Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Activation of Ras and Raf Proteins
2.1. Ras Activation by the Tyrosine Kinase, Interleukin (IL) Receptors, and G-Protein Coupled Receptors
2.2. Activation and Regulation of Raf Protein
2.3. Ras-Raf-MEK-ERK Pathway Interaction with p53
3. Ras-Raf Pathway Mutations
3.1. Ras Mutations
3.2. Raf Mutations
3.3. MEK and ERK Mutations
4. Ras-Raf Signaling in Various Types of Cancers
4.1. Leukemia/Lymphoma
4.2. Solid Tumors
5. Targeting of Ras-Raf Signaling in Cancers
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPK | Mitogen-activated protein kinase |
| ATLL | Adult T-cell leukemia/lymphoma |
| HTLV-1 | Human T-cell lymphotropic virus type 1 |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| EBV | Epstein–Barr virus |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HPV | Human papillomaviruses |
| GDP | Guanosine diphosphate |
| GTP | Guanosine triphosphate |
| Ras-GRF | Ras protein-specific guanine nucleotide releasing factor 1 |
| FTase | Farnesyl transferase |
| GGTase1 | Geranylgeranyl transferase 1 |
| Rce1 | Ras converting enzyme |
| Icmt | Isoprenylcysteine carboxyl methyltransferase |
| PDEδ | Phosphodiesterase delta |
| GPCR | G-protein-coupled receptors |
| IL | Interleukin |
| JAK2 | Janus kinase 2 |
| SH2 | Src homology 2 domain |
| Grb2 | Growth factor receptor bound protein 2 |
| SOS | Son of sevenless |
| GEF | Guanine nucleotide exchange factor |
| Gal-1 | Galectin-1 |
| EGFR | Epidermal growth factor receptor |
| PKA | Protein kinase A |
| PLCβ | Phospholipase C-β |
| RBD | Ras-binding domain |
| PAK | p21-activated protein kinase |
| PKC | Protein kinase C |
| BL | Burkitt’s lymphoma |
| NHL | non-Hodgkin’s lymphoma |
| HCC | Hepatocellular carcinoma |
| NSCLC | Non-small cell lung cancer |
| PAC | Pancreatic adenocarcinoma |
| CRC | Colorectal cancer |
| Shrp2 | Src homology region 2 domain-containing phosphatase 2 |
| Csk | C-terminal Src kinase |
| SFKs | Src family kinases (SFKs) |
| Epac-1 | Exchange protein directly activated by cAMP |
| PLCγ | Phospholipase C-γ |
| PAG | Phosphoprotein associated with glycosphingolipid-enriched microdomains |
| PP1 | Protein phosphatase 1 |
References
- Marshall, C.J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 1994, 4, 82–89. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Mor, A.; Philips, M.R. Compartmentalized ras/mapk signaling. Annu. Rev. Immunol. 2006, 24, 771–800. [Google Scholar] [CrossRef]
- Weiss, R.A. A perspective on the early days of RAS research. Cancer Metastasis Rev. 2020, 39, 1023–1028. [Google Scholar] [CrossRef]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Noh, S.J.; Zhou, G.; Dixon, J.E.; Guan, K.-L. Selective Activation of MEK1 but Not MEK2 by A-Raf from Epidermal Growth Factor-stimulated Hela Cells. J. Biol. Chem. 1996, 271, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Marais, R.; Light, Y.; Paterson, H.F.; Mason, C.S.; Marshall, C.J. Differential Regulation of Raf-1, A-Raf, and B-Raf by Oncogenic Ras and Tyrosine Kinases. J. Biol. Chem. 1997, 272, 4378–4383. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Messina, J.L.; Fedorenko, I.V.; Sondak, V.K.; Smalley, K.S.M. Paradoxical oncogenesis—The long-term effects of BRAF inhibition in melanoma. Nat. Rev. Clin. Oncol. 2013, 10, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.C.; Shaw, P.E. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene 2002, 21, 2236–2244. [Google Scholar] [CrossRef]
- Payne, D.M.; Rossomando, A.J.; Martino, P.; Erickson, A.K.; Her, J.H.; Shabanowitz, J.; Hunt, D.F.; Weber, M.J.; Sturgill, T.W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991, 10, 885–892. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ebisuya, M.; Ashida, F.; Okamoto, K.; Yonehara, S.; Nishida, E. Continuous ERK Activation Downregulates Antiproliferative Genes throughout G1 Phase to Allow Cell-Cycle Progression. Curr. Biol. 2006, 16, 1171–1182. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sewing, A.; Wiseman, B.; Lloyd, A.C.; Land, H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip. Mol. Cell. Biol. 1997, 17, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.; Parry, D.; Cherwinski, H.; Bosch, E.; Lees, E.; McMahon, M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip. Mol. Cell. Biol. 1997, 17, 5598–5611. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.M.; Gysin, S.; Malek, N.; Nakayama, K.-I.; Roberts, J.M.; McMahon, M. Cooperative Regulation of the Cell Division Cycle by the Protein Kinases RAF and AKT. Mol. Cell. Biol. 2004, 24, 10868–10881. [Google Scholar] [CrossRef][Green Version]
- Bertics, P.J.; Gill, G.N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J. Biol. Chem. 1985, 260, 14642–14647. [Google Scholar] [CrossRef]
- Batzer, A.G.; Rotin, D.; Ureña, J.M.; Skolnik, E.Y.; Schlessinger, J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 1994, 14, 5192–5201. [Google Scholar] [CrossRef]
- Margolis, B.; Skolnik, E.Y. Activation of Ras by receptor tyrosine kinases. J. Am. Soc. Nephrol. 1994, 5, 1288–1299. [Google Scholar] [CrossRef]
- Blaževitš, O.; Mideksa, Y.G.; Šolman, M.; Ligabue, A.; Ariotti, N.; Nakhaeizadeh, H.; Fansa, E.K.; Papageorgiou, A.; Wittinghofer, A.; Ahmadian, M.R.; et al. Galectin-1 dimers can scaffold Raf-effectors to increase H-ras nanoclustering. Sci. Rep. 2016, 6, 24165. [Google Scholar] [CrossRef] [PubMed]
- Vigil, D.; Cherfils, J.; Rossman, K.L.; Der, C.J. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat. Rev. Cancer 2010, 10, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Therrien, M. Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 2015, 16, 281–298. [Google Scholar] [CrossRef]
- James, B.P.; Bunch, T.A.; Krishnamoorthy, S.; Perkins, L.A.; Brower, D.L. Nuclear localization of the ERK MAP kinase mediated by Drosophila αPS2βPS integrin and importin-7. Mol. Biol. Cell 2007, 18, 4190–4199. [Google Scholar] [CrossRef]
- Lorenzen, J.; Baker, S.; Denhez, F.; Melnick, M.; Brower, D.; Perkins, L. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development 2001, 128, 1403–1414. [Google Scholar] [CrossRef]
- Chang, F.; Steelman, L.S.; Lee, J.T.; Shelton, J.G.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.A.; McCubrey, J. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia 2003, 17, 1263–1293. [Google Scholar] [CrossRef]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human Viral Oncogenesis: A Cancer Hallmarks Analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef]
- Olagnier, D.; Sze, A.; Hadj, S.B.; Chiang, C.; Steel, C.; Han, X.; Routy, J.-P.; Lin, R.; Hiscott, J.; Van Grevenynghe, J. HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4+ T Cells. PLOS Pathog. 2014, 10, e1004575. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Longnecker, R. Latent membrane protein 2A inhibits transforming growth factor-beta 1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 2004, 78, 1697–1705. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.-N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer 2015, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Jain, P.; Tsukasaki, K.; Bangham, C. HTLV-1 Infection and Its Associated Diseases. Leuk. Res. Treat. 2012, 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Khan, Z.K.; Wigdahl, B.; Jennings, S.R.; Tangy, F.; Jain, P. Murine FLT3 Ligand-Derived Dendritic Cell-Mediated Early Immune Responses Are Critical to Controlling Cell-Free Human T Cell Leukemia Virus Type 1 Infection. J. Immunol. 2010, 186, 390–402. [Google Scholar] [CrossRef]
- Mostoller, K.; Norbury, C.C.; Jain, P.; Wigdahl, B. Human T-cell leukemia virus type I Tax induces the expression of dendritic cell markers associated with maturation and activation. J. Neurovirol. 2004, 10, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Lavorgna, A.; Sehgal, M.; Gao, L.; Ginwala, R.; Sagar, D.; Harhaj, E.W.; Khan, Z.K. Myocyte enhancer factor (MEF)-2 plays essential roles in T-cell transformation associated with HTLV-1 infection by stabilizing complex between Tax and CREB. Retrovirology 2015, 12, 1–24. [Google Scholar] [CrossRef][Green Version]
