Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends
Abstract
Simple Summary
Abstract
1. Introduction
2. Targeting Agents
2.1. [18F]FDG
2.2. [11C/18F]Choline
2.3. [11C]Acetate
2.4. [68Ga]Ga-PSMA
2.5. [18F]PSMA
3. Other Agents
3.1. [99mTc]Tc-PSMA
3.2. [11C]Methionine
3.3. [18F]Fluciclovine (FACBC)
3.4. Androgen Receptor
3.5. Gastrin-Releasing Peptide Receptor
3.6. Urokinase Plasminogen Activator Ligand
3.7. VAPAC1-Targeting Agent
3.8. αvβ3 Integrin-Targeting Agent
4. PET/MR
5. Radiomics
6. Sentinel Lymph Node Biopsy
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Available online: https://uroweb.org/guideline/prostate-cancer/ (accessed on 28 June 2021).
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 16 October 2021).
- Samaržija, I. Site-Specific and Common Prostate Cancer Metastasis Genes as Suggested by Meta-Analysis of Gene Expression Data. Life 2021, 11, 636. [Google Scholar] [CrossRef]
- Humphrey, P.A. Histopathology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030411. [Google Scholar] [CrossRef]
- Trabulsi, E.J.; Rumble, R.B.; Jadvar, H.; Hope, T.; Pomper, M.; Turkbey, B.; Rosenkrantz, A.; Verma, S.; Margolis, D.J.; Froemming, A.; et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1963–1996. [Google Scholar] [CrossRef] [PubMed]
- Wynant, G.E.; Murphy, G.P.; Horoszewicz, J.S.; Neal, C.E.; Collier, B.D.; Mitchell, E.; Purnell, G.; Tyson, I.; Heal, A.; Abdel-Nabi, H.; et al. Immunoscintigraphy of prostatic cancer: Preliminary results with111in-labeled monoclonal antibody 7E11-C5.3 (CYT-356). Prostate 1991, 18, 229–241. [Google Scholar] [CrossRef]
- Shreve, P.D.; Grossman, H.B.; Gross, M.D.; Wahl, R.L. Metastatic prostate cancer: Initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology 1996, 199, 751–756. [Google Scholar] [CrossRef]
- Hara, T.; Kosaka, N.; Kishi, H. PET imaging of prostate cancer using carbon-11-choline. J. Nucl. Med. 1998, 39, 990–995. [Google Scholar] [PubMed]
- Oyama, N.; Akino, H.; Kanamaru, H.; Sadato, N.; Yonekura, Y.; Yamamoto, K.; Okada, K. C-11 Acetate Pet Imaging of Prostate Cancer. J. Urol. 2002, 43, 181–186. [Google Scholar] [CrossRef]
- Mapelli, P.; Picchio, M. Initial prostate cancer diagnosis and disease staging—The role of choline-PET–CT. Nat. Rev. Urol. 2015, 12, 510–518. [Google Scholar] [CrossRef]
- Mohsen, B.; Giorgio, T.; Rasoul, Z.S.; Werner, L.; Ali, G.R.M.; Reza, D.K.V.; Ramin, S. Application of11C-acetate positron-emission tomography (PET) imaging in prostate cancer: Systematic review and meta-analysis of the literature. BJU Int. 2013, 112, 1062–1072. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Ben Jemaa, A.; Bouraoui, Y.; Sallami, S.; Banasr, A.; Ben Rais, N.; Ouertani, L.; Nouira, Y.; Horchani, A.; Oueslati, R. Co-expression and impact of prostate specific membrane antigen and prostate specific antigen in prostatic pathologies. J. Exp. Clin. Cancer Res. 2010, 29, 171. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.A.; Coleman, R.E.; Goldsmith, S.J.; Vallabhajosula, S.; Petry, N.A.; Cho, S.; Armor, T.; Stubbs, J.B.; Maresca, K.P.; Stabin, M.G.; et al. First-in-Man Evaluation of 2 High-Affinity PSMA-Avid Small Molecules for Imaging Prostate Cancer. J. Nucl. Med. 2013, 54, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.M.; Maresca, K.P.; Lu, G.; Merkin, R.D.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen for Molecular Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Cordes, M.; Beck, M.; Goetz, T.I.; Schmidt, D.; Prante, O.; Bäuerle, T.; Uder, M.; Wullich, B.; Goebell, P.; et al. SPECT/CT With the PSMA Ligand 99mTc-MIP-1404 for Whole-Body Primary Staging of Patients With Prostate Cancer. Clin. Nucl. Med. 2018, 43, 225–231. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Osborne, J.; Nikolopoulou, A.; Lipai, I.; Tagawa, S.; Scherr, D.; Joyal, J.; Armor, T.; Goldsmith, S.; Babich, J. PSMA targeted SPECT imaging biomarker to detect local and metastatic prostate cancer (PCa): Phase I studies with 99mTc-MIP-1404. Soc. Nuclear Med. 2013, 54, 281. [Google Scholar]
- Hillier, S.M.; Maresca, K.P.; Femia, F.J.; Marquis, J.C.; Foss, C.A.; Nguyen, N.; Zimmerman, C.N.; Barrett, J.A.; Eckelman, W.C.; Pomper, M.G.; et al. Preclinical Evaluation of Novel Glutamate-Urea-Lysine Analogues That Target Prostate-Specific Membrane Antigen as Molecular Imaging Pharmaceuticals for Prostate Cancer. Cancer Res. 2009, 69, 6932–6940. [Google Scholar] [CrossRef]
- Mosayebnia, M.; Hajimahdi, Z.; Beiki, D.; Rezaeianpour, M.; Hajiramezanali, M.; Geramifar, P.; Sabzevari, O.; Amini, M.; Hatamabadi, D.; Shahhosseini, S. Design, synthesis, radiolabeling and biological evaluation of new urea-based peptides targeting prostate specific membrane antigen. Bioorganic Chem. 2020, 99, 103743. [Google Scholar] [CrossRef]
- Alberts, I.L.; Seide, S.E.; Mingels, C.; Bohn, K.P.; Shi, K.; Zacho, H.D.; Rominger, A.; Afshar-Oromieh, A. Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: A systematic review and network meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2978–2989. [Google Scholar] [CrossRef]
- Lamb, H.M.; Faulds, D. Capromab Pendetide. A review of its use as an imaging agent in prostate cancer. Drugs Aging 1998, 12, 293–304. [Google Scholar] [CrossRef]
- Beheshti, M.; Manafi-Farid, R.; Geinitz, H.; Vali, R.; Loidl, W.; Mottaghy, F.M.; Langsteger, W. Multiphasic 68Ga-PSMA PET/CT in the Detection of Early Recurrence in Prostate Cancer Patients with a PSA Level of Less Than 1 ng/mL: A Prospective Study of 135 Patients. J. Nucl. Med. 2020, 61, 1484–1490. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Shergill, I.S.; Vandal, M.T.; Gujral, S.S. ProstaScint™ and its role in the diagnosis of prostate cancer. Expert Rev. Mol. Diagn. 2007, 7, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Manyak, M.J.; Hinkle, G.H.; Olsen, J.O.; Chiaccherini, R.P.; Partin, A.W.; Piantadosi, S.; Burgers, J.K.; Texter, J.H.; Neal, C.E.; Libertino, J.A.; et al. Immunoscintigraphy with indium-111-capromab pendetide: Evaluation before definitive therapy in patients with prostate cancer. Urology 1999, 54, 1058–1063. [Google Scholar] [CrossRef]
- Feneley, M.; Chengazi, V.; Kirby, R.; Nimmon, C.; Granowska, M.; Mather, S.; Ellison, D.; Granowski, A.; Britton, K. Prostatic radioimmunoscintigraphy: Preliminary results using technetium-labelled monoclonal antibody, CYT-351. BJU Int. 1996, 77, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Krishnan, M.A.; Chattopadhyay, S.; Chelvam, V. Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer Rep. 2019, 2, e1169. [Google Scholar] [CrossRef]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Bander, N.H.; Trabulsi, E.J.; Kostakoglu, L.; Yao, D.; Vallabhajosula, S.; Smith-Jones, P.; Joyce, M.A.; Milowsky, M.; Nanus, D.M.; Goldsmith, S.J. Targeting Metastatic Prostate Cancer with Radiolabeled Monoclonal Antibody J591 to the Extracellular Domain of Prostate Specific Membrane Antigen. J. Urol. 2003, 170, 1717–1721. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Ruan, S.; Lyashchenko, S.K.; Carrasquillo, J.; Heller, G.; Martinez, D.F.; Cheal, S.M.; Lewis, J.; Fleisher, M.; et al. First-in-Human Imaging with 89Zr-Df-IAB2M Anti-PSMA Minibody in Patients with Metastatic Prostate Cancer: Pharmacokinetics, Biodistribution, Dosimetry, and Lesion Uptake. J. Nucl. Med. 2016, 57, 1858–1864. [Google Scholar] [CrossRef]
- Kapoor, V.; McCook, B.M.; Torok, F.S. An Introduction to PET-CT Imaging. Radiographics 2004, 24, 523–543. [Google Scholar] [CrossRef]
- Wallitt, K.L.; Khan, S.R.; Dubash, S.; Tam, H.H.; Khan, S.; Barwick, T.D. Clinical PET Imaging in Prostate Cancer. Radiographics 2017, 37, 1512–1536. [Google Scholar] [CrossRef]
- Salminen, E.; Hogg, A.; Binns, D.; Frydenberg, M.; Hicks, R. Investigations with FDG-PET Scanning in Prostate Cancer Show Limited Value for Clinical Practice. Acta Oncol. 2002, 41, 425–429. [Google Scholar] [CrossRef]
- Kitajima, K.; Murphy, R.C.; Nathan, M.A.; Sugimura, K. Update on positron emission tomography for imaging of prostate cancer. Int. J. Urol. 2014, 21, 12–23. [Google Scholar] [CrossRef]
- Jadvar, H. Molecular Imaging of Prostate Cancer: PET Radiotracers. Am. J. Roentgenol. 2012, 199, 278–291. [Google Scholar] [CrossRef]
- Morris, M.J.; Akhurst, T.; Osman, I.; Nunez, R.; Macapinlac, H.; Siedlecki, K.; Verbel, D.; Schwartz, L.; Larson, S.M.; Scher, H.I. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology 2002, 59, 913–918. [Google Scholar] [CrossRef]
- Zukotynski, K.A.; Kim, C.K.; Gerbaudo, V.H.; Hainer, J.; Taplin, M.E.; Kantoff, P.; den Abbeele, A.D.; Seltzer, S.; Sweeney, C.J. (18)F-FDG-PET/CT and (18)F-NaF-PET/CT in men with castrate-resistant prostate cancer. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 72–82. [Google Scholar]
