Diabetes and Cancer: Risk, Challenges, Management and Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Literature Search
3. Classification
4. Epidemiology
5. Link between Diabetes and Cancer
5.1. Mechanism of Cancer Risk in Diabetes
5.2. Role of the Microbiome in Cancer and Diabetes
5.3. Epidemiological Link between Diabetes and Cancer
5.4. Diabetic Pharmacotherapy and Risk of Cancer
6. Diabetes Mellitus & Cancer Outcomes
7. Anti-Cancer Therapy & Hyperglycemia
7.1. Immunotherapy
7.2. Targeted Therapy
7.2.1. PI3K/mTOR Inhibition
Mammalian Target of Rapamycin (mTOR) Inhibitors
Phosphatidylinositol 3-Kinase (PI3K) Inhibitors
AKT Inhibitors
7.2.2. Insulin-like Growth Factor Type 1 Receptor (IGF-1R)
IGF-1R Monoclonal Antibodies
Small Molecule Inhibitors of IGF-IR and the Insulin Receptor
Other IGF-1R Inhibitors
7.2.3. Epidermal Growth Factor Receptor (EGFR) Inhibitors
7.2.4. BCR-ABL Multi-Targeted Tyrosine-Kinase Inhibitors
7.3. Hormone Therapy
7.3.1. Anti-Estrogen Therapy
7.3.2. Androgen Deprivation Therapy
7.3.3. Somatostatin Analogs
7.4. Corticosteroids
7.5. Miscellaneous Compounds
8. Factors That Influence the Choice of Cancer Therapy in Patients with Diabetes
8.1. Renal Disease
8.2. Cardiovascular Disease
8.3. Neuropathy
9. Screening & Diagnosis of Diabetes in Cancer Patients
10. Management of Diabetes in Cancer Patients
10.1. Multidisciplinary Management
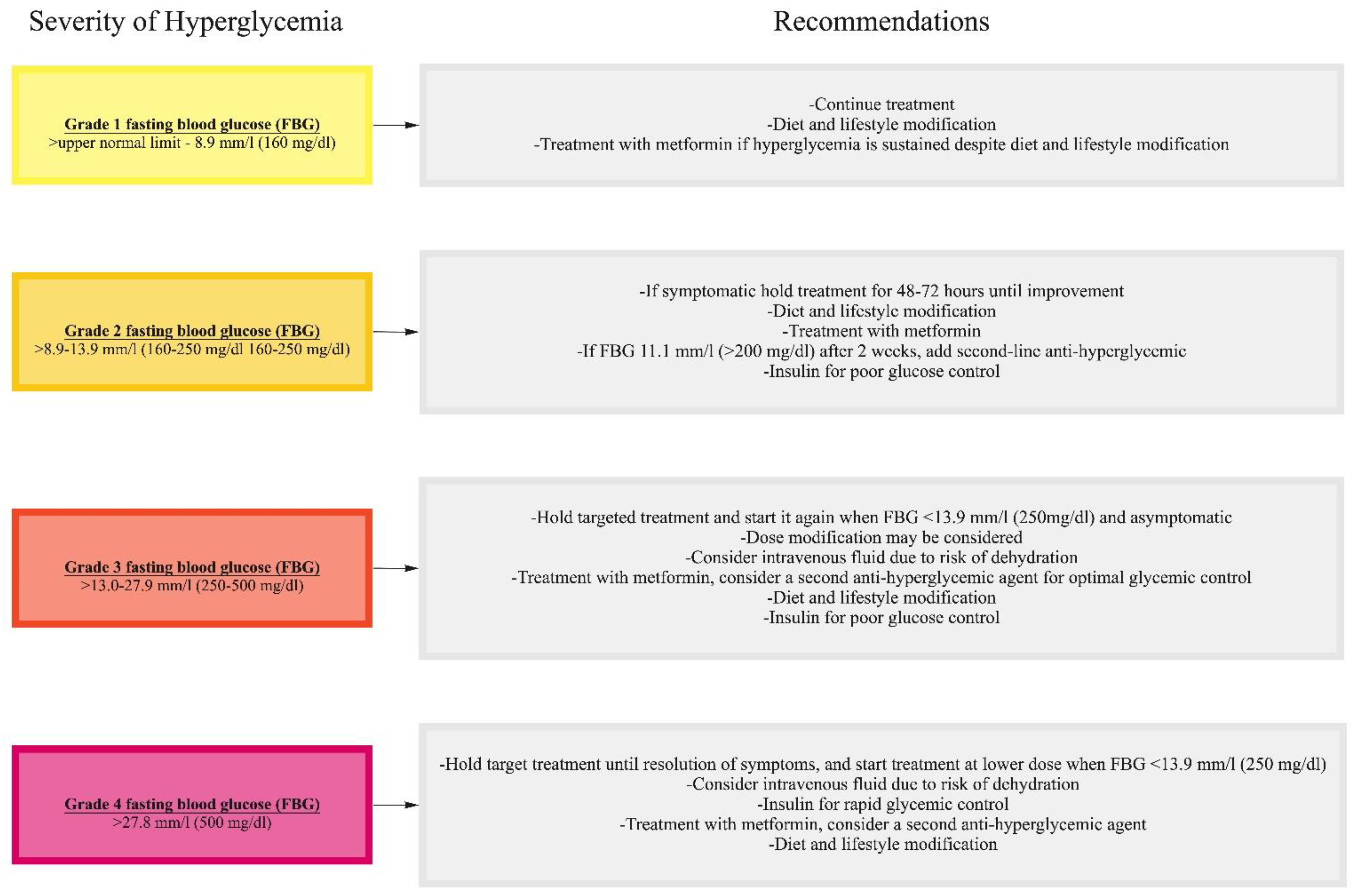
10.2. Management of Hyperglycemia
10.3. Management of Fluid and Electrolyte Balance
10.4. Management of Hypertension
10.5. Management of Cardiovascular Complications
10.6. Management of Infection
10.7. Management of Diabetic Autonomic Neuropathy of the Gastrointestinal Tract
11. Survivorship Care
12. Future Directions
13. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. About “NIOSH”. Available online: https://www.cdc.gov/niosh/about/default.html (accessed on 21 September 2020).