- Jain, P.; Manuel, S.L.; Khan, Z.K.; Ahuja, J.; Quann, K.; Wigdahl, B. DC-SIGN Mediates Cell-Free Infection and Transmission of Human T-Cell Lymphotropic Virus Type 1 by Dendritic Cells. J. Virol. 2009, 83, 10908–10921. [Google Scholar] [CrossRef]
- Jain, P.; Ahuja, J.; Khan, Z.K.; Shimizu, S.; Meucci, O.; Jennings, S.R.; Wigdahl, B. Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type. J. Leukoc. Biol. 2007, 82, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Caruso, B.; Khan, Z.K.; Pattekar, A.; Chew, G.M.; Corley, M.J.; Loonawat, R.; Jacobson, S.; Sreedhar, S.; Ndhlovu, L.C.; et al. HTLV-1 Infection and Neuropathogenesis in the Context of Rag1(-/-)gammac(-/-) (RAG1-Hu) and BLT Mice. J. Neuroimmune Pharmacol. 2017, 12, 504–520. [Google Scholar] [CrossRef]
- Janes, M.R.; Zhang, J.; Li, L.S.; Hansen, R.; Peters, U.; Guo, X.; Chen, Y.; Babbar, A.; Firdaus, S.J.; Darjania, L.; et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell 2018, 172, 578–589. [Google Scholar] [CrossRef]
- Hallin, J.; Engstrom, L.D.; Hargis, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRAS(G12C) Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Adachi, Y.; Ito, K.; Hayashi, Y.; Kimura, R.; Tan, T.Z.; Yamaguchi, R.; Ebi, H. Epithelial-to-Mesenchymal Transition is a Cause of Both Intrinsic and Acquired Resistance to KRAS G12C Inhibitor in KRAS G12C–Mutant Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 5962–5973. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1268. [Google Scholar] [CrossRef]
- Brock, E.J.; Ji, K.; Reiners, J.J.; Mattingly, R.R. How to Target Activated Ras Proteins: Direct Inhibition vs. Induced Mislocalization. Mini-Rev. Med. Chem. 2016, 16, 358–369. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Huang, J. New tricks for human farnesyltransferase inhibitor: Cancer and beyond. MedChemComm 2017, 8, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission Possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef] [PubMed]
- Porru, M.; Pompili, L.; Caruso, C.; Biroccio, A.; Leonetti, C. Targeting KRAS in metastatic colorectal cancer: Current strategies and emerging opportunities. J. Exp. Clin. Cancer Res. 2018, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dharmaiah, S.; Bindu, L.; Tran, T.H.; Gillette, W.K.; Frank, P.H.; Ghirlando, R.; Nissley, D.V.; Esposito, D.; McCormick, F.; Stephen, A.G.; et al. Structural basis of recognition of farnesylated and methylated KRAS4b by PDEdelta. Proc. Natl. Acad. Sci. USA 2016, 113, E6766–E6775. [Google Scholar] [CrossRef]
- Choy, E.; Chiu, V.K.; Silletti, J.; Feoktistov, M.; Morimoto, T.; Michaelson, D.; Ivanov, I.E.; Philips, M.R. Endomembrane Trafficking of Ras: The CAAX Motif Targets Proteins to the ER and Golgi. Cell 1999, 98, 69–80. [Google Scholar] [CrossRef]
- Wang, G.; Deschenes, R.J. Plasma Membrane Localization of Ras Requires Class C Vps Proteins and Functional Mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 2006, 26, 3243–3255. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.J.; Philips, M.R. Targeting RAS Membrane Association: Back to the Future for Anti-RAS Drug Discovery? Clin. Cancer Res. 2015, 21, 1819–1827. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef]
- Broughton, S.; Dhagat, U.; Hercus, T.; Nero, T.; Grimbaldeston, M.A.; Bonder, C.S.; Lopez, A.F.; Parker, M.W. The GM-CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition to initiation of signaling. Immunol. Rev. 2012, 250, 277–302. [Google Scholar] [CrossRef]
- Reddy, E.P.; Korapati, A.; Chaturvedi, P.; Rane, S. IL-3 signaling and the role of Src kinases, JAKs and STATs: A covert liaison unveiled. Oncogene 2000, 19, 2532–2547. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Prignent, S. Insights into the Shc Family of Adaptor Proteins. J. Mol. Signal. 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.C.; Monkhouse, J.L.; Hamilton, J.A.; Csar, X.F. Direct binding of Shc, Grb2, SHP-2 and p40 to the murine granulocyte colony-stimulating factor receptor. Biochim. Biophys. Acta Bioenerg. 1998, 1448, 70–76. [Google Scholar] [CrossRef][Green Version]
- Kerr, D.L.; Haderk, F.; Bivona, T.G. Allosteric SHP2 inhibitors in cancer: Targeting the intersection of RAS, resistance, and the immune microenvironment. Curr. Opin. Chem. Biol. 2021, 62, 1–12. [Google Scholar] [CrossRef]
- Hanafusa, H.; Torii, S.; Yasunaga, T.; Nishida, E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 2002, 4, 850–858. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Toering, S.J.; Simon, M.A.; Krasnow, M.A.; Smith-Bolton, R. Sprouty proteins are in vivo targets of Corkscrew/SHP-2 tyrosine phosphatases. Development 2006, 133, 1133–1142. [Google Scholar] [CrossRef]
- Montagner, A.; Yart, A.; Dance, M.; Perret, B.; Salles, J.-P.; Raynal, P. A Novel Role for Gab1 and SHP2 in Epidermal Growth Factor-induced Ras Activation. J. Biol. Chem. 2005, 280, 5350–5360. [Google Scholar] [CrossRef]
- Agazie, Y.M.; Hayman, M.J. Molecular Mechanism for a Role of SHP2 in Epidermal Growth Factor Receptor Signaling. Mol. Cell. Biol. 2003, 23, 7875–7886. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein–tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 2004, 324, 1155–1164. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, M.; Zhang, H.; Yu, B. Double-edged roles of protein tyrosine phosphatase SHP2 in cancer and its inhibitors in clinical trials. Pharmacol. Ther. 2021, 107966. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Feng, X.; Zhou, W.; Knyazev, P.G.; Ullrich, A.; Chen, Z. Csk-binding protein (Cbp) negatively regulates epidermal growth factor-induced cell transformation by controlling Src activation. Oncogene 2006, 25, 5495–5506. [Google Scholar] [CrossRef] [PubMed]
- Hrdinka, M.; Horejsi, V. PAG—A multipurpose transmembrane adaptor protein. Oncogene 2013, 33, 4881–4892. [Google Scholar] [CrossRef] [PubMed]
- Bivona, T.G.; De Castro, I.P.; Ahearn, I.M.; Grana, T.M.; Chiu, V.K.; Lockyer, P.J.; Cullen, P.J.; Pellicer, A.; Cox, A.D.; Philips, M.R. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature 2003, 424, 694–698. [Google Scholar] [CrossRef]
- McKay, M.M.; Morrison, D.K. Integrating signals from RTKs to ERK/MAPK. Oncogene 2007, 26, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Fernández-Medarde, A.; Santos, E. The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim. Biophys. Acta Bioenerg. 2011, 1815, 170–188. [Google Scholar] [CrossRef]
- Le, D.T.; Shannon, K.M. Ras processing as a therapeutic target in hematologic malignancies. Curr. Opin. Hematol. 2002, 9, 308–315. [Google Scholar] [CrossRef] [PubMed]
- De Mora, J.F.; Esteban, L.M.; Burks, D.J.; Núñez, A.; Garcés, C.; García-Barrado, M.J.; Iglesias-Osma, M.C.; Moratinos, J.; Ward, J.M.; Santos, E. Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J. 2003, 22, 3039–3049. [Google Scholar] [CrossRef]
- Ruiz, S.; Santos, E.; Bustelo, X.R. RasGRF2, a Guanosine Nucleotide Exchange Factor for Ras GTPases, Participates in T-Cell Signaling Responses. Mol. Cell. Biol. 2007, 27, 8127–8142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stone, J.C. Regulation and Function of the RasGRP Family of Ras Activators in Blood Cells. Genes Cancer 2011, 2, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Pan, C.; He, S.-M.; Lang, B.; Gao, G.-D.; Wang, X.-L.; Wang, Y. The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases. Front. Mol. Neurosci. 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Gideon, P.; John, J.; Frech, M.; Lautwein, A.; Clark, R.; Scheffler, J.E.; Wittinghofer, A. Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: The C-terminal domain of GAP is not sufficient for full activity. Mol. Cell. Biol. 1992, 12, 2050–2056. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rudack, T.; Xia, F.; Schlitter, J.; Kötting, C.; Gerwert, K. Ras and GTPase-activating protein (GAP) drive GTP into a precatalytic state as revealed by combining FTIR and biomolecular simulations. Proc. Natl. Acad. Sci. USA 2012, 109, 15295–15300. [Google Scholar] [CrossRef]
