Variable Expression of the Disialoganglioside GD2 in Breast Cancer Molecular Subtypes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Data and Histopathological Assessment
2.3. Assessment of GD2 Expression
2.4. GD2 Staining by Immunohistochemistry
2.5. GD2 Staining by Immunofluorescence
2.6. GD2 Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Patients
3.2. GD2 Expression in Invasive Breast Cancer
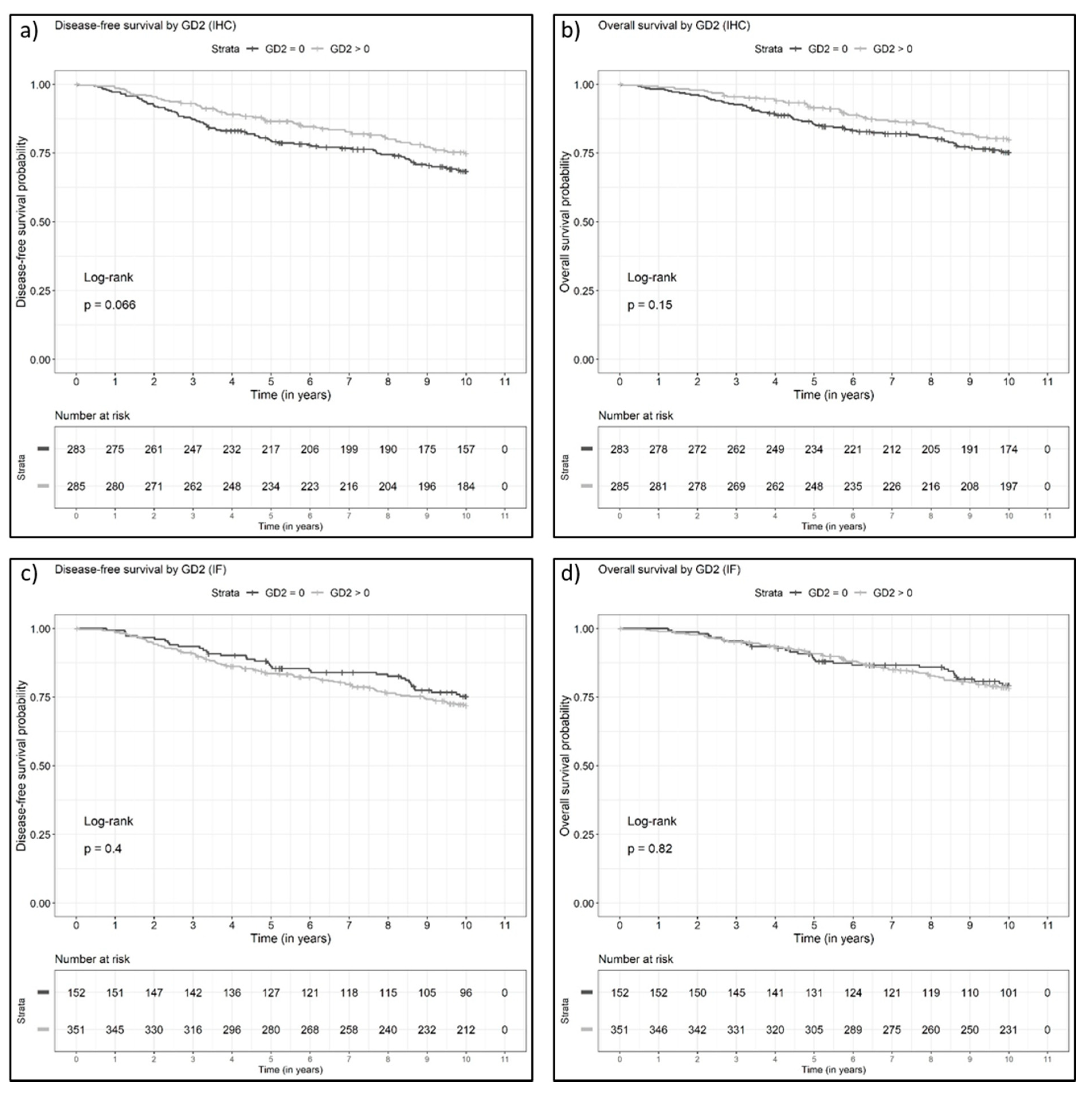
3.3. Disease-Free and Overall Survival of Patients with GD2-Positive Versus GD2-Negative Invasive Breast Cancers
3.4. GD2 Expression in Non-Neoplastic Breast Parenchyma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartkopf, A.D.; Müller, V.; Wöckel, A.; Lux, M.P.; Janni, W.; Nabieva, N.; Taran, F.-A.; Ettl, J.; Lüftner, D.; Belleville, E.; et al. Update breast cancer 2019 Part 1–Implementation of Study results of novel study designs in clinical practice in patients with early breast cancer. Geburtshilfe Frauenheilkd. 2019, 79, 256–267. [Google Scholar] [CrossRef]
- Janni, W.; Schneeweiss, A.; Müller, V.; Wöckel, A.; Lux, M.P.; Hartkopf, A.D.; Nabieva, N.; Taran, F.-A.; Tesch, H.; Overkamp, F.; et al. Update breast cancer 2019 Part 2–Implementation of novel diagnostics and therapeutics in advanced breast cancer patients in clinical practice. Geburtshilfe Frauenheilkd. 2019, 79, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Kolberg, H.-C.; Schneeweiss, A.; Fehm, T.N.; Wöckel, A.; Huober, J.; Pontones, C.; Titzmann, A.; Belleville, E.; Lux, M.P.; Janni, W.; et al. Update breast cancer 2019 Part 3–Current developments in early breast cancer: Review and critical assessment by an international expert panel. Geburtshilfe Frauenheilkd. 2019, 79, 470–482. [Google Scholar] [CrossRef] [Green Version]
- Schütz, F.; Fasching, P.A.; Welslau, M.; Hartkopf, A.D.; Wöckel, A.; Lux, M.P.; Janni, W.; Ettl, J.; Lüftner, D.; Belleville, E.; et al. Update breast cancer 2019 Part 4–Diagnostic and therapeutic challenges of new, personalised therapies for patients with early breast cancer. Geburtshilfe Frauenheilkd. 2019, 79, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welslau, M.; Hartkopf, A.D.; Müller, V.; Wöckel, A.; Lux, M.P.; Janni, W.; Ettl, J.; Lüftner, D.; Belleville, E.; Schütz, F.; et al. Update breast cancer 2019 Part 5–Diagnostic and therapeutic challenges of new, personalised therapies in patients with advanced breast cancer. Geburtshilfe Frauenheilkd. 