- Liu, J.; Ren, Z.H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the incidence of diabetes mellitus: Results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef]
- Ling, S.; Brown, K.; Miksza, J.K.; Howells, L.; Morrison, A.; Issa, E.; Yates, T.; Khunti, K.; Davies, M.J.; Zaccardi, F. Association of Type 2 Diabetes with Cancer: A Meta-analysis with Bias Analysis for Unmeasured Confounding in 151 Cohorts Comprising 32 Million People. Diabetes Care 2020, 43, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, H.H.; Rawshani, A.; Rawshani, A.; Franzén, S.; Svensson, A.-M.; Sattar, N.; Gudbjörnsdottir, S. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Clore, J.N.; Thurby-Hay, L. Glucocorticoid-Induced Hyperglycemia. Endocr. Pract. 2009, 15, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Vudattu, N.; Sznol, M.; Gettinger, S.; Kluger, H.; Lupsa, B.; Herold, K.C. Precipitation of Autoimmune Diabetes with Anti-PD-1 Immunotherapy. Diabetes Care 2015, 38, e55–e57. [Google Scholar] [PubMed]
- Morviducci, L.; Rota, F.; Rizza, L.; Di Giacinto, P.; Ramponi, S.; Nardone, M.; Tubili, C.; Lenzi, A.; Zuppi, P.; Baldelli, R. Everolimus is a new anti-cancer molecule: Metabolic side effects as lipid disorders and hyperglycemia. Diabetes Res. Clin. Pract. 2018, 143, 428–431. [Google Scholar] [CrossRef]
- Coughlin, S.S.; E Calle, E.; Teras, L.R.; Petrelli, J.; Thun, M.J. Diabetes Mellitus as a Predictor of Cancer Mortality in a Large Cohort of US Adults. Am. J. Epidemiol. 2004, 159, 1160–1167. [Google Scholar] [CrossRef]
- Saydah, S.H.; Loria, C.M.; Eberhardt, M.S.; Brancati, F.L. Abnormal glucose tolerance and the risk of cancer death in the United States. Am. J. Epidemiol. 2003, 157, 1092–1100. [Google Scholar] [CrossRef]
- Liu, X.; Ji, J.; Sundquist, K.; Sundquist, J.; Hemminki, K. The impact of type 2 diabetes mellitus on cancer-specific survival: A follow-up study in Sweden. Cancer 2012, 118, 1353–1361. [Google Scholar] [CrossRef]
- Hansen, L.J.; Olivarius, N.D.F.; Siersma, V. 16-year excess all-cause mortality of newly diagnosed type 2 diabetic patients: A cohort study. BMC Public Health 2009, 9, 400. [Google Scholar] [CrossRef]
- Jansson, S.P.; Andersson, D.K.; Svardsudd, K. Mortality Trends in Subjects with and without Diabetes during 33 Years of Follow-up. Diabetes Care 2010, 33, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Salehidoost, R.; Mansouri, A.; Amini, M.; Yamini, S.A.; Aminorroaya, A. Diabetes and all-cause mortality, a 18-year follow-up study. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- van de Poll-Franse, L.V.; Houterman, S.; Janssen-Heijnen, M.L.; Dercksen, M.W.; Coebergh, J.W.; Haak, H.R. Less aggressive treatment and worse overall survival in cancer patients with diabetes: A large population based analysis. Int. J. Cancer 2007, 120, 1986–1992. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2016, 66, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ariaans, G.; de Jong, S.; Gietema, J.; Lefrandt, J.; de Vries, E.; Jalving, M. Cancer-drug induced insulin resistance: Innocent bystander or unusual suspect. Cancer Treat. Rev. 2015, 41, 376–384. [Google Scholar] [CrossRef]
- Gallo, M.; Muscogiuri, G.; Felicetti, F.; Faggiano, A.; Trimarchi, F.; Arvat, E.; Vigneri, R.; Colao, A. Adverse glycaemic effects of cancer therapy: Indications for a rational approach to cancer patients with diabetes. Metabolism 2018, 78, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, N.; Argentiero, A.; Beretta, G.D.; Di Bartolo, P.; Montagnani, M.; Danesi, R.; Ferrari, P.; D’Oronzo, S.; Gori, S.; Russo, A.; et al. Management of metabolic adverse events of targeted therapies and immune checkpoint inhibitors in cancer patients: An Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. Crit. Rev. Oncol. Hematol. 2020, 154, 103066. [Google Scholar]
- A Hart, P.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; E Forsmark, C.; O Goodarzi, M.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, F.B. The global implications of diabetes and cancer. Lancet 2014, 383, 1947–1948. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in Older Adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Ransom, J.; de Andrade, M.; Petersen, G.M. Probability of Pancreatic Cancer Following Diabetes: A Population-Based Study. Gastroenterology 2005, 129, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Kim, K.-W. Diabetes and Cancer: Cancer Should Be Screened in Routine Diabetes Assessment. Diabetes Metab. J. 2019, 43, 733. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, L.; Vigneri, R.; Tumminia, A.; Frasca, F.; Squatrito, S.; Frittitta, L. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 808–815. [Google Scholar] [CrossRef]
- Hirsch, I.B.; Juneja, R.; Beals, J.M.; Antalis, C.J.; E Wright, E. The Evolution of Insulin and How it Informs Therapy and Treatment Choices. Endocr. Rev. 2020, 41, 733–755. [Google Scholar] [CrossRef]
