Involvement of Kynurenine Pathway in Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Primary Liver Cancer
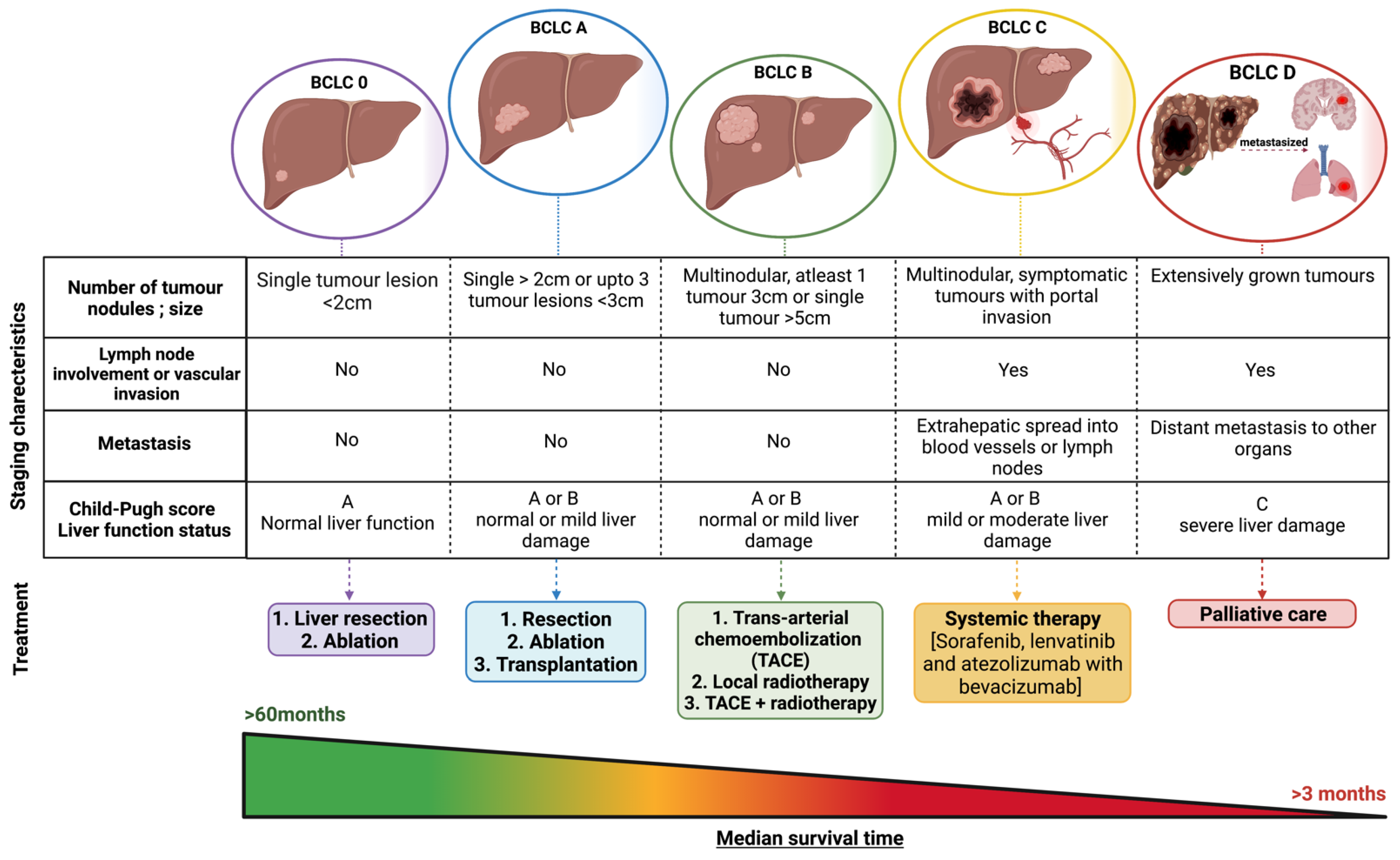
HCC Stages and Its Prognosis
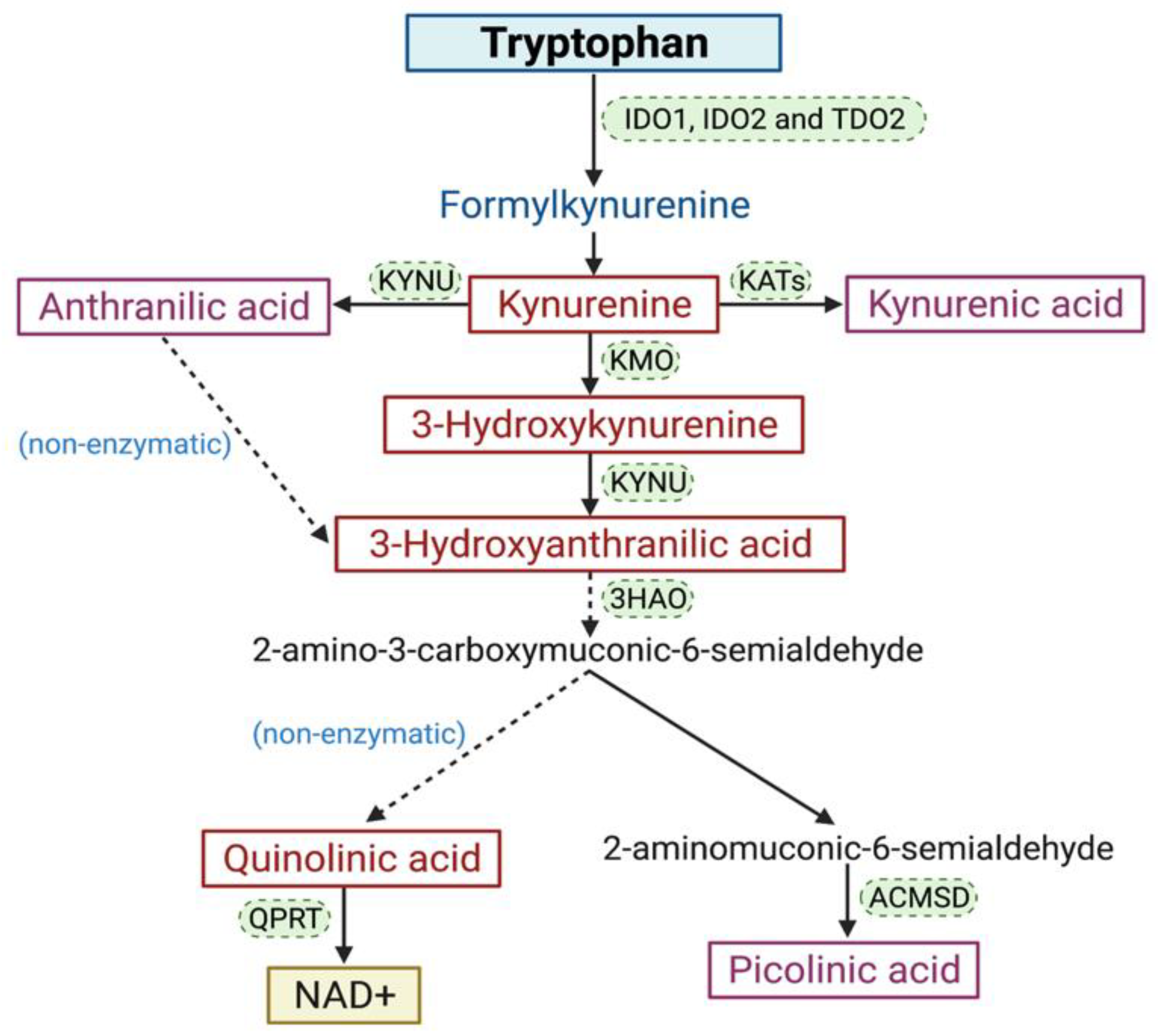
2. The KP
Involvement of the KP in Cancer
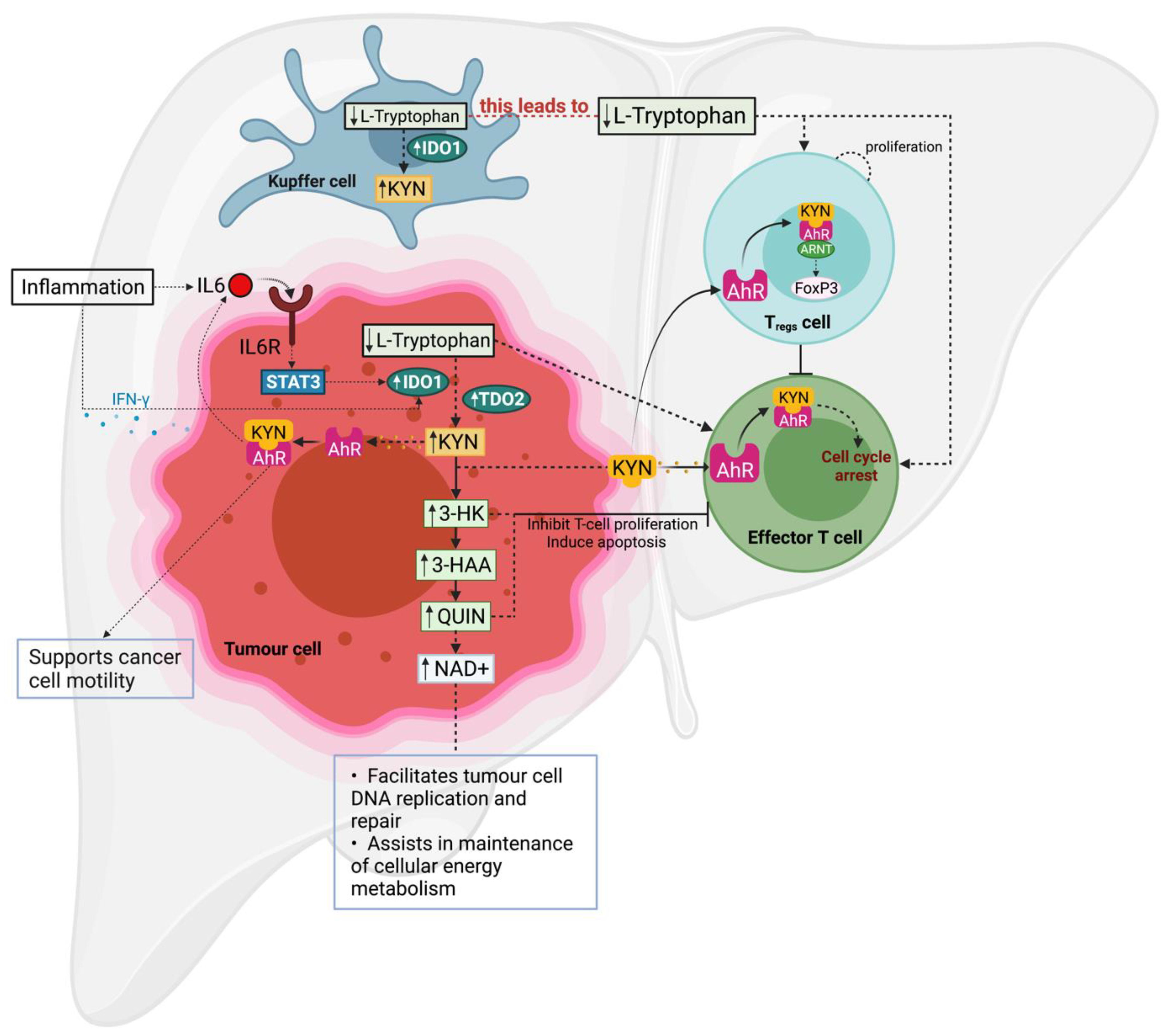
3. Involvement of the KP in Chronic Liver Disease and HCC
3.1. IDO1
3.2. TDO2
3.3. KYN Levels in Patient Sera
3.4. KMO
3.5. Clinical Trials: IDO1 Inhibitors as HCC Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1-MT | 1-methyltryptophan |
| 3-HAA | 3-hydroxyanthranilic acid |
| 3HAO | 3-hydroxyanthranilate 3,4-dioxygenase |
| 3-HK | 3-hydroxykynurenine |
| ACMSD | 2-amino-3-carboxymuconate semialdehyde decarboxylase |
| AFP | Alpha-fetoprotein |
| AhR | Aryl hydrocarbon receptor |
| BCLC | Barcelona clinic liver cancer |
| bHLH | basic helix–loop–helix |
| CRC | Colorectal cancer |
| GPC-3 | Glypican-3 |
| HCC | Hepatocarcinoma |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| ICC | Intrahepatic cholangiocarcinoma |
| IDO1 | Indoleamine 2,3 dioxygenase 1 |
| IDO2 | Indoleamine 2,3 dioxygenase 2 |
| IL4I1 | Interleukin-4 induced gene 1 |
| IL-6 | Interleukin 6 |
| KMO | Kynurenine-3-monooxygenase |
| KP | Kynurenine pathway |
| KYN | Kynurenine |
| KYNA | Kynurenic acid |
| KYNU | Kynureninase |
| NAD+ | Nicotinamide adenine dinucleotide |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| QPRT | Quinolate phosphoribosyltransferase |
| QUIN | Quinolinic acid |
| PAS | Per–Arnt–Sim |
| TACE | Trans-arterial chemoembolization |
| Tc | Cytotoxic T-cells |
| TDO2 | Tryptophan 2,3 dioxygenase 2 |
| Th | T helper cells |
| Tregs | CD4+CD25+Foxp3+ Regulatory T-cells |
| TRP | Tryptophan |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Olubuyide, I. Pattern of metastasis of primary liver cancer at autopsy: An African series. Trop. Gastroenterol. 1991, 12, 67–72. [Google Scholar] [PubMed]
- Lee, Y.-T.M.; Geer, D.A. Primary liver cancer: Pattern of metastasis. J. Surg. Oncol. 1987, 36, 26–31. [Google Scholar] [CrossRef]
- Jiang, X.-B.; Ke, C.; Zhang, G.-H.; Zhang, X.-H.; Sai, K.; Chen, Z.-P.; Mou, Y.-G. Brain metastases from hepatocellular carcinoma: Clinical features and prognostic factors. BMC Cancer 2012, 12, 49. [Google Scholar] [CrossRef]
