In Vivo Reflectance Confocal Microscopy as a Response Monitoring Tool for Actinic Keratoses Undergoing Cryotherapy and Photodynamic Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Treatment Protocol
2.3.1. Cryotherapy
2.3.2. PDT
2.4. Reflectance Confocal Microscopy Evaluation
2.5. Statistical Methods
3. Results
3.1. Patient Population
3.2. Clinical Assessment of AKs
3.3. RCM Interobserver Agreement
3.4. Baseline RCM Scoring
3.5. RCM Evaluation over Time
3.5.1. Stratum Corneum
3.5.2. Epidermis
3.5.3. Dermis
3.6. Comparison between Treatment Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callen, J.P.; Bickers, D.R.; Moy, R.L. Actinic Keratoses. J. Am. Acad. Dermatol. 1997, 36, 650–653. [Google Scholar] [CrossRef]
- Cockerell, C.J. Histopathology of Incipient Intraepidermal Squamous Cell Carcinoma (“actinic Keratosis”). J. Am. Acad. Dermatol. 2000, 42, 11–17. [Google Scholar] [CrossRef]
- Novak, B.; DuBois, J.; Chahrour, O.; Papusha, T.; Hirt, S.; Philippi, T.; Zogel, C.; Osenberg, K.; Schmitz, B.; Lübbert, H. Clinical Pharmacokinetics and Safety of a 10% Aminolevulinic Acid Hydrochloride Nanoemulsion Gel (BF-200 ALA) in Photodynamic Therapy of Patients Extensively Affected with Actinic Keratosis: Results of 2 Maximal Usage Pharmacokinetic Trials. Clin. Pharmacol. Drug Dev. 2021. [Google Scholar] [CrossRef]
- Crow, L.D.; Saylor, D.; Griffith-Bauer, K.; Tello, T.; Aroyan, C.; Lazar, A.A.; Arron, S.T. Comparative Tolerability and Efficacy of Daylight, Conventional, and Combination Aminolevulinic Acid Photodynamic Therapy for Treatment of Actinic Keratosis. J. Am. Acad. Dermatol. 2021, 85, 967–969. [Google Scholar] [CrossRef]
- Rubel, D.M.; Spelman, L.; Murrell, D.F.; See, J.-A.; Hewitt, D.; Foley, P.; Bosc, C.; Kerob, D.; Kerrouche, N.; Wulf, H.C.; et al. Daylight Photodynamic Therapy with Methyl Aminolevulinate Cream as a Convenient, Similarly Effective, Nearly Painless Alternative to Conventional Photodynamic Therapy in Actinic Keratosis Treatment: A Randomized Controlled Trial. Br. J. Dermatol. 2014, 171, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Aghassi, D.; Anderson, R.R.; González, S. Confocal Laser Microscopic Imaging of Actinic Keratoses in Vivo: A Preliminary Report. J. Am. Acad. Dermatol. 2000, 43, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richtig, E.; Ahlgrimm-Siess, V.; Koller, S.; Gerger, A.; Horn, M.; Smolle, J.; Hofmann-Wellenhof, R. Follow-up of Actinic Keratoses after Shave Biopsy by in-Vivo Reflectance Confocal Microscopy—A Pilot Study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Krueger-Corcoran, D.; Roewert-Huber, J.; Sterry, W.; Stockfleth, E.; Astner, S. Reflectance Confocal Microscopy for Noninvasive Monitoring of Therapy and Detection of Subclinical Actinic Keratoses. Dermatology 2010, 220, 15–24. [Google Scholar] [CrossRef]