2019, 79, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early Triple-Negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Svennerholm, L. Chromatographlc separation of human brain gangliosides. J. Neurochem. 1963, 10, 613–623. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.K.; Tsai, Y.-T.; Ariga, T.; Yanagisawa, M. Structures, biosynthesis, and functions of Gangliosides—An overview. J. Oleo Sci. 2011, 60, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammie, G.; Cheung, N.; Gerald, W.; Rosenblum, M.; Cordoncardo, C. Ganglioside gd(2) expression in the human nervous-system and in neuroblastomas-an immunohistochemical study. Int. J. Oncol. 1993, 3, 909–915. [Google Scholar] [CrossRef]
- Svennerholm, L.; Boström, K.; Fredman, P.; Jungbjer, B.; Lekman, A.; Månsson, J.-E.; Rynmark, B.-M. Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1994, 1214, 115–123. [Google Scholar] [CrossRef]
- Schengrund, C.L.; Shochat, S.J. Gangliosides in neuroblastomas. Neurochem. Pathol. 1988, 8, 189–202. [Google Scholar] [CrossRef]
- Cahan, L.D.; Irie, R.F.; Singh, R.; Cassidenti, A.; Paulson, J.C. Identification of a human neuroectodermal tumor antigen (OFA-I-2) as ganglioside GD2. Proc. Natl. Acad. Sci. USA 1982, 79, 7629–7633. [Google Scholar] [CrossRef] [Green Version]
- Kailayangiri, S.; Altvater, B.; Meltzer, J.; Pscherer, S.; Luecke, A.; Dierkes, C.; Titze, U.; Leuchte, K.; Landmeier, S.; Hotfilder, M.; et al. The ganglioside antigen GD2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br. J. Cancer 2012, 106, 1123–1133. [Google Scholar] [CrossRef]
- Chang, H.R.; Cordon-Cardo, C.; Houghton, A.N.; Cheung, N.K.; Brennan, M.F. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer 1992, 70, 633–638. [Google Scholar] [CrossRef]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.-K.V. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr. Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef] [Green Version]
- Ziebarth, A.J.; Felder, M.A.; Harter, J.; Connor, J.P. Uterine leiomyosarcoma diffusely express disialoganglioside GD2 and bind the therapeutic immunocytokine 14.18-IL2: Implications for immunotherapy. Cancer Immunol. Immunother. 2012, 61, 1149–1153. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, Interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.-K. Humanized 3F8 Anti-GD2Monoclonal antibody dosing with granulocyte-macrophage colony-stimulating factor in patients with resistant neuroblastoma. JAMA Oncol. 2018, 4, 1729–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, R.M.; Sotillo, E.; Majzner, R.G. CAR T cell therapy for neuroblastoma. Front. Immunol. 2018, 9, 2380. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, K.O.; Old, L.J. Human monoclonal antibodies to glycolipids and other carbohydrate antigens: Dissection of the humoral immune response in cancer patients. Cancer Res. 1989, 49, 3445–3451. [Google Scholar] [PubMed]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.-Y.; Spaeth, E.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, M.; Roudi, R.; Abbasi, A.; Abolhasani, M.; Rad, I.A.; Shariftabrizi, A.; Madjd, Z. High GD2 expression defines breast cancer cells with enhanced invasiveness. Exp. Mol. Pathol. 2019, 109, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.; Barbolini, M.; Ficarra, G.; Tazzioli, G.; Manni, P.; Petrachi, T.; Mastrolia, I.; Orvieto, E.; Spano, C.; Prapa, M.; et al. GD2 expression in breast cancer. Oncotarget 2017, 8, 31592–31600. [Google Scholar] [CrossRef]
- De Giorgi, U.; Cohen, E.; Gao, H.; Mego, M.; Lee, B.-N.; Lodhi, A.; Cristofanilli, M.; Lucci, A.; Reuben, J.M. Mesenchymal stem cells expressing GD2 and CD271 correlate with breast cancer-initiating cells in bone marrow. Cancer Biol. Ther. 2011, 11, 812–815. [Google Scholar] [CrossRef] [Green Version]
- Seitz, C.M.; Schroeder, S.; Knopf, P.; Krahl, A.-C.; Hau, J.; Schleicher, S.; Martella, M.; Quintanilla-Martinez, L.; Kneilling, M.; Pichler, B.; et al. GD2-targeted chimeric antigen receptor T cells prevent metastasis formation by elimination of breast cancer stem-like cells. OncoImmunology 2020, 9, 1683345. [Google Scholar] [CrossRef] [Green Version]
- Fasching, P.A.; Weihbrecht, S.; Haeberle, L.; Gasparyan, A.; Villalobos, I.E.; Ma, Y.; Ekici, A.B.; Wachter, D.L.; Hartmann, A.; Beckmann, M.W.; et al. HER2 and TOP2A amplification in a hospital-based cohort of breast cancer patients: Associations with patient and tumor characteristics. Breast Cancer Res. Treat. 2014, 145, 193–203. [Google Scholar] [CrossRef]
- Serce, N.B.; Boesl, A.; Klaman, I.; Von Serényi, S.; Noetzel, E.; Press, M.F.; Dimmler, A.; Hartmann, A.; Sehouli, J.; Knuechel, R.; et al. Overexpression of SERBP1 (Plasminogen activator inhibitor 1 RNA binding protein) in human breast cancer is correlated with favourable prognosis. BMC Cancer 2012, 12, 597. [Google Scholar] [CrossRef] [Green Version]
- Bektas, N.; Noetzel, E.; Veeck, J.; Press, M.F.; Kristiansen, G.; Naami, A.; Hartmann, A.; Dimmler, A.; Beckmann, M.W.; Knüchel, R.; et al. The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res. 2008, 10, R58. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.; Gass, P.; Häberle, L.; Wang, D.; Hartmann, A.; Lux, M.P.; Beckmann, M.W.; Untch, M.; Fasching, P.A. The effect of participation in neoadjuvant clinical trials on outcomes in patients with early breast cancer. Breast Cancer Res. Treat. 2018, 171, 747–758. [Google Scholar] [CrossRef]