- Tang, D.; Nagano, H.; Nakamura, M.; Wada, H.; Marubashi, S.; Miyamoto, A.; Takeda, Y.; Umeshita, K.; Dono, K.; Monden, M. Clinical and Pathological Features of Allen’s Type C Classification of Resected Combined Hepatocellular and Cholangiocarcinoma: A Comparative Study with Hepatocellular Carcinoma and Cholangiocellular Carcinoma. J. Gastrointest. Surg. 2006, 10, 987–998. [Google Scholar] [CrossRef]
- Chen, G.; Lin, W.; Shen, F.; Iloeje, U.H.; London, W.T.; Evans, A.A. Past HBV Viral Load as Predictor of Mortality and Morbidity from HCC and Chronic Liver Disease in a Prospective Study. Am. J. Gastroenterol. 2006, 101, 1797–1803. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. GLOBOCAN 2018. IARC 2020. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=11&type=0&statistic=5&prevalence=0&population_groupearth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&rotate=%255B10%252C0%255D (accessed on 30 August 2021).
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2020, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Cancer in Australia: Actual incidence data from 1982 to 2013 and mortality data from 1982 to 2014 with projections to 2017. Asia-Pac. J. Clin. Oncol. 2018, 14, 5–15. [Google Scholar] [CrossRef]
- Wallace, M.C.; Preen, D.; Short, M.W.; Adams, L.A.; Jeffrey, G.P. Hepatocellular carcinoma in Australia 1982–2014: Increasing incidence and improving survival. Liver Int. 2018, 39, 522–530. [Google Scholar] [CrossRef]
- Wu, E.M.; Wong, L.L.; Hernandez, B.Y.; Ji, J.-F.; Jia, W.; Kwee, S.A.; Kalathil, S. Gender differences in hepatocellular cancer: Disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018, 4, 66. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Trinchet, J.-C.; Bourcier, V.; Chaffaut, C.; Ahmed, M.A.; Allam, S.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; De Lédinghen, V.; et al. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology 2015, 62, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Ringelhan, M.; McKeating, J.A.; Protzer, U. Viral hepatitis and liver cancer. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160274. [Google Scholar] [CrossRef]