- Maertens, O.; Cichowski, K. An expanding role for RAS GTPase activating proteins (RAS GAPs) in cancer. Adv. Biol. Regul. 2014, 55, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Trahey, M.; McCormick, F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science 1987, 238, 542–545. [Google Scholar] [CrossRef]
- Patil, S.; Chamberlain, R.S. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist 2012, 17, 101–116. [Google Scholar] [CrossRef]
- McGillicuddy, L.T.; Fromm, J.A.; Hollstein, P.E.; Kubek, S.; Beroukhim, R.; De Raedt, T.; Johnson, B.W.; Williams, S.M.; Nghiemphu, P.; Liau, L.M.; et al. Proteasomal and Genetic Inactivation of the NF1 Tumor Suppressor in Gliomagenesis. Cancer Cell 2009, 16, 44–54. [Google Scholar] [CrossRef]
- Dote, H.; Toyooka, S.; Tsukuda, K.; Yano, M.; Ota, T.; Murakami, M.; Naito, M.; Toyota, M.; Gazdar, A.F.; Shimizu, N. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in gastrointestinal tumour. Br. J. Cancer 2005, 92, 1117–1125. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Tu, S.-W.; Hsieh, J.-T. Down-regulation of Human DAB2IP Gene Expression Mediated by Polycomb Ezh2 Complex and Histone Deacetylase in Prostate Cancer. J. Biol. Chem. 2005, 280, 22437–22444. [Google Scholar] [CrossRef]
- Yano, M.; Toyooka, S.; Tsukuda, K.; Dote, H.; Ouchida, M.; Hanabata, T.; Aoe, M.; Date, H.; Gazdar, A.F.; Shimizu, N. Aberrant promoter methylation of human DAB2 interactive protein (hDAB2IP) gene in lung cancers. Int. J. Cancer 2005, 113, 59–66. [Google Scholar] [CrossRef]
- Xie, D.; Gore, C.; Liu, J.; Pong, R.-C.; Mason, R.; Hao, G.; Long, M.; Kabbani, W.; Yu, L.; Zhang, H.; et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc. Natl. Acad. Sci. USA 2010, 107, 2485–2490. [Google Scholar] [CrossRef]
- Min, J.; Zaslavsky, A.; Fedele, G.; McLaughlin, S.K.; Reczek, E.E.; De Raedt, T.; Guney, I.; Strochlic, D.E.; MacConaill, L.E.; Beroukhim, R.; et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat. Med. 2010, 16, 286–294. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Li, X.; Zhao, W.; Qiao, Y.; Liang, L.; Ding, Y. Low expression of DAB2IP contributes to malignant development and poor prognosis in hepatocellular car-cinoma. J. Gastroenterol. Hepatol. 2012, 27, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.; Yao, B.; Liu, Q.; Guo, C. miR-1307-3p promotes tumor growth and metastasis of hepatocellular carcinoma by repressing DAB2 inter-acting protein. Biomed. Pharmacother. 2019, 117, 109055. [Google Scholar] [CrossRef]
- Prior, I.; Muncke, C.; Parton, R.G.; Hancock, J.F. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 2003, 160, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Henis, Y.; Hancock, J.; Prior, I.A. Ras acylation, compartmentalization and signaling nanoclusters (Review). Mol. Membr. Biol. 2009, 26, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Rotblat, B.; Prior, I.A.; Muncke, C.; Parton, R.G.; Kloog, Y.; Henis, Y.; Hancock, J.F. Three Separable Domains Regulate GTP-Dependent Association of H-ras with the Plasma Membrane. Mol. Cell. Biol. 2004, 24, 6799–6810. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Harding, A.; Yan, J.; Sluimer, J.; Parton, R.G.; Hancock, J.F. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 2001, 3, 368–375. [Google Scholar] [CrossRef]
- Weise, K.; Triola, G.; Brunsveld, L.; Waldmann, H.; Winter, R. Influence of the Lipidation Motif on the Partitioning and Association of N-Ras in Model Membrane Subdomains. J. Am. Chem. Soc. 2009, 131, 1557–1564. [Google Scholar] [CrossRef]
- Tebar, F.; Enrich, C.; Rentero, C.; Grewal, T. GTPases Rac1 and Ras Signaling from Endosomes. Mol. Evol. Evid. Monophyly Metazoa 2018, 57, 65–105. [Google Scholar] [CrossRef]
- Cristea, S.; Sage, J. Is the Canonical RAF/MEK/ERK Signaling Pathway a Therapeutic Target in SCLC? J. Thorac. Oncol. 2016, 11, 1233–1241. [Google Scholar] [CrossRef]
- Goldsmith, Z.G.; Dhanasekaran, D.N. G Protein regulation of MAPK networks. Oncogene 2007, 26, 3122–3142. [Google Scholar] [CrossRef]
- Dubrovska, A.; Cojoc, M.; Peitzsch, C.; Trautmann, F.; Polishchuk, L.; Telegeev, G.D. Emerging targets in cancer management: Role of the CXCL12/CXCR4 axis. OncoTargets Ther. 2013, 6, 1347–1361. [Google Scholar] [CrossRef]
- Gesty-Palmer, D.; Chen, M.; Reiter, E.; Ahn, S.; Nelson, C.D.; Wang, S.; Eckhardt, A.E.; Cowan, C.L.; Spurney, R.F.; Luttrell, L.M.; et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone recep-tor-stimulated ERK1/2 activation. J. Biol. Chem. 2006, 281, 10856–10864. [Google Scholar] [CrossRef] [PubMed]
- Vossler, M.R.; Yao, H.; York, R.D.; Pan, M.-G.; Rim, C.S.; Stork, P.J. cAMP Activates MAP Kinase and Elk-1 through a B-Raf- and Rap1-Dependent Pathway. Cell 1997, 89, 73–82. [Google Scholar] [CrossRef]
- Winitz, S.; Russell, M.; Qian, N.X.; Gardner, A.; Dwyer, L.; Johnson, G.L. Involvement of Ras and Raf in the Gi-coupled acetylcholine muscarinic m2 receptor activation of mito-gen-activated protein (MAP) kinase kinase and MAP kinase. J. Biol. Chem. 1993, 268, 19196–19199. [Google Scholar] [CrossRef]
- Della Rocca, G.J.; van Biesen, T.; Daaka, Y.; Luttrell, D.K.; Luttrell, L.; Lefkowitz, R.J. Ras-dependent Mitogen-activated Protein Kinase Activation by G Protein-coupled Receptors. J. Biol. Chem. 1997, 272, 19125–19132. [Google Scholar] [CrossRef] [PubMed]
- Gutkind, J.S. The Pathways Connecting G Protein-coupled Receptors to the Nucleus through Divergent Mitogen-activated Protein Kinase Cascades. J. Biol. Chem. 1998, 273, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Kwan, D.H.; Yung, L.Y.; Ye, R.D.; Wong, Y.H. Activation of ras-dependent signaling pathways by G 14 -coupled receptors requires the adaptor protein TPR. J. Cell. Biochem. 2012, 113, 3486–3497. [Google Scholar] [CrossRef]
- Nugent, A.; Proia, R.L. The role of G protein-coupled receptors in lymphoid malignancies. Cell. Signal. 2017, 39, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, M.; Kurosawa, A.; Tani, Y.; Tsuji, T.; Takeda, S. GPR31 and GPR151 are activated under acidic conditions. J. Biochem. 2019, 166, 317–322. [Google Scholar] [CrossRef]
- Fehrenbacher, N.; Philips, M.R. Targeting RAS—Will GPR31 deliver us a new path forward? Mol. Cell. Oncol. 2017, 4, e1359228. [Google Scholar] [CrossRef]
- Fehrenbacher, N.; Da Silva, I.T.; Ramirez, C.; Zhou, Y.; Cho, K.-J.; Kuchay, S.; Shi, J.; Thomas, S.; Pagano, M.; Hancock, J.; et al. The G protein–coupled receptor GPR31 promotes membrane association of KRAS. J. Cell Biol. 2017, 216, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eishingdrelo, A.; Kongsamut, S.; Eishingdrelo, H. G-protein-coupled receptors mediate 14-3-3 signal transduction. Signal. Transduct. Target. Ther. 2016, 1, 16018. [Google Scholar] [CrossRef]
- McCudden, C.R.; Hains, M.D.; Kimple, R.J.; Siderovski, D.; Willard, F.S. G-protein signaling: Back to the future. Cell. Mol. Life Sci. 2005, 62, 551–577. [Google Scholar] [CrossRef]
- Ito, A.; Satoh, T.; Kaziro, Y.; Itoh, H. G protein βγ subunit activates Ras, Raf, and MAP kinase in HEK 293 cells. FEBS Lett. 1995, 368, 183–187. [Google Scholar] [CrossRef]
- Harden, T.K. G Protein-dependent Regulation of Phospholipase C by Cell Surface Receptors. Am. Rev. Respir. Dis. 1990, 141, 119–122. [Google Scholar] [CrossRef]
- Bivona, T.G.; Quatela, S.E.; Bodemann, B.O.; Ahearn, I.M.; Soskis, M.J.; Mor, A.; Miura, J.; Wiener, H.H.; Wright, L.; Saba, S.G.; et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mito-chondria and induces apoptosis. Mol. Cell 2006, 21, 481–493. [Google Scholar] [CrossRef]
- Alvarez-Moya, B.; López-Alcalá, C.; Drosten, M.; Bachs, O.; Agell, N. K-Ras4B phosphorylation at Ser181 is inhibited by calmodulin and modulates K-Ras activity and function. Oncogene 2010, 29, 5911–5922. [Google Scholar] [CrossRef]
- Kohno, T.; Matsuda, E.; Sasaki, H.; Sasaki, T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem. J. 2008, 410, 513–523. [Google Scholar] [CrossRef]
- Blaukat, A.; Ivankovic-Dikic, I.; Gronroos, E.; Dolfi, F.; Tokiwa, G.; Vuori, K.; Dikic, I. Adaptor Proteins Grb2 and Crk Couple Pyk2 with Activation of Specific Mitogen-activated Protein Kinase Cascades. J. Biol. Chem. 1999, 274, 14893–14901. [Google Scholar] [CrossRef]