- Giovannucci, E.L.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Liao, Z. Diabetes and cancer: Epidemiological and biological links. World J. Diabetes 2020, 11, 227–238. [Google Scholar] [CrossRef]
- Duan, W.; Shen, X.; Lei, J.; Xu, Q.; Yu, Y.; Li, R.; Wu, E.; Ma, Q. Hyperglycemia, a Neglected Factor during Cancer Progression. BioMed Res. Int. 2014, 2014, 461917. [Google Scholar] [CrossRef]
- Masur, K.; Vetter, C.; Hinz, A.; Tomas, N.; Henrich, H.; Niggemann, B.; Zänker, K.S. Diabetogenic glucose and insulin concentrations modulate transcriptom and protein levels involved in tumour cell migration, adhesion and proliferation. Br. J. Cancer 2010, 104, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.M.; de Sousa, F.R.; Barbosa, A.L.; Martins, S.C.; Araújo, R.L.; Soares, R.; Abreu, C. Metabolic syndrome and risk of cancer: Which link? Metabolism 2015, 64, 182–189. [Google Scholar] [CrossRef]
- Lee, K.A.; Luong, M.K.; Shaw, H.; Nathan, P.; Bataille, V.; Spector, T.D. The gut microbiome: What the oncologist ought to know. Br. J. Cancer 2021, 125, 1197–1209. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Kounatidis, D.; Christodoulatos, G.; Panagopoulos, F.; Karampela, I.; Dalamaga, M. Mycobiome and Cancer: What Is the Evidence? Cancers 2021, 13, 3149. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef]
- Chung, L.-M.; Liang, J.-A.; Lin, C.-L.; Sun, L.-M.; Kao, C.-H. Cancer risk in patients with candidiasis: A nationwide population-based cohort study. Oncotarget 2017, 8, 63562–63573. [Google Scholar] [CrossRef]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar]
- Carstensen, B.; Read, S.H.; Friis, S.; Sund, R.; Keskimäki, I.; Svensson, A.-M.; Ljung, R.; Wild, S.H.; Kerssens, J.J.; Harding, J.L.; et al. Diabetes and Cancer Research Consortium. Cancer incidence in persons with type 1 diabetes: A five-country study of 9000 cancers in type 1 diabetic individuals. Diabetologia 2016, 59, 980–988. [Google Scholar] [CrossRef]
- Harding, J.L.; Shaw, J.E.; Peeters, A.; Cartensen, B.; Magliano, D.J. Cancer Risk among People with Type 1 and Type 2 Diabetes: Disentangling True Associations, Detection Bias, and Reverse Causation. Diabetes Care 2014, 38, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Stocks, T.; Rapp, K.; Bjørge, T.; Stocks, T.; Rapp, K.; Bjørge, T.; Manjer, J.; Ulmer, H.; Selmer, R.; Lukanova, A.; et al. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (Me-Can): Analysis of six prospective cohorts. PLoS Med. 2009, 6, 100020. [Google Scholar] [CrossRef]
- Cannata, D.; Fierz, Y.; Vijayakumar, A.; Le Roith, D. Type 2 diabetes and cancer: What is the connection? Mt. Sinai J. Med. 2010, 77, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, X.; Ma, Y.; Yuan, C.; Wang, M.; Wu, K.; Tabung, F.K.; Tobias, D.; Hu, F.B.; Giovannucci, E.; et al. Incident Type 2 Diabetes Duration and Cancer Risk: A Prospective Study in Two US Cohorts. J. Natl. Cancer Inst. 2021, 113, 381–389. [Google Scholar] [CrossRef] [PubMed]
- A Davila, J.; O Morgan, R.; Shaib, Y.; A McGlynn, K.; El-Serag, H.B. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut 2005, 54, 533–539. [Google Scholar] [CrossRef]
- Boyle, P.; Boniol, M.; Koechlin, A.; Robertson, C.; Valentini, F.; Coppens, K.; Fairley, L.-L.; Zheng, T.; Zhang, Y.; Pasterk, M.; et al. Diabetes and breast cancer risk: A meta-analysis. Br. J. Cancer 2012, 107, 1608–1617. [Google Scholar] [CrossRef]
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937. [Google Scholar] [CrossRef]
- Wang, F.; Gupta, S.; Holly, E.A. Diabetes Mellitus and Pancreatic Cancer in a Population-Based Case-Control Study in the San Francisco Bay Area, California. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1458–1463. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; E Ntzani, E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Currie, C.J.; Johnson, J. The safety profile of exogenous insulin in people with type 2 diabetes: Justification for concern. Diabetes Obes. Metab. 2011, 14, 1–4. [Google Scholar] [CrossRef]
- Smith, U.; Gale, E.A.M. Does diabetes therapy influence the risk of cancer? Diabetology 2009, 52, 1699–1708. [Google Scholar] [CrossRef]
- ORIGIN Trial Investigators; Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Díaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar]
- Home, P.; DeFronzo, R.A.; Eldor, R.; Abdul-Ghani, M. Insulin Therapy and Cancer. Diabetes Care 2013, 36, S240–S244. [Google Scholar] [CrossRef]
- Shlomai, G.; Neel, B.; Leroith, D.; Gallagher, E.J. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J. Clin. Oncol. 2016, 34, 4261–4269. [Google Scholar] [CrossRef] [PubMed]