- Yang, J.D.; Kim, W.R.; Coelho, R.; Mettler, T.A.; Benson, J.T.; Sanderson, S.O.; Therneau, T.M.; Kim, B.; Roberts, L. Cirrhosis Is Present in Most Patients with Hepatitis B and Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zamor, P.J.; Delemos, A.S.; Russo, M.W. Viral hepatitis and hepatocellular carcinoma: Etiology and management. J. Gastrointest. Oncol. 2017, 8, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Chang, M.-H. Hepatitis B virus and cancer prevention. Recent Results Cancer Res. 2011, 188, 75–84. [Google Scholar] [CrossRef]
- Hoshida, Y.; Fuchs, B.C.; Bardeesy, N.; Baumert, T.F.; Chung, R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014, 61, S79–S90. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef]
- Blonski, W.; Kotlyar, D.S.; Forde, K.A. Non-viral causes of hepatocellular carcinoma. World J. Gastroenterol. 2010, 16, 3603–3615. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Said, A.; Ghufran, A. Epidemic of non-alcoholic fatty liver disease and hepatocellular carcinoma. World J. Clin. Oncol. 2017, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.-R.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014, 60, 1767. [Google Scholar] [CrossRef]
- Yu, M.-L.; Chuang, W.-L. Treatment of chronic hepatitis C in Asia: When East meets West. J. Gastroenterol. Hepatol. 2009, 24, 336–345. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Henley, S.J.; Cucinelli, J.E.; McGlynn, K.A. Changing Hepatocellular Carcinoma Incidence and Liver Cancer Mortality Rates in the United States. Am. J. Gastroenterol. 2014, 109, 542–553. [Google Scholar] [CrossRef]
- Lan, X.; Li, L. Association between hepatitis B virus/hepatitis C virus infection and primary hepatocellular carcinoma risk: A meta-analysis based on Chinese population. J. Cancer Res. Ther. 2016, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yan, L.; Liu, Y.; Qu, C.; Ni, J.; Li, H. The Burden and Trends of Primary Liver Cancer Caused by Specific Etiologies from 1990 to 2017 at the Global, Regional, National, Age, and Sex Level Results from the Global Burden of Disease Study 2017. Liver Cancer 2020, 9, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Liver Cancer Survival Rates. American Cancer Society. Available online: https://www.cancer.org/cancer/liver-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 30 August 2021).