- Jadvar, H. Is There Use for FDG-PET in Prostate Cancer? Semin. Nucl. Med. 2016, 46, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Espiritu, J.; Segall, G.; Terris, M. Fluorodeoxyglucose positron emission tomography studies in the diagnosis and staging of clinically advanced prostate cancer. BJU Int. 2003, 92, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Akino, H.; Suzuki, Y.; Kanamaru, H.; Sadato, N.; Yonekura, Y.; Okada, K. The Increased Accumulation of [18F]Fluorodeoxyglucose in Untreated Prostate Cancer. Jpn. J. Clin. Oncol. 1999, 29, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Meziou, S.; Goulet, C.R.; Hovington, H.; Lefebvre, V.; Lavallée, É.; Bergeron, M.; Brisson, H.; Champagne, A.; Neveu, B.; Lacombe, D.; et al. GLUT1 expression in high-risk prostate cancer: Correlation with 18F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020, 23, 441–448. [Google Scholar] [CrossRef]
- Beauregard, J.-M.; Blouin, A.-C.; Fradet, V.; Caron, A.; Fradet, Y.; Lemay, C.; Lacombe, L.; Dujardin, T.; Tiguert, R.; Rimac, G.; et al. FDG-PET/CT for pre-operative staging and prognostic stratification of patients with high-grade prostate cancer at biopsy. Cancer Imaging 2015, 15, 2. [Google Scholar] [CrossRef]
- Kanamaru, H.; Oyama, N.; Akino, H.; Okada, K. [Evaluation of prostate cancer using FDG-PET][Abstract]. Hinyokika Kiyo 2000, 46, 851–853. [Google Scholar]
- Sadeghi, R.; Giovanella, L.; Treglia, G.; Bertagna, F. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland. Systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin 2014, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H. Prostate Cancer: PET with 18F-FDG, 18F- or 11C-Acetate, and 18F- or 11C-Choline. J. Nucl. Med. 2011, 52, 81–89. [Google Scholar] [CrossRef]
- Beheshti, M.; Manafi-Farid, R.; Rezaee, A.; Langsteger, W. PET/CT and PET/MRI, Normal Variations, and Artifacts. In Clinical Nuclear Medicine, 2nd ed.; Ahmadzadehfar, H., Biersack, H.-J., Freeman, L.M., Zuckier, L.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 549–584. [Google Scholar]
- Brogsitter, C.; Zöphel, K.; Kotzerke, J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: Comparative analysis for imaging prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Farsad, M.; Schiavina, R.; Castellucci, P.; Nanni, C.; Corti, B.; Martorana, G.; Canini, R.; Grigioni, W.; Boschi, S.; Marengo, M.; et al. Detection and localization of prostate cancer: Correlation of (11)C-choline PET/CT with histopathologic step-section analysis. J. Nucl. Med. 2005, 46, 1642–1649. [Google Scholar]
- De Jong, I.J.; Pruim, J.; Elsinga, P.H.; Vaalburg, W.; Mensink, H.J. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J. Nucl. Med. 2003, 44, 331–335. [Google Scholar]
- Price, D.T.; Coleman, R.E.; Liao, R.P.; Robertson, C.N.; Polascik, T.J.; DeGrado, T.R. Comparison of [18 F]fluorocholine and [18 F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J. Urol. 2002, 168, 273–280. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Coleman, R.E.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2001, 61, 110–117. [Google Scholar]
- Hara, T.; Kosaka, N.; Kishi, H. Development of (18)F-fluoroethylcholine for cancer imaging with PET: Synthesis, biochemistry, and prostate cancer imaging. J. Nucl. Med. 2002, 43, 187–199. [Google Scholar] [PubMed]
- Igerc, I.; Kohlfürst, S.; Gallowitsch, H.J.; Matschnig, S.; Kresnik, E.; Gomez-Segovia, I.; Lind, P. The value of 18F-Choline PET/CT in patients with elevated PSA-level and negative prostate needle biopsy for localisation of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Cervino, A.R.; Burei, M.; Gregianin, M.; Saladini, G.; Marzola, M.C.; Chondrogianis, S.; Rubello, D. Comparative studies of radiolabeled choline positron emission tomography, histology of primary tumor and other imaging modalities in prostate cancer: A systematic review and meta-analysis. Clin. Transl. Imaging 2013, 1, 99–109. [Google Scholar] [CrossRef]
- Beheshti, M.; Langsteger, W. PET Imaging of Prostate Cancer Using Radiolabeled Choline. PET Clin. 2009, 4, 173–184. [Google Scholar] [CrossRef]
- Beheshti, M.; Imamovic, L.; Broinger, G.; Vali, R.; Waldenberger, P.; Stoiber, F.; Nader, M.; Gruy, B.; Janetschek, G.; Langsteger, W. 18F Choline PET/CT in the Preoperative Staging of Prostate Cancer in Patients with Intermediate or High Risk of Extracapsular Disease: A Prospective Study of 130 Patients 1. Radiology 2010, 254, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Palard-Novello, X.; Blin, A.-L.; Bourhis, D.; Garin, E.; Salaun, P.-Y.; Devillers, A.; Querellou, S.; Bourguet, P.; Le Jeune, F.; Saint-Jalmes, H. Comparison of choline influx from dynamic 18F-Choline PET/CT and clinicopathological parameters in prostate cancer initial assessment. Ann. Nucl. Med. 2018, 32, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Guttilla, A.; Zattoni, F.; Muzzio, P.C.; Zattoni, F. Utility of Choline Positron Emission Tomography/Computed Tomography for Lymph Node Involvement Identification in Intermediate- to High-risk Prostate Cancer: A Systematic Literature Review and Meta-analysis. Eur. Urol. 2013, 63, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Yin, L.; Yue, J.-L.; Li, Y.-F.; Yang, Y.; Lin, Z.-C. Direct comparison of choline PET/CT and MRI in the diagnosis of lymph node metastases in patients with prostate cancer. Medicine 2018, 97, e13344. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Vali, R.; Waldenberger, P.; Fitz, F.; Nader, M.; Hammer, J.; Loidl, W.; Pirich, C.; Fogelman, I.; Langsteger, W. The Use of F-18 Choline PET in the Assessment of Bone Metastases in Prostate Cancer: Correlation with Morphological Changes on CT. Mol. Imaging Biol. 2009, 11, 446–454. [Google Scholar] [CrossRef]
- Beheshti, M.; Vali, R.; Waldenberger, P.; Fitz, F.; Nader, M.; Loidl, W.; Broinger, G.; Stoiber, F.; Foglman, I.; Langsteger, W. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET–CT: A comparative study. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Hu, J.; Feng, D.; Xu, L. Diagnostic performance of choline PET/CT for the detection of bone metastasis in prostate cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0203400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gou, Z.; Wu, R.; Yuan, Y.; Yu, G.; Zhao, Y. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A systematic review and meta-analysis. Skelet. Radiol. 2019, 48, 1915–1924. [Google Scholar] [CrossRef]
- Wetter, A.; Nensa, F.; Schenck, M.; Heusch, P.; Pöppel, T.; Bockisch, A.; Forsting, M.; Schlosser, T.W.; Lauenstein, T.C.; Nagarajah, J. Combined PET Imaging and Diffusion-Weighted Imaging of Intermediate and High-Risk Primary Prostate Carcinomas with Simultaneous [18F] Choline PET/MRI. PLoS ONE 2014, 9, e101571. [Google Scholar] [CrossRef]
- De Perrot, T.; Rager, O.; Scheffler, M.; Lord, M.; Pusztaszeri, M.; Iselin, C.; Ratib, O.; Vallee, J.-P. Potential of hybrid 18F-fluorocholine PET/MRI for prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Piert, M.; Montgomery, J.; Kunju, L.P.; Siddiqui, J.; Rogers, V.; Rajendiran, T.; Johnson, T.D.; Shao, X.; Davenport, M.S. 18F-Choline PET/MRI: The Additional Value of PET for MRI-Guided Transrectal Prostate Biopsies. J. Nucl. Med. 2016, 57, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Cheon, G.J.; Paeng, J.C.; Cho, J.Y.; Kwak, C.; Kang, K.W.; Chung, J.-K.; Kim, E.E.; Lee, D.S. Usefulness of MRI-assisted metabolic volumetric parameters provided by simultaneous 18F-fluorocholine PET/MRI for primary prostate cancer characterization. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Vagnoni, V.; Brunocilla, E.; Bianchi, L.; Porreca, A.; Borghesi, M.; Pultrone, C.V.; Angelo, P.; Chessa, F.; Ceci, F.; Mengoni, F.; et al. State of the art of PET/CT with 11-choline and 18F-fluorocholine in the diagnosis and follow-up of localized and locally advanced prostate cancer. Arch. Españoles Urol. (Ed. Impresa) 2015, 68, 354–370. [Google Scholar]
- Yoshimoto, M.; Waki, A.; Yonekura, Y.; Sadato, N.; Murata, T.; Omata, N.; Takahashi, N.; Welch, M.J.; Fujibayashi, Y. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: Acetate metabolism in tumor cells. Nucl. Med. Biol. 2001, 28, 117–122. [Google Scholar] [CrossRef]
- Kato, T.; Tsukamoto, E.; Kuge, Y.; Takei, T.; Shiga, T.; Shinohara, N.; Katoh, C.; Nakada, K.; Tamaki, N. Accumulation of [ 11 C]acetate in normal prostate and benign prostatic hyperplasia: Comparison with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1492–1495. [Google Scholar] [CrossRef]