- Boniol, M.; Franchi, M.; Bota, M.; Leclercq, A.; Guillaume, J.; van Damme, N.; Corrao, G.; Autier, P.; Boyle, P. Incretin-Based Therapies and the Short-term Risk of Pancreatic Cancer: Results From Two Retrospective Cohort Studies. Diabetes Care 2017, 41, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Forsmark, C.E. Incretins, Diabetes, Pancreatitis and Pancreatic Cancer: What the GI specialist needs to know. Pancreatology 2016, 16, 10–13. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Tianpei, H.; Yang, J.; Lu, R.; Zhan, S.; Haukka, J.; Hong, T. Incretin-based therapies and risk of pancreatic cancer in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2018, 20, 910–920. [Google Scholar] [CrossRef]
- Morales, D.R.; Morris, A.D. Metformin in Cancer Treatment and Prevention. Annu. Rev. Med. 2015, 66, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Rizos, C.V.; Elisaf, M.S. Metformin and cancer. Eur. J. Pharmacol. 2013, 705, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Long-term All-Cause Mortality in Cancer Patients With Preexisting Diabetes Mellitus. A Systematic Review and Meta-analysis. JAMA 2008, 300, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Trudeau, M.E.; Koo, J.; Madarnas, Y.; Hartwick, W.; Hoffman, B.; Hood, N. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J. Clin. Oncol. 2002, 20, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Seshasai, S.R.K.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar]
- Zhou, X.H.; Qiao, Q.; Zethelius, B.; Pyörälä, K.; Söderberg, S.; Pajak, A.; Stehouwer, C.D.A.; Heine, R.J.; Jousilahti, P.; Ruotolo, G.; et al. DECODE Study Group. Diabetes, prediabetes and cancer mortality. Diabetology 2010, 53, 1867–1876. [Google Scholar] [CrossRef]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Postoperative Mortality in Cancer Patients With Preexisting Diabetes: Systematic review and meta-analysis. Diabetes Care 2010, 33, 931–939. [Google Scholar] [CrossRef]
- Bergen, E.S.; Christou, N.; Malicot, K.L.; Canton, C.; Di Batolomeo, M.; Galli, F.; Galli, F.; Labianca, R.; Shi, Q.; Alberts, S.R.; et al. 391MO Impact of diabetes and metformin use on recurrence and outcome in early colon cancer (CC) patients—A pooled analysis of 3 adjuvant trials. Ann. Oncol. 2021, 32, S530–S582. [Google Scholar] [CrossRef]
- Bertoni, A.G.; Saydah, S.; Brancati, F.L. Diabetes and the Risk of Infection-Related Mortality in the U.S. Diabetes Care 2001, 24, 1044–1049. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Isaacs, S.D.; Bazargan, N.; You, X.; Thaler, L.M.; Kitabchi, A.E. Hyperglycemia: An Independent Marker of In-Hospital Mortality in Patients with Undiagnosed Diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 978–982. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Macdonald, J.S.; Benson, A.B., 3rd; Fuchs, C.S. Impact of diabetes mellitus on outcomes in patients with colon cancer. J. Clin. Oncol. 2003, 21, 433–440. [Google Scholar] [CrossRef]
- Shariff, A.I.; Syed, S.; A Shelby, R.; Force, J.; Clarke, J.M.; D’Alessio, D.; Corsino, L. Novel cancer therapies and their association with diabetes. J. Mol. Endocrinol. 2019, 62, R187–R199. [Google Scholar] [CrossRef]
- Liu, M.; Guo, F. Recent updates on cancer immunotherapy. Precis. Clin. Med. 2018, 1, 65–74. [Google Scholar] [CrossRef]
- Padma, V.V. An overview of targeted cancer therapy. BioMedicine 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.X.; Loh, K.; Yap, Y.-S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol. Med. 2015, 12, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.Y.; Shameem, R.; Wu, S. Risk of hyperglycemia attributable to everolimus in cancer patients: A meta-analysis. Acta Oncol. 2016, 55, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Szczylik, C.; Feingold, J.; Strahs, A.; Berkenblit, A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Ann. Oncol. 2008, 19, 1387–1392. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; et al. SOLAR-1 Study Group. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Doi, T.; Fujiwara, Y.; Matsubara, N.; Tomomatsu, J.; Iwasa, S.; Tanaka, A.; Endo-Tsukude, C.; Nakagawa, S.; Takahashi, S. Phase I study of ipatasertib as a single agent and in combination with abiraterone plus prednisolone in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2019, 84, 393–404. [Google Scholar] [CrossRef]
- Smyth, L.M.; Tamura, K.; Oliveira, M.; Ciruelos, E.M.; Mayer, I.A.; Sablin, M.-P.; Biganzoli, L.; Ambrose, H.J.; Ashton, J.; Barnicle, A.; et al. Capivasertib, an AKT Kinase Inhibitor, as Monotherapy or in Combination with Fulvestrant in Patients with AKT1E17K-Mutant, ER-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2020, 26, 3947–3957. [Google Scholar] [CrossRef]
- van der Veeken, J.; Oliveira, S.; Schiffelers, R.M.; Storm, G.; van Bergen En Henegouwen, P.M.; Roovers, R.C. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: Implications for cancer therapy. Curr. Cancer Drug Targets 2009, 9, 748–760. [Google Scholar] [CrossRef]