- Ulrich, M.; Lange-Asschenfeldt, S.; González, S. In Vivo Reflectance Confocal Microscopy for Early Diagnosis of Nonmelanoma Skin Cancer. Actas Dermo-Sifiliográficas 2012, 103, 784–789. [Google Scholar] [CrossRef]
- Pellacani, G.; Ulrich, M.; Casari, A.; Prow, T.W.; Cannillo, F.; Benati, E.; Losi, A.; Cesinaro, A.M.; Longo, C.; Argenziano, G.; et al. Grading Keratinocyte Atypia in Actinic Keratosis: A Correlation of Reflectance Confocal Microscopy and Histopathology. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2216–2221. [Google Scholar] [CrossRef]
- Ishioka, P.; Maia, M.; Rodrigues, S.B.; Lellis, R.F.; Hirata, S.H. In Vivo Confocal Laser Microscopy for Monitoring of Actinic Keratosis Treatment: A Comparison with Histopathologic Assessment after Treatment with Topical 5% 5-Fluorouracil. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1155–1163. [Google Scholar] [CrossRef]
- Pasquali, P.; Segurado-Miravalles, G.; González, S. Sequential Treatment of Actinic Keratosis with Cryotherapy and Ingenol Mebutate: Reflectance Confocal Microscopy Monitoring of Efficacy and Local Skin Reaction. Int. J. Dermatol. 2018, 57, 1178–1181. [Google Scholar] [CrossRef]
- Benati, E.; Longhitano, S.; Pampena, R.; Mirra, M.; Raucci, M.; Pellacani, G.; Longo, C. Digital Follow-up by Means of Dermatoscopy and Reflectance Confocal Microscopy of Actinic Keratosis Treated with Imiquimod 3.75% Cream. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1471–1477. [Google Scholar] [CrossRef]
- Horn, M.; Gerger, A.; Ahlgrimm-Siess, V.; Weger, W.; Koller, S.; Kerl, H.; Samonigg, H.; Smolle, J.; Hofmann-Wellenhof, R. Discrimination of Actinic Keratoses from Normal Skin with Reflectance Mode Confocal Microscopy. Dermatol. Surg. 2008, 34, 620–625. [Google Scholar] [CrossRef]
- Mota, A.; Piñeiro-Maceira, J.M.; Barcaui, C. Evaluation of Diagnostic Criteria of Actinic Keratosis through Reflectance Confocal Microscopy. Skin Res. Technol. 2020, 26, 883–890. [Google Scholar] [CrossRef]
- Rishpon, A.; Kim, N.; Scope, A.; Porges, L.; Oliviero, M.C.; Braun, R.P.; Marghoob, A.A.; Fox, C.A.; Rabinovitz, H.S. Reflectance Confocal Microscopy Criteria for Squamous Cell Carcinomas and Actinic Keratoses. Arch. Dermatol. 2009, 145, 766–772. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.M.; Lambie, D.; Sinnya, S.; Sahebian, A.; Soyer, H.P.; Prow, T.W.; Ardigò, M. Histopathology and Reflectance Confocal Microscopy Features of Photodamaged Skin and Actinic Keratosis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Maltusch, A.; Rius-Diaz, F.; Röwert-Huber, J.; González, S.; Sterry, W.; Stockfleth, E.; Astner, S. Clinical Applicability of in Vivo Reflectance Confocal Microscopy for the Diagnosis of Actinic Keratoses. Dermatol. Surg. 2008, 34, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Lisa Abernethy, M.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E. A Double-Blind, Vehicle-Controlled Study Evaluating Masoprocol Cream in the Treatment of Actinic Keratoses on the Head and Neck. J. Am. Acad. Dermatol. 1991, 24, 738–743. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Anderson, R.R. In Vivo Confocal Scanning Laser Microscopy of Human Skin: Melanin Provides Strong Contrast. J. Investig. Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajadhyaksha, M.; González, S.; Zavislan, J.M.; Anderson, R.R.; Webb, R.H. In Vivo Confocal Scanning Laser Microscopy of Human Skin II: Advances in Instrumentation and Comparison with Histology. J. Investig. Dermatol. 1999, 113, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardigo, M.; Longo, C.; Gonzalez, S. The International Confocal Working Group Inflammatory Skin Diseases Project Multicentre Study on Inflammatory Skin Diseases from The International Confocal Working Group: Specific Confocal Microscopy Features and an Algorithmic Method of Diagnosis. Br. J. Dermatol. 2016, 175, 364–374. [Google Scholar] [CrossRef] [PubMed]
- da Silva Sousa, A.C.; Campos, M.A.C.; Baptista, A.M.; Menezes, N.M.B.V.N. Daylight Photodynamic Therapy in 25 Patients with Actinic Keratosis and Evaluation of Efficacy by Confocal Microscopy. Photodiagn. Photodyn. Ther. 2019, 25, 414–416. [Google Scholar] [CrossRef]
- Malvehy, J.; Roldán-Marín, R.; Iglesias-García, P.; Díaz, A.; Puig, S. Monitoring Treatment of Field Cancerisation with 3% Diclofenac Sodium 2.5% Hyaluronic Acid by Reflectance Confocal Microscopy: A Histologic Correlation. Acta Derm. Venerol. 2015, 95, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Malvehy, J.; Alarcon, I.; Montoya, J.; Rodríguez-Azeredo, R.; Puig, S. Treatment Monitoring of 0.5% 5-Fluorouracil and 10% Salicylic Acid in Clinical and Subclinical Actinic Keratoses with the Combination of Optical Coherence Tomography and Reflectance Confocal Microscopy. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 258–265. [Google Scholar] [CrossRef]
