Long-Term Follow-Up of Patients Receiving Neoadjuvant Treatment Modalties for Soft Tissue Sarcomas of the Extremities
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
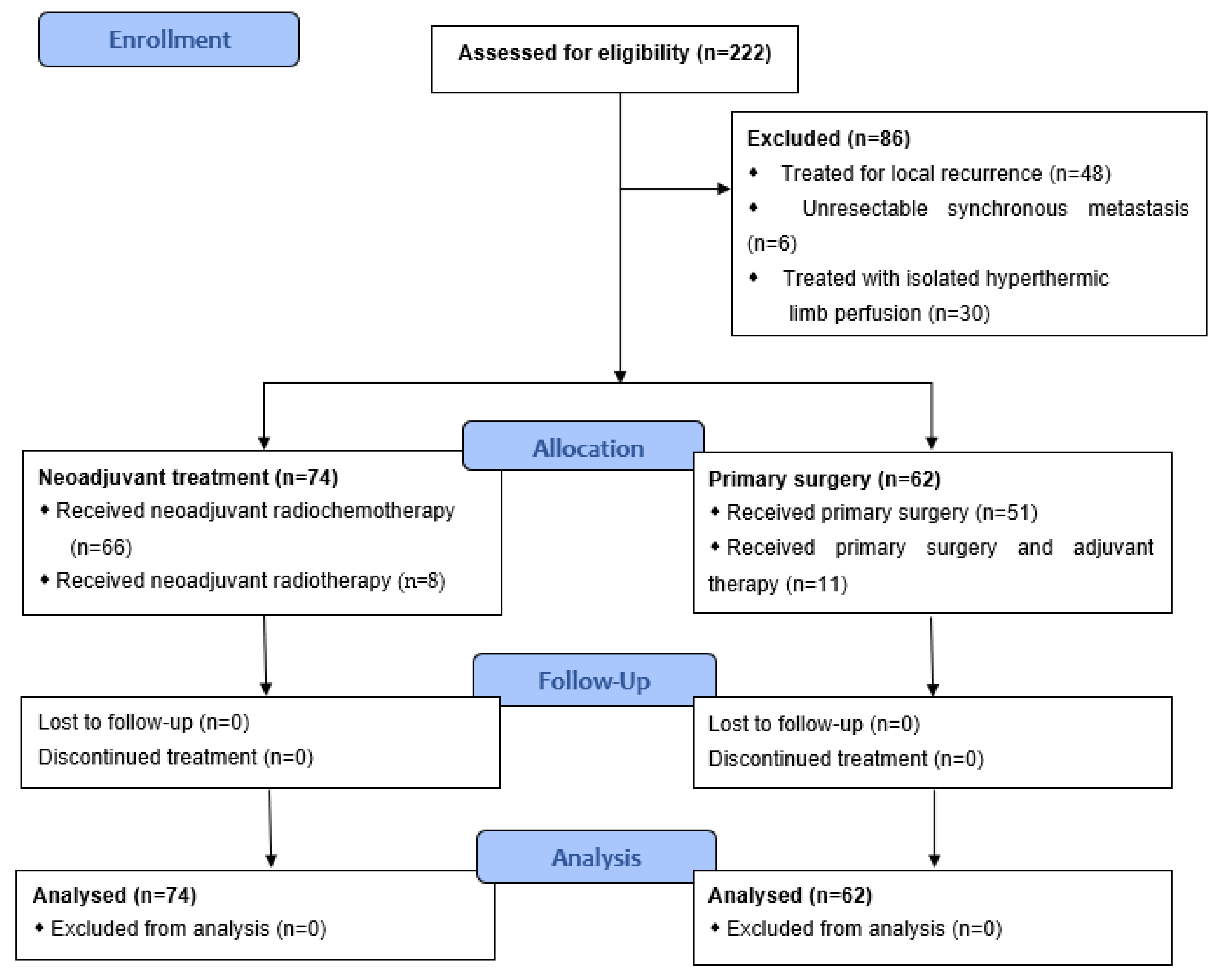
2.1. Patient Selection and Data
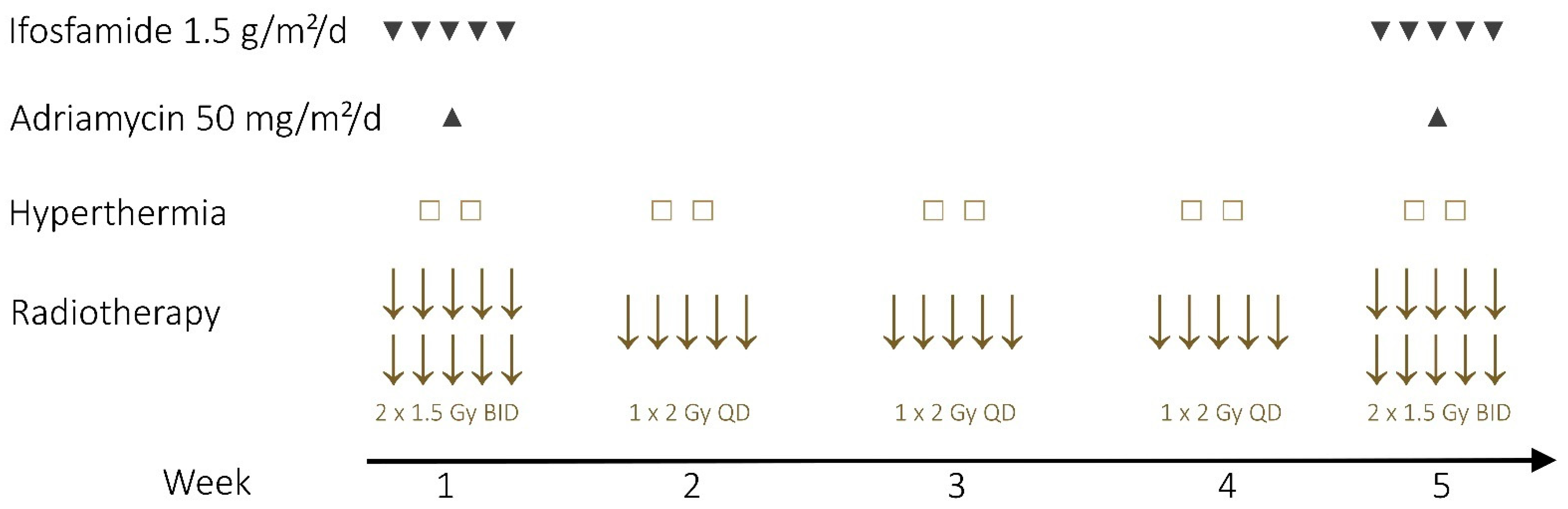
2.2. Neoadjuvant Therapy
2.3. Conduct of Surgery and Response Assessment
2.4. Follow Up and Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Therapeutic Characteristics
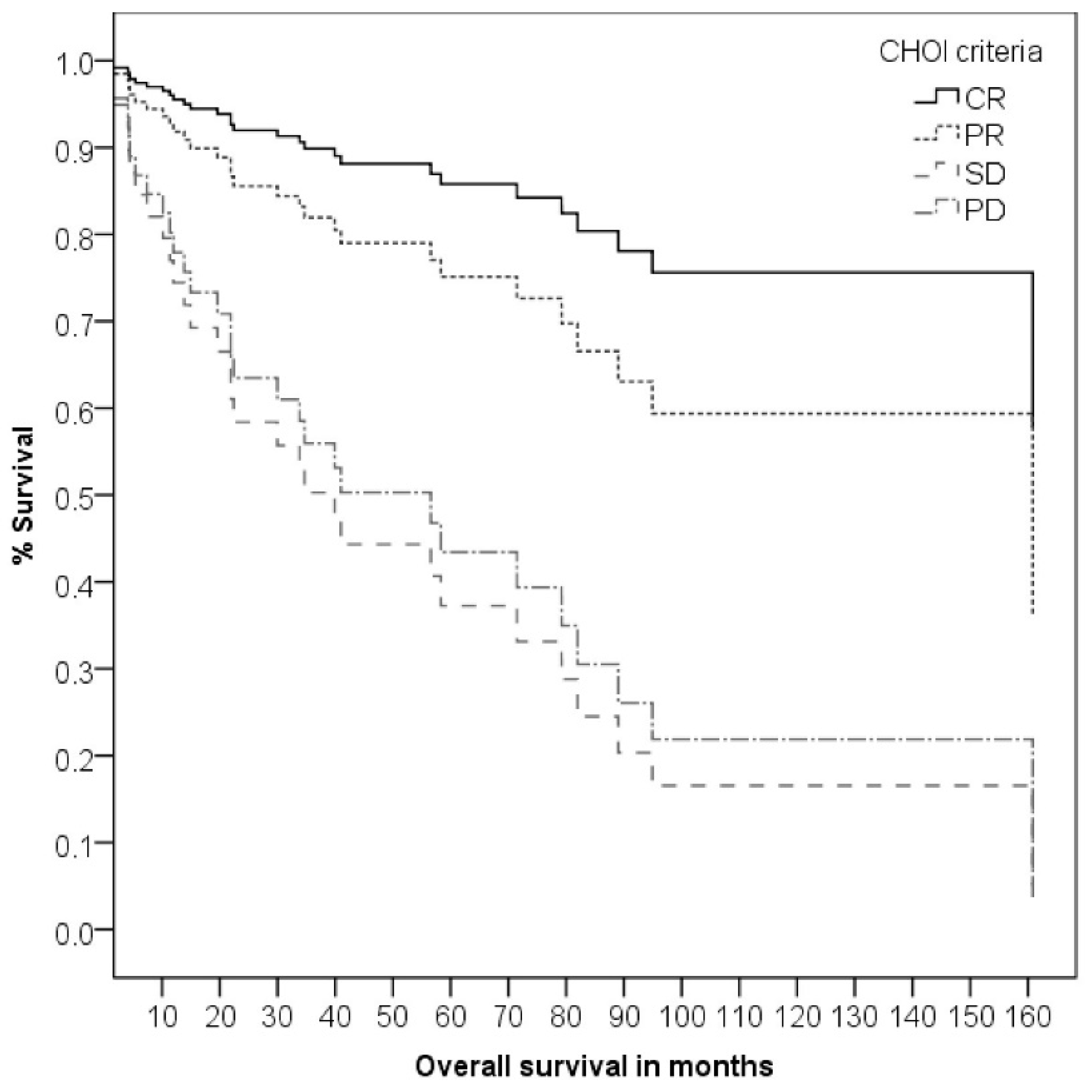
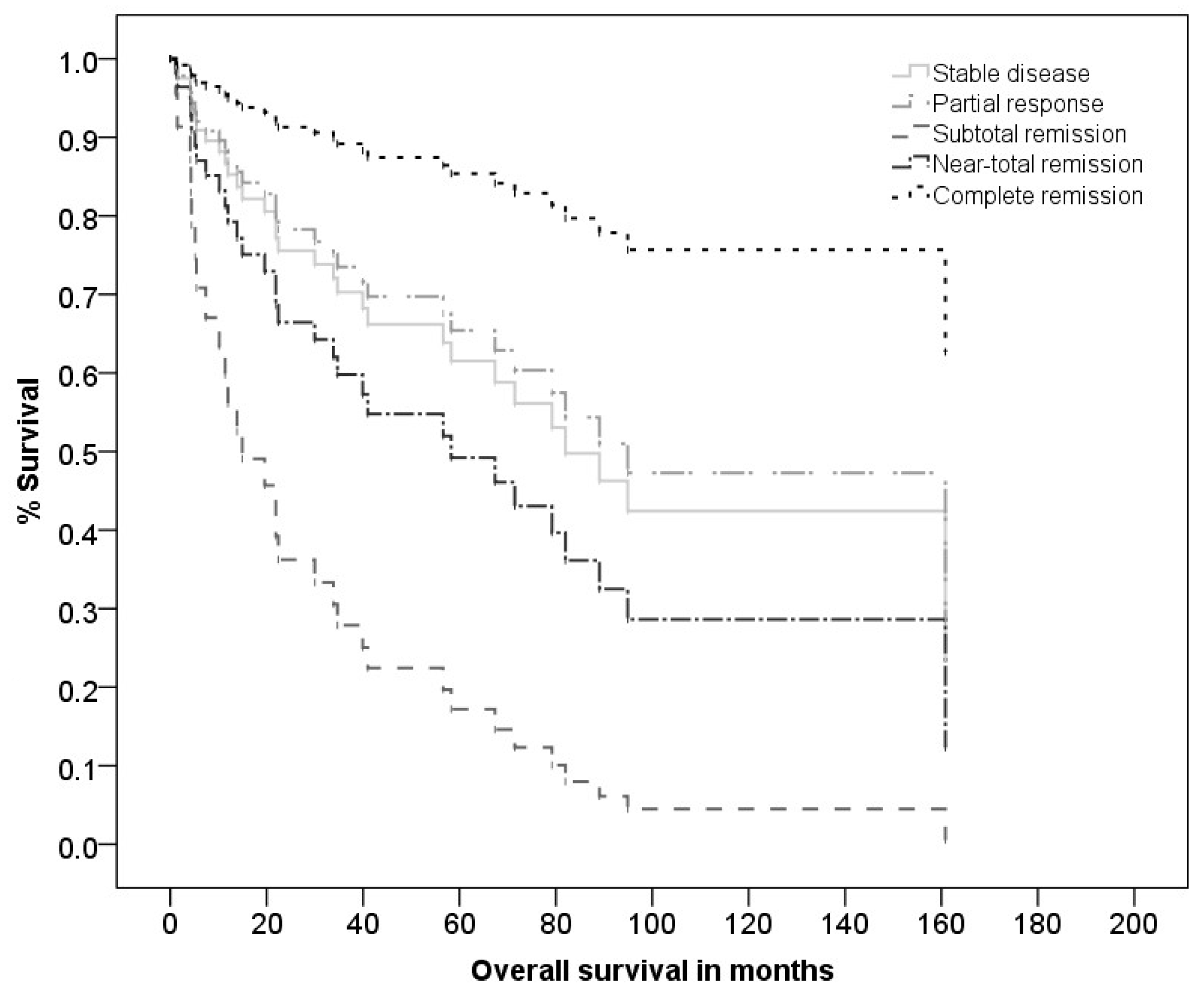
3.3. Clinical and Pathological Response to the Neoadjuvant Therapy
3.4. Follow-Up
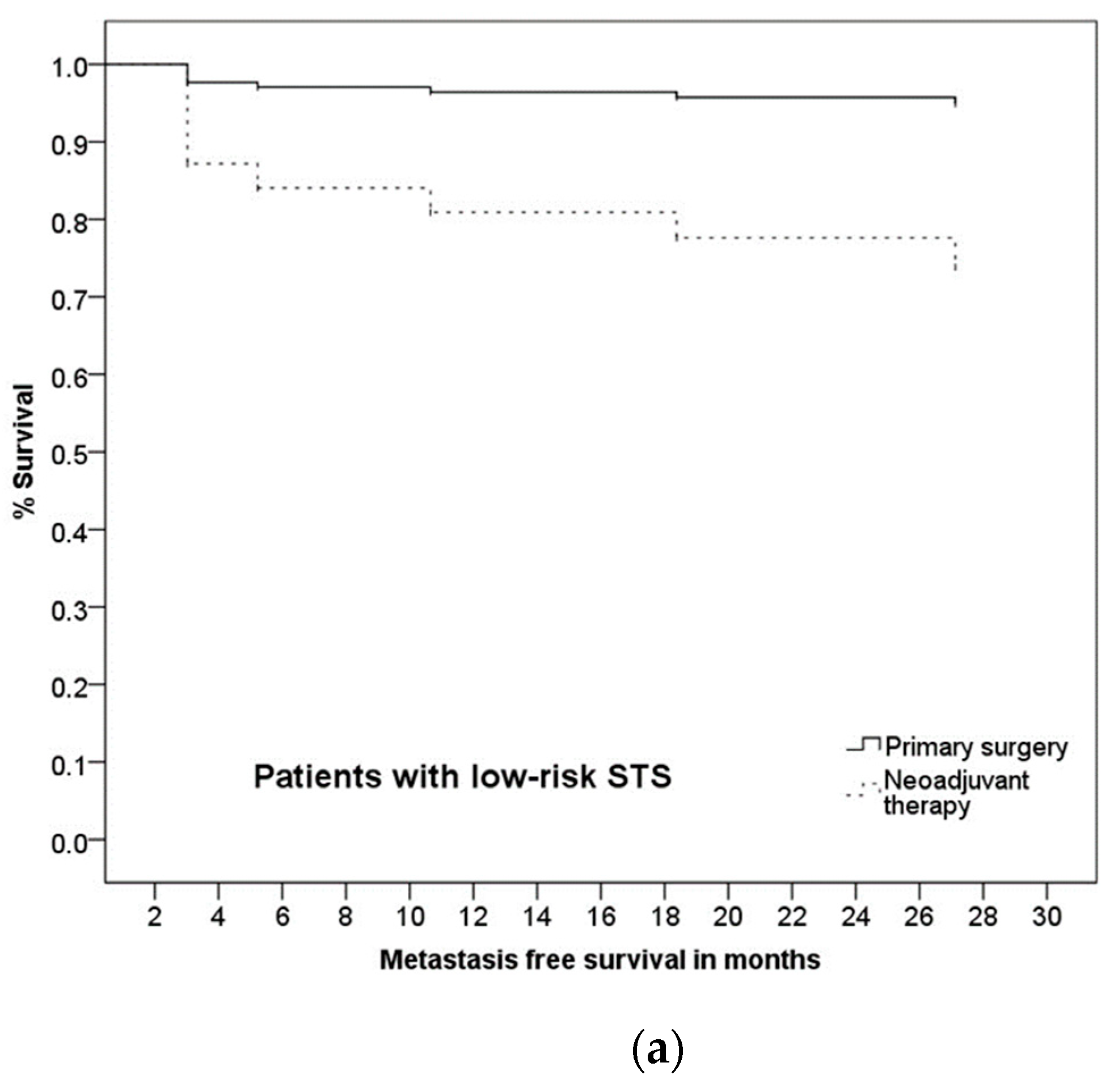
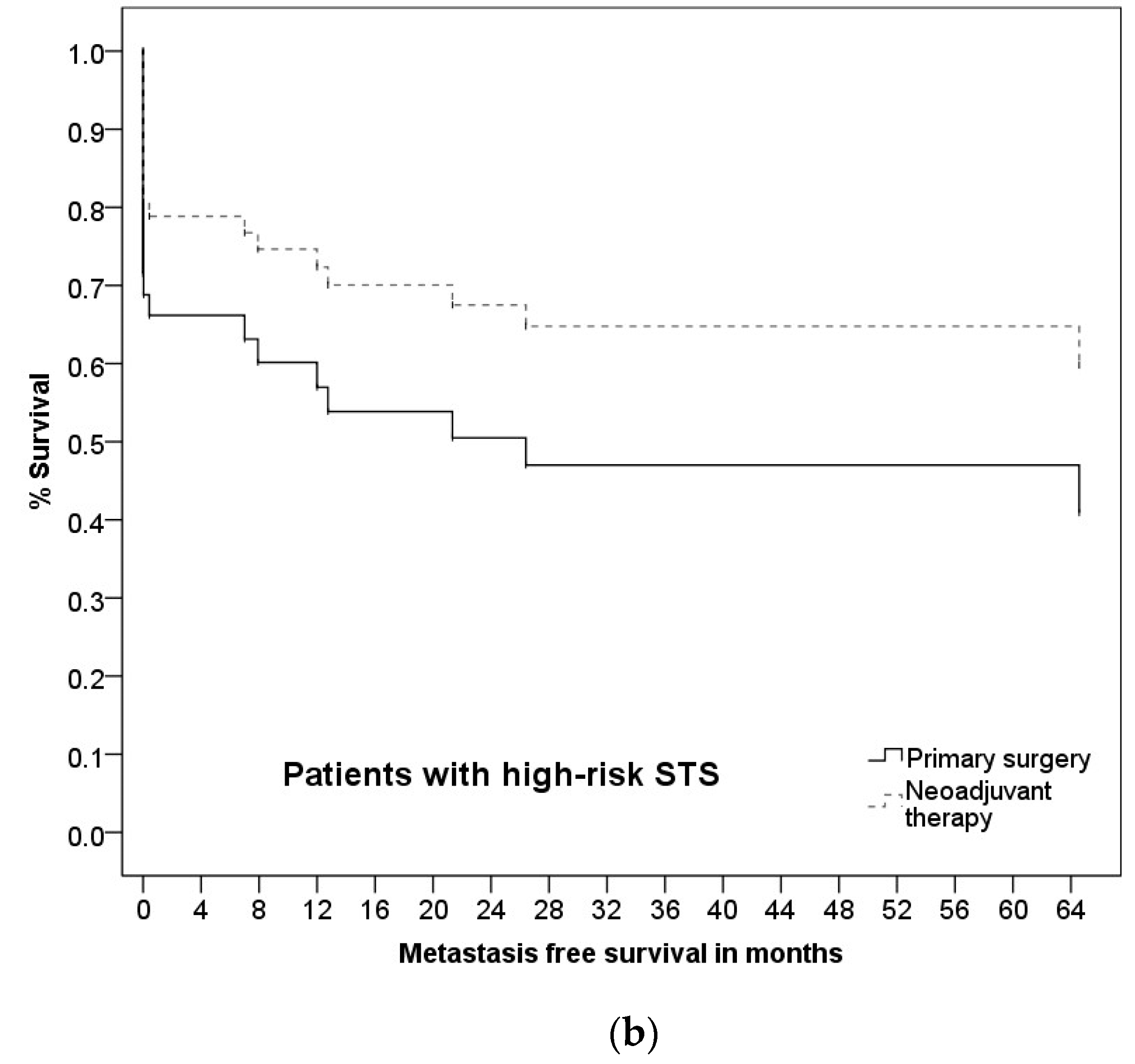
3.5. Prognostic Factors and Group Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panwar, U.; Sankaye, P. Preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma: A changing trend towards preoperative radiotherapy in the UK. Clin. Oncol. 2015, 27, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Lindner, L.H.; Ismann, B.; Abdel-Rahman, S.; Schuebbe, G.; Alig, A.; Bücklein, V.; Gioia, D.D.; Issels, R.D. Neoadjuvante chemotherapie und regionale tiefenhyperthermie für die behandlung von patienten mit hochrisiko-weichteilsarkom. In Knochentumoren und Weichteilsarkome; Lindner, L., Ed.; W. Zuckschwerdt Verlag: München, Germany, 2017; pp. 116–121. [Google Scholar]
- Bramwell, V.H.C. Intraarterial chemotherapy of soft-tissue sarcomas. Semin. Surg. Oncol. 1988, 4, 66–72. [Google Scholar] [CrossRef]
- Davis, L.E.; Ryan, C.W. Preoperative therapy for extremity soft tissue sarcomas. Curr. Treat. Options Oncol. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eilber, F.; Eckardt, J.; Rosen, G.; Forscher, C.; Selch, M.; Fu, Y.S. Preoperative therapy for soft tissue sarcoma. Hematol. Clin. N. Am. 1995, 9, 817–823. [Google Scholar] [CrossRef]
- Eilber, F.R.; Morton, D.L.; Eckardt, J.; Grant, T.; Weisenburger, T. Limb salvage for skeletal and soft tissue sarcomas multidisciplinary preoperative therapy. Cancer 1984, 53, 2579–2584. [Google Scholar] [CrossRef]
- Hoekstra, H.J.; Koops, H.S.; Molenaar, W.M.; Mehta, D.M.; Sleijfer, D.T.; Dijkhuis, G.; Oldhoff, J. A combination of intraarterial chemotherapy, preoperative and postoperative radiotherapy, and surgery as limb-saving treatment of primarily unresectable high-grade soft tissue sarcomas of the extremities. Cancer 1989, 63, 59–62. [Google Scholar] [CrossRef]