- Guo, F.F.; Kumahara, E.; Saffen, D. A CalDAG-GEFI/Rap1/B-Raf cassette couples M(1) muscarinic acetylcholine receptors to the activation of ERK1/2. J. Biol. Chem. 2001, 276, 25568–25581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wang, R.C.; Cheng, K.; Ring, B.Z.; Su, L. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer. Biol. Med. 2017, 14, 90–99. [Google Scholar]
- Feng, H.; Liu, K.W.; Guo, P.; Zhang, P.; Cheng, T.; McNiven, M.A.; Johnson, G.R.; Hu, B.; Cheng, S.Y. Dynamin 2 mediates PDGFRalpha-SHP-2-promoted glioblastoma growth and invasion. Oncogene 2012, 31, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, L.; Faragó, A.; Buday, L. Characterization of Interactions of Nck with Sos and Dynamin. Cell. Signal. 1999, 11, 25–29. [Google Scholar] [CrossRef]
- Kranenburg, O.; Verlaan, I.; Hordijk, P.L.; Moolenaar, W.H. Gi-mediated activation of the Ras/MAP kinase pathway involves a 100 kDa tyrosine-phosphorylated Grb2 SH3 binding protein, but not Src nor Shc. EMBO J. 1997, 16, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, O.; Verlaan, I.; Moolenaar, W.H. Gi-mediated tyrosine phosphorylation of Grb2 (growth-factor-receptor-bound protein 2)-bound dynamin-II by lysophosphatidic acid. Biochem. J. 1999, 339 Pt 1, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- Ster, J.; De Bock, F.; Guerineau, N.; Janossy, A.; Barrere-Lemaire, S.; Bos, J.L.; Bockaert, J.; Fagni, L. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 2519–2524. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Stork, P.J. PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol. Cell 2002, 9, 85–94. [Google Scholar] [CrossRef]
- Schramm, K.; Niehof, M.; Radziwill, G.; Rommel, C.; Moelling, K. Phosphorylation of c-Raf-1 by Protein Kinase A Interferes with Activation. Biochem. Biophys. Res. Commun. 1994, 201, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Negishi, M.; Katoh, H. RasGRF1 mediates brain-derived neurotrophic factor-induced axonal growth in primary cultured cortical neurons. Biochem. Biophys. Rep. 2018, 17, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Nazmun, N.; Hassan, S.; Liu, X.; Yang, J. BRAF mutation and its inhibitors in sarcoma treatment. Cancer Med. 2020, 9, 4881–4896. [Google Scholar] [CrossRef]
- Park, E.; Rawson, S.; Li, K.; Kim, B.W.; Ficarro, S.B.; Gonzalez-Del Pino, G.; Sharif, H.; Marto, J.A.; Jeon, H.; Eck, M.J. Architecture of autoinhibited and active BRAF-MEK1-14-3-3 complexes. Nature 2019, 575, 545–550. [Google Scholar] [CrossRef]
- Cutler, R.E.; Stephens, R.M.; Saracino, M.R.; Morrison, D.K. Autoregulation of the Raf-1 serine/threonine kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 9214–9219. [Google Scholar] [CrossRef] [PubMed]
- Obsilova, V.; Obsil, T. The 14-3-3 Proteins as Important Allosteric Regulators of Protein Kinases. Int. J. Mol. Sci. 2020, 21, 8824. [Google Scholar] [CrossRef]
- Liau, N.P.D.; Venkatanarayan, A.; Quinn, J.G.; Phung, W.; Malek, S.; Hymowitz, S.G.; Sudhamsu, J. Dimerization Induced by C-Terminal 14–3–3 Binding Is Sufficient for BRAF Kinase Activation. Biochemistry 2020, 59, 3982–3992. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.L.; Chan, T.Y.; Torres, M.; Andersen, J.L. The dynamic and stress-adaptive signaling hub of 14-3-3: Emerging mechanisms of regulation and context-dependent protein–protein interactions. Oncogene 2018, 37, 5587–5604. [Google Scholar] [CrossRef]
- Yaffe, M.B. How do 14-3-3 proteins work?—Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002, 513, 53–57. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Pollock, C.; Steen, H.; Shaw, P.E.; Mischak, H.; Kolch, W. Cyclic AMP-Dependent Kinase Regulates Raf-1 Kinase Mainly by Phosphorylation of Serine. Mol. Cell. Biol. 2002, 22, 3237–3246. [Google Scholar] [CrossRef]
- Rommel, C.; Clarke, B.A.; Zimmermann, S.; Nuñez, L.; Rossman, R.; Reid, K.; Moelling, K.; Yancopoulos, G.D.; Glass, D.J. Differentiation Stage-Specific Inhibition of the Raf-MEK-ERK Pathway by Akt. Science 1999, 286, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Moelling, K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 1999, 286, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ng, W.H.; Yap, J.; Chia, B.; Huang, X.; Wang, M.; Hu, J. The AMPK inhibitor overcomes the paradoxical effect of RAF inhibitors through blocking phospho–Ser-621 in the C terminus of CRAF. J. Biol. Chem. 2018, 293, 14276–14284. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Yuan, P.; Perez-Lorenzo, R.; Zhang, Y.; Lee, S.X.; Ou, Y.; Asara, J.M.; Cantley, L.; Zheng, B. Phosphorylation of BRAF by AMPK Impairs BRAF-KSR1 Association and Cell Proliferation. Mol. Cell 2013, 52, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Nguyen, L.; Matallanas, D.; Halasz, M.; Doherty, C.; Kholodenko, B.; Kolch, W. Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nat. Cell Biol. 2014, 16, 673–684. [Google Scholar] [CrossRef]
- Tran, T.H.; Chan, A.H.; Young, L.C.; Bindu, L.; Neale, C.; Messing, S.; Dharmaiah, S.; Taylor, T.; Denson, J.-P.; Esposito, D.; et al. KRAS interaction with RAF1 RAS-binding domain and cysteine-rich domain provides insights into RAS-mediated RAF activation. Nat. Commun. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Luo, Z.; Diaz, B.; Marshall, M.S.; Avruch, J. An intact Raf zinc finger is required for optimal binding to processed Ras and for ras-dependent Raf activation in situ. Mol. Cell. Biol. 1997, 17, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Podar, K.; Pacher, M.; Kubicek, M.; Welzel, N.; Hemmings, B.A.; Dilworth, S.M.; Mischak, H.; Kolch, W.; Baccarini, M. Raf-1-associated Protein Phosphatase 2A as a Positive Regulator of Kinase Activation. J. Biol. Chem. 2000, 275, 22300–22304. [Google Scholar] [CrossRef]
- Jaumot, M.; Hancock, J.F. Protein phosphatases 1 and 2A promote Raf-1 activation by regulating 14-3-3 interactions. Oncogene 2001, 20, 3949–3958. [Google Scholar] [CrossRef]
- Ory, S.; Zhou, M.; Conrads, T.P.; Veenstra, T.D.; Morrison, D.K. Protein Phosphatase 2A Positively Regulates Ras Signaling by Dephosphorylating KSR1 and Raf-1 on Critical 14-3-3 Binding Sites. Curr. Biol. 2003, 13, 1356–1364. [Google Scholar] [CrossRef]
- Young, L.C.; Hartig, N.; del Río, I.B.; Sari, S.; Ringham-Terry, B.; Wainwright, J.R.; Jones, G.G.; McCormick, F.; Rodriguez-Viciana, P. SHOC2–MRAS–PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E10576–E10585. [Google Scholar] [CrossRef]
- Yin, Q.; Han, T.; Fang, B.; Zhang, G.; Zhang, C.; Roberts, E.R.; Izumi, V.; Zheng, M.; Jiang, S.; Yin, X.; et al. K27-linked ubiquitination of BRAF by ITCH engages cytokine response to maintain MEK-ERK signaling. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Abankwa, D.; Gorfe, A. Mechanisms of Ras Membrane Organization and Signaling: Ras Rocks Again. Biomolecules 2020, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Stites, E.C.; Yu, H.; Germino, E.A.; Meharena, H.S.; Stork, P.J.; Kornev, A.; Taylor, S.S.; Shaw, A.S. Allosteric Activation of Functionally Asymmetric RAF Kinase Dimers. Cell 2013, 154, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Drosten, M.; Sum, E.Y.M.; Lechuga, C.G.; Simón-Carrasco, L.; Jacob, H.K.C.; García-Medina, R.; Huang, S.; Beijersbergen, R.; Bernards, R.; Barbacid, M. Loss of p53 induces cell proliferation via Ras-independent activation of the Raf/Mek/Erk signaling pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 15155–15160. [Google Scholar] [CrossRef] [PubMed]
- Drosten, M.; Barbacid, M. Ras and p53: An unsuspected liaison. Mol. Cell. Oncol. 2015, 3, e996001. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras Provokes Premature Cell Senescence Associated with Accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Z.; Yin, H.; Yang, G. Interaction between p53 and Ras signaling controls cisplatin resistance via HDAC4- and HIF-1alpha-mediated reg-ulation of apoptosis and autophagy. Theranostics 2019, 9, 1096–1114. [Google Scholar] [CrossRef]
- Lu, L.; Zeng, J. Evaluation of K-ras and p53 expression in pancreatic adenocarcinoma using the cancer genome atlas. PLoS ONE 2017, 12, e0181532. [Google Scholar] [CrossRef]