- Pollack, M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Becerra, C.R.; Salazar, R.; Garcia-Carbonero, R.; Thomas, A.L.; Vázquez-Mazón, F.J.; Cassidy, J.; Maughan, T.; Castillo, M.G.; Iveson, T.; Yin, N.; et al. Figitumumab in patients with refractory metastatic colorectal cancer previously treated with standard therapies: A nonrandomized, open-label, phase II trial. Cancer Chemother. Pharmacol. 2014, 73, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Berlin, J.; Gordon, M.; Lorusso, P.; Tang, S.; Dontabhaktuni, A.; Schwartz, J.D.; Cosaert, J.; Mehnert, J.M. Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors. Investig. New Drugs 2015, 33, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, F.; Kim, T.Y.; Cunningham, D.; Tabernero, J.; Schmoll, H.J.; Roh, J.K.; Kim, S.Y.; Park, Y.S.; Guren, T.K.; Hawkes, E.; et al. A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRASWild-Type, Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2015, 107, djv258. [Google Scholar] [CrossRef] [PubMed]
- Scartozzi, M.; Bianconi, M.; Maccaroni, E.; Giampieri, R.; Berardi, R.; Cascinu, S. Dalotuzumab, a recombinant humanized mAb targeted against IGFR1 for the treatment of cancer. Curr. Opin. Mol. Ther. 2010, 12, 361–371. [Google Scholar]
- Fassnacht, M.; Berruti, A.; Baudin, E.; Demeure, M.J.; Gilbert, J.; Haak, H.; Kroiss, M.; Quinn, D.I.; Hesseltine, E.; Ronchi, C.L.; et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015, 16, 426–435. [Google Scholar] [CrossRef]
- Goyal, L.; Wadlow, R.; Blaszkowsky, L.S.; Wolpin, B.M.; Abrams, T.A.; McCleary, N.J.; Sheehan, S.; Sundaram, E.; Karol, M.D.; Chen, J.; et al. A phase I and pharmacokinetic study of ganetespib (STA-9090) in advanced hepatocellular carcinoma. Investig. New Drugs 2015, 33, 128–137. [Google Scholar] [CrossRef]
- Pillai, R.N.; Fennell, D.A.; Kovcin, V.; Ciuleanu, T.E.; Ramlau, R.; Kowalski, D.; Schenker, M.; Yalcin, I.; Teofilovici, F.; Vukovic, V.M. Randomized Phase III Study of Ganetespib, a Heat Shock Protein 90 Inhibitor, With Docetaxel Versus Docetaxel in Advanced Non-Small-Cell Lung Cancer (GALAXY-2). J. Clin. Oncol. 2020, 38, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.-W.; Mehra, R.; Tan, D.S.; Felip, E.; Chow, L.Q.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; et al. Ceritinib inALK-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef]
- Dassonville, O.; Bozec, A.; Fischel, J.L.; Milano, G. EGFR targeting therapies: Monoclonal antibodies versus tyrosine kinase inhibitors: Similarities and differences. Crit. Rev. Oncol. 2007, 62, 53–61. [Google Scholar] [CrossRef]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017, 36, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Soria, J.-C.; Goldman, J.W.; Wakelee, H.A.; Gadgeel, S.M.; Varga, A.; Papadimitrakopoulou, V.; Solomon, B.J.; Oxnard, G.R.; Dziadziuszko, R.; et al. Rociletinib in EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, J.; Huang, X.; Jiang, Q. Adverse effects of dasatinib on glucose-lipid metabolism in patients with chronic myeloid leukaemia in the chronic phase. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Saglio, G.; Kim, D.-W.; Issaragrisil, S.; Le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.; et al. Nilotinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Racil, Z.; Razga, F.; Drapalova, J.; Buresova, L.; Zackova, D.; Palackova, M.; Semerad, L.; Malaskova, L.; Haluzik, M.; Mayer, J. Mechanism of impaired glucose metabolism during nilotinib therapy in patients with chronic myelogenous leukemia. Haematology 2013, 98, e124–e126. [Google Scholar] [CrossRef]
- Gibb, F.W.; Dixon, J.M.; Clarke, C.; Homer, N.Z.; Faqehi, A.M.M.; Andrew, R.; Walker, B.R. Higher Insulin Resistance and Adiposity in Postmenopausal Women with Breast Cancer Treated with Aromatase Inhibitors. J. Clin. Endocrinol. Metab. 2019, 104, 3670–3678. [Google Scholar] [CrossRef]
- Hamood, R.; Hamood, H.; Merhasin, I.; Keinan-Boker, L. Diabetes after Hormone Therapy in Breast Cancer Survivors: A Case-Cohort Study. J. Clin. Oncol. 2018, 36, 2061–2069. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Zhao, L.; Chen, X.; Zhao, J. Androgen deprivation therapy is associated with diabetes: Evidence from meta-analysis. J. Diabetes Investig. 2016, 7, 629–636. [Google Scholar] [CrossRef]
- Yu, I.-C.; Lin, H.-Y.; Sparks, J.; Yeh, S.; Chang, C. Androgen Receptor Roles in Insulin Resistance and Obesity in Males: The Linkage of Androgen-Deprivation Therapy to Metabolic Syndrome. Diabetes 2014, 63, 3180–3188. [Google Scholar] [CrossRef]
- Angeletti, S.; Corleto, V.D.; Schillaci, O.; Marignani, M.; Annibale, B.; Moretti, A.; Silecchia, G.; Scopinaro, F.; Basso, N.; Bordi, C.; et al. Use of the somatostatin analogue octreotide to localise and manage somatostatin-producing tumours. Gut 1998, 42, 792–794. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, P.J.; Kris, M.G.; Basch, E.; Bohlke, K.; Barbour, S.Y.; Clark-Snow, R.A.; Danso, M.A.; Dennis, K.; Dupuis, L.L.; Dusetzina, S.B.; et al. Antiemetics: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 2782–2797. [Google Scholar] [CrossRef]