- Levine, E.A.; Trippon, M.; das Gupta, T.K. Preoperative multimodality treatment for soft tissue sarcomas. Cancer 1993, 71, 3685–3689. [Google Scholar] [CrossRef]
- Mack, L.A.; Crowe, P.J.; Yang, J.L.; Schachar, N.S.; Morris, D.G.; Kurien, E.C.; Temple, C.L.F.; Lindsay, R.L.; Magi, E.; DeHaas, W.G.; et al. Preoperative chemoradiotherapy (modified eilber protocol) provides maximum local control and minimal morbidity in patients with soft tissue sarcoma. Ann. Surg. Oncol. 2005, 12, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Temple, W.J.; Russell, J.A.; Arthur, K.; Schachar, N.S.; Crabtree, T.S.; Anderson, M.A. Neoadjuvant treatment in conservative surgery of peripheral sarcomas. Can. J. Surg. 1989, 32, 361–365. [Google Scholar]
- DeLaney, T.F.; Spiro, I.J.; Suit, H.D.; Gebhardt, M.C.; Hornicek, F.J.; Mankin, H.J.; Rosenberg, A.L.; Rosenthal, D.I.; Miryousefi, F.; Ancukiewicz, M.; et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int. J. Radiat. Oncol. 2003, 56, 1117–1127. [Google Scholar] [CrossRef]
- Mullen, J.T.; Kobayashi, W.; Wang, J.J.; Harmon, D.C.; Choy, E.; Hornicek, F.J.; Rosenberg, A.E.; Chen, Y.-L.; Spiro, I.J.; Delaney, T.F. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer 2012, 118, 3758–3765. [Google Scholar] [CrossRef]
- Kraybill, W.G.; Ms, J.H.; Spiro, I.J.; Ettinger, D.S.; Delaney, T.F.; Blum, R.H.; Lucas, D.R.; Harmon, D.C.; Letson, G.D.; Eisenberg, B.L. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall. Cancer 2010, 116, 4613–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraybill, W.G.; Harris, J.; Spiro, I.J.; Ettinger, D.S.; Delaney, T.F.; Blum, R.H.; Lucas, D.R.; Harmon, D.C.; Letson, G.D.; Eisenberg, B. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation therapy oncology group trial 9514. J. Clin. Oncol. 2006, 24, 619–625. [Google Scholar] [CrossRef]