- Segurado-Miravalles, G.; Jiménez-Gómez, N.; Moreno-Arrones, O.; Alarcón-Salazar, I.; Alegre-Sánchez, A.; Saceda-Corralo, D.; Jaén-Olasolo, P.; González-Rodríguez, S. Assessment of the Effect of 3% Diclofenac Sodium on Photodamaged Skin by Means of Reflectance Confocal Microscopy. Acta Derm. Venerol. 2018, 98, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Seyed Jafari, S.M.; Timchik, T.; Hunger, R.E. In Vivo Confocal Microscopy Efficacy Assessment of Daylight Photodynamic Therapy in Actinic Keratosis Patients. Br. J. Dermatol. 2016, 175, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Reinhold, U.; Falqués, M.; Rodriguez Azeredo, R.; Stockfleth, E. Use of Reflectance Confocal Microscopy to Evaluate 5-Fluorouracil 0.5%/Salicylic Acid 10% in the Field-Directed Treatment of Subclinical Lesions of Actinic Keratosis: Subanalysis of a Phase III, Randomized, Double-Blind, Vehicle-Controlled Trial. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, C.; Greco, V.; Costa, C.; Scalvenzi, M.; Russo, D.; Savastano, R.; Staibano, S.; Fabbrocini, G. Management of Clinical and Subclinical Actinic Keratoses with Histological and Immunohistochemical Assessments by Confocal Microscopy. Dermatol. Ther. 2018, 31, e12672. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.; Reiner, G.; Cremer, C.; Stelzer, E.H.K. Aberrations in Confocal Fluorescence Microscopy Induced by Mismatches in Refractive Index. J. Microsc. 1993, 169, 391–405. [Google Scholar] [CrossRef]
- Fernández-Figueras, M.T.; Carrato, C.; Sáenz, X.; Puig, L.; Musulen, E.; Ferrándiz, C.; Ariza, A. Actinic Keratosis with Atypical Basal Cells (AK I) Is the Most Common Lesion Associated with Invasive Squamous Cell Carcinoma of the Skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Farnetani, F.; Scope, A.; Braun, R.P.; Gonzalez, S.; Guitera, P.; Malvehy, J.; Manfredini, M.; Marghoob, A.A.; Moscarella, E.; Oliviero, M.; et al. Skin Cancer Diagnosis with Reflectance Confocal Microscopy: Reproducibility of Feature Recognition and Accuracy of Diagnosis. JAMA Dermatol. 2015, 151, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Paquet, M.; Villanueva, E.; Brintnell, W. Interventions for Actinic Keratoses. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Schmitz, L.; Gupta, G.; Stücker, M.; Doerler, M.; Gambichler, T.; Welzel, J.; Szeimies, R.M.; Bierhoff, E.; Stockfleth, E.; Dirschka, T. Evaluation of Two Histological Classifications for Actinic Keratoses—PRO Classification Scored Highest Inter-rater Reliability. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1092–1097. [Google Scholar] [CrossRef]
- Schmitz, L.; Gambichler, T.; Gupta, G.; Stücker, M.; Stockfleth, E.; Szeimies, R.M.; Dirschka, T. Actinic Keratoses Show Variable Histological Basal Growth Patterns—A Proposed Classification Adjustment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Gambichler, T.; Kost, C.; Gupta, G.; Stücker, M.; Stockfleth, E.; Dirschka, T. Cutaneous Squamous Cell Carcinomas Are Associated with Basal Proliferating Actinic Keratoses. Br. J. Dermatol. 2019, 180, 916–921. [Google Scholar] [CrossRef]