- Ries, S.; Biederer, C.; Woods, D.; Shifman, O.; Shirasawa, S.; Sasazuki, T.; McMahon, M.; Oren, M.; McCormick, F. Opposing Effects of Ras on p53: Transcriptional Activation of mdm2 and Induction of p19ARF. Cell 2000, 103, 321–330. [Google Scholar] [CrossRef]
- Sui, X.; Shin, S.; Zhang, R.; Firozi, P.F.; Yang, L.; Abbruzzese, J.L.; Reddy, S.A.G. Hdm2 is regulated by K-Ras and mediates p53-independent functions in pancreatic cancer cells. Oncogene 2008, 28, 709–720. [Google Scholar] [CrossRef]
- Esteller, M.; Tortola, S.; Toyota, M.; Capella, G.; Peinado, M.A.; Baylin, S.B.; Herman, J.G. Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res. 2000, 60, 129–133. [Google Scholar]
- Li, L.; Zhao, G.-D.; Shi, Z.; Qi, L.-L.; Zhou, L.-Y.; Fu, Z.-X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016, 12, 3045–3050. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A Comprehensive Survey of Ras Mutations in Cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.R.M.; Baig, M.; Mohamoud, H.S.A.; Ul-Haq, Z.; Hoessli, D.C.; Khogeer, G.S.; Al-Sayed, R.R.; Al-Aama, J.Y. BRAF gene: From human cancers to developmental syndromes. Saudi J. Biol. Sci. 2015, 22, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Niihori, T.; Inoue, S.-I.; Matsubara, Y. Recent advances in RASopathies. J. Hum. Genet. 2016, 61, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.; Der, C.J.; Rossman, K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Khrenova, M.; Mironov, V.; Grigorenko, B.; Nemukhin, A.V. Modeling the Role of G12V and G13V Ras Mutations in the Ras-GAP-Catalyzed Hydrolysis Reaction of Guanosine Triphosphate. Biochemistry 2014, 53, 7093–7099. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Stone, R.M.; Al-Kali, A.; Barta, S.K.; Bejar, R.; Bennett, J.M.; Carraway, H.; De Castro, C.M.; Deeg, H.J.; DeZern, A.E.; et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 15, 60–87. [Google Scholar] [CrossRef]
- Steensma, D.P. Myelodysplastic syndromes current treatment algorithm. Blood Cancer J. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Ma, X. Epidemiology of Myelodysplastic Syndromes. Am. J. Med. 2012, 125, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L. ras oncogenes in human cancer: A review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar]
- Kadia, T.M.; Kantarjian, H.; Kornblau, S.; Borthakur, G.; Faderl, S.; Freireich, E.J.; Luthra, R.; Garcia-Manero, G.; Pierce, S.; Cortes, J.; et al. Clinical and proteomic characterization of acute myeloid leukemia with mutated RAS. Cancer 2012, 118, 5550–5559. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Wang, E.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; Coutre, S.E.; De Lima, M.; Fathi, A.T.; Fiorella, M.; et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 721–749. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef]
- Neubauer, A.; Maharry, K.; Mrózek, K.; Thiede, C.; Marcucci, G.; Paschka, P.; Mayer, R.J.; Larson, R.A.; Liu, E.T.; Bloomfield, C.D. Patients with Acute Myeloid Leukemia and RAS Mutations Benefit Most From Postremission High-Dose Cytarabine: A Cancer and Leukemia Group B Study. J. Clin. Oncol. 2008, 26, 4603–4609. [Google Scholar] [CrossRef]
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Pérez, J.C.; Jiménez-Castillo, R.A.; Herrera-Garza, J.L.; Gutiérrez-Aguirre, H.; Marfil-Rivera, L.J.; Gómez-Almaguer, D. Survival Rates of Adults with Acute Lymphoblastic Leukemia in a Low-Income Population: A Decade of Experience at a Single Institution in Mexico. Clin. Lymphoma Myeloma Leuk. 2017, 17, 60–68. [Google Scholar] [CrossRef]
- Knight, T.; Irving, J.A. Ras/Raf/MEK/ERK Pathway Activation in Childhood Acute Lymphoblastic Leukemia and Its Therapeutic Targeting. Front. Oncol. 2014, 4, 160. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.; Angelini, S.; Sander, B.; Christensson, B.; Hemminki, K.; Kumar, R. Mutations in the BRAF and N-ras genes in childhood acute lymphoblastic leukaemia. Leukemia 2004, 19, 310–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pleyer, L.; Germing, U.; Sperr, W.R.; Linkesch, W.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Schreder, M.; Pfeilstocker, M.; Lang, A.H.; et al. Azacitidine in CMML: Matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk. Res. 2014, 38, 475–483. [Google Scholar] [CrossRef]
- Gelsi-Boyer, V.; Trouplin, V.; Adélaïde, J.; Aceto, N.; Remy, V.; Pinson, S.; Houdayer, C.; Arnoulet, C.; Sainty, D.; Bentires-Alj, M.; et al. Genome profiling of chronic myelomonocytic leukemia: Frequent alterations of RAS and RUNX1genes. BMC Cancer 2008, 8, 299. [Google Scholar] [CrossRef]
- Onida, F.; Kantarjian, H.M.; Smith, T.L.; Ball, G.; Keating, M.J.; Estey, E.H.; Glassman, A.B.; Albitar, M.; Kwari, M.I.; Beran, M. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: A retrospective analysis of 213 patients. Blood 2002, 99, 840–849. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. Cytogenetic and molecular abnormalities in chronic myelomonocytic leukemia. Blood Cancer J. 2016, 6, e393. [Google Scholar] [CrossRef]
- Zhang, L.; Singh, R.R.; Patel, K.P.; Stingo, F.; Routbort, M.; You, M.J.; Miranda, R.N.; Garcia-Manero, G.; Kantarjian, H.M.; Medeiros, L.J.; et al. BRAF kinase domain mutations are present in a subset of chronic myelomonocytic leukemia with wild-type RAS. Am. J. Hematol. 2014, 89, 499–504. [Google Scholar] [CrossRef]
- Radich, J.P.; Deininger, M.; Abboud, C.N.; Altman, J.K.; Berman, E.; Bhatia, R.; Bhatnagar, B.; Curtin, P.; DeAngelo, D.J.; Gotlib, J.; et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1108–1135. [Google Scholar] [CrossRef]
- Liu, E.; Hjelle, B.; Bishop, J.M. Transforming genes in chronic myelogenous leukemia. Proc. Natl. Acad. Sci. USA 1988, 85, 1952–1956. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Chiorean, E.G.; Czito, B.; Scaife, C.; Narang, A.K.; Fountzilas, C.; Wolpin, B.M.; Al-Hawary, M.; Asbun, H.; et al. Pancreatic Adenocarcinoma, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 202–210. [Google Scholar] [CrossRef]
- Le, N.; Sund, M.; Vinci, A.; Beyer, G.; Javed, M.A.; Krug, S.; Neessee, A.; Schober, M. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig. Liver Dis. 2016, 48, 223–230. [Google Scholar] [CrossRef]
- Caldas, C.; Kern, S.E. K-ras mutation and pancreatic adenocarcinoma. Int. J. Pancreatol. 1995, 18, 1–6. [Google Scholar] [CrossRef]
- Schultz, N.A.; Roslind, A.; Christensen, I.J.; Horn, T.; Høgdall, E.; Pedersen, L.N.; Kruhøffer, M.; Burcharth, F.; Wøjdemann, M.; Johansen, J.S. Frequencies and Prognostic Role of KRAS and BRAF Mutations in Patients with Localized Pancreatic and Ampullary Adenocarcinomas. Pancreas 2012, 41, 759–766. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 2004, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.J.; Yohn, J.J.; Morelli, J.G.; Norris, D.A.; Golitz, L.E.; Hoeffler, J.P. RAS Mutations in Human Melanoma: A Marker of Malignant Progression. J. Investig. Dermatol. 1994, 102, 285–290. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef]
- Riely, G.J.; Marks, J.; Pao, W. KRAS Mutations in Non-Small Cell Lung Cancer. Proc. Am. Thorac. Soc. 2009, 6, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, J.M.; Viteri, S.; Molina, M.A.; Rosell, R. BRAF mutant non-small cell lung cancer and treatment with BRAF inhibitors. Transl. Lung Cancer Res. 2013, 2, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Viallet, J.; Mulshine, J.; Linnoila, R.I.; Minna, J.D.; Gazdar, A.F. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene 1991, 6, 1353–1362. [Google Scholar] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.-J.; Tveit, K.-M.; Gibson, F. A Review of the Evolution of Systemic Chemotherapy in the Management of Colorectal Cancer. Clin. Color. Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Breivik, J.; Meling, G.I.; Spurkland, A.; Rognum, T.O.; Gaudernack, G. K-ras mutation in colorectal cancer: Relations to patient age, sex and tumour location. Br. J. Cancer 1994, 69, 367–371. [Google Scholar] [CrossRef]
- Oldenburg, J.; Fosså, S.D.; Nuver, J.; Heidenreich, A.; Schmoll, H.-J.; Bokemeyer, C.; Horwich, A.; Beyer, J.; Kataja, V. Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi125–vi132. [Google Scholar] [CrossRef]