- Mahmoud, O.; Tunceroglu, A.; Chokshi, R.; Benevenia, J.; Beebe, K.; Patterson, F.; DeLaney, T.F. Overall survival advantage of chemotherapy and radiotherapy in the perioperative management of large extremity and trunk soft tissue sarcoma; a large database analysis. Radiother. Oncol. 2017, 124, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Schuchardt, U.; Hohenberger, W.; Wittekind, C.; Papadopoulos, T.; Grabenbauer, G.G.; Fietkau, R. Neoadjuvante radiochemotherapie von weichteilsarkomen—Optimierung der lokalen funktionserhaltenden tumorkontrolle. Strahlenther. Onkol. 1999, 175, 259–266. [Google Scholar] [CrossRef]
- Stubbe, F.; Agaimy, A.; Ott, O.; Lettmaier, S.; Vassos, N.; Croner, R.; Hohenberger, W.; Fietkau, R.; Semrau, S. Effective local control of advanced soft tissue sarcoma with neoadjuvant chemoradiotherapy and surgery: A single institutional experience. Cancer/Radiothérapie 2016, 20, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Ballo, M.T.; Zagars, G.K.; Mirza, A.N.; Lin, P.P.; Lewis, V.O.; Yasko, A.W.; Benjamin, R.S.; Pisters, P.W. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006, 107, 2455–2461. [Google Scholar] [CrossRef]
- Cheng, E.; Dusenbery, K.E.; Winters, M.R.; Thompson, R.C. Soft tissue sarcomas: Preoperative versus postoperative radiotherapy. J. Surg. Oncol. 1996, 61, 90–99. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Msc, A.M.G.; Dickie, C.I.; Sharpe, M.B.; Chung, P.W.M.; Catton, C.N.; Ferguson, P.C.; Wunder, J.S.; Deheshi, B.M.; White, L.M.; et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer 2013, 119, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Ball, A.; Schofield, J.; Fisher, C.; Harmer, C.; Thomas, J. Preoperative radiotherapy for initially inoperable extremity soft tissue sarcomas. Clin. Oncol. 1992, 4, 36–43. [Google Scholar] [CrossRef]
- Tseng, J.F.; Ballo, M.T.; Langstein, H.N.; Wayne, J.D.; Cormier, J.N.; Hunt, K.K.; Feig, B.W.; Yasko, A.W.; Lewis, V.O.; Lin, P.P.; et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann. Surg. Oncol. 2006, 13, 1209–1215. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Wittekind, C. The pathologist and the residual tumor (R) classification. Pathol.—Res. Pr. 1994, 190, 115–123. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Gail, M.; Krickeberg, K.; Samet, J.; Tsiatis, A. Statistics for Biology and Health, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Ziegler, A.; Lange, S.; Bender, R. Überlebenszeitanalyse: Die cox-regression. DMW—Dtsch. Med. Wochenschr. 2007, 132, e42–e44. [Google Scholar] [CrossRef] [Green Version]