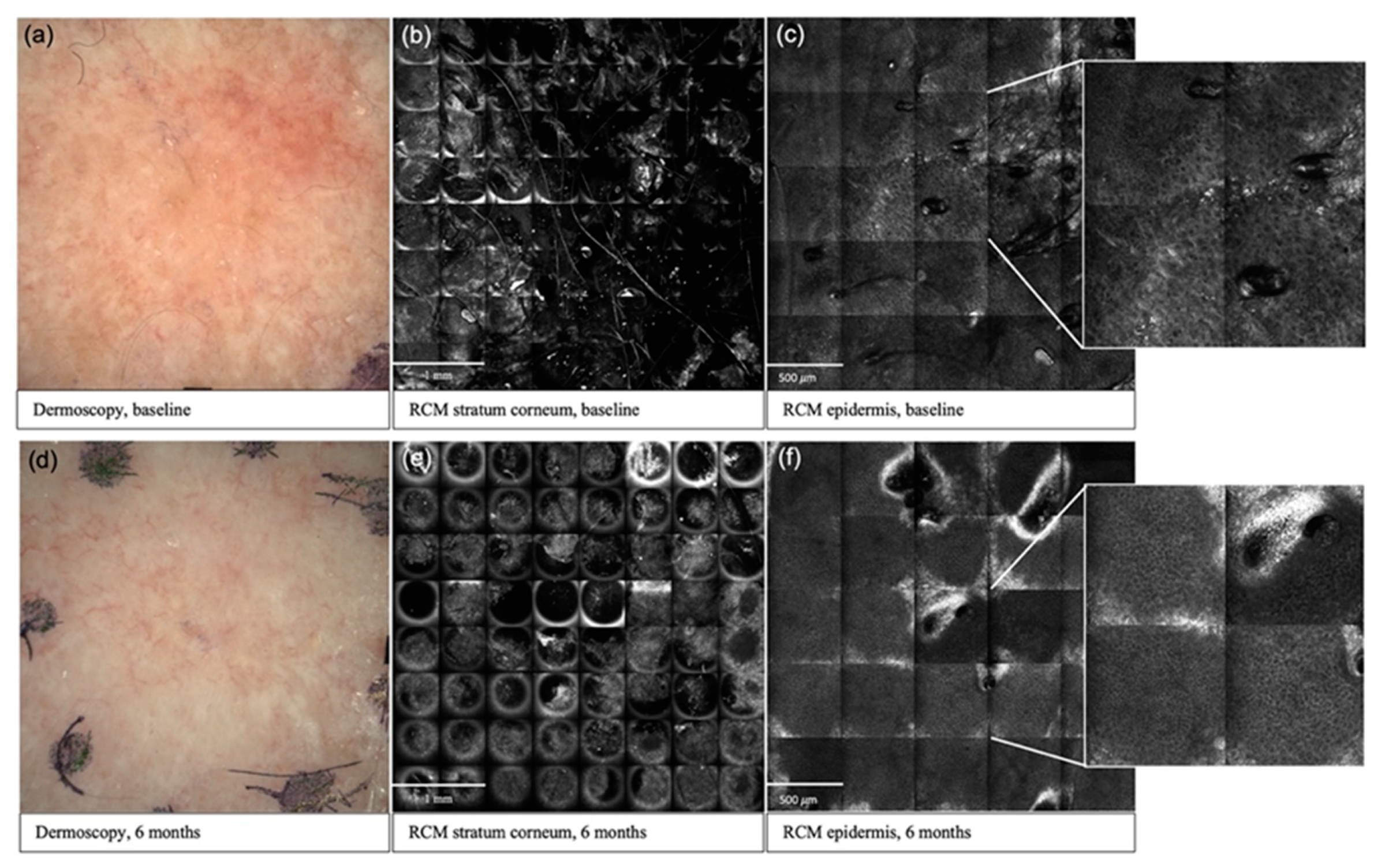
| Grade 1 AK | Grade 2 AK | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID | Baseline Erythema 1 | 3 Mo Erythema | 6 Mo Erythema | Baseline Hyperkeratosis 1 | 3 Mo Hyperkeratosis | 6 Mo Hyperkeratosis | Baseline Erythema 1 | 3 Mo Erythema | 6 Mo Erythema | Baseline Hyperkeratosis 1 | 3 Mo Hyperkeratosis | 6 Mo Hyperkeratosis |
| C1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| C2 | 1 | 0 | 1 | 1 | 0 | 0.5 | 2 | 1 | 0 | 2 | 2 | 0.5 |
| C3 | 1 | 0 | 0 | 1 | 0.5 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| C4 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| C5 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| C6 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| C7 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| C8 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| C9 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 2 | 0 | 0 |
| C10 | 1 | 0 | 0.5 | 1 | 1 | 0 | 2 | 0 | 1 | 2 | 1 | 2.5 |
| P1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| P2 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | - | 0 | 2 | - | 0 |
| P3 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 0 |
| P4 | 1 | 0 | 0 | 1 | 1 | 0.5 | 2 | 0 | 1 | 2 | 0 | 1 |
| P5 | 1 | 0.5 | 0.5 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| P6 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0.5 |
| P7 | 1 | - | - | 1 | - | - | 2 | - | - | 2 | - | - |
| P8 | 1 | 0 | 0 | 1 | 0.5 | 0 | 2 | 0 | 1 | 2 | 1 | 1 |
| P9 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| P10 | 1 | 0 | 0 | 1 | 0.5 | 0 | 2 | 0 | 0 | 2 | 0.5 | 0.5 |
| Skin Layer | RCM Parameter | Pre-Standardization Proportion of Agreement 1 (95% CI) | Post-Standardization Proportion of Agreement (95% CI) |
|---|---|---|---|
| Stratum Corneum | Parakeratosis | 54 (45–63) | 65 (60–69) |
| Hyperkeratosis | 63 (53–71) | 63 (58–68) | |
| Stratum Corneum Disruption | 37 (24–52) | 61 (57–65) SS | |
| Epidermis | Atypical Honeycomb Pattern 2 | 80 (68–88) | 86 (83–88) |
| Round Nucleated Cells | 52 (42–61) | 79 (76–82) SS | |
| Disarranged Epidermal Pattern | 37 (29–47) | 63 (59–66) SS | |
| Presence of Inflammatory Cells | 61 (55–67) | 62 (58–66) | |
| Presence of Dendritic Cells | 87 (80–92) | 80 (77–83) | |
| Dermis | Presence of Inflammatory Cells 2 | 85 (82–89) | 83 (80–86) |
| Solar Elastosis | N/A | 73 (69–76) | |
| Round Blood Vessels 2 | 69 (57–78) | 64 (60–67) | |
| Polymorphous Blood Vessels 2 | 53 (74–65) | 81 (78–84) SS |
| Skin Layer | RCM Parameter | Grade 1 AK (95% CI) | Grade 2 AK (95% CI) | All AK (95% CI) |
|---|---|---|---|---|