- Pons, F.; Varela, M.; Llovet, J.M. Staging systems in hepatocellular carcinoma. HPB 2005, 7, 35–41. [Google Scholar] [CrossRef]
- Wege, H.; Li, J.; Ittrich, H. Treatment Lines in Hepatocellular Carcinoma. Visc. Med. 2019, 35, 266–272. [Google Scholar] [CrossRef]
- Hibi, T.; Cherqui, D.; Geller, D.A.; Itano, O.; Kitagawa, Y.; Wakabayashi, G. Expanding indications and regional diversity in laparoscopic liver resection unveiled by the International Survey on Technical Aspects of Laparoscopic Liver Resection (INSTALL) study. Surg. Endosc. 2015, 30, 2975–2983. [Google Scholar] [CrossRef]
- Fuks, D.; Dokmak, S.; Paradis, V.; Diouf, M.; Durand, F.; Belghiti, J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: An intention-to-treat analysis. Hepatology 2011, 55, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Rhim, H.; Lim, H.K. Radiofrequency Ablation of Hepatocellular Carcinoma: Pros and Cons. Gut Liver 2010, 4, S113–S118. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.I.; Khan, S.A.; Leen, E.L.S.; Waked, I.; Taylor-Robinson, S.D. Diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 2009, 15, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.K.; Dziadek, M.; Tamaoki, T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J. Biol. Chem. 1982, 257, 5148–5153. [Google Scholar] [CrossRef]
- Tilghman, S.M.; Belayew, A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc. Natl. Acad. Sci. USA 1982, 79, 5254–5257. [Google Scholar] [CrossRef]
- Kelly, S.L.; Bird, T.G. The Evolution of the Use of Serum Alpha-fetoprotein in Clinical Liver Cancer Surveillance. J. Immunobiol. 2016, 1. [Google Scholar] [CrossRef]
- Colombo, M. Screening for Cancer in Viral Hepatitis. Clin. Liver Dis. 2001, 5, 109–122. [Google Scholar] [CrossRef]
- Filmus, J.; Selleck, S.B. Glypicans: Proteoglycans with a surprise. J. Clin. Investig. 2001, 108, 497–501. [Google Scholar] [CrossRef]
- Iglesias, B.V.; Centeno, G.; Pascuccelli, H.; Ward, F.; Peters, M.G.; Filmus, J.; Puricelli, L.; Joffé, E.B.D.K. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol. Histopathol. 2008, 23, 1333–1340. [Google Scholar]
- Filmus, J. Glypicans in growth control and cancer. Glycobiology 2001, 11, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, N.; Watanabe, A.; Hishinuma, M.; Ohashi, K.-I.; Midorikawa, Y.; Morishita, Y.; Niki, T.; Shibahara, J.; Mori, M.; Makuuchi, M.; et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod. Pathol. 2005, 18, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Wang, F.H.; Yip, Y.C.; Zhang, M.; Vong, H.T.; Chan, K.I.; Wai, K.C.; Wen, J.M. Diagnostic utility of glypican-3 for hepatocellular carcinoma on liver needle biopsy. J. Clin. Pathol. 2010, 63, 599–603. [Google Scholar] [CrossRef]
- Hippo, Y.; Watanabe, K.; Watanabe, A.; Midorikawa, Y.; Yamamoto, S.; Ihara, S.; Tokita, S.; Iwanari, H.; Ito, Y.; Nakano, K.; et al. Identification of Soluble NH2-Terminal Fragment of Glypican-3 as a Serological Marker for Early-Stage Hepatocellular Carcinoma. Cancer Res. 2004, 64, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gao, Y.; Zhai, D.; Liu, J.; Cai, J.; Wang, Y.; Jing, L.; Du, Z. Assessment of the Clinical Utility of Glypican 3 as a Serum Marker for the Diagnosis of Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2016, 15, 780–786. [Google Scholar] [CrossRef]
- Yasuda, E.; Kumada, T.; Toyoda, H.; Kaneoka, Y.; Maeda, A.; Okuda, S.; Yoshimi, N.; Kozawa, O. Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma. Hepatol. Res. 2010, 40, 477–485. [Google Scholar] [CrossRef]
- Kubota, K.; Ina, H.; Okada, Y.; Irie, T. Growth Rate of Primary Single Hepatocellular Carcinoma: Determining Optimal Screening Interval with Contrast Enhanced Computed Tomography. Dig. Dis. Sci. 2003, 48, 581–586. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Geschwind, J.-F.; Liapi, E.; Salem, R. Transcatheter Intraarterial Therapies: Rationale and Overview. Radiology 2011, 259, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267. [Google Scholar] [CrossRef]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef]
- Lubel, J.S.; Roberts, S.K.; Strasser, S.I.; Thompson, A.J.; Philip, J.; Goodwin, M.; Clarke, S.; Crawford, D.H.; Levy, M.T.; Shackel, N. Australian recommendations for the management of hepatocellular carcinoma: A consensus statement. Med. J. Aust. 2020, 214, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Pembrolizumab (Keytruda) in Advanced Hepatocellular Carcinoma. ClinicalTrials.gov U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT02658019 (accessed on 30 August 2021).
- Nivolumab and Bevacizumab in Patients with Advanced and or Metastatic Hepatocellular Carcinoma (NUANCE). ClinicalTrials.gov U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03382886?term=Nivolumab&cond=Hepatocellular+Carcinoma&draw=2&rank=3 (accessed on 30 August 2021).