- Spick, C.; Herrmann, K.; Czernin, J. Evaluation of Prostate Cancer with 11C-Acetate PET/CT. J. Nucl. Med. 2016, 57, 30S–37S. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Turkbey, B.; Mani, H.; Adler, S.; Valera, V.A.; Bernardo, M.; Shah, V.; Pohida, T.; McKinney, Y.; Kwarteng, G.; et al. 11C-Acetate PET/CT in Localized Prostate Cancer: A Study with MRI and Histopathologic Correlation. J. Nucl. Med. 2012, 53, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Jambor, I.; Borra, R.; Kemppainen, J.; Lepomäki, V.; Parkkola, R.; Dean, K.; Alanen, K.; Arponen, E.; Nurmi, M.; Aronen, H.J.; et al. Functional Imaging of Localized Prostate Cancer Aggressiveness Using 11C-Acetate PET/CT and 1H-MR Spectroscopy. J. Nucl. Med. 2010, 51, 1676–1683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jambor, I.; Borra, R.; Kemppainen, J.; Lepomäki, V.; Parkkola, R.; Dean, K.; Alanen, K.; Arponen, E.; Nurmi, M.; Aronen, H.J.; et al. Improved detection of localized prostate cancer using co-registered MRI and 11C-acetate PET/CT. Eur. J. Radiol. 2012, 81, 2966–2972. [Google Scholar] [CrossRef]
- Haseebuddin, M.; Dehdashti, F.; Siegel, B.A.; Liu, J.; Roth, E.B.; Nepple, K.G.; Siegel, C.L.; Fischer, K.C.; Kibel, A.S.; Andriole, G.L.; et al. 11C-Acetate PET/CT Before Radical Prostatectomy: Nodal Staging and Treatment Failure Prediction. J. Nucl. Med. 2013, 54, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.C.; Radecka, E.; Hellström, M.; Jacobsson, H.; Sundin, A. [11C]Acetate positron emission tomography-computed tomography imaging of prostate cancer lymph-node metastases correlated with histopathological findings after extended lymphadenectomy. Scand. J. Urol. 2014, 49, 35–42. [Google Scholar] [CrossRef]
- Strandberg, S.; Karlsson, C.T.; Ogren, M.; Axelsson, J.; Riklund, K. 11C-Acetate-PET/CT Compared to 99mTc-HDP Bone Scintigraphy in Primary Staging of High-risk Prostate Cancer. Anticancer Res. 2016, 36, 6475–6480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polanec, S.H.; Andrzejewski, P.; Baltzer, P.A.T.; Helbich, T.H.; Stiglbauer, A.; Georg, D.; Karanikas, G.; Susani, M.; Wadsak, W.; Margreiter, M.; et al. Multiparametric [11C]Acetate positron emission tomography-magnetic resonance imaging in the assessment and staging of prostate cancer. PLoS ONE 2017, 12, e0180790. [Google Scholar] [CrossRef]
- García, J.; Soler, M.; Blanch, M.; Ramírez, I.; Riera, E.; Lozano, P.; Pérez, X.; Delgado, E.; Carrio, I.; Lomeña, F. [PET/CT with (11)C-choline and (18)F-FDG in patients with elevated PSA after radical treatment of a prostate cancer]. Rev. Española Med. Nucl. 2009, 28, 95–100. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Uprimny, C.; Kroiss, A.S.; Decristoforo, C.; Fritz, J.; von Guggenberg, E.; Kendler, D.; Scarpa, L.; di Santo, G.; Roig, L.G.; Maffey-Steffan, J.; et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Aslan, G.; Çelik, S.; Sözen, S.; Akdoğan, B.; Izol, V.; Bilen, C.Y.; Sahin, B.; Türkeri, L.; Koparal, M.Y.; Yazıcı, S.; et al. Comparison of TRUS and combined MRI-targeted plus systematic prostate biopsy for the concordance between biopsy and radical prostatectomy pathology. Int. J. Clin. Pract. 2021, 75, e13797. [Google Scholar] [CrossRef] [PubMed]
- Von Eyben, F.E.; Picchio, M.; von Eyben, R.; Rhee, H.; Bauman, G. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Piert, M. Performance of 68Ga-PSMA PET/CT for Prostate Cancer Management at Initial Staging and Time of Biochemical Recurrence. Curr. Urol. Rep. 2017, 18, 84. [Google Scholar] [CrossRef]
- Cem, O.; Torun, N.; Guler, O.C.; Reyhan, M.; Yildrim, B.A.; Yapar, A.F. Is there a correlation between Gleason score and maximum standardized uptake value in locally advanced prostate cancer patients? J. Clin. Oncol. 2019, 37, 68. [Google Scholar] [CrossRef]
- Harsini, S.; Fallahi, B.; Ziarati, N.K.; Razi, A.; Amini, E.; Ardekani, A.E.; Esfehani, A.F.; Parizi, M.K.; Farzanehfar, S.; Beiki, D. A Prospective Study on [68Ga]-PSMA PET/CT Imaging in Newly Diagnosed Intermediate- and High-Risk Prostate Cancer. Asia Ocean. J. Nucl. Med. Biol. 2020, 9, 207–219. [Google Scholar] [CrossRef]
- Topuz, Ö.V.; Aksu, A.; Erinç, S.R.; Tamam, M. Correlations of (68)Ga-PSMA PET/CT in the initial staging of prostate cancer patients. Hell J. Nucl. Med. 2021, 24, 60–65. [Google Scholar] [CrossRef]
- Koerber, S.; Boesch, J.; Kratochwil, C.; Schlampp, I.; Ristau, J.; Winter, E.; Zschaebitz, S.; Hofer, L.; Herfarth, K.; Kopka, K.; et al. Predicting the Risk of Metastases by PSMA-PET/CT—Evaluation of 335 Men with Treatment-Naïve Prostate Carcinoma. Cancers 2021, 13, 1508. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Ghafoor, S.; Becker, A.S.; Han, S.; Wibmer, A.G.; Hricak, H.; Burger, I.A.; Schöder, H.; Vargas, H.A. Prostate-specific membrane antigen positron emission tomography (PSMA-PET) for local staging of prostate cancer: A systematic review and meta-analysis. Eur. J. Hybrid Imaging 2020, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Choudhury, P.S.; Hazarika, D.; Rawal, S. A comparative study of 68Gallium-prostate specific membrane antigen positron emission tomography-computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: An initial experience. World J. Nucl. Med. 2017, 16, 186. [Google Scholar] [CrossRef]
- Yaxley, J.W.; Raveenthiran, S.; Nouhaud, F.-X.; Samartunga, H.; Yaxley, A.J.; Coughlin, G.; Delahunt, B.; Egevad, L.; McEwan, L.; Wong, D. Outcomes of Primary Lymph Node Staging of Intermediate and High Risk Prostate Cancer with 68 Ga-PSMA Positron Emission Tomography/Computerized Tomography Compared to Histological Correlation of Pelvic Lymph Node Pathology. J. Urol. 2019, 201, 815–820. [Google Scholar] [CrossRef]
- Budäus, L.; Leyh-Bannurah, S.-R.; Salomon, G.; Michl, U.; Heinzer, H.; Huland, H.; Graefen, M.; Steuber, T.; Rosenbaum, C.M. Initial Experience of 68Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur. Urol. 2016, 69, 393–396. [Google Scholar] [CrossRef]
- Klingenberg, S.; Jochumsen, M.R.; Ulhøi, B.P.; Fredsøe, J.; Sørensen, K.D.; Borre, M.; Bouchelouche, K. 68Ga-PSMA PET/CT for Primary Lymph Node and Distant Metastasis NM Staging of High-Risk Prostate Cancer. J. Nucl. Med. 2021, 62, 214–220. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Peng, L.; Li, J.; Meng, C.; Li, J.; You, C.; Tang, D.; Wei, T.; Xiong, W.; Li, Y. Can 68Ga-prostate specific membrane antigen positron emission tomography/computerized tomography provide an accurate lymph node staging for patients with medium/high risk prostate cancer? A diagnostic meta-analysis. Radiat. Oncol. 2020, 15, 227. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Nie, L.; Qin, K.; Zhang, H.; Shi, H. The meta-analysis of the effect of 68Ga-PSMA-PET/CT diagnosis of prostatic cancer compared with bone scan. Medicine 2021, 100, e25417. [Google Scholar] [CrossRef]
- Simsek, D.H.; Sanli, Y.; Civan, C.; Engin, M.N.; Isik, E.G.; Ozkan, Z.G.; Kuyumcu, S. Does bone scintigraphy still have a role in the era of 68 Ga-PSMA PET/CT in prostate cancer? Ann. Nucl. Med. 2020, 34, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Shao, G.; Cui, C.; Li, T.-N.; Huang, Y.; Yao, X.; Fan, Q.; Chen, Z.; Du, J.; Jia, R.; et al. 68Ga-PSMA-11 PET/CT for prostate cancer staging and risk stratification in Chinese patients. Oncotarget 2017, 8, 12247–12258. [Google Scholar] [CrossRef]
- Soyluoglu, S.; Korkmaz, U.; Ozdemir, B.; Ustun, F.; Durmus-Altun, G. 68Ga-PSMA-I&T-PET/CT interobserver and intraobserver agreement for prostate cancer: A lesion based and subregional comparison study among observers with different levels of experience. Nucl. Med. Commun. 2021, 42, 1122–1129. [Google Scholar] [CrossRef]
- Chakraborty, P.S.; Kumar, R.; Tripathi, M.; Das, C.J.; Bal, C. Detection of Brain Metastasis With 68Ga-Labeled PSMA Ligand PET/CT: A novel radiotracer for imaging of prostate carcinoma. Clin. Nucl. Med. 2015, 40, 328–329. [Google Scholar] [CrossRef]
- Van Kalmthout, L.W.M.; Van Melick, H.H.E.; Lavalaye, J.; Meijer, R.P.; Kooistra, A.; De Klerk, J.M.H.; Braat, A.J.A.T.; Kaldeway, H.P.; De Bruin, P.C.; de Keizer, B.; et al. Prospective Validation of Gallium-68 Prostate Specific Membrane Antigen-Positron Emission Tomography/Computerized Tomography for Primary Staging of Prostate Cancer. J. Urol. 2020, 203, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Afaq, A.; Payne, H.; Davda, R.; Hines, J.; Cook, G.J.; Meagher, M.; Priftakis, D.; Warbey, V.S.; Kelkar, A.; Orczyk, C.; et al. A Phase II, Open-label study to assess safety and management change using 68Ga-THP PSMA PET/CT in patients with high risk primary prostate cancer or biochemical recurrence after radical treatment: The PRONOUNCED study. J. Nucl. Med. 2021, 62. [Google Scholar] [CrossRef]
- Wong, H.S.; Leung, J.; Bartholomeusz, D.; Sutherland, P.; Le, H.; Nottage, M.; Iankov, I.; Chang, J.H. Comparative study between 68 Ga-prostate-specific membrane antigen positron emission tomography and conventional imaging in the initial staging of prostate cancer. J. Med. Imaging Radiat. Oncol. 2018, 62, 816–822. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Prostate Cancer (Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 11 August 2021).