| Stratum Corneum | Parakeratosis | 5.5 ** (2.6–11.7) | 5.3 ** (2.3–11.9) | 5.3 ** (2.6–10.6) |
| Hyperkeratosis | 10.6 ** (3.8–29.0) | 18.3 ** (6.1–54.5) | 13.6 ** (5.3–34.9) | |
| Stratum Corneum Disruption | 7.1 ** (2.9–17.4) | 8.9 ** (3.5–22.8) | 7.8 ** (3.5–17.3) | |
| Epidermis | Atypical Honeycomb Pattern 1 | 9.7 ** (3.8–24.5) | 18.0 ** (7.7–41.8) | 12.7 ** (5.7–28.1) |
| Round Nucleated Cells | 3.0 * (1.01–8.9) | 1.6 (0.5–5.1) | 2.3 (0.8–6.5) | |
| Disarranged Epidermal Pattern | 5.4 ** (2.2–12.3) | 8.3 ** (3.2–21.5) | 6.5 ** (2.9–14.8) | |
| Presence of Inflammatory Epidermal Cells | 1.9 (0.9–4.1) | 2.4 * (1.1–5.2) | 2.1 * (1.1–4.0) | |
| Presence of Dendritic Cells | 0.8 (0.2–2.6) | 1.1 (0.3–3.5) | 0.9 (0.4–2.6) | |
| Dermis | Inflammatory Infiltrate Dermis 1 | 3.6 ** (1.5–8.6) | 2.2 (0.8–5.8) | 2.8 * (1.2–6.5) |
| Solar Elastosis | 3.5 (0.9–12.5) | 1.6 (0.6–4.4) | 2.2 * (0.9–5.5) | |
| Round Blood Vessels 1 | 1.5 (0.8–2.7) | 1.8 * (1.02–3.2) | 1.6 * (1.0–2.6) | |
| Polymorphous Blood Vessels 1 | 3.8 ** (1.6–8.7) | 3.0 * (1.1–7.7) | 3.3 ** (1.5–7.4) |
| Treatment | Grade 1 AK | Grade 2 AK | PD Skin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | 3 mos | 6 mos | BL | 3 mos | 6 mos | BL | 3 mos | 6 mos | |||
| Clinical Parameter | Erythema | Cryo | 1.00 1 | 0.20 (0.42) ** | 0.15 (0.34) ** | 2.00 1 | 0.20 (0.42) ** | 0.10 (0.32) ** | N/A | N/A | N/A |
| PDT | 1.00 1 | 0.55 (0.17) ** | 0.17 (0.35) ** | 2.00 1 | 0.00 (0.00) ** | 0.22 (0.44) ** 2 | N/A | N/A | N/A | ||
| Hyperkeratosis | Cryo | 1.00 1 | 0.35 (0.47) ** | 0.05 (0.16) ** | 2.00 1 | 0.30 (0.67) ** | 0.30 (0.79) ** | N/A | N/A | N/A | |
| PDT | 1.00 1 | 0.33 (0.43) ** | 0.05 (0.17) ** | 2.00 1 | 0.31 (0.46) ** | 0.33 (0.43) ** 3 | N/A | N/A | N/A | ||
| RCM Parameter: Stratum Corneum | Parakeratosis | Cryo | 85% | 43% ** | 57% * | 58% | 50% | 70% | 22% | 31% | 37% |
| PDT | 52% | 29% | 29% | 79% | 16% ** | 50% | 33% | 12% | 44% | ||
| Hyperkeratosis | Cryo | 71% | 45% * | 23% ** | 80% | 47% ** | 43% ** | 19% | 21% | 17% | |
| PDT | 45% | 22% | 20% | 63% | 33% * | 56% | 5% | 6% | 11% | ||
| Stratum Corneum Disruption | Cryo | 88% | 47% ** | 40% ** | 83% | 60% * | 67% | 26% | 46% | 40% | |
| PDT | 56% | 39% | 12% * | 69% | 24% * | 47% | 26% | 22% | 13% | ||
| Epidermis | Atypical Honeycomb Pattern 4 | Cryo | 100% | 67% * | 63% ** | 96% | 83% * | 83% ** | 65% | 57% * | 57% |
| PDT | 97% | 63% * | 53% ** | 100% | 69% ** | 78% ** | 73% | 59% | 59% | ||
| Round Nucleated Cells | Cryo | 23% | 23% | 7% | 13% | 7% | 7% | 4% | 7% | 7% | |
| PDT | 23% | 4% | 6% | 15% | 19% | 33% | 14% | 0% | 6% | ||
| Disarranged Pattern | Cryo | 48% | 23% * | 13% ** | 59% | 38% * | 13% ** | 11% | 7% | 17% | |