- Ly, K.I.; Wen, P.Y. Clinical Relevance of Steroid Use in Neuro-Oncology. Curr. Neurol. Neurosci. Rep. 2017, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.I.; Cidlowski, J.A. Corticosteroids in the Treatment of Neoplasms. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., III, Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Barbour, S.Y. Corticosteroids in the Treatment of Chemotherapy-Induced Nausea and Vomiting. J. Natl. Compr. Cancer Netw. 2012, 10, 493–499. [Google Scholar] [CrossRef]
- Haywood, A.; Good, P.; Khan, S.; Leupp, A.; Jenkins-Marsh, S.; Rickett, K.; Hardy, J.R. Corticosteroids for the management of cancer-related pain in adults. Cochrane Database Syst. Rev. 2015, 2021, CD010756. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Shafiq, I. Diabetic ketoacidosis following PEG-asparaginase therapy. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 18-0064. [Google Scholar] [CrossRef]
- Komatsu, Y.; Nakamura, A.; Takihata, M.; Inoue, Y.; Yahagi, S.; Tajima, K.; Tsuchiya, H.; Takano, T.; Yamakawa, T.; Yoshida, M.; et al. Safety and tolerability of diazoxide in Japanese patients with hyperinsulinemic hypoglycemia. Endocr. J. 2016, 63, 311–314. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2015, 92, 63–69. [Google Scholar] [CrossRef]
- Bennis, Y.; Savry, A.; Rocca, M.; Gauthier-Villano, L.; Pisano, P.; Pourroy, B. Cisplatin dose adjustment in patients with renal impairment, which recommendations should we follow? Int. J. Clin. Pharm. 2014, 36, 420–429. [Google Scholar] [CrossRef]
- Poole, C.; Gardiner, J.; Twelves, C.; Johnston, P.; Harper, P.; Cassidy, J.; Monkhouse, J.; Banken, L.; Weidekamm, E.; Reigner, B. Effect of renal impairment on the pharmacokinetics and tolerability of capecitabine (Xeloda) in cancer patients. Cancer Chemother. Pharmacol. 2002, 49, 225–234. [Google Scholar] [CrossRef]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015, 12, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sami, A.; Xiang, J. HER2-directed therapy: Current treatment options for HER2-positive breast cancer. Breast Cancer 2015, 22, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic neuropathy: Mechanisms to management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Zukas, A.M.; Schiff, D. Neurological complications of new chemotherapy agents. Neuro-Oncology 2018, 20, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Gallois, C. Adjuvant Chemotherapy for Stage III Colon Cancer. Cancers 2020, 12, 2679. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Tamez-Pérez, H.E.; Quintanilla-Flores, D.L.; Rodríguez-Gutiérrez, R.; González-González, J.G.; Tamez-Peña, A.L. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J. Diabetes 2015, 6, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.V.; Bishop, G.; Benito-Herrero, M. Diabetic Ketoacidosis in Type 2 Diabetics: A Novel Presentation of Pancreatic Adenocarcinoma. J. Gen. Intern. Med. 2010, 25, 369–373. [Google Scholar] [CrossRef]
- Markabawi, D.; Kondapi, D.; Tambe, V.; Seth, R. When it is not just DKA; diabetic ketoacidosis as a first presentation of pancreatic adenocarcinoma. Am. J. Emerg. Med. 2018, 36, 1720.e1–1720.e2. [Google Scholar] [CrossRef]
- Busaidy, N.L.; Farooki, A.; Dowlati, A.; Perentesis, J.P.; Dancey, J.E.; Doyle, L.A.; Brell, J.M.; Siu, L.L. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J. Clin. Oncol. 2012, 30, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2014, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.J.; Masri, N.; Ghanem, H.; Azar, M. Diabetic Ketoacidosis Associated With Alpelisib Treatment of Metastatic Breast Cancer. AACE Clin. Case Rep. 2020, 6, e349–e351. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Morgan, A.; Shah, S.; Ebeling, P.R. Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 18–0021. [Google Scholar] [CrossRef] [PubMed]
- Kapila, V.; Topf, J. Sodium-Glucose Co-transporter 2 Inhibitor-Associated Euglycemic Diabetic Ketoacidosis after Bariatric Surgery: A Case and Literature Review. Cureus 2021, 13, 17093. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Antonuzzo, A.; Cherny, N.; Rosengarten, O.; Pernot, S.; Trippa, F.; Schuler, U.; Snegovoy, A.; Jordan, K.; Ripamonti, C. Diarrhoea in adult cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv126–iv142. [Google Scholar] [CrossRef] [PubMed]
- Roila, F.; Molassiotis, A.; Herrstedt, J.; Aapro, M.; Gralla, R.J.; Bruera, E.; Clark-Snow, R.A.; Dupuis, L.L.; Einhorn, L.H.; Feyer, P.; et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann. Oncol. 2016, 27, v119–v133. [Google Scholar] [CrossRef]
- Katsi, V.; Magkas, N.; Georgiopoulos, G.; Athanasiadi, E.; Virdis, A.; Masi, S.; Kliridis, P.; Hatziyanni, A.; Tsioufis, C.; Tousoulis, D. Arterial hypertension in patients under antineoplastic therapy: A systematic review. J. Hypertens. 2019, 37, 884–901. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Chang, H.M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 1. J. Am. Coll. Cardiol. 2017, 70, 2536–2551. [Google Scholar] [CrossRef]