- Beckmann, M.W.; Brucker, C.; Hanf, V.; Rauh, C.; Bani, M.R.; Knob, S.; Petsch, S.; Schick, S.; Fasching, P.A.; Hartmann, A.; et al. Quality assured health care in certified breast centers and improvement of the prognosis of breast cancer patients. Oncol. Res. Treat. 2011, 34, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Wöckel, A.; Festl, J.; Stüber, T.; Brust, K.; Stangl, S.; Heuschmann, P.U.; Albert, U.-S.; Budach, W.; Follmann, M.; Janni, W.; et al. Interdisciplinary Screening, Diagnosis, Therapy and Follow-up of breast cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017)–Part 1 with recommendations for the screening, diagnosis and therapy of breast cancer. Geburtshilfe Frauenheilkd. 2018, 78, 927–948. [Google Scholar] [CrossRef] [Green Version]
- Wöckel, A.; Festl, J.; Stüber, T.; Brust, K.; Krockenberger, M.; Heuschmann, P.U.; Jírů-Hillmann, S.; Albert, U.-S.; Budach, W.; Follmann, M.; et al. Interdisciplinary screening, diagnosis, therapy and follow-up of breast cancer. guideline of the DGGG and the DKG (S3-Level, AWMF registry number 032/045OL, December 2017)–Part 2 with recommendations for the therapy of primary, recurrent and advanced breast cancer. Geburtshilfe Frauenheilkd. 2018, 78, 1056–1088. [Google Scholar] [CrossRef] [Green Version]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Salmen, J.; Neugebauer, J.; Fasching, P.A.; Haeberle, L.; Huober, J.; Wöckel, A.; Rauh, C.; Schuetz, F.; Weissenbacher, T.; Kost, B.; et al. Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res. Treat. 2014, 148, 143–151. [Google Scholar] [CrossRef]
- Lin, L.I.-K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255. [Google Scholar] [CrossRef]
- Maccioni, H.J.F. Glycosylation of glycolipids in the Golgi complex. J. Neurochem. 2007, 103, 81–90. [Google Scholar] [CrossRef]
- Richman, S.A.; Nunez-Cruz, S.; Moghimi, B.; Li, L.; Gershenson, Z.T.; Mourelatos, Z.; Barrett, D.M.; Grupp, S.A.; Milone, M.C. High-Affinity GD2-Specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol. Res. 2018, 6, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kailayangiri, S.; Altvater, B.; Spurny, C.; Jamitzky, S.; Schelhaas, S.; Jacobs, A.H.; Wiek, C.; Roellecke, K.; Hanenberg, H.; Hartmann, W.; et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. OncoImmunology 2017, 6, e1250050. [Google Scholar] [CrossRef] [Green Version]