- Spałek, M.J.; Kozak, K.; Czarnecka, A.M.; Bartnik, E.; Borkowska, A.; Rutkowski, P. Neoadjuvant treatment options in soft tissue sarcomas. Cancers 2020, 12, 2061. [Google Scholar] [CrossRef]
- Chowdhary, M.; Chowdhary, A.; Sen, N.; Zaorsky, N.G.; Patel, K.R.; Wang, D. Does the addition of chemotherapy to neoadjuvant radiotherapy impact survival in high-risk extremity/trunk soft-tissue sarcoma? Cancer 2019, 125, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Hazell, S.Z.; Hu, C.; Alcorn, S.R.; Asiedu, K.O.; Pulido, G.; Frassica, D.A.; Meyer, C.; Levin, A.S.; Morris, C.D.; Terezakis, S.A. Neoadjuvant chemoradiation compared with neoadjuvant radiation alone in the management of high-grade soft tissue extremity sarcomas. Adv. Radiat. Oncol. 2020, 5, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bedi, M.; King, D.M.; Shivakoti, M.; Wang, T.; Zambrano, E.V.; Charlson, J.; Hackbarth, D.; Neilson, J.; Whitfield, R.; Wang, D. Prognostic variables in patients with primary soft tissue sarcoma of the extremity and trunk treated with neoadjuvant radiotherapy or neoadjuvant sequential chemoradiotherapy. Radiat. Oncol. 2013, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronchi, A.; de Paoli, A.; Dani, C.; Merlo, D.F.; Quagliuolo, V.; Grignani, G.; Bertola, G.; Navarria, P.; Sangalli, C.; Buonadonna, A.; et al. Preoperative chemo-radiation therapy for localised retroperitoneal sarcoma: A phase I–II study from the Italian Sarcoma Group. Eur. J. Cancer 2014, 50, 784–792. [Google Scholar] [CrossRef]
- Haas, R.L.; Gronchi, A.; van de Sande, M.; Baldini, E.H.; Gelderblom, H.; Messiou, C.; Wardelmann, E.; le Cesne, A. Perioperative management of extremity soft tissue sarcomas. J. Clin. Oncol. 2018, 36, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Haas, R.L.; Miah, A.B.; LePechoux, C.; DeLaney, T.F.; Baldini, E.H.; Alektiar, K.; O’Sullivan, B. Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother. Oncol. 2016, 119, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Gannon, N.P.; Stemm, M.H.; King, D.M.; Bedi, M. Pathologic necrosis following neoadjuvant radiotherapy or chemoradiotherapy is prognostic of poor survival in soft tissue sarcoma. J. Cancer Res. Clin. Oncol. 2019, 145, 1321–1330. [Google Scholar] [CrossRef]