- Dong, W.; Gang, W.; Liu, M.; Zhang, H. Analysis of the prognosis of patients with testicular seminoma. Oncol. Lett. 2016, 11, 1361–1366. [Google Scholar] [CrossRef]
- Mulder, M.P.; Keijzer, W.; Verkerk, A.; Boot, A.J.; Prins, M.E.; Splinter, T.A.; Bos, J.L. Activated ras genes in human seminoma: Evidence for tumor heterogeneity. Oncogene 1989, 4, 1345–1351. [Google Scholar]
- DeGeorge, K.C.; Holt, H.R.; Hodges, S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician 2017, 96, 507–514. [Google Scholar]
- Abdollah, F.; Gandaglia, G.; Thuret, R.; Schmitges, J.; Tian, Z.; Jeldres, C.; Passoni, N.M.; Briganti, A.; Shariat, S.F.; Perrotte, P.; et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: A trend analysis. Cancer Epidemiol. 2013, 37, 219–225. [Google Scholar] [CrossRef]
- Oxford, G.; Theodorescu, D. Review Article: The Role of Ras Superfamily Proteins in Bladder Cancer Progression. J. Urol. 2003, 170, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Bladder Cancer: New Insights into Its Molecular Pathology. Cancers 2018, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Turati, F.; La Vecchia, C. Hepatocellular carcinoma epidemiology. Best Pr. Res. Clin. Gastroenterol. 2014, 28, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Taketomi, A.; Shirabe, K.; Muto, J.; Yoshiya, S.; Motomura, T.; Mano, Y.; Ikegami, T.; Yoshizumi, T.; Sugio, K.; Maehara, Y. A rare point mutation in the Ras oncogene in hepatocellular carcinoma. Surg. Today 2013, 43, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Clin. North. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Protani, M.M.; Nagle, C.M.; Webb, P. Obesity and Ovarian Cancer Survival: A Systematic Review and Meta-analysis. Cancer Prev. Res. 2012, 5, 901–910. [Google Scholar] [CrossRef]
- Alsharedi, M.; Katz, H. Check point inhibitors a new era in renal cell carcinoma treatment. Med. Oncol. 2018, 35, 85. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Michaelson, M.D.; Nandagopal, L.; Gore, J.L.; George, S.; Alva, A.; Haas, N.; Harrison, M.R.; Plimack, E.R.; et al. NCCN Guidelines Insights: Kidney Cancer, Version 2.2020. J. Natl. Compr. Cancer Netw. 2019, 17, 1278–1285. [Google Scholar] [CrossRef]
- Bayrak, O.; Sen, H.; Bulut, E.; Cengiz, B.; Karakok, M.; Erturhan, S.; Seckiner, I. Evaluation of EGFR, KRAS and BRAF gene mutations in renal cell carcinoma. J. Kidney Cancer VHL 2014, 1, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wiesweg, M.; Kasper, S.; Worm, K.; Herold, T.; Reis, H.; Sara, L.; Metzenmacher, M.; Abendroth, A.; Darwiche, K.; Aigner, C.; et al. Impact of RAS mutation subtype on clinical outcome—A cross-entity comparison of patients with advanced non-small cell lung cancer and colorectal cancer. Oncogene 2019, 38, 2953–2966. [Google Scholar] [CrossRef] [PubMed]
- Terrell, E.M.; Durrant, D.E.; Ritt, D.A.; Sealover, N.E.; Sheffels, E.; Spencer-Smith, R.; Esposito, D.; Zhou, Y.; Hancock, J.F.; Kortum, R.; et al. Distinct Binding Preferences between Ras and Raf Family Members and the Impact on Oncogenic Ras Signaling. Mol. Cell 2019, 76, 872–884.e5. [Google Scholar] [CrossRef] [PubMed]
- Barbacid, M. ras oncogenes: Their role in neoplasia. Eur. J. Clin. Investig. 2008, 20, 225–235. [Google Scholar] [CrossRef]
- Cook, J.H.; Melloni, G.E.M.; Gulhan, D.C.; Park, P.J.; Haigis, K.M. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Nair, S.G.; Loppnow, G.R. Comparison of K-Ras and N-Ras Mutagenic Hot Spots for UVC Damage. ACS Omega 2019, 4, 3469–3475. [Google Scholar] [CrossRef]
- Holderfield, M.; Deuker, M.M.; McCormick, F.; McMahon, M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer 2014, 14, 455–467. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Cancer Genome Project; Jones, C.M.; Marshall, C.J.; Springer, C.J.; et al. Mechanical acts RAF-Erk signal pathway by oncological mutations B-Raf. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Hanrahan, A.J.; Sylvester, B.E.; Chang, M.T.; ElZein, A.; Gao, J.; Han, W.; Liu, Y.; Xu, D.; Gao, S.P.; Gorelick, A.N.; et al. Leveraging Systematic Functional Analysis to Benchmark an In Silico Framework Distinguishes Driver from Passenger MEK Mutants in Cancer. Cancer Res. 2020, 80, 4233–4243. [Google Scholar] [CrossRef]
- Greger, J.G.; Eastman, S.D.; Zhang, V.; Bleam, M.R.; Hughes, A.M.; Smitheman, K.N.; Dickerson, S.H.; Laquerre, S.G.; Liu, L.; Gilmer, T.M. Combinations of BRAF, MEK, and PI3K/mTOR Inhibitors Overcome Acquired Resistance to the BRAF Inhibitor GSK2118436 Dabrafenib, Mediated by NRAS or MEK Mutations. Mol. Cancer Ther. 2012, 11, 909–920. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Wagle, N.; Sucker, A.; Treacy, D.J.; Johannessen, C.M.; Goetz, E.M.; Place, C.S.; Taylor-Weiner, A.; Whittaker, S.; Kryukov, G.V.; et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014, 4, 94–109. [Google Scholar] [CrossRef]
- Rodriguez-Viciana, P.; Rauen, K.A. Biochemical characterization of novel germline BRAF and MEK mutations in car-dio-facio-cutaneous syndrome. Methods Enzymol. 2008, 438, 277–289. [Google Scholar]
- Smorodinsky-Atias, K.; Soudah, N.; Engelberg, D. Mutations That Confer Drug-Resistance, Oncogenicity and Intrinsic Activity on the ERK MAP Kinases—Current State of the Art. Cells 2020, 9, 129. [Google Scholar] [CrossRef]
- Emery, C.M.; Vijayendran, K.G.; Zipser, M.C.; Sawyer, A.M.; Niu, L.; Kim, J.J.; Hatton, C.; Chopra, R.; Oberholzer, P.A.; Karpova, M.B.; et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl. Acad. Sci. USA 2009, 106, 20411–20416. [Google Scholar] [CrossRef] [PubMed]
- Goetz, E.M.; Ghandi, M.; Treacy, D.J.; Wagle, N.; Garraway, L.A. ERK Mutations Confer Resistance to Mitogen-Activated Protein Kinase Pathway Inhibitors. Cancer Res. 2014, 74, 7079–7089. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, B.S.; Durinck, S.; Stawiski, E.W.; Yin, J.; Wang, W.; Lin, E.; Moffat, J.G.; Martin, S.E.; Modrusan, Z.; Seshagiri, S. ERK Mutations and Amplification Confer Resistance to ERK-Inhibitor Therapy. Clin. Cancer Res. 2018, 24, 4044–4055. [Google Scholar] [CrossRef]
- Phillips, A.A.; Harewood, J.C.K. Adult T Cell Leukemia-Lymphoma (ATL): State of the Art. Curr. Hematol. Malign. Rep. 2018, 13, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mehta-Shah, N.; Ratner, L.; Horwitz, S.M. Adult T-Cell Leukemia/Lymphoma. J. Oncol. Pract. 2017, 13, 487–492. [Google Scholar] [CrossRef]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene 2005, 24, 5926–5930. [Google Scholar] [CrossRef]
- Yamagishi, M.; Watanabe, T. Molecular Hallmarks of Adult T Cell Leukemia. Front. Microbiol. 2012, 3, 334. [Google Scholar] [CrossRef]
- Krump, N.A.; You, J. Molecular mechanisms of viral oncogenesis in humans. Nat. Rev. Genet. 2018, 16, 684–698. [Google Scholar] [CrossRef]
- Bangham, C.R.; Ratner, L. How does HTLV-1 cause adult T-cell leukaemia/lymphoma (ATL)? Curr. Opin. Virol. 2015, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, J.-I. Strategies of Human T-Cell Leukemia Virus Type 1 for Persistent Infection: Implications for Leukemogenesis of Adult T-Cell Leukemia-Lymphoma. Front. Microbiol. 2020, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, W.; Li, M.; Liu, Y.; Zheng, D. Tax1 enhances cancer cell proliferation via Ras-Raf-MEK-ERK signaling pathway. IUBMB Life 2009, 61, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.-I.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Stoppa, G.; Rumiato, E.; Saggioro, D. Ras signaling contributes to survival of human T-cell leukemia/lymphoma virus type 1 (HTLV-1) Tax-positive T-cells. Apoptosis 2011, 17, 219–228. [Google Scholar] [CrossRef]
- Ziegler, J.L. Burkitt’s Lymphoma. N. Engl. J. Med. 1981, 305, 735–745. [Google Scholar] [CrossRef]
- Dunleavy, K.; Little, R.F.; Wilson, W.H. Update on Burkitt Lymphoma. Hematol. Clin. North. Am. 2016, 30, 1333–1343. [Google Scholar] [CrossRef]
- De-Thé, G.; Geser, A.; Day, N.E.; Tukei, P.M.; Williams, E.H.; Beri, D.P.; Smith, P.G.; Dean, A.G.; Bornkamm, G.W.; Feorino, P.; et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature 1978, 274, 756–761. [Google Scholar] [CrossRef]