- Pule, M.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270. [Google Scholar] [CrossRef]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, L.F.; Makhoul, I.; Emanuel, P.D.; Pennisi, A.; Siegel, E.R.; Jousheghany, F.; Guo, X.; Pashov, A.; Monzavi-Karbassi, B.; Kieber-Emmons, T. Targeting tumor-associated carbohydrate antigens: A phase I study of a carbohydrate mimetic-peptide vaccine in stage IV breast cancer subjects. Oncotarget 2017, 8, 99161–99178. [Google Scholar] [CrossRef] [Green Version]
- Cheal, S.M.; Xu, H.; Guo, H.-F.; Zanzonico, P.B.; Larson, S.; Cheung, N.-K. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 Using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol. Cancer Ther. 2014, 13, 1803–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheal, S.M.; Xu, H.; Guo, H.-F.; Patel, M.; Punzalan, B.; Fung, E.K.; Lee, S.-G.; Bell, M.; Singh, M.; Jungbluth, A.A.; et al. Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: Curative treatment of HER2-positive breast carcinoma. Theranostics 2018, 8, 5106–5125. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Nguyen, K.; Sun, J.; Pitner, M.K.; Yuan, B.; Bartholomeusz, C.; Hail, N.; Andreeff, M. IKK inhibition by BMS-345541 suppresses breast tumorigenesis and metastases by targeting GD2+ cancer stem cells. Oncotarget 2017, 8, 36936–36949. [Google Scholar] [CrossRef] [Green Version]
- Elston, C.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Gelber, R.D.; Coates, A.S.; Thürlimann, B.; Senn, H.-J. Meeting highlights: Updated international expert consensus on the primary therapy of early breast cancer. J. Clin. Oncol. 2003, 21, 3357–3365. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Glick, J.H.; Gelber, R.D.; Coates, A.S.; Thürlimann, B.; Senn, H.-J. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann. Oncol. 2005, 16, 1569–1583. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med. 2010, 134, e48–e72. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Wolff, A.C. Clinical notice for American society of clinical oncology-college of American pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J. Clin. Oncol. 2011, 29, e458. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Glick, J.H.; Gelber, R.D.; Coates, A.S.; Senn, H.-J. Meeting highlights: International consensus panel on the treatment of primary breast cancer. J. Clin. Oncol. 2001, 19, 3817–3827. [Google Scholar] [CrossRef] [PubMed]