- Gronchi, A.; Verderio, P.; de Paoli, A.; Ferraro, A.; Tendero, O.; Majò, J.; Martin-Broto, J.; Comandone, A.; Grignani, G.; Pizzamiglio, S.; et al. Quality of surgery and neoadjuvant combined therapy in the ISG-GEIS trial on soft tissue sarcomas of limbs and trunk wall. Ann. Oncol. 2013, 24, 817–823. [Google Scholar] [CrossRef]
- Menendez, L.R.; Ahlmann, E.R.; Savage, K.; Cluck, M.; Fedenko, A.N. Tumor necrosis has no prognostic value in neoadjuvant chemotherapy for soft tissue sarcoma. Clin. Orthop. Relat. Res. 2007, 455, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.T.; Hornicek, F.J.; Harmon, D.C.; Raskin, K.A.; Chen, Y.-L.; Szymonifka, J.; Yeap, B.Y.; Choy, E.; Delaney, T.F.; Nielsen, G.P. Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer 2014, 120, 3676–3682. [Google Scholar] [CrossRef] [Green Version]
- Roberge, D.; Skamene, T.; Nahal, A.; Turcotte, R.E.; Powell, T.; Freeman, C. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother. Oncol. 2010, 97, 404–407. [Google Scholar] [CrossRef]
- Schaefer, I.-M.; Hornick, J.L.; Barysauskas, C.M.; Raut, C.P.; Patel, S.A.; Royce, T.J.; Fletcher, C.D.; Baldini, E.H. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: Assessment of the European Organization for Research and Treatment of Cancer—Soft Tissue and Bone Sarcoma Group Response Score. Int. J. Radiat. Oncol. 2017, 98, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Götzl, R.; Sterzinger, S.; Semrau, S.; Vassos, N.; Hohenberger, W.; Grützmann, R.; Agaimy, A.; Arkudas, A.; Horch, R.E.; Beier, J.P. Patient’s quality of life after surgery and radiotherapy for extremity soft tissue sarcoma—A retrospective single-center study over ten years. Health Qual. Life Outcomes 2019, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.A.; Esther, R.J.; Erfanian, K.; Green, R.; Kim, H.J.; Sweeting, R.; Tepper, J.E. Wound complications in preoperatively irradiated soft-tissue sarcomas of the extremities. Int. J. Radiat. Oncol. 2013, 85, 432–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgeri, A.; Klein, A.; Lindner, L.H.; Nachbichler, S.; Knösel, T.; Birkenmaier, C.; Jansson, V.; Baur-Melnyk, A.; Dürr, H.R. The effect of resection margin on local recurrence and survival in high grade soft tissue sarcoma of the extremities: How far is far enough? Cancers 2020, 12, 2560. [Google Scholar] [CrossRef]