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Robinson, M.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Platten, M.; Wick, W.; Eynde, B.J.V.D. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012, 72, 5435–5440. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef]
- Moffett, J.R.; Namboodiri, M.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003, 81, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Colabroy, K.L.; Begley, T.P. Tryptophan Catabolism: Identification and Characterization of a New Degradative Pathway. J. Bacteriol. 2005, 187, 7866–7869. [Google Scholar] [CrossRef] [PubMed]
- Heng, R.B.; Lim, E.; Lovejoy, D.B.; Bessede, A.; Gluch, L.; Guillemin, G.J. Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget 2015, 7, 6506–6520. [Google Scholar] [CrossRef]
- Takikawa, O.; Yoshida, R.; Kido, R.; Hayaishi, O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J. Biol. Chem. 1986, 261, 3648–3653. [Google Scholar] [CrossRef]
- Ball, H.J.; Sanchez-Perez, A.; Weiser, S.; Austin, C.J.; Astelbauer, F.; Miu, J.; McQuillan, J.A.; Stocker, R.; Jermiin, L.; Hunt, N.H. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 2007, 396, 203–213. [Google Scholar] [CrossRef]
- Ball, H.J.; Yuasa, H.J.; Austin, C.J.; Weiser, S.; Hunt, N.H. Indoleamine 2, 3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int. J. Biochem. Cell Biol. 2009, 41, 467–471. [Google Scholar] [CrossRef]
- Ren, S.; Liu, H.; Licad, E.; Correia, M.A. Expression of Rat Liver Tryptophan 2, 3-Dioxygenase inEscherichia coli: Structural and Functional Characterization of the Purified Enzyme. Arch. Biochem. Biophys. 1996, 333, 96–102. [Google Scholar] [CrossRef]
- Théate, I.; Van Baren, N.; Pilotte, L.; Moulin, P.; Larrieu, P.; Renauld, J.-C.; Hervé, C.; Gutierrez-Roelens, I.; Marbaix, E.; Sempoux, C.; et al. Extensive Profiling of the Expression of the Indoleamine 2,3-Dioxygenase 1 Protein in Normal and Tumoral Human Tissues. Cancer Immunol. Res. 2014, 3, 161–172. [Google Scholar] [CrossRef]
- Fukunaga, M.; Yamamoto, Y.; Kawasoe, M.; Arioka, Y.; Murakami, Y.; Hoshi, M.; Saito, K. Studies on tissue and cellular distribution of indoleamine 2, 3-dioxygenase 2: The absence of IDO1 upregulates IDO2 expression in the epididymis. J. Histochem. Cytochem. 2012, 60, 854–860. [Google Scholar] [CrossRef]
- Metz, R.; Smith, C.; DuHadaway, J.B.; Chandler, P.; Baban, B.; Merlo, L.; Pigott, E.; Keough, M.P.; Rust, S.; Mellor, A.L.; et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int. Immunol. 2014, 26, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.; Hazelwood, R.; Pogson, C.I.; Iyer, R.; Madge, D.J. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem. Pharmacol. 1995, 49, 1435–1442. [Google Scholar] [CrossRef]
- Kanai, M.; Funakoshi, H.; Takahashi, H.; Hayakawa, T.; Mizuno, S.; Matsumoto, K.; Nakamura, T. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol. Brain 2009, 2, 1–16. [Google Scholar] [CrossRef]
- Ren, S.; Correia, M.A. Heme: A regulator of rat hepatic tryptophan 2, 3-dioxygenase? Arch. Biochem. Biophys. 2000, 377, 195–203. [Google Scholar] [CrossRef]
- Kudo, Y.; Boyd, C.A.R. Characterisation of L-tryptophan transporters in human placenta: A comparison of brush border and basal membrane vesicles. J. Physiol. 2001, 531, 405–416. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. Tryptophan catabolism and T-cell tolerance: Immunosuppression by starvation? Immunol. Today 1999, 20, 469–473. [Google Scholar] [CrossRef]
- Van Baren, N.; van den Eynde, B.J. Tryptophan-degrading enzymes in tumoral immune resistance. Front. Immunol. 2016, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Van Baren, N.; Van den Eynde, B.J. Tumoral immune resistance mediated by enzymes that degrade tryptophan. Cancer Immunol. Res. 2015, 3, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Eynde, B.J.V.D. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Spranger, S.; Spaapen, R.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef] [PubMed]
- Friberg, M.; Jennings, R.; Alsarraj, M.; Dessureault, S.; Cantor, A.; Extermann, M.; Mellor, A.L.; Munn, D.H.; Antonia, S.J. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer 2002, 101, 151–155. [Google Scholar] [CrossRef]
- Muller, A.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Li, S.; Chen, Z.; Wu, J.; Cao, W.; Wo, Q.; Qin, X.; Xu, J. TDO2 Promotes the EMT of Hepatocellular Carcinoma Through Kyn-AhR Pathway. Front. Oncol. 2021, 10, 3008. [Google Scholar]
- Opitz, C.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Liu, Q.; Zhai, J.; Kong, X.; Wang, X.; Wang, Z.; Fang, Y.; Wang, J. Comprehensive Analysis of the Expression and Prognosis for TDO2 in Breast Cancer. Mol. Ther. Oncolytics 2020, 17, 153–168. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, B.; Yang, C.; Lin, Y.; Zhang, Z.; Wang, S.; Ye, Y.; Shen, Z. TDO2 knockdown inhibits colorectal cancer progression via TDO2-KYNU-AhR pathway. Gene 2021, 792, 145736. [Google Scholar] [CrossRef]