- Sauter, G.; Lee, J.; Bartlett, J.M.; Slamon, D.J.; Press, M.F. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J. Clin. Oncol. 2009, 27, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef]
- Wunderle, M.; Pretscher, J.; Brucker, S.Y.; Volz, B.; Hartmann, A.; Fiessler, C.; Hein, A.; Häberle, L.; Jud, S.M.; Lux, M.P.; et al. Association between breast cancer risk factors and molecular type in postmenopausal patients with hormone receptor-positive early breast cancer. Breast Cancer Res. Treat. 2019, 174, 453–461. [Google Scholar] [CrossRef]
- Bethune, G.C.; Van Zanten, D.V.; MacIntosh, R.F.; Rayson, D.; Younis, T.; Thompson, K.; Barnes, P.J. Impact of the 2013 American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 (HER2) testing of invasive breast carcinoma: A focus on tumours assessed as ‘equivocal’ for HE. Histopathology 2015, 67, 880–887. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [Green Version]

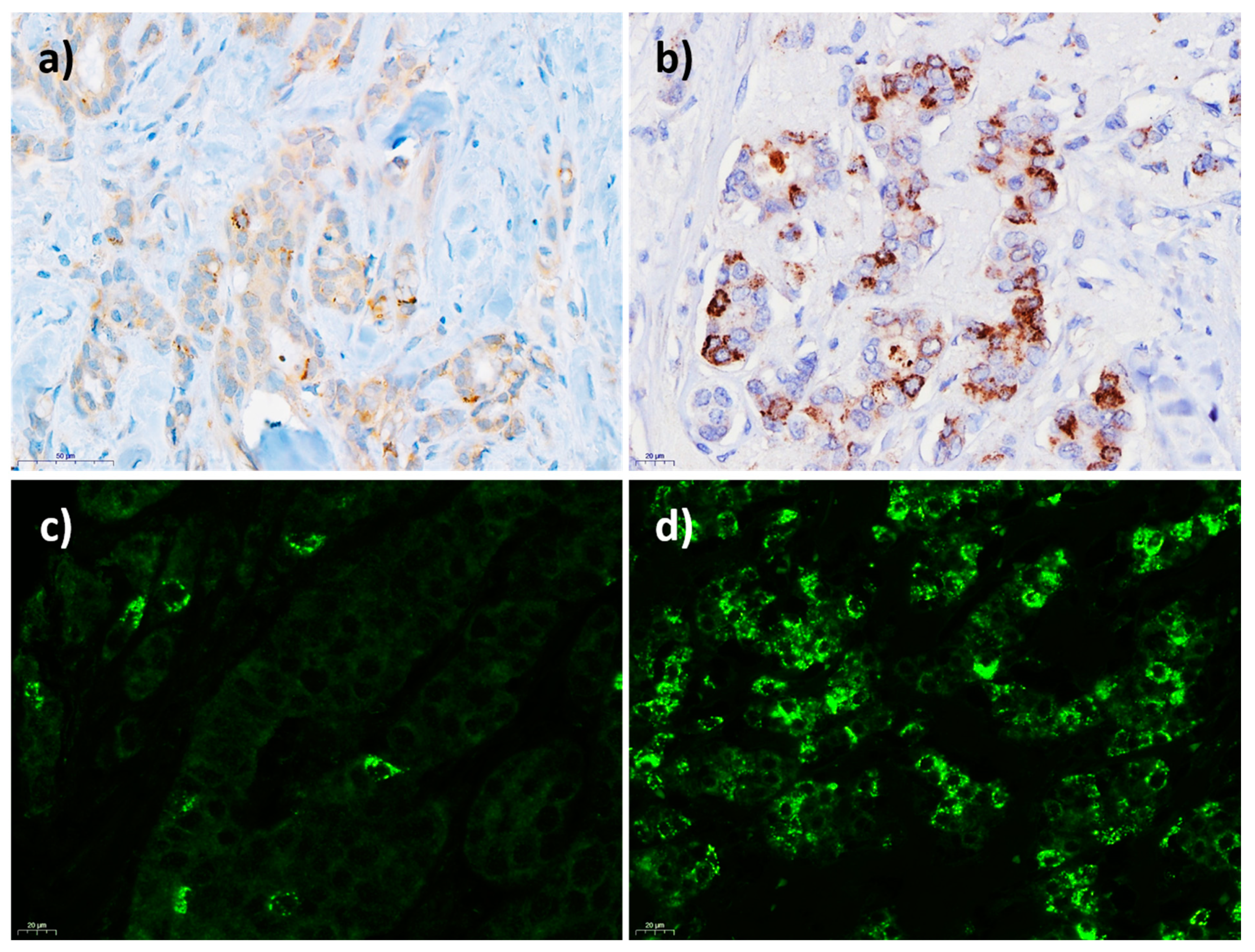
| Characteristic | IHC (n = 568) | IF (n = 503) |
|---|---|---|
| Age at diagnosis (years; mean, SD) | 58.2 (12.4) | 58.2 (12.5) |
| BMI (kg/m2; median, IQR) | 25.4 (22.7–28.6) | 25.4 (22.7–28.7) |
| Grading | ||
| G1 | 70 (12.3) | 61 (12.1) |
| G2 | 367 (64.6) | 320 (63.6) |
| G3 | 131 (23.1) | 122 (24.3) |
| Lymph-node status | ||
| N0 | 340 (59.9) | 304 (60.4) |
| N1 | 228 (40.1) | 199 (39.6) |
| Tumor stage | ||
| T1 | 297 (52.3) | 273 (54.3) |
| T2 | 220 (38.7) | 186 (37.0) |
| T3 | 28 (4.9) | 24 (4.8) |
| T4 | 23 (4.0) | 20 (4.0) |
| Breast cancer subtype | ||
| TNBC | 76 (13.4) | 69 (13.7) |
| Luminal A | 228 (40.1) | 196 (39.0) |
| Luminal B | 198 (34.9) | 182 (36.2) |
| HER2 | 66 (11.6) | 56 (11.1) |
| Histologic subtype | ||
| NST/IDC | 417 (73.4) | 366 (72.8) |
| ILC | 70 (12.3) | 66 (13.1) |
| Medullary pattern | 21 (3.7) | 23 (4.6) |
| Tubular | 15 (2.6) | 14 (2.8) |
| Other/Unknown | 14 (2.5) | 23 (4.6) |
| Micropapillary | 13 (2.3) | 0 |
| Mucinous | 9 (1.6) | 6 (1.2) |
| Metaplastic | 4 (0.7) | 1 (0.2) |
| Apocrine | 4 (0.7) | 4 (0.8) |
| Papillary (invasive) | 1 (0.2) | |
| GD2 percentage (%; median, IQR) | 1 (0–5) | 1 (0–3) |
| GD2 intensity | ||
| 0 | 283 (49.8) | 152 (30.2) |
| 1 | 63 (11.1) | 113 (22.5) |
| 2 | 105 (18.5) | 82 (16.3) |
| 3 | 117 (20.6) | 156 (31.0) |
| Intensity | Breast Cancer Subtypes | p * | |||
|---|---|---|---|---|---|
| TNBC | Luminal A | Luminal B | HER2 | ||
| GD2 immunohistochemistry | |||||
| 0 | 62 (81.6) | 92 (40.4) | 93 (47.0) | 36 (54.5) | <0.0001 |
| 1 | 5 (6.6) | 33 (14.5) | 20 (10.1) | 5 (7.6) | |
| 2 | 4 (5.3) | 44 (19.3) | 46 (23.2) | 11 (16.7) | |