- Casulo, C.; Friedberg, J. Treating Burkitt Lymphoma in Adults. Curr. Hematol. Malign. Rep. 2015, 10, 266–271. [Google Scholar] [CrossRef]
- Dalla-Favera, R.; Bregni, M.; Erikson, J.; Patterson, D.; Gallo, R.C.; Croce, C.M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc. Natl. Acad. Sci. USA 1982, 79, 7824–7827. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, L.; Jiang, W.; Zhang, H.; Zhou, A.; Zeng, N. Identification of clinical molecular targets for childhood Burkitt lymphoma. Transl. Oncol. 2020, 13, 100855. [Google Scholar] [CrossRef]
- Strati, P.; Nastoupil, L.J.; Davis, R.E.; Fayad, L.E.; Fowler, N.; Hagemeister, F.B.; Kwak, L.; Oki, Y.; Wang, M.; Westin, J.; et al. A phase 1 trial of alisertib and romidepsin for relapsed/refractory aggressive B-cell and T-cell lymphomas. Haematologica 2019, 105, e26–e28. [Google Scholar] [CrossRef] [PubMed]
- Wever, C.M.; Geoffrion, D.; Grande, B.M.; Yu, S.; Alcaide, M.; Lemaire, M.; Riazalhosseini, Y.; Hébert, J.; Gavino, C.; Vinh, D.; et al. The genomic landscape of two Burkitt lymphoma cases and derived cell lines: Comparison between primary and relapse samples. Leuk. Lymphoma 2018, 59, 2159–2174. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Viatour, P. Hepatocellular carcinoma: Old friends and new tricks. Exp. Mol. Med. 2020, 52, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D., III; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-N.; Bai, T.; Chen, Z.-S.; Wu, F.-X.; Chen, Y.-Y.; De Xiang, B.; Peng, T.; Han, Z.-G.; Li, L.-Q. The p53 mutation spectrum in hepatocellular carcinoma from Guangxi, China: Role of chronic hepatitis B virus infection and aflatoxin B1 exposure. Liver Int. 2014, 35, 999–1009. [Google Scholar] [CrossRef]
- Hou, W.; Liu, J.; Chen, P.; Wang, H.; Ye, B.C.; Qiang, F. Mutation analysis of key genes in RAS/RAF and PI3K/PTEN pathways in Chinese patients with hepatocellular carcinoma. Oncol. Lett. 2014, 8, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Choi, J.Y.; Kim, S.; Chung, E.S.; Kim, T.; Koh, S.S.; Lee, B.; Bae, S.H.; Kim, J.; Park, Y.M. Over-expression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol. Res. 2004, 29, 113–121. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Jiang, C.Y.; Wei, L.X.; Wang, Y.L.; Dai, G.H. Expression and prognostic role of pan-Ras, Raf-1, pMEK1 and pERK1/2 in patients with hepatocellular carcinoma. Eur. J. Surg. Oncol. 2011, 37, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.; Nguyen, T.T.T.; Chow, K.-H.K.-P.; Tan, P.H.; Soo, K.C.; Tran, E. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: Its role in tumor progression and apoptosis. BMC Gastroenterol. 2003, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Sasaki, Y.; Horimoto, M.; Wada, S.; Tanaka, Y.; Kasahara, A.; Ueki, T.; Hirano, T.; Yamamoto, H.; Fujimoto, J.; et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology 1998, 27, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hisamoto, T.; Akiba, J.; Koga, H.; Nakamura, K.; Tokunaga, Y.; Hanada, S.; Kumemura, H.; Maeyama, M.; Harada, M.; et al. Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene 2006, 25, 6056–6066. [Google Scholar] [CrossRef]
- Vareedayah, A.A.; Alkaade, S.; Taylor, J.R. Pancreatic Adenocarcinoma. MO. Med. 2018, 115, 230–235. [Google Scholar]
- Jonckheere, N.; Vasseur, R.; Van Seuningen, I. The cornerstone K-RAS mutation in pancreatic adenocarcinoma: From cell sig-naling network, target genes, biological processes to therapeutic targeting. Crit. Rev. Oncol. Hematol. 2017, 111, 7–19. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2140–2141. [Google Scholar] [CrossRef]
- Neuzillet, C.; Hammel, P.; Tijeras-Raballand, A.; Couvelard, A.; Raymond, E. Targeting the Ras–ERK pathway in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2012, 32, 147–162. [Google Scholar] [CrossRef]
- Drosten, M.; Barbacid, M. Targeting the MAPK Pathway in KRAS-Driven Tumors. Cancer Cell 2020, 37, 543–550. [Google Scholar] [CrossRef]
- Goebel, C.; Louden, C.L.; McKenna, R.; Onugha, O.; Wachtel, A.; Long, T. Diagnosis of Non-small Cell Lung Cancer for Early Stage Asymptomatic Patients. Cancer Genom. Proteom. 2019, 16, 229–244. [Google Scholar] [CrossRef]
- Kerr, E.M.; Gaude, E.; Turrell, F.K.; Frezza, C.; Martins, C.P. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nat. Cell Biol. 2016, 531, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ricciuti, B.; Nguyen, T.; Li, X.; Rabin, M.S.; Awad, M.M.; Lin, X.; Johnson, B.E.; Christiani, D.C. Association between Smoking History and Tumor Mutation Burden in Advanced Non–Small Cell Lung Cancer. Cancer Res. 2021, 81, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Shen, R.; Ang, D.C.; Johnson, M.L.; D’Angelo, S.P.; Paik, P.K.; Brzostowski, E.B.; Riely, G.J.; Kris, M.; Zakowski, M.F.; et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3,026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012, 18, 6169–6177. [Google Scholar] [CrossRef] [PubMed]
- Ihle, N.T.; Byers, L.A.; Kim, E.S.; Saintigny, P.; Lee, J.J.; Blumenschein, G.R.; Tsao, A.; Liu, S.; Larsen, J.; Wang, J.; et al. Effect of KRAS Oncogene Substitutions on Protein Behavior: Implications for Signaling and Clinical Outcome. J. Natl. Cancer Inst. 2012, 104, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Yuan, M.; Li, X.; Chen, L.; Yang, J.; Zhao, X.; Ma, W.; Xin, J. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: A systematic review with meta-analysis. Lung Cancer 2013, 81, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Sima, C.S.; Shen, R.; Kass, S.; Gainor, J.; Shaw, A.; Hames, M.; Iams, W.; Aston, J.; Lovly, C.; et al. Prognostic Impact of KRAS Mutation Subtypes in 677 Patients with Metastatic Lung Adenocarcinomas. J. Thorac. Oncol. 2015, 10, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Domerg, C.; Hainaut, P.; Jänne, P.A.; Pignon, J.-P.; Graziano, S.; Douillard, J.-Y.; Brambilla, E.; Le Chevalier, T.; Seymour, L.; et al. Pooled Analysis of the Prognostic and Predictive Effects of KRAS Mutation Status and KRAS Mutation Subtype in Early-Stage Resected Non–Small-Cell Lung Cancer in Four Trials of Adjuvant Chemotherapy. J. Clin. Oncol. 2013, 31, 2173–2181. [Google Scholar] [CrossRef]
- Glodde, N.; Hölzel, M. RAS and PD-L1: A Masters’ Liaison in Cancer Immune Evasion. Immunity 2017, 47, 1007–1009. [Google Scholar] [CrossRef]
- Coelho, M.A.; Trécesson, S.D.C.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K.; et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity 2017, 47, 1083–1099.e6. [Google Scholar] [CrossRef]
- Zdanov, S.; Mandapathil, M.; Abu Eid, R.; Adamson-Fadeyi, S.; Wilson, W.; Qian, J.; Carnie, A.; Tarasova, N.; Mkrtichyan, M.; Berzofsky, J.A.; et al. Mutant KRAS Conversion of Conventional T Cells into Regulatory T Cells. Cancer Immunol. Res. 2016, 4, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H.; Imabayashi, F.; Iwata, T.; Kawakami, Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 2006, 203, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Khalili, J.S.; Liu, S.; Rodríguez-Cruz, T.G.; Whittington, M.; Wardell, S.; Liu, C.; Zhang, M.; Cooper, Z.; Frederick, D.T.; Li, Y.; et al. Oncogenic BRAF(V600E) Promotes Stromal Cell-Mediated Immunosuppression Via Induction of Interleukin-1 in Melanoma. Clin. Cancer Res. 2012, 18, 5329–5340. [Google Scholar] [CrossRef]
- Boni, A.; Cogdill, A.; Dang, P.; Udayakumar, D.; Njauw, C.-N.J.; Sloss, C.M.; Ferrone, C.R.; Flaherty, K.T.; Lawrence, D.P.; Fisher, D.E.; et al. Selective BRAFV600E Inhibition Enhances T-Cell Recognition of Melanoma without Affecting Lymphocyte Function. Cancer Res. 2010, 70, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.; Melendez, B.; Talukder, A.; Lizée, G. Trouble at the core: BRAF(V600E) drives multiple modes of T-cell suppression in melanoma. Oncoimmunology 2015, 5, e1078966. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Lawrence, D.; Atkinson, V.; Agarwal, S.; Miller, W.H.; Carlino, M.S.; Fisher, R.; Long, G.V.; Hodi, F.S.; Tsoi, J.; et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat. Med. 2019, 25, 936–940. [Google Scholar] [CrossRef]