- Chen, I.-C.; Lee, K.-H.; Hsu, Y.-H.; Wang, W.-R.; Chen, C.-M.; Cheng, Y.-W. Expression Pattern and Clinicopathological Relevance of the Indoleamine 2,3-Dioxygenase 1/Tryptophan 2,3-Dioxygenase Protein in Colorectal Cancer. Dis. Markers 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolušić, E.; Frédérick, R.; de Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2, 3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Dolusic, E.; Larrieu, P.; Moineaux, L.; Stroobant, V.; Pilotte, L.; Colau, D.; Pochet, L.; van den Eynde, B.; Masereel, B.; Wouters, J.; et al. Tryptophan 2, 3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl) ethenyl) indoles as potential anticancer immunomodulators. J. Med. Chem. 2011, 54, 5320–5334. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F. Targeting the Inhibition of Tryptophan 2,3-Dioxygenase (TDO-2) for Cancer Treatment. ACS Med. Chem. Lett. 2016, 8, 11–13. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2–KYN–AhR pathway for cancer immunotherapy–challenges and opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Löb, S.; Königsrainer, A.; Zieker, D.; Brücher, B.L.D.M.; Rammensee, H.-G.; Opelz, G.; Terness, P. IDO1 and IDO2 are expressed in human tumors: Levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol. Immunother. 2008, 58, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Nevler, A.; Muller, A.J.; Sutanto-Ward, E.; DuHadaway, J.B.; Nagatomo, K.; Londin, E.; O’Hayer, K.; Cozzitorto, J.A.; Lavu, H.; Yeo, T.P.; et al. Host IDO2 Gene Status Influences Tumor Progression and Radiotherapy Response in KRAS-Driven Sporadic Pancreatic Cancers. Clin. Cancer Res. 2018, 25, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Mandarano, M.; Bellezza, G.; Belladonna, M.L.; Vannucci, J.; Gili, A.; Ferri, I.; Lupi, C.; Ludovini, V.; Falabella, G.; Metro, G.; et al. Indoleamine 2,3-Dioxygenase 2 Immunohistochemical Expression in Resected Human Non-small Cell Lung Cancer: A Potential New Prognostic Tool. Front. Immunol. 2020, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, R.B.; Køllgaard, T.; Andersen, R.S.; Berg, J.H.V.D.; Svane, I.M.; Straten, P.T.; Andersen, M.H. Spontaneous Cytotoxic T-Cell Reactivity against Indoleamine 2,3-Dioxygenase-2. Cancer Res. 2011, 71, 2038–2044. [Google Scholar] [CrossRef]
- Zhou, L. AHR Function in Lymphocytes: Emerging Concepts. Trends Immunol. 2015, 37, 17–31. [Google Scholar] [CrossRef]
- Leclerc, D.; Pires, A.C.S.; Guillemin, G.J.; Gilot, D. Detrimental activation of AhR pathway in cancer: An overview of therapeutic strategies. Curr. Opin. Immunol. 2021, 70, 15–26. [Google Scholar] [CrossRef]
- Kewley, R.J.; Whitelaw, M.L.; Chapman-Smith, A. The mammalian basic helix–loop–helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004, 36, 189–204. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009, 206, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Z.; Kijlstra, A.; Zhou, Y.; Yang, P. Activation of the aryl hydrocarbon receptor affects activation and function of human monocyte-derived dendritic cells. Clin. Exp. Immunol. 2014, 177, 521–530. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Dong, W.; Fang, Y.; Lv, J.; Zhang, T.; Fiskesund, R.; Xie, J.; Liu, J.; Yin, X.; et al. Tumor-Repopulating Cells Induce PD-1 Expression in CD8+ T Cells by Transferring Kynurenine and AhR Activation. Cancer Cell 2018, 33, 480–494.e7. [Google Scholar] [CrossRef]
- Safe, S.; Lee, S.-O.; Jin, U.-H. Role of the Aryl Hydrocarbon Receptor in Carcinogenesis and Potential as a Drug Target. Toxicol. Sci. 2013, 135, 1–16. [Google Scholar] [CrossRef]
- Xue, P.; Fu, J.; Zhou, Y. The Aryl Hydrocarbon Receptor and Tumor Immunity. Front. Immunol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Routy, J.-P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Hjortsø, M.D.; Larsen, S.K.; Kongsted, P.; Met, Ö.; Frøsig, T.M.; Andersen, G.H.; Ahmad, S.M.; Svane, I.M.; Becker, J.C.; Straten, P.T.; et al. Tryptophan 2,3-dioxygenase (TDO)-reactive T cells differ in their functional characteristics in health and cancer. OncoImmunology 2015, 4, e968480. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, U.M.; Opitz, C.A.; Sahm, F.; Rauschenbach, K.J.; Trump, S.; Winter, M.; Ott, M.; Ochs, K.; Lutz, C.; Liu, X.; et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 2014, 5, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand that Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Sadik, A.; Patterson, L.F.S.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef]
- Castellano, F.; Prevost-Blondel, A.; Cohen, J.L.; Molinier-Frenkel, V. What role for AHR activation in IL4I1-mediated immunosuppression? Oncoimmunology 2021, 10, 1924500. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Hayashi, T.; Mo, J.-H.; Gong, X.; Rossetto, C.; Jang, A.; Beck, L.; Elliott, G.I.; Kufareva, I.; Abagyan, R.; Broide, D.H.; et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 18619–18624. [Google Scholar] [CrossRef]
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2, 3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010, 2, 32ra36. [Google Scholar] [CrossRef]
- Zaher, S.S.; Germain, C.; Fu, H.; Larkin, D.F.; George, A.J. 3-hydroxykynurenine suppresses CD4+ T-cell proliferation, induces T-regulatory-cell development, and prolongs corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2640–2648. [Google Scholar] [CrossRef]
- Asghar, K.; Brain, J.; Palmer, J.M.; Douglass, S.; Naemi, F.M.A.; O’Boyle, G.; Kirby, J.; Ali, S. Potential role of indoleamine 2,3-dioxygenase in primary biliary cirrhosis. Oncol. Lett. 2017, 14, 5497–5504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, R.; Gao, N.; Chang, Q.; Meng, X.; Wang, W. The role of IDO, IL-10, and TGF-β in the HCV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J. Med. Virol. 2019, 91, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Moreau, R.; Fenaille, F.; Amorós, A.; Junot, C.; Gronbaek, H.; Coenraad, M.J.; Pruvost, A.; Ghettas, A.; Chu-Van, E.; et al. Orchestration of Tryptophan-Kynurenine pathway, acute decompensation, and Acute-on-Chronic liver failure in cirrhosis. Hepatology 2019, 69, 1686–1701. [Google Scholar] [CrossRef]