| 3 | 5 (6.6) | 59 (25.9) | 39 (19.7) | 14 (21.2) | |
| GD2 immunofluorescence | |||||
| 0 | 38 (55.1) | 43 (21.9) | 46 (25.3) | 25 (44.6) | <0.0001 |
| 1 | 14 (20.3) | 43 (21.9) | 44 (24.2) | 12 (21.4) | |
| 2 | 8 (11.6) | 36 (18.4) | 29 (15.9) | 9 (16.1) | |
| 3 | 9 (13.0) | 74 (37.8) | 63 (34.6) | 10 (17.9) | |
| Outcome | Biomarker | Unadjusted HR (95% CI) | p Value | Adjusted HR * (95% CI) | p Value |
|---|---|---|---|---|---|
| GD2 immunohistochemistry | |||||
| DFS | GD2 binary (0 vs. >0) | 0.74 (0.54, 1.02) | 0.07 | 0.79 (0.56, 1.10) | 0.16 |
| GD2 percentage | 1.00 (0.99, 1.01) | 0.94 | 1.00 (0.99, 1.01) | 0.76 | |
| OS | GD2 binary (0 vs. >0) | 0.77 (0.54, 1.10) | 0.15 | 0.84 (0.57, 1.23) | 0.36 |
| GD2 percentage | 1.00 (0.99, 1.01) | 0.67 | 1.00 (0.99, 1.01) | 0.93 | |
| GD2 immunofluorescence | |||||
| DFS | GD2 binary (0 vs. >0) | 1.18 (0.80, 1.73) | 0.40 | 1.12 (0.75, 1.67) | 0.59 |
| GD2 percentage | 1.00 (0.98, 1.01) | 0.65 | 0.99 (0.98, 1.01) | 0.27 | |
| OS | GD2 binary (0 vs. >0) | 1.05 (0.69, 1.61) | 0.82 | 0.99 (0.63, 1.55) | 0.96 |
| GD2 percentage | 1.00 (0.98, 1.02) | 0.94 | 0.99 (0.98, 1.01) | 0.54 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erber, R.; Kailayangiri, S.; Huebner, H.; Ruebner, M.; Hartmann, A.; Häberle, L.; Meyer, J.; Völkl, S.; Mackensen, A.; Landgraf, L.; et al. Variable Expression of the Disialoganglioside GD2 in Breast Cancer Molecular Subtypes. Cancers 2021, 13, 5577. https://doi.org/10.3390/cancers13215577
Erber R, Kailayangiri S, Huebner H, Ruebner M, Hartmann A, Häberle L, Meyer J, Völkl S, Mackensen A, Landgraf L, et al. Variable Expression of the Disialoganglioside GD2 in Breast Cancer Molecular Subtypes. Cancers. 2021; 13(21):5577. https://doi.org/10.3390/cancers13215577
Chicago/Turabian StyleErber, Ramona, Sareetha Kailayangiri, Hanna Huebner, Matthias Ruebner, Arndt Hartmann, Lothar Häberle, Julia Meyer, Simon Völkl, Andreas Mackensen, Laura Landgraf, and et al. 2021. "Variable Expression of the Disialoganglioside GD2 in Breast Cancer Molecular Subtypes" Cancers 13, no. 21: 5577. https://doi.org/10.3390/cancers13215577
APA StyleErber, R., Kailayangiri, S., Huebner, H., Ruebner, M., Hartmann, A., Häberle, L., Meyer, J., Völkl, S., Mackensen, A., Landgraf, L., Geppert, C. I., Schulz-Wendtland, R., Beckmann, M. W., Fasching, P. A., Farwick, N., Rossig, C., & Gass, P. (2021). Variable Expression of the Disialoganglioside GD2 in Breast Cancer Molecular Subtypes. Cancers, 13(21), 5577. https://doi.org/10.3390/cancers13215577