- Chang, H.M.; Okwuosa, T.M.; Scarabelli, T.; Moudgil, R.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J. Am. Coll. Cardiol. 2017, 70, 2552–2565. [Google Scholar] [CrossRef]
- Yeh, E.T.; Bickford, C.L. Cardiovascular Complications of Cancer Therapy: Incidence, Pathogenesis, Diagnosis, and Management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Hosking, F.J.; Cook, D.G. Glycemic Control and Risk of Infections Among People With Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care 2018, 41, 2127–2135. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Candida sp. Infections in Patients with Diabetes Mellitus. J. Clin. Med. 2019, 8, 76. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; van de Wetering, M.; Kremer, L.; Leibovici, L. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst. Rev. 2012, 1, CD004386. [Google Scholar] [CrossRef]
- Renner, P.; Milazzo, S.; Liu, J.P.; Zwahlen, M.; Birkmann, J.; Horneber, M. Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database Syst. Rev. 2012, 10, CD007913. [Google Scholar] [CrossRef]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic Neuropathy in Diabetes Mellitus. Front. Endocrinol. 2014, 5, 205. [Google Scholar] [CrossRef]
- Vinik, A.I.; Maser, R.E.; Michell, B.D.; Freeman, R. Diabetic Autonomic Neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Donald, M.; Stankowski, R.V.; Engel, J.M.; Williams, G.; Doi, S. Breast and Prostate Cancer Survivors in a Diabetic Cohort: Results from the Living with Diabetes Study. Clin. Med. Res. 2013, 11, 210–218. [Google Scholar] [CrossRef][Green Version]
- Stava, C.J.; Beck, M.L.; Feng, L.; Lopez, A.; Busaidy, N.; Vassilopoulou-Sellin, R. Diabetes mellitus among cancer survivors. J. Cancer Surviv. 2007, 1, 108–115. [Google Scholar] [CrossRef]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2013, 142, 155–170. [Google Scholar] [CrossRef]
- Edgington, A.; Morgan, M.A. Looking Beyond Recurrence: Comorbidities in Cancer Survivors. Clin. J. Oncol. Nurs. 2011, 15, E3–E12. [Google Scholar] [CrossRef]
- Ligibel, J. Lifestyle Factors in Cancer Survivorship. J. Clin. Oncol. 2012, 30, 3697–3704. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.S.; Grant, S.F.; Herman, M.E. Intersections and Clinical Translations of Diabetes Mellitus with Cancer Promotion, Progression and Prognosis. Postgrad. Med. 2019, 131, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020, 6, 05954. [Google Scholar] [CrossRef] [PubMed]
- Klil-Drori, A.; Azoulay, L.; Pollak, M.N. Cancer, obesity, diabetes, and antidiabetic drugs: Is the fog clearing? Nat. Rev. Clin. Oncol. 2016, 14, 85–99. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef]
| Anticancer Drugs | Mechanism of Action |
|---|---|
| Targeted Agents | |
| IGF-1R inhibitor IGF-1R-specific monoclonal antibodies (dalotuzumab) Small-molecule inhibitors of IGF-1R and IR (linsitinib) Other inhibitors of IGF-1R (ganetespib, ceritinib) | Inhibit IGF-1R, which is partially homologous to the IR, and block the activation of the Ras/MAPK/ERK and PI3K/AKT/mTOR pathways and thereby block cancer cell growth and proliferation |
| PI3K/mTOR inhibition mTOR inhibitors (everolimus, temsirolimus) PI3K3CA inhibitor (alpelisib) AKT inhibitors (ipatasertib, capivasertib) | Inhibit the PI3K/Akt/mTOR pathway and thus interfere with malignant cell growth, but may lead to hyperglycemia by interrupting the intracellular response to insulin, causing decreased glucose transport, decreased glycogen synthesis, and increased glycolysis |
| BCR-ABL inhibitors (nilotinib, dasatinib, and ponatinib) | Multi-targeted TKIs that inhibit BCR-ABL and other TKIs such as KIT, PDGFR, DDR, and CSF-1R |
| Anti-EFGR (rociletinib) | Block EGFR pathway and affect downstream signaling cascades, namely the RAS/MAPK/ERK, PI3K/Akt, and JAK/STAT pathways |
| Immunotherapy | |
| Anti CTLA-4 (ipilimumab) Immune checkpoint inhibitors (nivolumab, pembrolizumab) | Activate T cells and enhance the immune response against malignant cells; treatment could cause autoimmune phenomena including autoimmune diabetes mellitus |
| Hormone Therapy | |
| Somatostatin analogues (octreotide and lanreotide) | Bind predominantly to the somatostatin receptors and suppress insulin secretion |
| Anti-estrogen therapy (tamoxifen, aromatase inhibitors) | Decrease insulin secretion via inhibition of antiapoptotic effects of estradiol on pancreatic β-cells and increase insulin resistance via elevated triglyceride levels and fatty liver |
| Anti-androgen therapy (bicalutamide) | Increase insulin resistance by modulating androgen and androgen receptor signaling pathways in the liver and adipose tissue |
| Miscellaneous | |
| Asparaginase | Hydrolyzes serum asparagine to nonfunctional aspartic acid and ammonia, depriving tumor cells of asparagine |
| Diazoxide | Nondiuretic benzothiadiazine that inhibits insulin secretion |