- Choong, P.; Petersen, I.A.; Nascimento, A.G.; Sim, F.H. Is radiotherapy important for low-grade soft tissue sarcoma of the extremity? Clin. Orthop. Relat. Res. 2001, 387, 191–199. [Google Scholar] [CrossRef]
- Jebsen, N.L.; Trovik, C.S.; Bauer, H.C.; Rydholm, A.; Monge, O.R.; Hall, K.S.; Alvegård, T.; Bruland, Ø.S. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: A Scandinavian Sarcoma Group Study. Int. J. Radiat. Oncol. 2008, 71, 1196–1203. [Google Scholar] [CrossRef]
- Pennington, J.D.; Eilber, F.C.; Eilber, F.R.; Singh, A.S.; Reed, J.P.; Chmielowski, B.; Eckardt, J.J.; Bukata, S.V.; Bernthal, N.M.; Federman, N.; et al. Long-term outcomes with ifosfamide-based hypofractionated preoperative chemoradiotherapy for extremity soft tissue sarcomas. Am. J. Clin. Oncol. 2018, 41, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Sadoski, C.; Suit, H.D.; Rosenberg, A.; Mankin, H.; Efird, J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J. Surg. Oncol. 1993, 52, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Wardelmann, E.; Merkelbach-Bruse, S.; Schildhaus, H.; Büttner, R. Brauchen wir in Deutschland Sarkomzentren? Der Pathol. 2010, 32, 72–75. [Google Scholar] [CrossRef] [PubMed]
| nRT (n = 74) | PS (n = 62) | Total (n = 136) | p-Value ** | |
|---|---|---|---|---|
| Sex, no. (%) | ||||
| Male | 43 (58.1%) | 30 (48.4%) | 73 (53.7%) | 0.302 a |
| Female | 31 (41.9%) | 32 (51.6%) | 63 (46.3%) | |
| Age in years | ||||
| Mean (±SD) | 61.69 (±13.57) | 61.89(±16.61) | 61.8 (±15.0) | 0.940 b |
| Median | 64.0 | 63.5 | 64.0 | |
| Range (min.-max.) | 24–87 | 21–93 | 21–93 | |
| Histological Type, no. (%) | ||||
| Undifferentiated pleomorph sarcoma | 28 (37.8%) | 7 (11.3%) | 35 (25.7%) | <0.001 a |
| Well differentiated Liposarcoma | 3 (4.1%) | 29 (46.8%) | 32 (23.5%) | <0.001 c |
| Myxofibrosarcoma | 8 (10.9%) | 8 (12.9%) | 16 (11.8%) | 0.706 a |
| Leiomyosarcoma | 5 (6.7%) | 5 (8.1%) | 10 (7.4%) | 0.771 a |
| Myxoid liposarcoma | 5 (6.7%) | 4 (6.5%) | 9 (6.7%) | 1.000 c |
| Synovialsarcoma | 5 (6.7%) | 2 (3.2%) | 7 (5.1%) | 0.454 c |
| Spindle cell sarcoma | 5 (6.7%) | 1 (1.6%) | 6 (4.4%) | 0.219 c |
| Dedifferentiated liposarcoma | 3 (4.1%) | 0 (0%) | 3 (2.2%) | 0.250 c |
