Bisphenol A Alters the Energy Metabolism of Stromal Cells and Could Promote Bladder Cancer Progression
Abstract
:Simple Summary
Abstract
1. Introduction
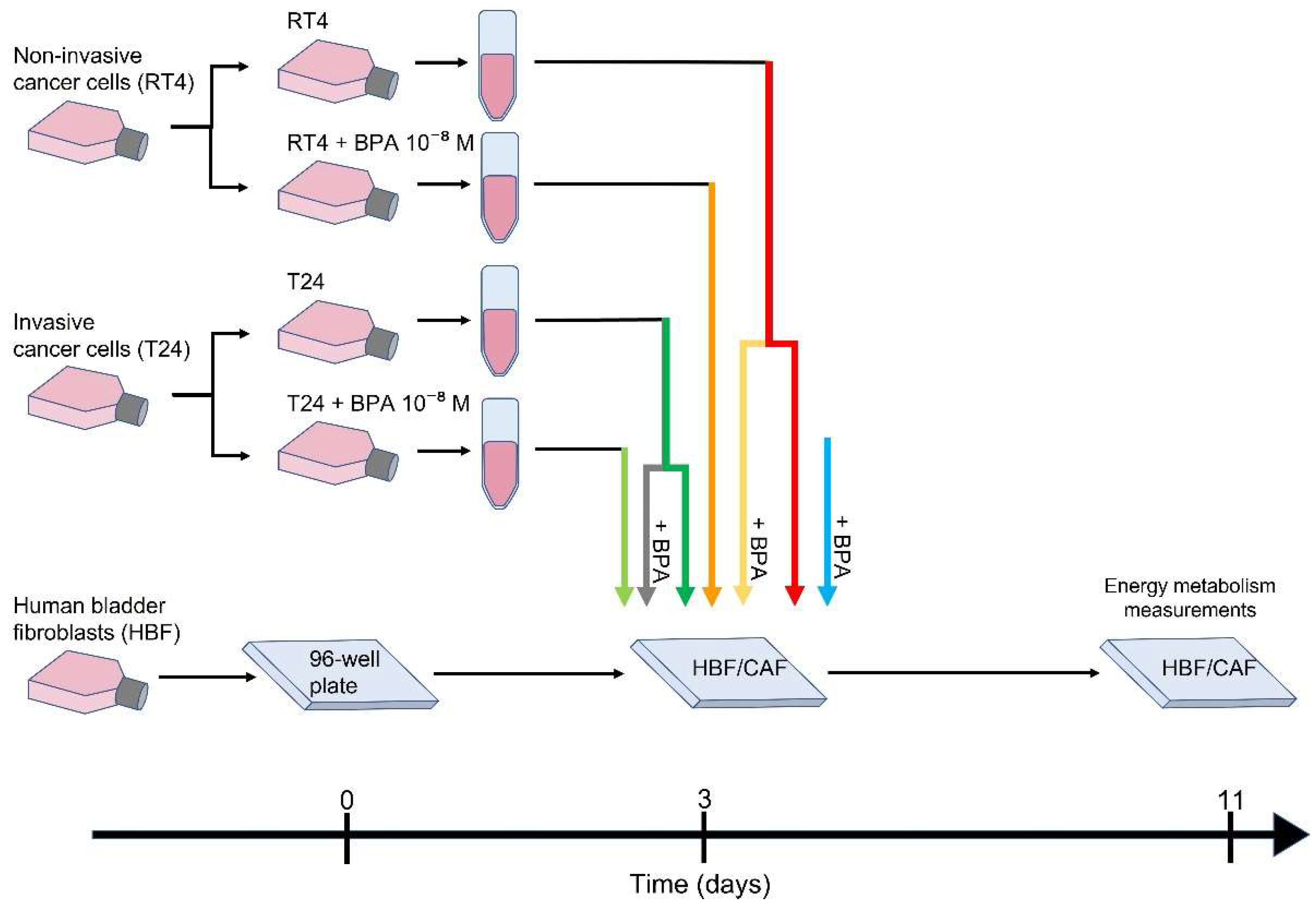
2. Materials and Methods
2.1. Cell Lines
2.2. CAF Induction
2.3. Seahorse Energy Metabolism Measurements
2.4. Statistical Analysis
3. Results
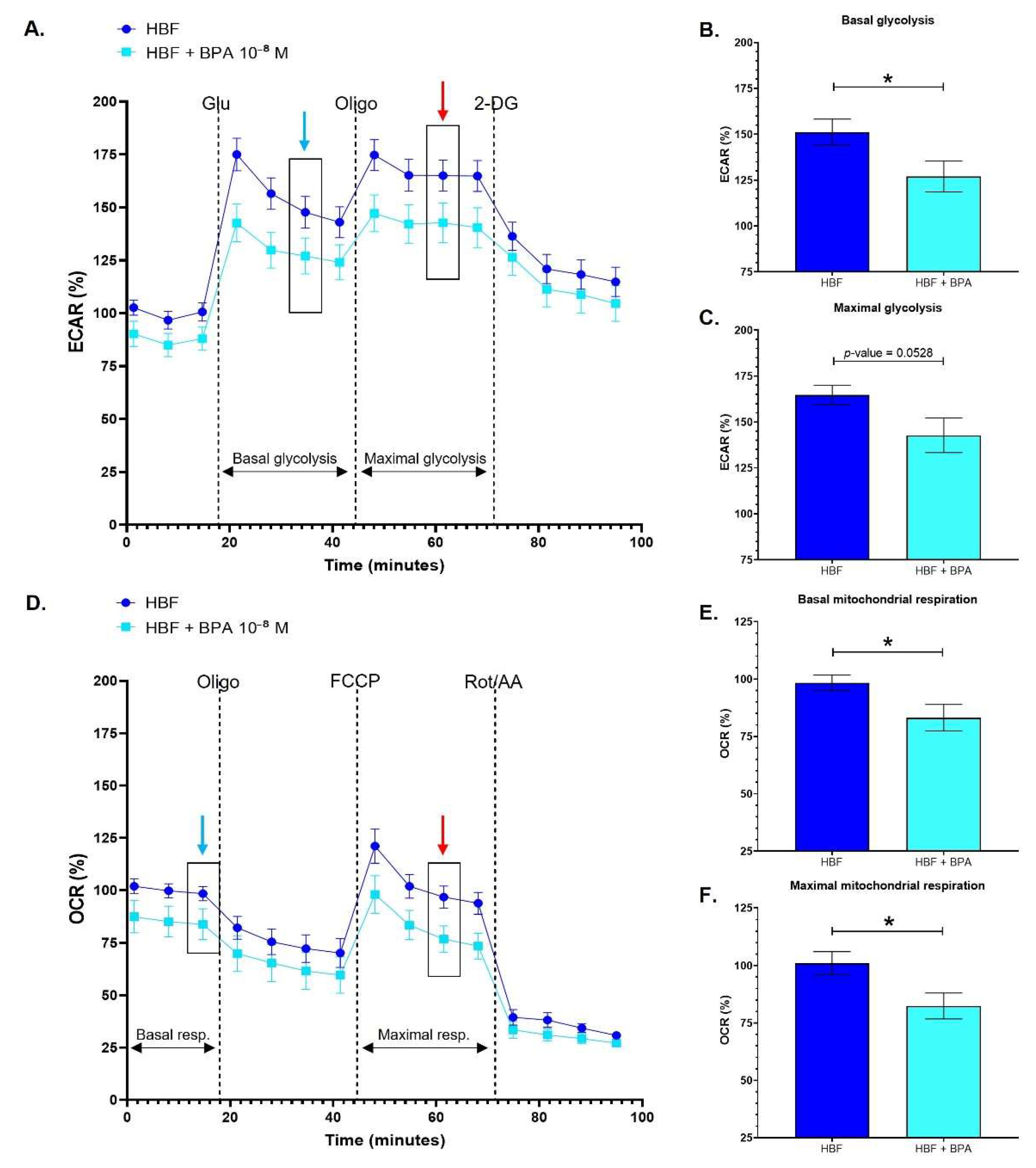
3.1. Healthy Human Bladder Fibroblasts Exhibit Decreased Glycolytic and Mitochondrial Metabolism following Chronic Exposure to BPA
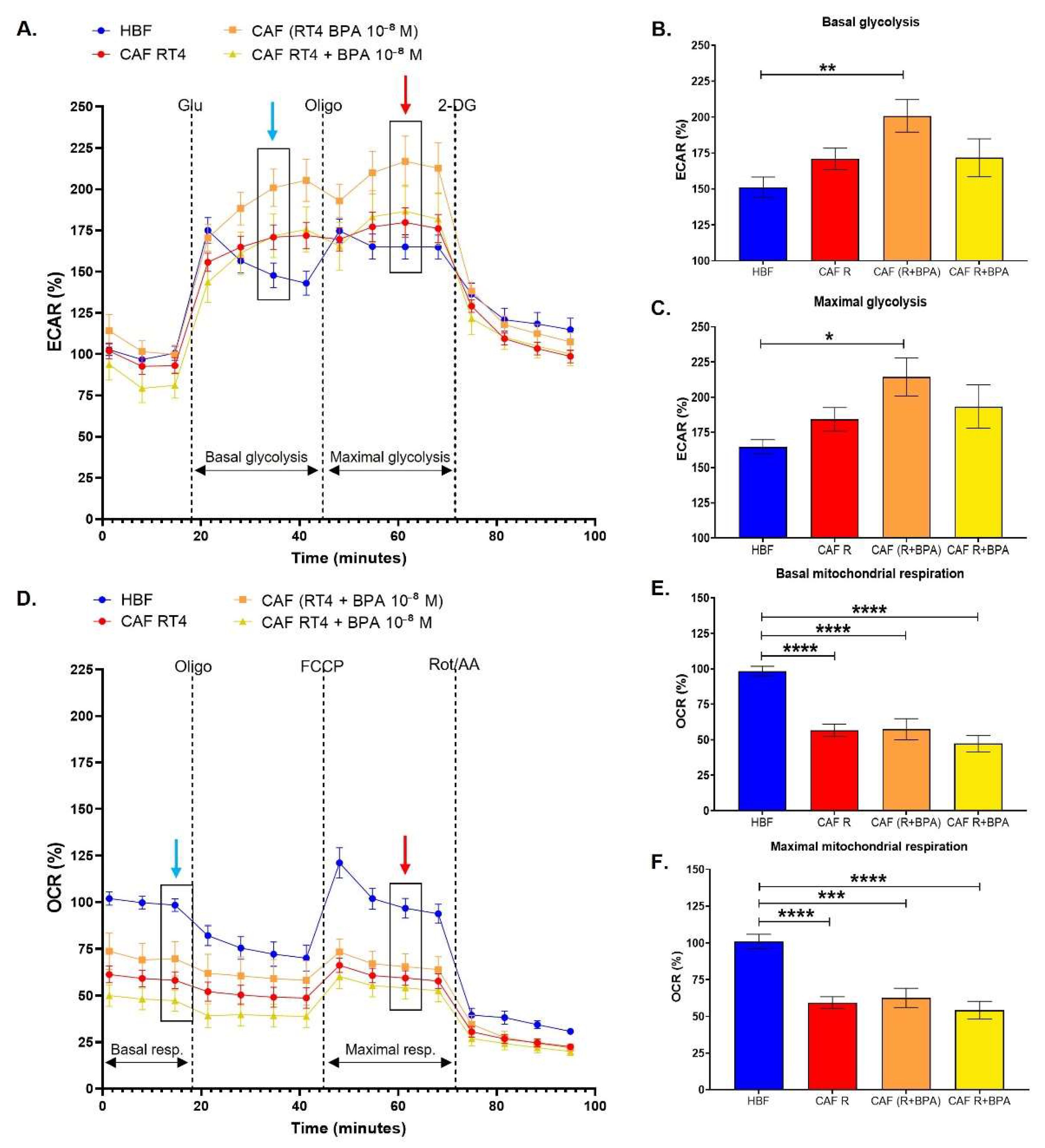
3.2. Cancer-Associated Fibroblasts Conditioned by Non-Invasive Bladder Cancer Cells Exhibit a Metabolic Switch, Characterized by a Decreased Mitochondrial Metabolism and Increased Glycolysis, Accentuated by BPA
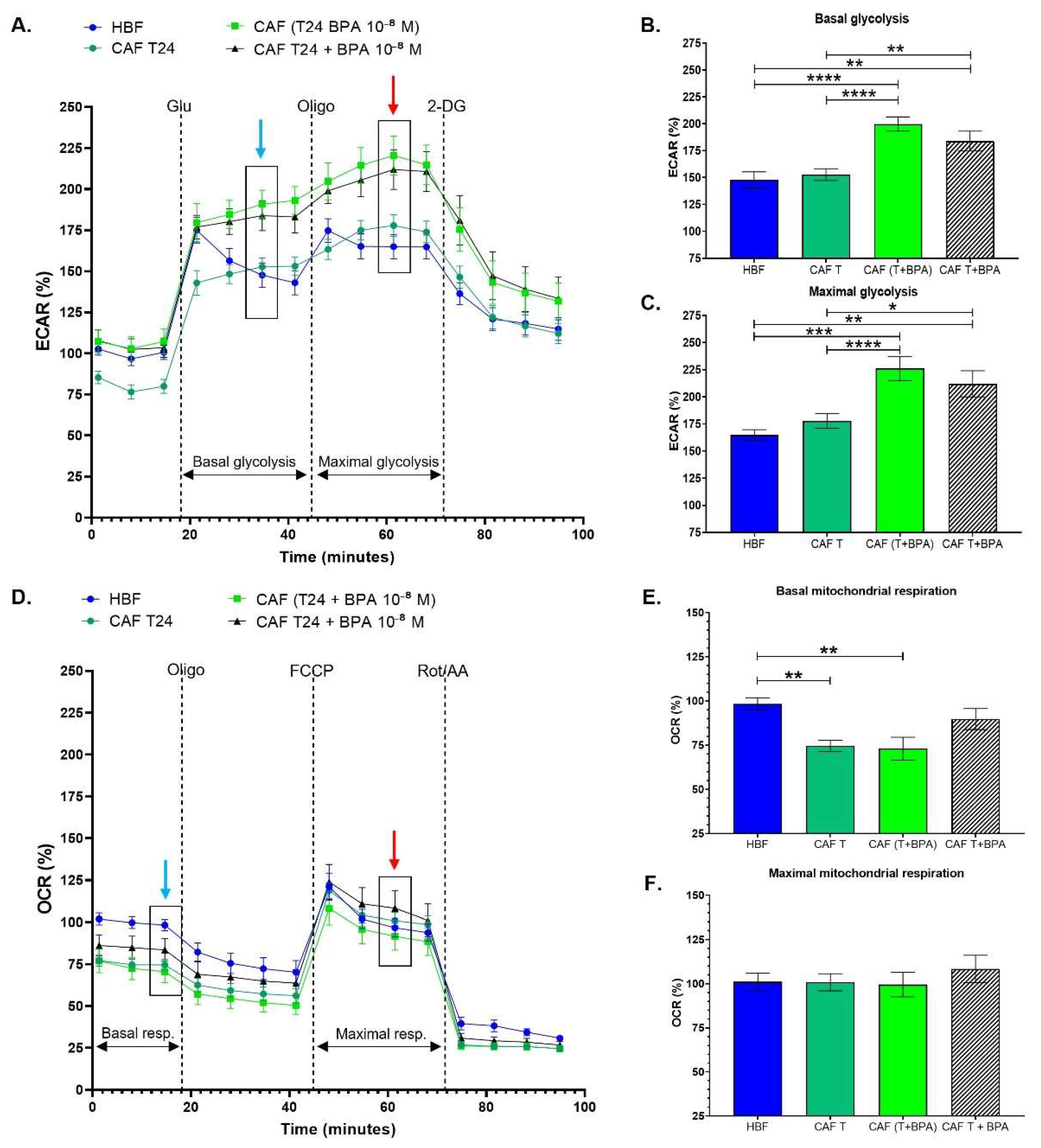
3.3. Cancer-Associated Fibroblasts Conditioned with Invasive Bladder Cancer Cells in the Presence of BPA Exhibit an Increased Glycolytic Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murata, M.; Kang, J.-H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Birkholz, D.; Lobo, R.A. Human Excretion of Bisphenol A: Blood, Urine, and Sweat (BUS) Study. J. Environ. Public Health 2011, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushnik, T.; Haines, D.; Levallois, P.; Levesque, J.; Van Oostdam, J.; Viau, C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010, 21, 7–18. [Google Scholar] [PubMed]