| Medication | Mechanism of Action |
|---|---|
| Biguanides (metformin) | Improve hepatic insulin resistance via decreasing the hepatic glucose output |
| Second-generation sulfonylureas Glyburide, glipizide, glimepiride | Stimulate endogenous insulin secretion through inhibition of potassium channels in pancreatic cells; most effective in early stages of diabetes when insulin secretion is still working |
| Meglitinides Repaglinide, nateglinide | Insulin secretagogues that stimulate insulin release by inhibiting potassium channels in the pancreas on a different site from sulfonylureas; work much faster than other secretagogues and can be taken more effectively before meals |
| Thiazolidinediones Rosiglitazone, pioglitazone | Activate PPARG and improve metabolic control in type 2 diabetes through the improvement of insulin sensitivity in adipose tissue, muscle, and the liver |
| Glucosidase inhibitors Acarbose, miglitol, voglibose | Inhibit α-glucosidase at the brush border of the small intestine and affect the digestion of complex carbohydrates, resulting in lower postprandial blood glucose |
| Bromocriptine mesylate | A sympatholytic dopamine D2 receptor agonist that exerts inhibitory effects on serotonin turnover in the central nervous system |
| GLP-1 agonists Exenatide, liraglutide, lixisenatide, dulaglutide, semaglutide | Bind to GLP-1 receptors to restore pancreatic β-cell sensitivity to glucose and to increase β-cell mass |
| DPP-4 inhibitors (gliptins) Sitagliptin, saxagliptin, linagliptin, vildagliptin, alogliptin | Block GLP-1 degradation |
| SGLT-2 inhibitors Empagliflozin, canagliflozin, dapagliflozin, ertugliflozin | SGLT2 is expressed in the proximal renal tubules and mediates glucose reabsorption; SGLT2 inhibitors promote the renal excretion of glucose and thereby reduce the serum glucose level |
| Amylinomimetics Pramlintide | Regulate postprandial spikes in blood glucose by slowing gastric emptying and digestion, promoting satiety, and inhibiting glucagon secretion |
| Insulin/insulin analogs | Similarly to endogenous insulin, exogenous insulin increases the uptake of glucose into cells, stimulates glycogen synthesis, and inhibits glucagon |
| Organ System | Infection Type | Organisms |
|---|---|---|
| Soft Tissues and bones | Diabetic foot, osteomyelitis & septic arthritis | Aerobic Gram-positive cocci including staphylococcus aureus, streptococcus agalactiae, streptococcus pyogenes, gram-negative bacilli, and anaerobic organisms |
| Necrotizing fasciitis | S. aureus, S. pyogenes, enterobacteriaceae, vibrio species, aeromonas species, salmonella species, and anaerobic organisms | |
| Cellulitis | S. aureus, S. pyogenes, corynebacterium jeikeium, pseudomonas aeruginosa (ecthyma gangrenosum) | |
| Genito-urinary tract | Urinary tract infection: cystitis, urethritis, pyelonephritis, | Escherichia coli, klebsiella species and other enterobacteria, acinetobacter species, P. aeruginosa. S. agalactiae, candida albicans, and other yeasts |
| vulvovaginitis | Candida albicans, and other yeasts, gardnerella vaginalis, mycoplasma hominis | |
| Respiratory tract | Pneumonia | S. pneumoniae, S. aureus, K. pneumoniae and other Gram-negative, bacilli, legionella species, influenza virus, mycobacterium tuberculosis complex |
| Head and neck | Mucormycosis (zygomycosis) | Rhizopus species, mucor species |
| Malignant otitis externa | P. aeruginosa, aspergillus species, and other fungi | |
| Endophthalmitis | E. coli, K. pneumoniae | |
| Periodontal disease | Oral commensals organisms, porphyromonas gingivalis, tannerella forsythia, | |
| Gastrointestinal | Cholecystitis | Enterobacteriaceae: E. coli, other species, anaerobic organisms, Candida species |
| Typhlitis | Clostridium species, enterobacteriacae, bacteroides fragilis, candida species | |
| Perianal abscess | Polymicrobial (Gram-positive cocci, gram-negative bacilli and anaerobes) | |
| Bacteremia and sepsis | Community-acquired and hospital-acquired | viridans Streptococci, S. aureus, S. pneumoniae, enterobacteriacae, E. coli, klebsiella species, pseudomonas aeruginosa, candida albican, enterococci, and others |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, R.K.; Ahmed, S.; Le, D.; Yadav, S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers 2021, 13, 5735. https://doi.org/10.3390/cancers13225735
Shahid RK, Ahmed S, Le D, Yadav S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers. 2021; 13(22):5735. https://doi.org/10.3390/cancers13225735
Chicago/Turabian StyleShahid, Rabia K., Shahid Ahmed, Duc Le, and Sunil Yadav. 2021. "Diabetes and Cancer: Risk, Challenges, Management and Outcomes" Cancers 13, no. 22: 5735. https://doi.org/10.3390/cancers13225735
APA StyleShahid, R. K., Ahmed, S., Le, D., & Yadav, S. (2021). Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers, 13(22), 5735. https://doi.org/10.3390/cancers13225735