- Yoshio, S.; Sugiyama, M.; Shoji, H.; Mano, Y.; Mita, E.; Okamoto, T.; Matsuura, Y.; Okuno, A.; Takikawa, O.; Mizokami, M.; et al. Indoleamine-2,3-dioxygenase as an effector and an indicator of protective immune responses in patients with acute hepatitis B. Hepatology 2015, 63, 83–94. [Google Scholar] [CrossRef]
- Ishio, T.; Goto, S.; Tahara, K.; Tone, S.; Kawano, K.; Kitano, S. Immunoactivative role of indoleamine 2, 3-dioxygenase in human hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2004, 19, 319–326. [Google Scholar] [CrossRef]
- Pan, K.; Wang, H.; Chen, M.; Zhang, H.; Weng, D.; Zhou, J.; Huang, W.; Li, J.; Song, H.; Xia, J. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2008, 134, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, X.; Lyu, N.; Xie, Q.; Deng, H.; Mu, L.; Pan, T.; Huang, X.; Wang, X.; Shi, Y.; et al. Mechanism and prognostic value of indoleamine 2,3-dioxygenase 1 expressed in hepatocellular carcinoma. Cancer Sci. 2018, 109, 3726–3736. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Yu, S.J.; Heinrich, B.; Ma, C.; Fu, Q.; Sandhu, M.; Agdashian, D.; Zhang, Q.; Korangy, F.; Greten, T.F. Indoleamine 2,3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol. Immunother. 2018, 67, 1305–1315. [Google Scholar] [CrossRef]
- Hoffmann, D.; Dvorakova, T.; Stroobant, V.; Bouzin, C.; Daumerie, A.; Solvay, M.; Klaessens, S.; Letellier, M.-C.; Renauld, J.C.; Van Baren, N.; et al. Tryptophan 2,3-Dioxygenase Expression Identified in Human Hepatocellular Carcinoma Cells and in Intratumoral Pericytes of Most Cancers. Cancer Immunol. Res. 2019, 8, 19–31. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Wu, J.; Song, F.; Qin, Z.; Hou, L.; Xiao, C.; Weng, J.; Qin, X.; Xu, J. TDO Promotes Hepatocellular Carcinoma Progression. OncoTargets Ther. 2020, 13, 5845–5855. [Google Scholar] [CrossRef]
- Bekki, S.; Hashimoto, S.; Yamasaki, K.; Komori, A.; Abiru, S.; Nagaoka, S.; Saeki, A.; Suehiro, T.; Kugiyama, Y.; Beppu, A.; et al. Serum kynurenine levels are a novel biomarker to predict the prognosis of patients with hepatocellular carcinoma. PLoS ONE 2020, 15, e0241002. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; You, H.; Tao, X.; Wang, C.; Jin, G.; Wang, N.; Ruan, H.; Gu, D.; Huo, X.; et al. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration and invasion of human hepatocellular carcinoma. Sci. Rep. 2015, 5, srep10466. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Nucci, N.; Menicali, E.; Morelli, S.; Bini, V.; Colella, R.; Mandarano, M.; Sidoni, A.; Puxeddu, E. The Aryl Hydrocarbon Receptor Is Expressed in Thyroid Carcinoma and Appears to Mediate Epithelial-Mesenchymal-Transition. Cancers 2020, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Guo, L.; Li, Z. Molecular mechanisms of 3,3′4,4′,5-pentachlorobiphenyl-induced epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Toxicol. Appl. Pharmacol. 2017, 322, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.; Chevallier, A.; Teixeira-Clerc, F.; Ambolet-Camoit, A.; Bui, L.-C.; Bats, A.-S.; Fournet, J.-C.; Fernandez-Salguero, P.M.; Aggerbeck, M.; Lotersztajn, S.; et al. Aryl Hydrocarbon Receptor–Dependent Induction of Liver Fibrosis by Dioxin. Toxicol. Sci. 2013, 137, 114–124. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Zhang, F.; Han, L.; Bao, G.; He, X.; Xu, Z. AhR expression is increased in hepatocellular carcinoma. J. Mol. Histol. 2013, 44, 455–461. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Wang, L.-T.; Chai, C.-Y.; Wu, C.-C.; Hsi, E.; Chiou, S.-S.; Wang, S.-N. Aryl hydrocarbon receptor promotes hepatocellular carcinoma tumorigenesis by targeting intestine-specific homeobox expression. Mol. Carcinog. 2017, 56, 2167–2177. [Google Scholar] [CrossRef]
- Giorgini, F.; Möller, T.; Kwan, W.; Zwilling, D.; Wacker, J.L.; Hong, S.; Tsai, L.-C.L.; Cheah, C.S.; Schwarcz, R.; Guidetti, P.; et al. Histone Deacetylase Inhibition Modulates Kynurenine Pathway Activation in Yeast, Microglia, and Mice Expressing a Mutant Huntingtin Fragment. J. Biol. Chem. 2008, 283, 7390–7400. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Kerr, S.; Smythe, G.A.; Smith, D.G.; Kapoor, V.; Armati, P.J.; Croitoru, J.; Brew, B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001, 78, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Heyes, M.P.; Saito, K.; Markey, S.P. Human macrophages convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1992, 283, 633–635. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, T.-T.; Lee, C.-H.; Wang, W.-L.; Lee, H.-C.; Tseng, L.-M. Kynurenine-3-monooxygenase (KMO) protein promotes triple negative breast cancer progression. Ann. Oncol. 2017, 28, v3. [Google Scholar] [CrossRef]
- Lai, M.-H.; Liao, C.-H.; Tsai, N.-M.; Chang, K.-F.; Liu, C.-C.; Chiu, Y.-H.; Huang, K.-C.; Lin, C.-S. Surface Expression of Kynurenine 3-Monooxygenase Promotes Proliferation and Metastasis in Triple-Negative Breast Cancers. Cancer Control 2021, 28. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, T.-T.; Chen, J.-L.; Chu, P.-Y.; Lee, C.-H.; Lee, H.-C.; Lee, Y.-H.; Chang, Y.-Y.; Yang, S.-H.; Jiang, J.-K.; et al. Significance of Kynurenine 3-Monooxygenase Expression in Colorectal Cancer. Front. Oncol. 2021, 11, 620361. [Google Scholar] [CrossRef]
- Liu, X.; Shin, N.; Koblish, H.K.; Yang, G.; Wang, Q.; Wang, K.; Leffet, L.; Hansbury, M.J.; Thomas, B.; Rupar, M.; et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010, 115, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Study to Explore the Safety, Tolerability and Efficacy of MK-3475 in Combination with INCB024360 in Participants with Selected Cancers. ClinicalTrials.gov U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT02178722?term=Epacadostat&cond=Hepatocarcinoma&draw=2&rank=1 (accessed on 30 August 2021).