| Angiosarcoma | 0 (0%) | 2 (3.2%) | 2 (1.5%) | 0.206 c |
| Alveolar sarcoma | 1 (1.4%) | 1 (1.6%) | 2 (1.5%) | 1.000 c |
| Other sarcoma * | 11 (14.9%) | 3 (4.8%) | 14 (10.3%) | 0.087 c |
| Tumor location, no. (%) | ||||
| Upper Extremity | 15 (20.2%) | 15 (24.2%) | 30 (22%) | 0.679 a |
| Lower Extremity | 59 (79.8%) | 47 (75.8%) | 106 (78%) | |
| Tumor depth, no. (%) | ||||
| Epifascial Tumor | 15 (20.2%) | 18 (29.1%) | 33 (24.3%) | 0.248 a |
| Subfascial Tumor | 57 (77.1%) | 43 (69.3%) | 100 (73.5%) | |
| Missing value | 2 (2.7%) | 1 (1.6%) | 3 (2.2%) | |
| Initial tumorsize in cm | ||||
| Mean (±SD) | 11.3 (±6.3) | 12.0 (±8.8) | 11.6 (±7.5) | 0.617 b |
| Median | 10.0 | 9.8 | 10.0 | |
| Range (min.-max.) | 2.5–30.0 | 0.5–35.0 | 0.5–35 | |
| G-Grading (histological differentiation) | ||||
| G1 | 4 (5.4%) | 33 (53.3%) | 37 (27.2%) | <0.001 a |
| G2 | 20 (27.0%) | 14 (22.6%) | 34 (25.0%) | |
| G3 | 45 (60.8%) | 10 (16.1%) | 55 (40.4%) | |
| G4 | 1 (1.4%) | 1 (1.6%) | 2 (1.5%) | |
| GX | 3 (4.0%) | 3 (4.8%) | 6 (4.4%) | |
| Missing value | 1 (1.4%) | 1 (1.6%) | 2 (1.5%) | |
| High-/low grade STS | ||||
| Low-grade | 14 (18.9%) | 42 (67.7%) | 56 (42.4%) | <0.001 a |
| High-grade | 58 (78.4%) | 18 (29.1%) | 76 (57.6%) | |
| Missing value | 2 (2.7%) | 2 (3.2%) | 4 (2.9%) | |
| NT (n = 74) | PS (n = 62) | Total (n = 136) | p-Value * | |
|---|---|---|---|---|
| Group, no. (%) | ||||
| Primary surgery | -- | 62 (100%) | 62 (45.6%) | -- |
| Neoadjuvant therapy | 74 (100%) | -- | 74 (54.4%) | -- |
| RCT | 66 (89.2%) | -- | 66 (48.5%) | |
| With hyperthermia | 29 | -- | ||
| RT | 8 (10.8%) | -- | 8 (5.9%) | |
| With hyperthermia | 2 | |||
| Adjuvant therapy | -- | 11 (17.7%) | 11 (8.1%) | |
| RCT | -- | 6 (9.6%) | 6 (4.4%) | |
| RT | -- | 4 (6.5%) | 4 (2.9%) | |
| CTx | -- | 1 (1.6%) | 1 (0.7%) | |
| Outpatient practice primary R1-excision, no. (%) | ||||
| No outpatient practice primary excision | 53 (71.5%) | 48 (77.5%) | 101 (74.3%) | 0.441 |
| Outpatient practice primary excision | 21 (28.5%) | 14 (22.5%) | 35 (25.7%) | |
| Surgical procedure, no. (%) | ||||
| Wide resection | 46 (62.2%) | 45 (72.5%) | 91 (66.9%) | 0.198 |
| Compartmental resection | 28 (37.8%) | 17 (27.5%) | 45 (33.1%) | |
| R-Stage (residual tumor) | ||||