- Rauscher, I.; Maurer, T.; Fendler, W.P.; Sommer, W.H.; Schwaiger, M.; Eiber, M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Annunziata, S.; Pizzuto, D.A.; Giovanella, L.; Prior, J.O.; Ceriani, L. Detection Rate of 18F-Labeled PSMA PET/CT in Biochemical Recurrent Prostate Cancer: A Systematic Review and a Meta-Analysis. Cancers 2019, 11, 710. [Google Scholar] [CrossRef]
- Werner, R.A.; Derlin, T.; Lapa, C.; Sheikbahaei, S.; Higuchi, T.; Giesel, F.L.; Behr, S.; Drzezga, A.; Kimura, H.; Buck, A.K.; et al. 18F-Labeled, PSMA-Targeted Radiotracers: Leveraging the Advantages of Radiofluorination for Prostate Cancer Molecular Imaging. Theranostics 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Afshar-Oromieh, A.; Bögemann, M.; Wagner, S.; Schäfers, M.; Stegger, L.; Weckesser, M. 18F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: Biodistribution, tumour detection and activity kinetics. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1329–1334. [Google Scholar] [CrossRef]
- Kesch, C.; Kratochwil, C.; Mier, W.; Kopka, K.; Giesel, F.L. 68Ga or 18F for Prostate Cancer Imaging? J. Nucl. Med. 2017, 58, 687–688. [Google Scholar] [CrossRef]
- Wondergem, M.; van der Zant, F.M.; Broos, W.A.; Knol, R.J. Matched-Pair Comparison of 18F-DCFPyL PET/CT and 18F-PSMA-1007 PET/CT in 240 Prostate Cancer Patients: Interreader Agreement and Lesion Detection Rate of Suspected Lesions. J. Nucl. Med. 2021, 62, 1422–1429. [Google Scholar] [CrossRef]
- Giesel, F.L.; Will, L.; Lawal, I.; Lengana, T.; Kratochwil, C.; Vorster, M.; Neels, O.; Reyneke, F.; Haberkon, U.; Kopka, K.; et al. Intraindividual Comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J. Nucl. Med. 2018, 59, 1076–1080. [Google Scholar] [CrossRef]
- Cardinale, J.; Schäfer, M.; Benešová, M.; Bauder-Wüst, U.; Leotta, K.; Eder, M.; Neels, O.C.; Haberkorn, U.; Giesel, F.L.; Kopka, K. Preclinical Evaluation of 18F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for Prostate Cancer Imaging. J. Nucl. Med. 2017, 58, 425–431. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kesch, C.; Yun, M.; Cardinale, J.; Haberkorn, U.; Kopka, K.; Kratochwil, C.; Hadaschik, B. 18F-PSMA-1007 PET/CT Detects Micrometastases in a Patient With Biochemically Recurrent Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, e497–e499. [Google Scholar] [CrossRef]
- Szabo, Z.; Mena, E.; Rowe, S.P.; Plyku, D.; Nidal, R.; Eisenberger, M.A.; Antonarakis, E.S.; Fan, H.; Dannals, R.F.; Chen, Y.; et al. Initial Evaluation of [18F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol. Imaging Biol. 2015, 17, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Mease, R.C.; Dusich, C.L.; Foss, C.A.; Ravert, H.T.; Dannals, R.F.; Seidel, J.; Prideaux, A.; Fox, J.J.; Sgouros, G.; Kozikowski, A.P.; et al. N-[N-[(S)-1,3-Dicarboxypropyl]Carbamoyl]-4-[18F]Fluorobenzyl-l-Cysteine, [18F]DCFBC: A New Imaging Probe for Prostate Cancer. Clin. Cancer Res. 2008, 14, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-Head Comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J. Nucl. Med. 2019, 61, 527–532. [Google Scholar] [CrossRef]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef]
- Kesch, C.; Vinsensia, M.; Radtke, J.P.; Schlemmer, H.P.; Heller, M.; Ellert, E.; Holland-Letz, T.; Duensing, S.; Grabe, N.; Afshar-Oromieh, A.; et al. Intraindividual Comparison of 18F-PSMA-1007 PET/CT, Multiparametric MRI, and Radical Prostatectomy Specimens in Patients with Primary Prostate Cancer: A Retrospective, Proof-of-Concept Study. J. Nucl. Med. 2017, 58, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Awenat, S.; Piccardo, A.; Carvoeiras, P.; Signore, G.; Giovanella, L.; Prior, J.; Treglia, G. Diagnostic Role of 18F-PSMA-1007 PET/CT in Prostate Cancer Staging: A Systematic Review. Diagnostics 2021, 11, 552. [Google Scholar] [CrossRef]
- Tragardh, E.; Simoulis, A.; Bjartell, A.; Jogi, J. Tumor detection of 18F-PSMA-1007 in the prostate gland in patients with prostate cancer using prostatectomy specimens as reference method. J. Nucl. Med. 2021, 62. [Google Scholar] [CrossRef]
- Privé, B.M.; Israël, B.; Schilham, M.G.M.; Muselaers, C.H.J.; Zámecnik, P.; Mulders, P.F.A.; Witjes, J.A.; Sedelaar, M.; Mehra, N.; Verzijlbergen, F.; et al. Evaluating F-18-PSMA-1007-PET in primary prostate cancer and comparing it to multi-parametric MRI and histopathology. Prostate Cancer Prostatic Dis. 2021, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.A.; Debowski, M.; Gulhane, B.; Arnfield, E.G.; Pelecanos, A.M.; Garcia, P.L.; Latter, M.J.; Lin, C.Y.; Roberts, M.J.; Ramsay, S.C.; et al. Prospective intra-individual blinded comparison of [18F]PSMA-1007 and [68 Ga]Ga-PSMA-11 PET/CT imaging in patients with confirmed prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Sprute, K.; Kramer, V.; Koerber, S.A.; Meneses, M.; Fernandez, R.; Soza-Ried, C.; Eiber, M.; Weber, W.A.; Rauscher, I.; Rahbar, K.; et al. Diagnostic Accuracy of 18F-PSMA-1007 PET/CT Imaging for Lymph Node Staging of Prostate Carcinoma in Primary and Biochemical Recurrence. J. Nucl. Med. 2021, 62, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, S.; Anttinen, M.; Taimen, P.; Jambor, I.; Sandell, M.; Rinta-Kiikka, I.; Kajander, S.; Schildt, J.; Saukko, E.; Noponen, T.; et al. Prospective comparison of 18F-PSMA-1007 PET/CT, whole-body MRI and CT in primary nodal staging of unfavourable intermediate- and high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18 F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Jansen, B.H.E.; Bodar, Y.J.L.; Zwezerijnen, G.J.C.; Meijer, D.; van der Voorn, J.P.; Nieuwenhuijzen, J.A.; Wondergem, M.; Roeleveld, T.A.; Boellaard, R.; Hoekstra, O.S.; et al. Pelvic lymph-node staging with 18F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer—The SALT trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 509–520. [Google Scholar] [CrossRef]
- Keam, S.J. Piflufolastat F 18: Diagnostic First Approval. Mol. Diagn. Ther. 2021, 25, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Anttinen, M.; Ettala, O.; Malaspina, S.; Jambor, I.; Sandell, M.; Kajander, S.; Rinta-Kiikka, I.; Schildt, J.; Saukko, E.; Rautio, P.; et al. A Prospective Comparison of 18F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography, Whole-body 1.5 T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur. Urol. Oncol. 2021, 4, 635–644. [Google Scholar] [CrossRef]
- Arnfield, E.G.; Thomas, P.A.; Roberts, M.J.; Pelecanos, A.M.; Ramsay, S.C.; Lin, C.Y.; Latter, M.J.; Garcia, P.L.; Pattison, D.A. Clinical insignificance of [18F]PSMA-1007 avid non-specific bone lesions: A retrospective evaluation. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Okarvi, S.M. Recent developments of prostate-specific membrane antigen (PSMA)-specific radiopharmaceuticals for precise imaging and therapy of prostate cancer: An overview. Clin. Transl. Imaging 2019, 7, 189–208. [Google Scholar] [CrossRef]
- Vats, K.; Agrawal, K.; Sharma, R.; Sarma, H.D.; Satpati, D.; Dash, A. Preparation and clinical translation of 99mTc-PSMA-11 for SPECT imaging of prostate cancer. MedChemComm 2019, 10, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Urbán, S.; Meyer, C.; Dahlbom, M.; Farkas, I.; Sipka, G.; Besenyi, Z.; Czernin, J.; Calais, J.; Pávics, L. Radiation Dosimetry of 99mTc-PSMA I&S: A Single-Center Prospective Study. J. Nucl. Med. 2021, 62, 1075–1081. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Foss, C.A.; Castanares, M.; Mease, R.C.; Byun, Y.; Fox, J.J.; Hilton, J.; Lupold, S.E.; Kozikowski, A.P.; Pomper, M.G. Synthesis and Evaluation of Technetium-99m- and Rhenium-Labeled Inhibitors of the Prostate-Specific Membrane Antigen (PSMA). J. Med. Chem. 2008, 51, 4504–4517. [Google Scholar] [CrossRef] [PubMed]
- Nedrow-Byers, J.R.; Jabbes, M.; Jewett, C.; Ganguly, T.; He, H.; Liu, T.; Benny, P.; Bryan, J.; Berkman, C.E. A phosphoramidate-based prostate-specific membrane antigen-targeted SPECT agent. Prostate 2012, 72, 904–912. [Google Scholar] [CrossRef]