- Li, S.; Wang, B.; Tang, Q.; Liu, J.; Yang, X. Bisphenol A triggers proliferation and migration of laryngeal squamous cell carcinoma via GPER mediated upregulation of IL-6. Cell Biochem. Funct. 2017, 35, 209–216. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Liu, N.; Weng, S.-F.; Wang, H.-S. Bisphenol A Increases the Migration and Invasion of Triple-Negative Breast Cancer Cells via Oestrogen-related Receptor Gamma. Basic Clin. Pharmacol. Toxicol. 2016, 119, 389–395. [Google Scholar] [CrossRef]
- Dairkee, S.H.; Luciani-Torres, M.; Moore, D.H.; Goodson, W. Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis 2012, 34, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-M.; Rao, R.; To, S.; Schoch, E.; Tarapore, P. Bisphenol A and its analogues disrupt centrosome cycle and microtubule dynamics in prostate cancer. Endocr.-Relat. Cancer 2017, 24, 83–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy, G.; Gakis, G.; Smith, C.L.; Fahmy, O. Effects of Androgen and Estrogen Receptor Signaling Pathways on Bladder Cancer Initiation and Progression. Bladder Cancer 2016, 2, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Chen, J.; Miyamoto, H. Androgen Receptor Signaling in Bladder Cancer. Cancers 2017, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Pellerin, E.; Caneparo, C.; Chabaud, S.; Bolduc, S.; Pelletier, M. Endocrine-disrupting effects of bisphenols on urological cancers. Environ. Res. 2020, 195, 110485. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shestov, A.A.; Liu, X.; Ser, Z.; Cluntun, A.; Hung, Y.P.; Huang, L.; Kim, D.; Le, A.; Yellen, G.; Albeck, J.G.; et al. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. eLife 2014, 3, e03342. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet, C.R.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet, C.R.; Bernard, G.; Chabaud, S.; Couture, A.; Langlois, A.; Neveu, B.; Pouliot, F.; Bolduc, S. Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials 2017, 145, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.; Billingham, L.K.; Ramaswamy, M.; Siegel, R.M. Extracellular Flux Analysis to Monitor Glycolytic Rates and Mitochondrial Oxygen Consumption. Methods Enzymol. 2014, 542, 125–149. [Google Scholar] [CrossRef] [PubMed]
- Creta, M.; Celentano, G.; Napolitano, L.; La Rocca, R.; Capece, M.; Califano, G.; Ruvolo, C.C.; Mangiapia, F.; Morra, S.; Turco, C.; et al. Inhibition of Androgen Signalling Improves the Outcomes of Therapies for Bladder Cancer: Results from a Systematic Review of Preclinical and Clinical Evidence and Meta-Analysis of Clinical Studies. Diagnostics 2021, 11, 351. [Google Scholar] [CrossRef]
- Venugopal, P.; Moorthy, H.K.; Prabhu, G.G.L. Clinical and therapeutic implications of sex steroid hormone receptor status in urothelial bladder cancer. Indian J. Urol. 2020, 36, 171–178. [Google Scholar] [CrossRef]
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poolman, B.; Doeven, M.K.; Geertsma, E.R.; Biemans-Oldehinkel, E.; Konings, W.N.; Rees, D.C. Functional analysis of detergent-solubilized and membrane-reconstituted ATP-binding cassette transporters. Methods Enzymol. 2005, 400, 429–459. [Google Scholar] [PubMed] [Green Version]
- Kennedy, C.J. P-glycoprotein induction and its energetic costs in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2021, 47, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, E.; van Schijndel, M.; Voulgaris, D.; Di Criscio, M.; Ramsbottom, K.; Rigden, D.; Herland, A.; Rüegg, J. Bisphenol A Inhibits the Transporter Function of the Blood-Brain Barrier by Directly Interacting with the ABC Transporter Breast Cancer Resistance Protein (BCRP). Int. J. Mol. Sci. 2021, 22, 5534. [Google Scholar] [CrossRef] [PubMed]