- BMS-986205 and Nivolumab as First or Second Line Therapy in Treating Patients with Liver Cancer. ClinicalTrials.gov U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/results?recrs=&cond=Hepatocarcinoma&term=BMS-986205+&cntry=&state=&city=&dist= (accessed on 30 August 2021).
- Hamid, O.; Bauer, T.M.; Spira, A.I.; Smith, D.C.; Olszanski, A.J.; Tarhini, A.A.; Lara, P.; Gajewski, T.; Wasser, J.S.; Patel, S.P.; et al. Safety of epacadostat 100 mg bid plus pembrolizumab 200 mg Q3W in advanced solid tumors: Phase 2 data from ECHO-202/KEYNOTE-037. J. Clin. Oncol. 2017, 35, 3012. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients with Advanced Solid Tumors: Phase I Results from a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) [Ref] | Sample Type | Sample Size | Enzyme/Metabolite Studied | Technique Used | Finding |
|---|---|---|---|---|---|
| Ishio et al. (2004) [122] | Cell lines HCC tumour specimens | 4 HCC cell lines 21 HCC | IDO1 | RT-PCR IHC | IDO1 expression may play a role in antitumour immune response. |
| Pan et al. (2008) [123] | Cell lines Tumour and distant normal liver tissue | 6 HCC and human normal hepatocytes 138 HCC | IDO1 | RT-PCR IHC | HCC cancer cells and surrounding noncancerous tissue express high IDO1 expression. High IDO1 expression is confined to the tumour creating an immune suppressive microenvironment. |
| Li et al. (2018) [124] | Cell lines Tumour tissue | 2 HCC cell lines 112 HCC (HBV) * | IDO1 | RT-PCR WB IHC and IF | IDO1 is expressed in HCC cells on stimulation by IFN-γ via the JAK2-STAT1 signalling pathway. High IDO1 expression indicates antitumour immune response. IDO1 is a favourable prognostic indicator. |
| Brown et al. (2018) [125] | Cell lines | 2 HCC cell lines | IDO1 | RT-PCR | IDO1 inhibitors in combination with immune checkpoint inhibitors might be an effective treatment option for HCC patients. |
| Hoffman et al. (2020) [126] | Cell lines Tumour and normal tissue | 1 HCC cell line 171 tissue specimens | TDO2 | RT-PCR WB, HPLC IHC and IF | High TDO2 expression observed in HCC tumour cells. TDO2 may be a novel immunotherapeutic target for HCC. |
| Li et al. (2020) [127] | Cell lines Paired tumour and adjacent normal tissues | 5 HCC cell lines and 1 normal liver cell line 93 HCC | TDO2 | RT-PCR WB RT-PCR WB, IHC | TDO2 is overexpressed in HCC and may be facilitating HCC progression and invasion. TDO2 enzyme can be a novel prognostic biomarker for HCC patients. |
| Lei et al. (2021) [87] | Cell lines Paired tumour and adjacent normal tissue | 6 HCC cell lines and 1 normal liver cell 23 HCC | TDO2 | RT-PCR WB Knockdown using shRNAs HPLC IHC and IF | TDO2 supports EMT of HCC cells via the KYN-AhR pathway, facilitating HCC metastasis and invasion. |
| Bekki et al. (2020) [128] | Serum | 604 HCC * (HCV) 288 Control ** | KYN | ELISA | A high level of serum KYN correlated with poor prognosis of HCC. |
| Jin et al. (2015) [129] | Tumour and adjacent noncancerous liver tissue Cell lines | 120 matched HCC and adjacent tissue 205 HCC 5 HCC and 2 human normal liver cells | KMO | IHC RT-PCR WB Knockdown using siRNAs | High KMO expression correlated with HCC tumour aggression, recurrence, and shorter survival rate. KMO knockdown suppressed HCC progression in vitro. KMO overexpression enhanced HCC cell proliferation, migration, and invasion. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnamurthy, S.; Gilot, D.; Ahn, S.B.; Lam, V.; Shin, J.-S.; Guillemin, G.J.; Heng, B. Involvement of Kynurenine Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 5180. https://doi.org/10.3390/cancers13205180
Krishnamurthy S, Gilot D, Ahn SB, Lam V, Shin J-S, Guillemin GJ, Heng B. Involvement of Kynurenine Pathway in Hepatocellular Carcinoma. Cancers. 2021; 13(20):5180. https://doi.org/10.3390/cancers13205180
Chicago/Turabian StyleKrishnamurthy, Shivani, David Gilot, Seong Beom Ahn, Vincent Lam, Joo-Shik Shin, Gilles Jackie Guillemin, and Benjamin Heng. 2021. "Involvement of Kynurenine Pathway in Hepatocellular Carcinoma" Cancers 13, no. 20: 5180. https://doi.org/10.3390/cancers13205180
APA StyleKrishnamurthy, S., Gilot, D., Ahn, S. B., Lam, V., Shin, J.-S., Guillemin, G. J., & Heng, B. (2021). Involvement of Kynurenine Pathway in Hepatocellular Carcinoma. Cancers, 13(20), 5180. https://doi.org/10.3390/cancers13205180









