jaw | pT4apN0 cM0 | G2 | R0; cm (3 mm) | 3.33 | − | − | + | 68 (alive) | 40 | |
| 7 | male | 57 | Tongue | pT2pN0 cM0 | G2 | R0 | 2.00 | + | − | − | 16 | 4 | |
| 8 | male | 75 | Lower jaw | pT1pN2b cM0 | G2 | R0 * | 3.00 | + | + | (+) | 38 | 4 | Adjuvant therapy was carried out after nodal recurrence about 4 months after surgical therapy. Initially, it could not be performed due to a strongly reduced general condition. |
| 9 | female | 67 | Lower jaw, floor of mouth | pT3pN2c cM0 | G2 | R0; cm * | 3.00 | + | + | + | 127 (alive) | None | ERα IRS of corresponding lymph node metastasis: 0.5 |
| 10 | female | 71 | Lower jaw | pT2pN0 cM0 | G1 | R0 * | 2.00 | + | + | − | 48 | 47 | |
| 11 | male | 70 | Lower jaw, floor of mouth | pT4apN0 cM1 | G3 | R0; cm (4 mm) | 2.00 | + | + | − | 2 | None | Patient died within the inpatient stay after surgical therapy 2 months after first diagnosis. |
| No | Gender | Age | ERα IRS | Localization | Grading | R-Status | pTNM | Smoking | Alcohol | Adjuvant Therapy | Survival (Months) | Time to Recurrence (Months) | Comment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymph Node Metastasis | Primary Tumor | |||||||||||||
| 1 | female | 53 | 5.00 | 1.00 | Floor of mouth | G2 | R0; cm (4 mm) | pT1 pN2b cM0 | + | + | + | 69 (alive) | none | |
| 2 | male | 51 | 3.00 | 0 | Floor of mouth | G2 | R0; cm** | pT1 pN2c cM0 | + | + | + | 30 | none | |
| 3 | male | 51 | 3.75 | * | Lower jaw | G3 | R0 ** | pT2 pN2b cM0 | + | + | + | 13 | none | Adj. therapy was delayed due to a strongly reduced general condition. It was carried out 2.5 months after surgical approach. |
| 4 | female | 62 | 10.00 | 1.33 | Floor of mouth, lower jaw, tongue | G2 | R1 | pT4a pN2c cM0 | + | + | + | 16 | 10 | |
| 5 | female | 80 | 5.00 | 0 | Floor of mouth | G3 | R0; cm (4 mm) | pT1 pN2b cM0 | − | − | − | 76 | Patient lost to clinical follow-up | Patient refused recommendation for adj. therapy. The patient did not show up for regular clinical follow-up. However, the patient died 76 months after first diagnosis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doll, C.; Bestendonk, C.; Kreutzer, K.; Neumann, K.; Pohrt, A.; Trzpis, I.; Koerdt, S.; Dommerich, S.; Heiland, M.; Raguse, J.-D.; et al. Prognostic Significance of Estrogen Receptor Alpha in Oral Squamous Cell Carcinoma. Cancers 2021, 13, 5763. https://doi.org/10.3390/cancers13225763
Doll C, Bestendonk C, Kreutzer K, Neumann K, Pohrt A, Trzpis I, Koerdt S, Dommerich S, Heiland M, Raguse J-D, et al. Prognostic Significance of Estrogen Receptor Alpha in Oral Squamous Cell Carcinoma. Cancers. 2021; 13(22):5763. https://doi.org/10.3390/cancers13225763
Chicago/Turabian StyleDoll, Christian, Carolin Bestendonk, Kilian Kreutzer, Konrad Neumann, Anne Pohrt, Irena Trzpis, Steffen Koerdt, Steffen Dommerich, Max Heiland, Jan-Dirk Raguse, and et al. 2021. "Prognostic Significance of Estrogen Receptor Alpha in Oral Squamous Cell Carcinoma" Cancers 13, no. 22: 5763. https://doi.org/10.3390/cancers13225763
APA StyleDoll, C., Bestendonk, C., Kreutzer, K., Neumann, K., Pohrt, A., Trzpis, I., Koerdt, S., Dommerich, S., Heiland, M., Raguse, J.-D., & Jöhrens, K. (2021). Prognostic Significance of Estrogen Receptor Alpha in Oral Squamous Cell Carcinoma. Cancers, 13(22), 5763. https://doi.org/10.3390/cancers13225763