- Nedrow, J.; Moore, A.L.; Ganguly, T.; Hopkins, M.R.; Fulton, M.; Benny, P.D.; Berkman, C.E. PSMA-targeted SPECT agents: Mode of binding effect on in vitro performance. Prostate 2013, 73, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Maresca, K.P.; Hillier, S.M.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. Synthesis and SAR of 99mTc/Re-labeled small molecule prostate specific membrane antigen inhibitors with novel polar chelates. Bioorg. Med. Chem. Lett. 2013, 23, 1557–1563. [Google Scholar] [CrossRef]
- Knipper, S.; Tilki, D.; Mansholt, J.; Berliner, C.; Bernreuther, C.; Steuber, T.; Maurer, T.; Graefen, M. Metastases-yield and Prostate-specific Antigen Kinetics Following Salvage Lymph Node Dissection for Prostate Cancer: A Comparison Between Conventional Surgical Approach and Prostate-specific Membrane Antigen-radioguided Surgery. Eur. Urol. Focus 2019, 5, 50–53. [Google Scholar] [CrossRef]
- Su, H.-C.; Zhu, Y.; Hu, S.-L.; Liu, C.; Lin, G.-W.; Dai, B.; Zhang, Y.-J.; Ye, D.-W. The Value of 99mTc-PSMA SPECT/CT-Guided Surgery for Identifying and Locating Lymph Node Metastasis in Prostate Cancer Patients. Ann. Surg. Oncol. 2018, 26, 653–659. [Google Scholar] [CrossRef]
- Sergieva, S.; Mangaldgiev, R.; Dimcheva, M.; Nedev, K.; Zahariev, Z.; Robev, B. SPECT-CT Imaging with 99mTc-PSMAin Patients with Recurrent Prostate Cancer. Nucl. Med. Rev. 2021, 24, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, B.; Khademi, N.; Karamzade-Ziarati, N.; Fard-Esfahani, A.; Emami-Ardekani, A.; Farzanefar, S.; Eftekhari, M.; Beiki, D. 99mTc-PSMA SPECT/CT Versus 68Ga-PSMA PET/CT in the Evaluation of Metastatic Prostate Cancer. Clin. Nucl. Med. 2021, 46, e68–e74. [Google Scholar] [CrossRef]
- Goffin, K.E.; Joniau, S.; Tenke, P.; Slawin, K.; Klein, E.A.; Stambler, N.; Strack, T.; Babich, J.; Armor, T.; Wong, V. Phase 2 Study of 99mTc-Trofolastat SPECT/CT to Identify and Localize Prostate Cancer in Intermediate- and High-Risk Patients Undergoing Radical Prostatectomy and Extended Pelvic LN Dissection. J. Nucl. Med. 2017, 58, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.; Ankrah, A.; Mokgoro, N.P.; Vorster, M.; Maes, A.; Sathekge, M.M. Diagnostic sensitivity of Tc-99m HYNIC PSMA SPECT/CT in prostate carcinoma: A comparative analysis with Ga-68 PSMA PET/CT. Prostate 2017, 77, 1205–1212. [Google Scholar] [CrossRef]
- Green, D.; Osterberg, C.; Osborne, J.R.; Nikolopoulou, A.; Vallabhajosula, S.; Goldsmith, S.J.; Robinson, B.D.; Goldenberg, S.; Babich, J.; Scherr, D.S. 2200 A Phase 1 Pilot Study Of 99mtc-Mip-1404 Single Photon Emission Computed Tomography (Spect)/Ct Imaging In Men With Prostate Cancer Undergoing Radical Prostatectomy. J. Urol. 2013, 189, e902. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, S.; Bansal, P.; Hooda, M.; Singh, H.; Parihar, A.S.; Kumar, A.; Watts, A.; Mohan, R.; Singh, S.K. Comparison of the diagnostic utility of 99mTc-PSMA scintigraphy versus 68Ga-PSMA-11 PET/CT in the detection of metastatic prostate cancer and dosimetry analysis: A gamma-camera-based alternate prostate-specific membrane antigen imaging modality. Nucl. Med. Commun. 2021, 42, 482–489. [Google Scholar] [CrossRef]
- Albalooshi, B.; Al Sharhan, M.; Bagheri, F.; Miyanath, S.; Muhasin, M.; Ray, B.; Zakavi, S.R. Direct comparison of 99mTc-PSMA SPECT/CT and 68Ga-PSMA PET/CT in patients with prostate cancer. Asia Ocean. J. Nucl. Med. Biol. 2020, 8, 1–7. [Google Scholar] [PubMed]
- Nuñez, R.; Macapinlac, H.A.; Yeung, H.W.D.; Akhurst, T.; Cai, S.; Osman, I.; Gonen, M.; Riedel, E.; Scher, H.I.; Larson, S.M. Combined 18F-FDG and 11C-methionine PET scans in patients with newly progressive metastatic prostate cancer. J. Nucl. Med. 2002, 43, 46–55. [Google Scholar] [PubMed]
- Tóth, G.; Lengyel, Z.; Balkay, L.; Salah, M.A.; Trón, L.; Tóth, C. Detection Of Prostate Cancer With 11 C-Methionine Positron Emission Tomography. J. Urol. 2005, 173, 66–69. [Google Scholar] [CrossRef]
- Shiiba, M.; Ishihara, K.; Kimura, G.; Kuwako, T.; Yoshihara, N.; Sato, H.; Kondo, Y.; Tsuchiya, S.-I.; Kumita, S.-I. Evaluation of primary prostate cancer using 11C-methionine-PET/CT and 18F-FDG-PET/CT. Ann. Nucl. Med. 2011, 26, 138–145. [Google Scholar] [CrossRef]
- Turkbey, B.; Mena, E.; Shih, J.; Pinto, P.A.; Merino, M.J.; Lindenberg, M.L.; Bernardo, M.; McKinney, Y.L.; Adler, S.; Owenius, R.; et al. Localized prostate cancer detection with 18F FACBC PET/CT: Comparison with MR imaging and histopathologic analysis. Radiology 2014, 270, 849–856. [Google Scholar] [CrossRef]
- Schuster, D.M.; Taleghani, P.A.; Nieh, P.T.; Master, V.A.; Amzat, R.; Savir-Baruch, B.; Halkar, R.K.; Fox, T.; Osunkoya, A.O.; Moreno, C.S.; et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[18F] -fluorocyclobutane-1-carboxylic acid (anti-3-[18F] FACBC) uptake. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 85–96. [Google Scholar]
- Laudicella, R.; Albano, D.; Alongi, P.; Argiroffi, G.; Bauckneht, M.; Baldari, S.; Bertagna, F.; Boero, M.; De Vincentis, G.; Del Sole, A.; et al. 18F-Facbc in Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1348. [Google Scholar] [CrossRef]
- Suzuki, H.; Inoue, Y.; Fujimoto, H.; Yonese, J.; Tanabe, K.; Fukasawa, S.; Inoue, T.; Saito, S.; Ueno, M.; Otaka, A. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate cancer: Multicenter Phase IIb clinical trial. Jpn. J. Clin. Oncol. 2016, 46, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Selnæs, K.M.; Krüger-Stokke, B.; Elschot, M.; Willoch, F.; Størkersen, Ø.; Sandsmark, E.; Moestue, S.A.; Tessem, M.-B.; Halvorsen, D.; Kjøbli, E.; et al. 18F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur. Radiol. 2018, 28, 3151–3159. [Google Scholar] [CrossRef]
- Jambor, I.; Kuisma, A.; Kähkönen, E.; Kemppainen, J.; Merisaari, H.; Eskola, O.; Teuho, J.; Perez, I.M.; Pesola, M.; Aronen, H.J.; et al. Prospective evaluation of 18F-FACBC PET/CT and PET/MRI versus multiparametric MRI in intermediate- to high-risk prostate cancer patients (FLUCIPRO trial). Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 355–364. [Google Scholar] [CrossRef]
- Inoue, Y.; Asano, Y.; Satoh, T.; Tabata, K.-I.; Kikuchi, K.; Woodhams, R.; Baba, S.; Hayakawa, K. Phase IIa Clinical Trial of Trans-1-Amino-3-(18)F-Fluoro-Cyclobutane Carboxylic Acid in Metastatic Prostate Cancer. Asia Ocean. J. Nucl. Med. Biol. 2014, 2, 87–94. [Google Scholar]
- Nanni, C.; Zanoni, L.; Pultrone, C.; Schiavina, R.; Brunocilla, E.; Lodi, F.; Malizia, C.; Ferrari, M.; Rigatti, P.; Fonti, C.; et al. 18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: Results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1601–1610. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Huang, M.; Tian, R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer with Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 2021, 11, 684629. [Google Scholar] [CrossRef] [PubMed]
- Beattie, B.J.; Smith-Jones, P.M.; Jhanwar, Y.S.; Schöder, H.; Schmidtlein, C.R.; Morris, M.J.; Zanzonico, P.; Squire, O.; Meirelles, G.S.; Finn, R.; et al. Pharmacokinetic Assessment of the Uptake of 16β-18F-Fluoro-5α-Dihydrotestosterone (FDHT) in Prostate Tumors as Measured by PET. J. Nucl. Med. 2010, 51, 183–192. [Google Scholar] [CrossRef]
- Dehdashti, F.; Picus, J.; Michalski, J.M.; Dence, C.S.; Siegel, B.A.; Katzenellenbogen, J.A.; Welch, M.J. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 344–350. [Google Scholar] [CrossRef]
- Larson, S.M.; Morris, M.; Gunther, I.; Beattie, B.; Humm, J.L.; Akhurst, T.A.; Finn, R.D.; Erdi, Y.; Pentlow, K.; Dyke, J.; et al. Tumor localization of 16β-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J. Nucl. Med. 2004, 45, 366–373. [Google Scholar]
- Fox, J.J.; Schöder, H.; Larson, S.M. Molecular imaging of prostate cancer. Curr. Opin. Urol. 2012, 22, 320–327. [Google Scholar] [CrossRef]