| R0 | 65 (87.8%) | 51 (82.3%) | 116 (85.3%) | 0.482 |
| R1 | 8 (10.8%) | 9 (14.5%) | 17 (12.5%) | |
| RX | 1 (1.4%) | 2 (3.2%) | 3 (2.2%) | |
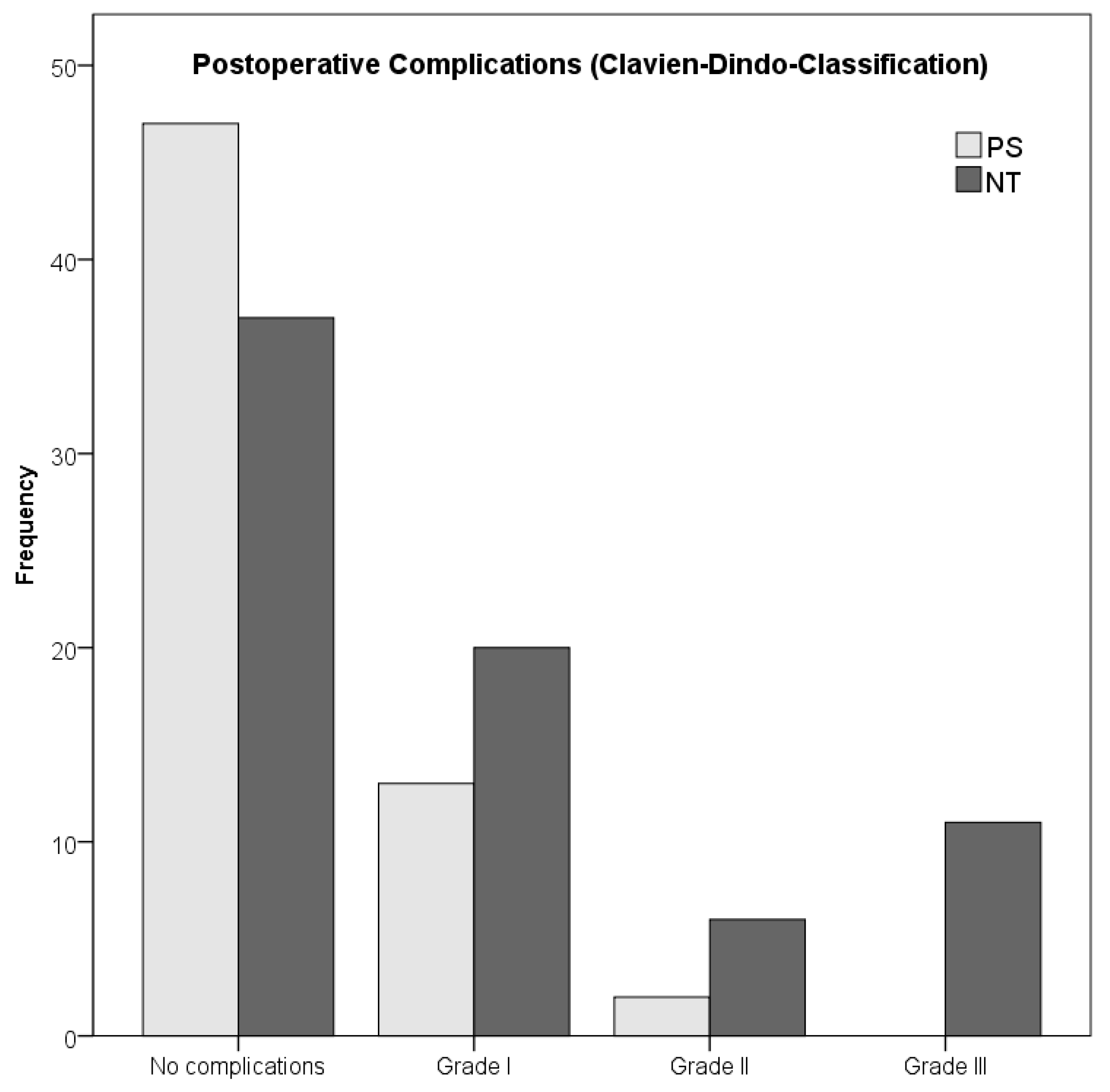
| Postoperative complications, no. (%) | ||||
| No complications | 37 (50.0%) | 47 (75.8%) | 83 (61.0%) | 0.003 |
| With Complications | 37 (50.0%) | 15 (24.2%) | 52 (38.2%) | |
| Grade I | 20 (27.0%) | 13 (20.9%) | 33 (24.3%) | |
| Grade II | 6 (8.1%) | 2 (3.3%) | 8 (5.9%) | |
| Grade III | 11 (14.9%) | 0 (0%) | 11 (8.1%) | |
| Grade IV | 0 (0%) | 0 (0%) | 0 (0%) | |
| Grade V | 0 (0%) | 0 (0%) | 0 (0%) | |
| Survival Rates | Low-Risk STS (n = 24) | High-Risk STS (n = 46) | p-Value * |
|---|---|---|---|
| OS | 111.97 (±10.00) | 92.93 (±13.22) | 0.018 * |
| LRFS | 127.01 (±7.64) | 176.19 (±9.32) | 0.962 |
| MFS | 104.91 (±11.83) | 120.79 (±14.51) | 0.346 |
| DFS | 99.21 (±12.46) | 115.65 (±14.76) | 0.442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauch, M.; Agaimy, A.; Semrau, S.; Willner, A.; Ott, O.; Fietkau, R.; Hohenberger, W.; Croner, R.S.; Grützmann, R.; Fechner, K.; et al. Long-Term Follow-Up of Patients Receiving Neoadjuvant Treatment Modalties for Soft Tissue Sarcomas of the Extremities. Cancers 2021, 13, 5244. https://doi.org/10.3390/cancers13205244
Rauch M, Agaimy A, Semrau S, Willner A, Ott O, Fietkau R, Hohenberger W, Croner RS, Grützmann R, Fechner K, et al. Long-Term Follow-Up of Patients Receiving Neoadjuvant Treatment Modalties for Soft Tissue Sarcomas of the Extremities. Cancers. 2021; 13(20):5244. https://doi.org/10.3390/cancers13205244
Chicago/Turabian StyleRauch, Miriam, Abbas Agaimy, Sabine Semrau, Alexander Willner, Oliver Ott, Rainer Fietkau, Werner Hohenberger, Roland S. Croner, Robert Grützmann, Katja Fechner, and et al. 2021. "Long-Term Follow-Up of Patients Receiving Neoadjuvant Treatment Modalties for Soft Tissue Sarcomas of the Extremities" Cancers 13, no. 20: 5244. https://doi.org/10.3390/cancers13205244
APA StyleRauch, M., Agaimy, A., Semrau, S., Willner, A., Ott, O., Fietkau, R., Hohenberger, W., Croner, R. S., Grützmann, R., Fechner, K., & Vassos, N. (2021). Long-Term Follow-Up of Patients Receiving Neoadjuvant Treatment Modalties for Soft Tissue Sarcomas of the Extremities. Cancers, 13(20), 5244. https://doi.org/10.3390/cancers13205244