- Quesnot, N.; Bucher, S.; Fromenty, B.; Robin, M.-A. Modulation of Metabolizing Enzymes by Bisphenol A in Human and Animal Models. Chem. Res. Toxicol. 2014, 27, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Beigh, S.; Chaudhari, B.P.; Sharma, S.; Abdi, S.A.H.; Ahmad, S.; Ahmad, F.; Parvez, S.; Raisuddin, S. Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats. Environ. Toxicol. 2015, 31, 1922–1934. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar]
- Bouhout, S.; Chabaud, S.; Bolduc, S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J. Urol. 2015, 34, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.E.; Carmean, N.; Bassuk, J.A. Extracellular Matrix Protein Coatings for Facilitation of Urothelial Cell Attachment. Tissue Eng. 2007, 13, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S. Human urinary carcinogen metabolites: Biomarkers for investigating tobacco and cancer. Carcinogenesis 2002, 23, 907–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, D.T.; Alguacil, J.; Rothman, N.; Real, F.X.; Garcia-Closas, M.; Cantor, K.P.; Malats, N.; Tardon, A.; Serra, C.; Garcia-Closas, R.; et al. Does increased urination frequency protect against bladder cancer? Int. J. Cancer 2008, 123, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [Green Version]
- Guido, C.; Whitaker-Menezes, D.; Capparelli, C.; Balliet, R.; Lin, Z.; Pestell, R.G.; Howell, A.; Aquila, S.; Andò, S.; Martinez-Outschoorn, U.; et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: Connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle 2012, 11, 3019–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, V.J.; Brown, J.K.; Maybin, J.; Saunders, P.T.K.; Duncan, W.C.; Horne, A.W. Transforming Growth Factor-β Induced Warburg-Like Metabolic Reprogramming May Underpin the Development of Peritoneal Endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, 3450–3459. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, E.M.; Tian, Y.; Nigdelioglu, R.; Witt, L.J.; Cetin-Atalay, R.; Meliton, A.Y.; Woods, P.S.; Kimmig, L.; Sun, K.A.; Gökalp, G.A.; et al. TGF-β Promotes Metabolic Reprogramming in Lung Fibroblasts via mTORC1-dependent ATF4 Activation. Am. J. Respir. Cell Mol. Biol. 2020, 63, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, S.; Moulin, V. Apoptosis Modulation as a Promising Target for Treatment of Systemic Sclerosis. Int. J. Rheumatol. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetze, K.; Walenta, S.; Ksiazkiewicz, M.; Kunz-Schughart, L.; Mueller-Klieser, W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 2011, 39, 453–463. [Google Scholar] [CrossRef]
- Niu, D.; Luo, T.; Wang, H.; Xia, Y.; Xie, Z. Lactic acid in tumor invasion. Clin. Chim. Acta 2021, 522, 61–69. [Google Scholar] [CrossRef]
- Kim, B.G.; Sung, J.S.; Jang, Y.; Cha, Y.J.; Kang, S.; Han, H.H.; Li, J.H.; Cho, N.H. Compression-induced expression of glycolysis genes in CAFs correlates with EMT and angiogenesis gene expression in breast cancer. Commun Biol. 2019, 2, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saal, F.S.V.; Welshons, W.V. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol. Cell. Endocrinol. 2014, 398, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Peillex, C.; Kerever, A.; Lachhab, A.; Pelletier, M. Bisphenol A, bisphenol S and their glucuronidated metabolites modulate glycolysis and functional responses of human neutrophils. Environ. Res. 2020, 196, 110336. [Google Scholar] [CrossRef]
- Song, P.; Fan, K.; Tian, X.; Wen, J. Bisphenol S (BPS) triggers the migration of human non-small cell lung cancer cells via upregulation of TGF-β. Toxicol. Vitr. 2018, 54, 224–231. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellerin, È.; Chabaud, S.; Pouliot, F.; Pelletier, M.; Bolduc, S. Bisphenol A Alters the Energy Metabolism of Stromal Cells and Could Promote Bladder Cancer Progression. Cancers 2021, 13, 5461. https://doi.org/10.3390/cancers13215461
Pellerin È, Chabaud S, Pouliot F, Pelletier M, Bolduc S. Bisphenol A Alters the Energy Metabolism of Stromal Cells and Could Promote Bladder Cancer Progression. Cancers. 2021; 13(21):5461. https://doi.org/10.3390/cancers13215461
Chicago/Turabian StylePellerin, Ève, Stéphane Chabaud, Frédéric Pouliot, Martin Pelletier, and Stéphane Bolduc. 2021. "Bisphenol A Alters the Energy Metabolism of Stromal Cells and Could Promote Bladder Cancer Progression" Cancers 13, no. 21: 5461. https://doi.org/10.3390/cancers13215461
APA StylePellerin, È., Chabaud, S., Pouliot, F., Pelletier, M., & Bolduc, S. (2021). Bisphenol A Alters the Energy Metabolism of Stromal Cells and Could Promote Bladder Cancer Progression. Cancers, 13(21), 5461. https://doi.org/10.3390/cancers13215461