- Vargas, H.A.; Wassberg, C.; Fox, J.J.; Wibmer, A.G.; Goldman, D.A.; Kuk, D.; Gonen, M.; Larson, S.; Morris, M.J.; Scher, H.I.; et al. Bone Metastases in Castration-Resistant Prostate Cancer: Associations between Morphologic CT Patterns, Glycolytic Activity, and Androgen Receptor Expression on PET and Overall Survival. Radiology 2014, 271, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niu, G.; Fan, X.; Lang, L.; Hou, G.; Chen, L.; Wu, H.; Zhu, Z.; Li, F.; Chen, X. PET Using a GRPR Antagonist 68Ga-RM26 in Healthy Volunteers and Prostate Cancer Patients. J. Nucl. Med. 2018, 59, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.; Loidl, W.C.; Beheshti, M.; Jambor, I.; Kemppainen, J.; Bostrom, P.; Kahkonen, E.; Berndt, M.; Mueller, A.; Minn, H.; et al. Detection of prostate cancer with the [68Ga]-labeled bombesin antagonist RM2 in patients undergoing radical prostatectomy. J. Clin. Oncol. 2016, 34, 80. [Google Scholar] [CrossRef]
- Mansi, R.; Fleischmann, A.; Mäcke, H.R.; Reubi, J.C. Targeting GRPR in urological cancers—from basic research to clinical application. Nat. Rev. Urol. 2013, 10, 235–244. [Google Scholar] [CrossRef]
- Aprikian, A.G.; Tremblay, L.; Han, K.; Chevalier, S. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int. J. Cancer 1997, 72, 498–504. [Google Scholar] [CrossRef]
- Hoosein, N.M.; Logothetis, C.J.; Chung, L.W. Differential Effects of Peptide Hormones Bombesin, Vasoactive Intestinal Polypeptide and Somatostatin Analog RC-160 on the Invasive Capacity of Human Prostatic Carcinoma Cells. J. Urol. 1993, 149, 1209–1213. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Wang, X.; Forrer, F.; Waser, B.; Cescato, R.; Graham, K.; Borkowski, S.; Reubi, J.C.; Maecke, H.R. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur. J. Nucl. Med. Mol. Imaging 2010, 38, 97–107. [Google Scholar] [CrossRef]
- Roivainen, A.; Kähkönen, E.; Luoto, P.; Borkowski, S.; Hofmann, B.; Jambor, I.; Lehtiö, K.; Rantala, T.; Rottmann, A.; Sipilä, H.; et al. Plasma Pharmacokinetics, Whole-Body Distribution, Metabolism, and Radiation Dosimetry of 68Ga Bombesin Antagonist BAY 86-7548 in Healthy Men. J. Nucl. Med. 2013, 54, 867–872. [Google Scholar] [CrossRef]
- Kähkönen, E.; Jambor, I.; Kemppainen, J.; Lehtiö, K.; Grönroos, T.J.; Kuisma, A.; Luoto, P.; Sipilä, H.J.; Tolvanen, T.; Alanen, K.; et al. In Vivo Imaging of Prostate Cancer Using [68Ga]-Labeled Bombesin Analog BAY86-7548. Clin. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef]
- Ilyushenkova, Y.; Sazonova, S.; Batalov, R.; Popov, S. The Utility of 99MTC-Pyrophosphate Spect in Combination with Left Atrium 64-MDTC in Diagnosis of Latent Myocarditis in Patients with Atrial Fibrillation: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 489–490. [Google Scholar] [CrossRef]
- Bakker, I.L.; Fröberg, A.C.; Busstra, M.B.; Verzijlbergen, J.F.; Konijnenberg, M.; van Leenders, G.J.L.H.; Schoots, I.G.; de Blois, E.; van Weerden, W.M.; Dalm, S.U.; et al. GRPr antagonist 68Ga-SB3 PET/CT-imaging of primary prostate cancer in therapy-naive patients. J. Nucl. Med. 2021, 62. [Google Scholar] [CrossRef]
- Maina, T.; Bergsma, H.; Kulkarni, H.R.; Mueller, D.; Charalambidis, D.; Krenning, E.P.; Nock, B.A.; De Jong, M.; Baum, R.P. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 964–973. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Liu, H.; Bu, L.; Ma, X.; Cheng, K.; Li, J.; Tian, M.; Zhang, H.; Cheng, Z. A Comparative Study of Radiolabeled Bombesin Analogs for the PET Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 2132–2138. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, G.; Lang, L.; Li, F.; Fan, X.; Yan, X.; Yao, S.; Yan, W.; Huo, L.; Chen, L.; et al. Clinical Translation of a Dual Integrin αvβ3– and Gastrin-Releasing Peptide Receptor–Targeting PET Radiotracer, 68Ga-BBN-RGD. J. Nucl. Med. 2017, 58, 228–234. [Google Scholar] [CrossRef]
- Rivera-Bravo, B.; Ramírez-Nava, G.; Mendoza-Figueroa, M.J.; Ocampo-García, B.; Ferro-Flores, G.; Ávila-Rodríguez, M.A.; Santos-Cuevas, C. [68Ga]Ga-iPSMA-Lys3-Bombesin: Biokinetics, dosimetry and first patient PET/CT imaging. Nucl. Med. Biol. 2021, 96–97, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lundmark, F.; Abouzayed, A.; Mitran, B.; Rinne, S.; Varasteh, Z.; Larhed, M.; Tolmachev, V.; Rosenström, U.; Orlova, A. Heterodimeric Radiotracer Targeting PSMA and GRPR for Imaging of Prostate Cancer—Optimization of the Affinity towards PSMA by Linker Modification in Murine Model. Pharmaceutics 2020, 12, 614. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, T.; Liu, L.; Zhao, L.; Guo, G.; Wang, N. Influence of Four Radiotracers in PET/CT on Diagnostic Accuracy for Prostate Cancer: A Bivariate Random-Effects Meta-Analysis. Cell. Physiol. Biochem. 2016, 39, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Hosseini, M.; Madsen, J.; Jørgensen, J.T.; Jensen, K.J.; Kjaer, A.; Ploug, M. Improved PET Imaging of uPAR Expression Using new 64Cu-labeled Cross-Bridged Peptide Ligands: Comparative in vitro and in vivo Studies. Theranostics 2013, 3, 618–632. [Google Scholar] [CrossRef]
- Persson, M.; Liu, H.; Madsen, J.; Cheng, Z.; Kjaer, A. First 18F-labeled ligand for PET imaging of uPAR: In vivo studies in human prostate cancer xenografts. Nucl. Med. Biol. 2013, 40, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, D.; Persson, M.; Brandt-Larsen, M.; Christensen, C.; Madsen, J.; Klausen, T.L.; Holm, S.; Andersen, F.L.; Loft, A.; Berthelsen, A.K.; et al. Safety, Dosimetry, and Tumor Detection Ability of 68 Ga-NOTA-AE105: First-in-Human Study of a Novel Radioligand for uPAR PET Imaging. J. Nucl. Med. 2017, 58, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Fosbøl, M.Ø.; Kurbegovic, S.; Johannesen, H.H.; Røder, M.A.; Hansen, A.E.; Mortensen, J.; Loft, A.; Petersen, P.M.; Madsen, J.; Brasso, K.; et al. Urokinase-Type Plasminogen Activator Receptor (uPAR) PET/MRI of Prostate Cancer for Noninvasive Evaluation of Aggressiveness: Comparison with Gleason Score in a Prospective Phase 2 Clinical Trial. J. Nucl. Med. 2021, 62, 354–359. [Google Scholar] [CrossRef]
- Reubi, J.C. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann. N. Y. Acad. Sci. 2006, 921, 1–25. [Google Scholar] [CrossRef]
- Truong, H.; Gomella, L.G.; Thakur, M.; Trabulsi, E.J. VPAC1-targeted PET/CT scan: Improved molecular imaging for the diagnosis of prostate cancer using a novel cell surface antigen. World J. Urol. 2018, 36, 719–726. [Google Scholar] [CrossRef]
- Israel, I.; Richter, D.; Stritzker, J.; Ooschot, M.; Donat, U.; Buck, A.; Samnick, S. PET Imaging with [68Ga]NOTA-RGD for Prostate Cancer: A Comparative Study with [18F]Fluorodeoxyglucose and [18F]Fluoroethylcholine. Curr. Cancer Drug Targets 2014, 14, 371–379. [Google Scholar] [CrossRef]
- Cheng, Z.; Wei, R.; Wu, C.; Qing, H.; Jiang, X.; Lu, H.; Chen, S.; Li, X.; Xu, G.; Ai, H. Ex-vivo biodistribution and micro-PET/CT imaging of 18F-FDG, 18F-FLT, 18F-FMISO, and 18F-AlF-NOTA-PRGD2 in a prostate tumor-bearing nude mouse model. Nucl. Med. Commun. 2015, 36, 914–921. [Google Scholar] [CrossRef]
- Zhang-Yin, J.; Provost, C.; Cancel-Tassin, G.; Rusu, T.; Penent, M.; Radulescu, C.; Comperat, E.; Cussenot, O.; Montravers, F.; Renard-Penna, R.; et al. A comparative study of peptide-based imaging agents [68Ga]Ga-PSMA-11, [68Ga]Ga-AMBA, [68Ga]Ga-NODAGA-RGD and [68Ga]Ga-DOTA-NT-20.3 in preclinical prostate tumour models. Nucl. Med. Biol. 2020, 84–85, 88–95. [Google Scholar] [CrossRef]
- Øen, S.K.; Aasheim, L.B.; Eikenes, L.; Karlberg, A.M. Image quality and detectability in Siemens Biograph PET/MRI and PET/CT systems—A phantom study. EJNMMI Phys. 2019, 6, 16. [Google Scholar] [CrossRef]
- Souvatzoglou, M.; Eiber, M.; Takei, T.; Fürst, S.; Maurer, T.; Gaertner, F.; Geinitz, H.; Drzezga, A.; Ziegler, S.; Nekolla, S.G.; et al. Comparison of integrated whole-body [11C]choline PET/MR with PET/CT in patients with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1486–1499. [Google Scholar] [CrossRef]
- Wiesmüller, M.; Quick, H.H.; Navalpakkam, B.; Lell, M.M.; Uder, M.; Ritt, P.; Schmidt, D.; Beck, M.; Kuwert, T.; Von Gall, C.C. Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 12–21. [Google Scholar] [CrossRef]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.-J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef]