| PDT | 53% | 16% * | 25% | 62% | 35% * | 44% | 20% | 11% | 12% | ||
| Inflammatory Cells Present | Cryo | 61% | 40% | 28% * | 43% | 24% | 53% | 35% | 27% | 30% | |
| PDT | 50% | 22% * | 22% | 74% | 35% ** | 41% * | 43% | 18% * | 29% | ||
| Dendritic Cells Present | Cryo | 18% | 10% | 17% | 13% | 10% | 23% | 11% | 10% | 23% | |
| PDT | 4% | 8% | 0% | 15% | 4% | 6% | 14% | 0% | 12% | ||
| Dermis | Inflammatory Cells Present 4 | Cryo | 61% | 54% | 27% * | 39% | 29% | 40% | 31% | 40% | 37% |
| PDT | 65% | 48% ** | 29% * | 54% | 50% | 21% | 34% | 37% | 29% | ||
| Solar Elastosis | Cryo | 96% | 79% | 90% | 81% | 68% | 87% | 70% | 83% | 63% | |
| PDT | 89% | 96% | 100% | 88% | 93% | 100% | 86% | 92% | 88% | ||
| Round Blood Vessels 4 | Cryo | 67% | 79% | 77% | 64% | 72% | 73% | 61% | 70% | 73% | |
| PDT | 72% | 85% * | 94% * | 86% | 81% | 100% | 62% | 84% ** | 94% ** | ||
| Polymorphous Blood Vessels 4 | Cryo | 44% | 31% | 13% | 26% | 21% | 17% | 19% | 13% | 13% | |
| PDT | 38% | 23% | 23% | 43% | 38% | 29% | 14% | 32% * | 23% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curiel-Lewandrowski, C.; Myrdal, C.N.; Saboda, K.; Hu, C.; Arzberger, E.; Pellacani, G.; Legat, F.J.; Ulrich, M.; Hochfellner, P.; Oliviero, M.C.; et al. In Vivo Reflectance Confocal Microscopy as a Response Monitoring Tool for Actinic Keratoses Undergoing Cryotherapy and Photodynamic Therapy. Cancers 2021, 13, 5488. https://doi.org/10.3390/cancers13215488
Curiel-Lewandrowski C, Myrdal CN, Saboda K, Hu C, Arzberger E, Pellacani G, Legat FJ, Ulrich M, Hochfellner P, Oliviero MC, et al. In Vivo Reflectance Confocal Microscopy as a Response Monitoring Tool for Actinic Keratoses Undergoing Cryotherapy and Photodynamic Therapy. Cancers. 2021; 13(21):5488. https://doi.org/10.3390/cancers13215488
Chicago/Turabian StyleCuriel-Lewandrowski, Clara, Caitlyn N. Myrdal, Kathylynn Saboda, Chengcheng Hu, Edith Arzberger, Giovanni Pellacani, Franz Josef Legat, Martina Ulrich, Petra Hochfellner, Margaret C. Oliviero, and et al. 2021. "In Vivo Reflectance Confocal Microscopy as a Response Monitoring Tool for Actinic Keratoses Undergoing Cryotherapy and Photodynamic Therapy" Cancers 13, no. 21: 5488. https://doi.org/10.3390/cancers13215488
APA StyleCuriel-Lewandrowski, C., Myrdal, C. N., Saboda, K., Hu, C., Arzberger, E., Pellacani, G., Legat, F. J., Ulrich, M., Hochfellner, P., Oliviero, M. C., Pasquali, P., Gill, M., & Hofmann-Wellenhof, R. (2021). In Vivo Reflectance Confocal Microscopy as a Response Monitoring Tool for Actinic Keratoses Undergoing Cryotherapy and Photodynamic Therapy. Cancers, 13(21), 5488. https://doi.org/10.3390/cancers13215488