- Lee, M.S.; Cho, J.Y.; Kim, S.Y.; Cheon, G.J.; Moon, M.H.; Oh, S.; Lee, J.; Lee, S.; Woo, S.; Kim, S.H. Diagnostic value of integrated PET/MRI for detection and localization of prostate cancer: Comparative study of multiparametric MRI and PET/CT. J. Magn. Reson. Imaging 2017, 45, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayati, M.; Grueneisen, J.; Lütje, S.; Sawicki, L.M.; Suntharalingam, S.; Tschirdewahn, S.; Forsting, M.; Rübben, H.; Herrmann, K.; Umutlu, L.; et al. Integrated 68Gallium Labelled Prostate-Specific Membrane Antigen-11 Positron Emission Tomography/Magnetic Resonance Imaging Enhances Discriminatory Power of Multi-Parametric Prostate Magnetic Resonance Imaging. Urol. Int. 2018, 100, 164–171. [Google Scholar] [CrossRef]
- Li, M.; Huang, Z.; Yu, H.; Wang, Y.; Zhang, Y.; Song, B. Comparison of PET/MRI with multiparametric MRI in diagnosis of primary prostate cancer: A meta-analysis. Eur. J. Radiol. 2019, 113, 225–231. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Yang, R.; Ma, X.; Tian, R. 68Ga-PSMA PET/MRI for the diagnosis of primary and biochemically recurrent prostate cancer: A meta-analysis. Eur. J. Radiol. 2020, 130, 109131. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Cassarino, G.; Artioli, P.; Cecchin, D.; Dal Moro, F.; Zucchetta, P. PET/MRI in prostate cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 859–873. [Google Scholar] [CrossRef]
- Kaufmann, S.; Kruck, S.; Gatidis, S.; Hepp, T.; Thaiss, W.M.; Hennenlotter, J.; Schwenck, J.; Scharpf, M.; Nikolaou, K.; Stenzl, A.; et al. Simultaneous whole-body PET/MRI with integrated multiparametric MRI for primary staging of high-risk prostate cancer. World J. Urol. 2020, 38, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Thalgott, M.; Düwel, C.; Rauscher, I.; Heck, M.M.; Haller, B.; Gafita, A.; Gschwend, J.E.; Schwaiger, M.; Maurer, T.; Eiber, M. One-Stop-Shop Whole-Body 68Ga-PSMA-11 PET/MRI Compared with Clinical Nomograms for Preoperative T and N Staging of High-Risk Prostate Cancer. J. Nucl. Med. 2018, 59, 1850–1856. [Google Scholar] [CrossRef]
- Grubmüller, B.; Baltzer, P.; Hartenbach, S.; D’Andrea, D.; Helbich, T.H.; Haug, A.; Goldner, G.M.; Wadsak, W.; Pfaff, S.; Mitterhauser, M.; et al. PSMA Ligand PET/MRI for Primary Prostate Cancer: Staging Performance and Clinical Impact. Clin. Cancer Res. 2018, 24, 6300–6307. [Google Scholar] [CrossRef] [PubMed]
- Muehlematter, U.J.; Burger, I.A.; Becker, A.S.; Schawkat, K.; Hötker, A.M.; Reiner, C.S.; Müller, J.; Rupp, N.J.; Rüschoff, J.H.; Eberli, D.; et al. Diagnostic Accuracy of Multiparametric MRI versus 68Ga-PSMA-11 PET/MRI for Extracapsular Extension and Seminal Vesicle Invasion in Patients with Prostate Cancer. Radiology 2019, 293, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.A.; Muehlematter, U.J.; Schüler, H.I.G.; Rupp, N.J.; Huellner, M.; Messerli, M.; Rüschoff, J.H.; ter Voert, E.; Hermanns, T.; Burger, I.A. 68Ga-PSMA-11 PET has the potential to improve patient selection for extended pelvic lymph node dissection in intermediate to high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 147–159. [Google Scholar] [CrossRef]
- Van Leeuwen, P.J.; Emmett, L.; Ho, B.; Delprado, W.; Ting, F.; Nguyen, Q.; Stricker, P. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2016, 119, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Domachevsky, L.; Bernstine, H.; Goldberg, N.; Nidam, M.; Stern, D.; Sosna, J.; Groshar, D. Early 68GA-PSMA PET/MRI acquisition: Assessment of lesion detectability and PET metrics in patients with prostate cancer undergoing same-day late PET/CT. Clin. Radiol. 2017, 72, 944–950. [Google Scholar] [CrossRef]
- Freitag, M.T.; Radtke, J.P.; Hadaschik, B.; Koppschneider, A.; Eder, M.; Kopka, K.; Haberkorn, U.; Roethke, M.; Schlemmer, H.-P.; Afshar-Oromieh, A. Comparison of hybrid 68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 70–83. [Google Scholar] [CrossRef]
- Lindenberg, L.; Ahlman, M.; Turkbey, B.; Mena, E.; Choyke, P. Advancement of MR and PET/MR in Prostate Cancer. Semin. Nucl. Med. 2016, 46, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Manafi-Farid, R.; Karamzade-Ziarati, N.; Vali, R.; Mottaghy, F.M.; Beheshti, M. 2-[18F]FDG PET/CT radiomics in lung cancer: An overview of the technical aspect and its emerging role in management of the disease. Methods 2021, 188, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; Bosaily, A.E.-S.; Brown, L.C.; Gabe, R.; Kaplan, R.S.; Parmar, M.K.; PROMIS Study Group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Smith, C.P.; Czarniecki, M.; Mehralivand, S.; Stoyanova, R.; Choyke, P.L.; Harmon, S.; Turkbey, B. Radiomics and radiogenomics of prostate cancer. Abdom. Radiol. 2018, 44, 2021–2029. [Google Scholar] [CrossRef]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Köber, G.; Schilling, O.; Ruf, J.; et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—A comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Moazemi, S.; Khurshid, Z.; Erle, A.; Lütje, S.; Essler, M.; Schultz, T.; Bundschuh, R.A. Machine Learning Facilitates Hotspot Classification in PSMA-PET/CT with Nuclear Medicine Specialist Accuracy. Diagnostics 2020, 10, 622. [Google Scholar] [CrossRef]
- Zamboglou, C.; Bettermann, A.S.; Gratzke, C.; Mix, M.; Ruf, J.; Kiefer, S.; Jilg, C.A.; Benndorf, M.; Spohn, S.; Fassbender, T.F.; et al. Uncovering the invisible—Prevalence, characteristics, and radiomics feature–based detection of visually undetectable intraprostatic tumor lesions in 68GaPSMA-11 PET images of patients with primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Cysouw, M.C.F.; Jansen, B.H.E.; van de Brug, T.; Oprea-Lager, D.E.; Pfaehler, E.; de Vries, B.M.; van Moorselaar, R.J.A.; Hoekstra, O.S.; Vis, A.N.; Boellaard, R. Machine learning-based analysis of [18F]DCFPyL PET radiomics for risk stratification in primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 340–349. [Google Scholar] [CrossRef]
- Acar, E.; Leblebici, A.; Ellidokuz, B.E.; Başbınar, Y.; Kaya, G. Çapa Machine learning for differentiating metastatic and completely responded sclerotic bone lesion in prostate cancer: A retrospective radiomics study. Br. J. Radiol. 2019, 92, 20190286. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.; Spielvogel, C.P.; Grubmüller, B.; Grahovac, M.; Krajnc, D.; Ecsedi, B.; Sareshgi, R.A.; Mohamad, D.; Hamboeck, M.; Rausch, I.; et al. Supervised machine learning enables non-invasive lesion characterization in primary prostate cancer with [68Ga]Ga-PSMA-11 PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1795–1805. [Google Scholar] [CrossRef]
- Tu, S.; Tran, V.T.; Teo, J.M.; Chong, W.C.; Tseng, J. Utility of radiomic zones for risk classification and clinical outcome predictions using supervised machine learning during simultaneous 11 C-choline PET/MRI acquisition in prostate cancer patients. Med. Phys. 2021, 48, 5192–5201. [Google Scholar] [CrossRef]
- Valdora, F.; Houssami, N.; Rossi, F.; Calabrese, M.; Tagliafico, A.S. Rapid review: Radiomics and breast cancer. Breast Cancer Res. Treat. 2018, 169, 217–229. [Google Scholar] [CrossRef]
- Mehralivand, S.; Van Der Poel, H.; Winter, A.; Choyke, P.L.; Pinto, P.A.; Turkbey, B. Sentinel lymph node imaging in urologic oncology. Transl. Androl. Urol. 2018, 7, 887–902. [Google Scholar] [CrossRef]
- Narayanan, R.; Wilson, T.G. Sentinel node evaluation in prostate cancer. Clin. Exp. Metastasis 2018, 35, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Wawroschek, F.; Vogt, H.; Weckermann, D.; Wagner, T.; Harzmann, R. The sentinel lymph node concept in prostate cancer—First results of gamma probe-guided sentinel lymph node identification. Eur. Urol. 1999, 36, 595–600. [Google Scholar] [CrossRef]
- Tabasi, K.; Bazaz, S.M.M.; Kakhki, V.R.D.; Massoom, A.F.; Gholami, H.; Zakavi, S.R.; Sadeghi, R. Sentinel node mapping in the prostate cancer. Nuklearmedizin 2011, 50, 107–115. [Google Scholar] [CrossRef]
- Wit, E.M.; Acar, C.; Grivas, N.; Yuan, C.; Horenblas, S.; Liedberg, F.; Olmos, R.A.V.; van Leeuwen, F.; Berg, N.S.V.D.; Winter, A.; et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur. Urol. 2017, 71, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Holl, G.; Dorn, R.; Wengenmair, H.; Weckermann, D.; Sciuk, J. Validation of sentinel lymph node dissection in prostate cancer: Experience in more than 2000 patients. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, H.G.; Buckle, T.; Brouwer, O.; Olmos, R.A.V.; van Leeuwen, F.W. Intraoperative Laparoscopic Fluorescence Guidance to the Sentinel Lymph Node in Prostate Cancer Patients: Clinical Proof of Concept of an Integrated Functional Imaging Approach Using a Multimodal Tracer. Eur. Urol. 2011, 60, 826–833. [Google Scholar] [CrossRef]
- Kleinjan, G.H.; van Werkhoven, E.; Berg, N.S.V.D.; Karakullukcu, M.B.; Zijlmans, H.J.M.A.A.; Van Der Hage, J.A.; Van De Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The best of both worlds: A hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, H.G.; Wit, E.M.; Acar, C.; Berg, N.S.V.D.; van Leeuwen, F.W.B.; Olmos, R.A.V.; Winter, A.; Wawroschek, F.; Liedberg, F.; Maclennan, S.; et al. Sentinel node biopsy for prostate cancer: Report from a consensus panel meeting. BJU Int. 2017, 120, 204–211. [Google Scholar] [CrossRef]
- Mazzone, E.; Dell’Oglio, P.; Grivas, N.; Wit, E.; Donswijk, M.; Briganti, A.; Van Leeuwen, F.; van der Poel, H. Diagnostic Value, Oncologic Outcomes, and Safety Profile of Image-Guided Surgery Technologies During Robot-Assisted Lymph Node Dissection with Sentinel Node Biopsy for Prostate Cancer. J. Nucl. Med. 2021, 62, 1363–1371. [Google Scholar] [CrossRef]
- Rousseau, C.; Rousseau, T.; Mathieu, C.; Lacoste, J.; Potiron, E.; Aillet, G.; Nevoux, P.; Le Coguic, G.; Campion, L.; Kraeber-Bodéré, F. Laparoscopic sentinel lymph node dissection in prostate cancer patients: The additional value depends on preoperative data. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1849–1856. [Google Scholar] [CrossRef]
- Grivas, N.; Wit, E.M.; Kuusk, T.; KleinJan, G.H.; Donswijk, M.L.; van Leeuwen, F.W.; van der Poel, H.G. The Impact of Adding Sentinel Node Biopsy to Extended Pelvic Lymph Node Dissection on Biochemical Recurrence in Prostate Cancer Patients Treated with Robot-Assisted Radical Prostatectomy. J. Nucl. Med. 2018, 59, 204–209. [Google Scholar] [CrossRef]
- Vermeeren, L.; Olmos, R.A.V.; Meinhardt, W.; Bex, A.; van der Poel, H.G.; Vogel, W.V.; Sivro, F.; Hoefnagel, C.A.; Horenblas, S. Value of SPECT/CT for Detection and Anatomic Localization of Sentinel Lymph Nodes Before Laparoscopic Sentinel Node Lymphadenectomy in Prostate Carcinoma. J. Nucl. Med. 2009, 50, 865–870. [Google Scholar] [CrossRef]
- Doughton, J.A.; Hofman, M.; Eu, P.; Hicks, R.J.; Williams, S.G. A First-in-Human Study of 68Ga-Nanocolloid PET/CT Sentinel Lymph Node Imaging in Prostate Cancer Demonstrates Aberrant Lymphatic Drainage Pathways. J. Nucl. Med. 2018, 59, 1837–1842. [Google Scholar] [CrossRef]
- Jilg, C.A.; Drendel, V.; Rischke, H.C.; Beck, T.I.R.; Reichel, K.; Krönig, M.; Wetterauer, U.; Schultze-Seemann, W.; Meyer, P.T.; Vach, W. Detection Rate of 18F-Choline PET/CT and 68Ga-PSMA-HBED-CC PET/CT for Prostate Cancer Lymph Node Metastases with Direct Link from PET to Histopathology: Dependence on the Size of Tumor Deposits in Lymph Nodes. J. Nucl. Med. 2019, 60, 971–977. [Google Scholar] [CrossRef]
- Schwenck, J.; Rempp, H.; Reischl, G.; Kruck, S.; Stenzl, A.; Nikolaou, K.; Pfannenberg, C.; La Fougère, C. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Miederer, M.; Wieler, H.J.; Ruf, C.; Jakobs, F.M.; Schreckenberger, M. Diagnostic performance of 68Gallium-PSMA-11 PET/CT to detect significant prostate cancer and comparison with 18FEC PET/CT. Oncotarget 2017, 8, 111073–111083. [Google Scholar] [CrossRef]
- Rauscher, I.; Kroenke, M.; König, M.; Gafita, A.; Maurer, T.; Horn, T.; Schiller, K.; Weber, W.; Eiber, M. Matched-Pair Comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: Frequency of Pitfalls and Detection Efficacy in Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2020, 61, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lestingi, J.F.; Guglielmetti, G.B.; Trinh, Q.-D.; Coelho, R.F.; Pontes, J.; Bastos, D.A.; Cordeiro, M.D.; Sarkis, A.S.; Faraj, S.F.; Mitre, A.I.; et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur. Urol. 2021, 79, 595–604. [Google Scholar] [CrossRef]
- Acar, C.; Kleinjan, G.H.; Berg, N.S.V.D.; Wit, E.M.; van Leeuwen, F.; Van Der Poel, H.G. Advances in sentinel node dissection in prostate cancer from a technical perspective. Int. J. Urol. 2015, 22, 898–909. [Google Scholar] [CrossRef]
- Yaxley, J.W.; Dagher, J.; Delahunt, B.; Egevad, L.; Srigley, J.; Samaratunga, H. Reconsidering the role of pelvic lymph node dissection with radical prostatectomy for prostate cancer in an era of improving radiological staging techniques. World J. Urol. 2017, 36, 15–20. [Google Scholar] [CrossRef]
- Fossati, N.; Willemse, P.-P.M.; Van den Broeck, T.; van den Bergh, R.C.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Wawroschek, F.; Vogt, H.; Wengenmair, H.; Weckermann, D.; Hamm, M.; Keil, M.; Graf, G.; Heidenreich, P.; Harzmann, R. Prostate Lymphoscintigraphy and Radio-Guided Surgery for Sentinel Lymph Node Identification in Prostate Cancer. Technique and results of the first 350 cases. Urol. Int. 2003, 70, 303–310. [Google Scholar] [CrossRef]
- Jeschke, S.; Nambirajan, T.; Leeb, K.; Ziegerhofer, J.; Sega, W.; Janetschek, G. Detection of early lymph node metastases in prostate cancer by laparoscopic radioisotope guided sentinel lymph node dissection. J. Urol. 2005, 173, 1943–1946. [Google Scholar] [CrossRef]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68 Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of 18F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef] [PubMed]
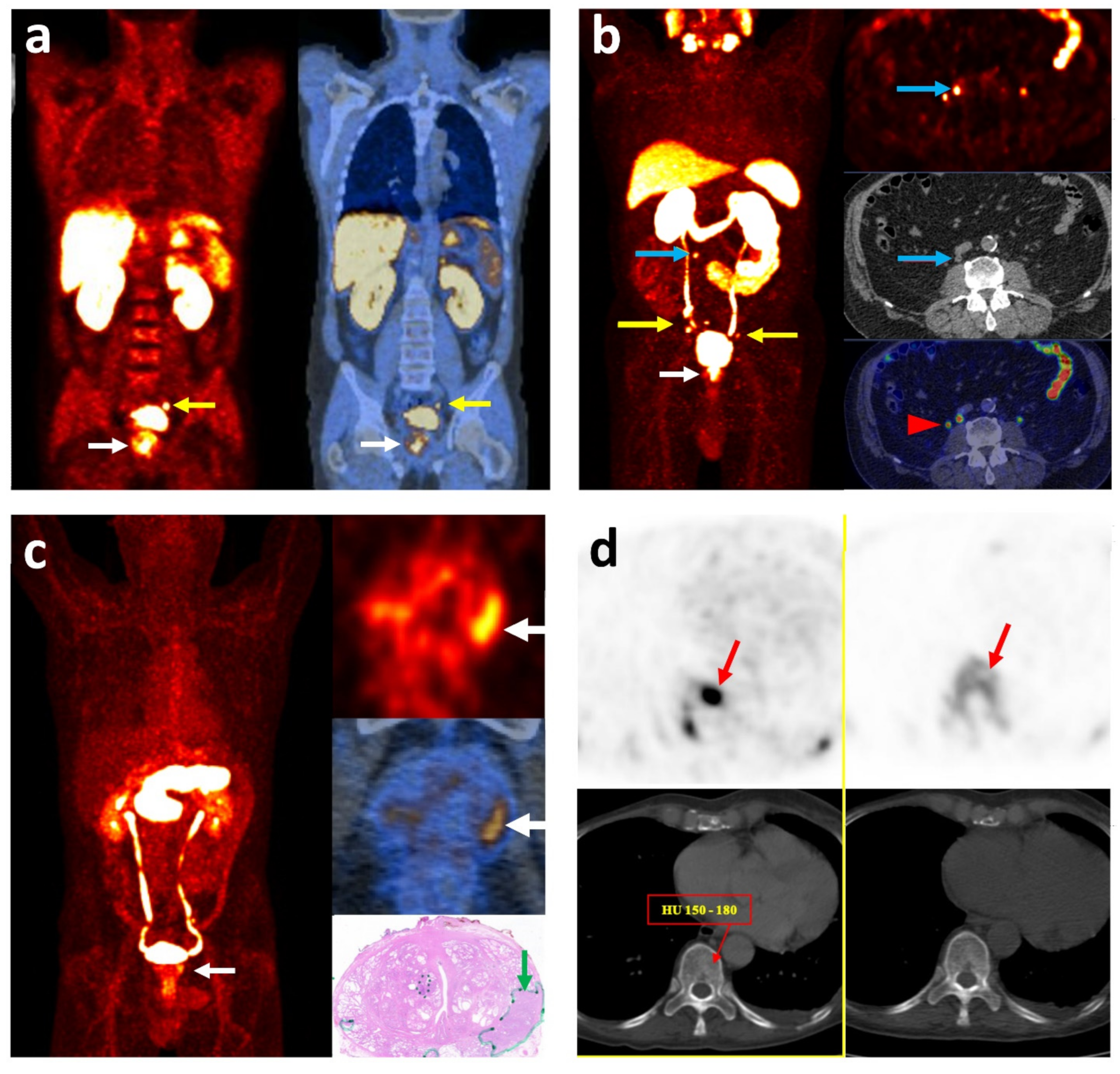
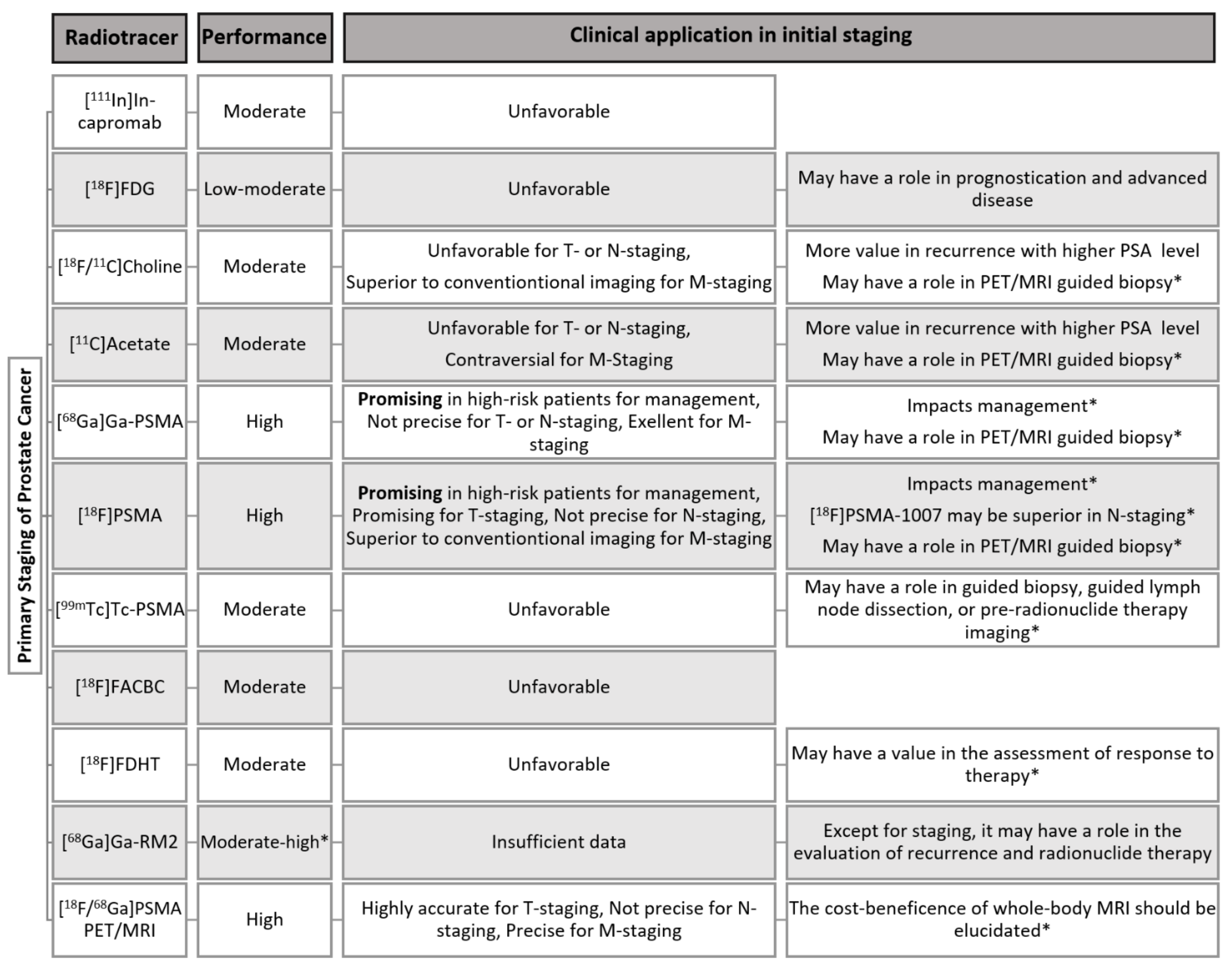
| Radiotracer | Lesion Site | Sensitivity (%) | Specificity (%) | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| [18F]FDG | T | 37–52 67~ | - 72~ |
|
| [32,34,35,38,41,44,181] |
| [11C/18F]Choline | T | 62 | 76 |
| [11,54,55,56,57] | |
| LN | 50–59 51 * | 92–95 99 * |
|
| [56,58,59] | |
| BM | 95 | 91 |
|
| [60,61,62,63] | |
| [11C]Acetate | T | 93 75 * | - 73 * |
|
| [12,46,70,71,72,73,74] |
| LN | 73 | - |
| [12,76] | ||
| [68Ga]Ga-PSMA | T | 70 * | 84 * |
|
| [81,83,84,85,87,88,89] |
| LN | 61–84 | 95–97 |
|
| [83,92,93,95] | |
| BM | 97 | 100 |
| [63,96] | ||
| [18F]PSMA | T | 95–100 | - |
| [107,108,109,111,112,120,121,122] | |
| LN | 87 § 71.2 *,§ 28.1–52.5 ¤ | 98 § 99.5 *,§ 94.0–99.4 ¤ |
| [108,112,124,126,127,128] | ||
| DM | 86–95% § | 76–90% § |
|
| [129,130] | |
| [18F]Fluciclovine | T | 86.3 | 75.5 |
| [151,153] | |
| LN | 40 | 100 |
|
| [154,155] | |
| [99mTc]PSMA | T | 94–100 | - |
|
| [17,143,144] |
| LN | 50 | 87 |
|
| [143] | |
| Na[18F]F | BM | 96 | 97 |
|
| [63] |
| [111In]In- capromab pendetide | LN | 62 | 72 |
| [25] | |
| [18F]FDHT | T | 63 | 86 |
|
| [161,162] |
| [68Ga]Ga-RM2 | T | 88 | 81 |
| [166] | |
| 70 | - |
| [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manafi-Farid, R.; Ranjbar, S.; Jamshidi Araghi, Z.; Pilz, J.; Schweighofer-Zwink, G.; Pirich, C.; Beheshti, M. Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends. Cancers 2021, 13, 5360. https://doi.org/10.3390/cancers13215360
Manafi-Farid R, Ranjbar S, Jamshidi Araghi Z, Pilz J, Schweighofer-Zwink G, Pirich C, Beheshti M. Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends. Cancers. 2021; 13(21):5360. https://doi.org/10.3390/cancers13215360
Chicago/Turabian StyleManafi-Farid, Reyhaneh, Shaghayegh Ranjbar, Zahra Jamshidi Araghi, Julia Pilz, Gregor Schweighofer-Zwink, Christian Pirich, and Mohsen Beheshti. 2021. "Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends" Cancers 13, no. 21: 5360. https://doi.org/10.3390/cancers13215360
APA StyleManafi-Farid, R., Ranjbar, S., Jamshidi Araghi, Z., Pilz, J., Schweighofer-Zwink, G., Pirich, C., & Beheshti, M. (2021). Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends. Cancers, 13(21), 5360. https://doi.org/10.3390/cancers13215360