- Pratilas, C.A.; Hanrahan, A.J.; Halilovic, E.; Persaud, Y.; Soh, J.; Chitale, D.; Shigematsu, H.; Yamamoto, H.; Sawai, A.; Janakiraman, M.; et al. Genetic Predictors of MEK Dependence in Non–Small Cell Lung Cancer. Cancer Res. 2008, 68, 9375–9383. [Google Scholar] [CrossRef]
- Karreth, F.A.; Frese, K.K.; DeNicola, G.; Baccarini, M.; Tuveson, D.A. C-Raf Is Required for the Initiation of Lung Cancer by K-RasG12D. Cancer Discov. 2011, 1, 128–136. [Google Scholar] [CrossRef]
- Blasco, R.B.; Francoz, S.; Santamaría, D.; Cañamero, M.; Dubus, P.; Charron, J.; Baccarini, M.; Barbacid, M. c-Raf, but Not B-Raf, Is Essential for Development of K-Ras Oncogene-Driven Non-Small Cell Lung Carcinoma. Cancer Cell 2011, 19, 652–663. [Google Scholar] [CrossRef]
- Takezawa, K.; Okamoto, I.; Yonesaka, K.; Hatashita, E.; Yamada, Y.; Fukuoka, M.; Nakagawa, K. Sorafenib Inhibits Non–Small Cell Lung Cancer Cell Growth by Targeting B-RAF in KRAS Wild-Type Cells and C-RAF in KRAS Mutant Cells. Cancer Res. 2009, 69, 6515–6521. [Google Scholar] [CrossRef]
- Hofmann, M.H.; Gmachl, M.; Ramharter, J.; Savarese, F.; Gerlach, D.; Marszalek, J.R.; Sanderson, M.P.; Kessler, D.; Trapani, F.; Arnhof, H.; et al. BI-3406, a Potent and Selective SOS1–KRAS Interaction Inhibitor, Is Effective in KRAS-Driven Cancers through Combined MEK Inhibition. Cancer Discov. 2021, 11, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Koczywas, M.; Haura, E.; Janne, P.A.; Pacheco, J.M.; Ulahannan, S.; Wang, J.S.; Burris, H.A.; Riess, J.W.; McCoach, C.; Gordon, M.S.; et al. Abstract LB001: Anti-tumor activity and tolerability of the SHP2 inhibitor RMC-4630 as a single agent in patients with RAS-addicted solid cancers. Cancer Res. 2021, 81. [Google Scholar] [CrossRef]
- Karp, J.E.; Lancet, J.E. Development of the farnesyltransferase inhibitor tipifarnib for therapy of hematologic malignancies. Futur. Oncol. 2005, 1, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Hidalgo, M.; Canon, J.; Macarulla, T.; Bazin, I.; Poddubskaya, E.; Manojlovic, N.; Radenkovic, D.; Verslype, C.; Raymond, E.; et al. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int. J. Cancer 2018, 143, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Brana, I.; Haddad, R.; Bauman, J.; Bible, K.; Oosting, S.; Wong, D.J.; Ahn, M.-J.; Boni, V.; Even, C.; et al. Tipifarnib in Head and Neck Squamous Cell Carcinoma with HRAS Mutations. J. Clin. Oncol. 2021, 39, 1856–1864. [Google Scholar] [CrossRef]
- Untch, B.R.; Dos Anjos, V.; Garcia-Rendueles, M.E.R.; Knauf, J.A.; Krishnamoorthy, G.P.; Saqcena, M.; Bhanot, U.K.; Socci, N.D.; Ho, A.L.; Ghossein, R.; et al. Tipifarnib Inhibits HRAS-Driven Dedifferentiated Thyroid Cancers. Cancer Res. 2018, 78, 4642–4657. [Google Scholar] [CrossRef]
- Dunnett-Kane, V.; Nicola, P.; Blackhall, F.; Lindsay, C. Mechanisms of Resistance to KRAS(G12C) Inhibitors. Cancers 2021, 13, 151. [Google Scholar] [CrossRef]
- Witkiewicz, A.K.; McMillan, E.A.; Balaji, U.; Baek, G.; Lin, W.-C.; Mansour, J.C.; Mollaee, M.; Wagner, K.-U.; Koduru, P.; Yopp, A.C.; et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015, 6, 6744. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kamada, Y.; Sameshima, T.; Yaguchi, M.; Niida, A.; Sasaki, S.; Miwa, M.; Ohkubo, S.; Sakamoto, J.-I.; Kamaura, M.; et al. K-Ras(G12D)-selective inhibitory peptides generated by random peptide T7 phage display technology. Biochem. Biophys. Res. Commun. 2017, 484, 605–611. [Google Scholar] [CrossRef]
- Carlino, M.S.; Kwan, V.; Miller, D.K.; Saunders, C.A.; Yip, D.; Nagrial, A.M.; Tomlinson, J.; Grimmond, S.M.; Scolyer, R.A.; Kefford, R.F.; et al. New RAS-Mutant Pancreatic Adenocarcinoma with Combined BRAF and MEK Inhibition for Metastatic Melanoma. J. Clin. Oncol. 2015, 33, e52–e56. [Google Scholar] [CrossRef][Green Version]
- Boussemart, L.; Girault, I.; Malka-Mahieu, H.; Mateus, C.; Routier, E.; Rubington, M.; Kamsu-Kom, N.; Thomas, M.; Tomasic, G.; Agoussi, S.; et al. Secondary Tumors Arising in Patients Undergoing BRAF Inhibitor Therapy Exhibit Increased BRAF–CRAF Heterodimerization. Cancer Res. 2016, 76, 1476–1484. [Google Scholar] [CrossRef]
- Mellema, W.W.; Burgers, S.A.; Smit, E.F. Tumor flare after start of RAF inhibition in KRAS mutated NSCLC: A case report. Lung Cancer 2015, 87, 201–203. [Google Scholar] [CrossRef]
- Miyauchi, S.; Shien, K.; Takeda, T.; Araki, K.; Nakata, K.; Miura, A.; Takahashi, Y.; Kurihara, E.; Ogoshi, Y.; Namba, K.; et al. Antitumor Effects of Pan-RAF Inhibitor LY3009120 Against Lung Cancer Cells Harboring Oncogenic BRAF Mutation. Anticancer Res. 2020, 40, 2667–2673. [Google Scholar] [CrossRef]
- Semrad, T.J.; Gandara, D.R.; Lara, P.N. Enhancing the clinical activity of sorafenib through dose escalation: Rationale and current experience. Ther. Adv. Med. Oncol. 2011, 3, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, H.; Ming, L. Multiple Roles of Autophagy in the Sorafenib Resistance of Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017, 44, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Choi, G.H.; Jung, Y.; Kim, K.M.; Jang, S.-J.; Yu, E.S.; Lee, H.C. Downregulation of Raf-1 kinase inhibitory protein as a sorafenib resistance mechanism in hepatocellular carcinoma cell lines. J. Cancer Res. Clin. Oncol. 2018, 144, 1487–1501. [Google Scholar] [CrossRef]
- Ye, L.; Mayerle, J.; Ziesch, A.; Reiter, F.P.; Gerbes, A.L.; De Toni, E.N. The PI3K inhibitor copanlisib synergizes with sorafenib to induce cell death in hepatocellular carcinoma. Cell Death Discov. 2019, 5, 86. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, C.-R.; Yin, H.; Wang, K.; Gao, J.-J. Synergistic antitumor activity of sorafenib and artesunate in hepatocellular carcinoma cells. Acta Pharmacol. Sin. 2020, 41, 1609–1620. [Google Scholar] [CrossRef]
- Gedaly, R.; Angulo, P.; Hundley, J.; Daily, M.; Chen, C.; Koch, A.; Evers, B.M. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010, 30, 4951–4958. [Google Scholar]
- Gedaly, R.; Angulo, P.; Hundley, J.; Daily, M.; Chen, C.; Evers, B.M. PKI-587 and Sorafenib Targeting PI3K/AKT/mTOR and Ras/Raf/MAPK Pathways Synergistically Inhibit HCC Cell Proliferation. J. Surg. Res. 2012, 176, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Daouti, S.; Li, W.H.; Wen, Y.; Rizzo, C.; Higgins, B.; Packman, K.; Rosen, N.; Boylan, J.F.; Heimbrook, D.; et al. Identification of the MEK1(F129L) activating mutation as a potential mechanism of acquired resistance to MEK inhi-bition in human cancers carrying the B-RafV600E mutation. Cancer Res. 2011, 71, 5535–5545. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Bilal, F.; Morales, C.B.; Salcedo, M.T.; Macarulla, T.; Massó-Vallés, D.; Mohan, V.; Vivancos, A.; Carreras, M.-J.; Serres-Créixams, X.; et al. Pancreatic cancer heterogeneity and response to Mek inhibition. Oncogene 2017, 36, 5639–5647. [Google Scholar] [CrossRef] [PubMed]
- Kun, E.; Tsang, Y.; Ng, C.; Gershenson, D.; Wong, K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 2021, 92, 102137. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.K.; Neel, N.F.; Hu, C.; Gautam, P.; Chenard, M.; Long, B.; Aziz, M.; Kassner, M.; Bryant, K.L.; Pierobon, M.; et al. Long-Term ERK Inhibition in KRAS-Mutant Pancreatic Cancer Is Associated with MYC Degradation and Senes-cence-like Growth Suppression. Cancer Cell 2016, 29, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Lake, D.; Correa, S.A.; Muller, J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell. Mol. Life Sci. 2016, 73, 4397–4413. [Google Scholar] [CrossRef]
- Yan, Z.; Ohuchida, K.; Fei, S.; Zheng, B.; Guan, W.; Feng, H.; Kibe, S.; Ando, Y.; Koikawa, K.; Abe, T.; et al. Inhibition of ERK1/2 in cancer-associated pancreatic stellate cells suppresses cancer-stromal interaction and metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 221. [Google Scholar] [CrossRef]
- Bhagwat, S.V.; McMillen, W.T.; Cai, S.; Zhao, B.; Whitesell, M.; Shen, W.; Kindler, L.; Flack, R.S.; Wu, W.; Anderson, B.; et al. ERK Inhibitor LY3214996 Targets ERK Pathway–Driven Cancers: A Therapeutic Approach Toward Precision Medicine. Mol. Cancer Ther. 2019, 19, 325–336. [Google Scholar] [CrossRef]
- Hatzivassiliou, G.; Liu, B.; O’Brien, C.; Spoerke, J.M.; Hoeflich, K.P.; Haverty, P.M.; Soriano, R.; Forrest, W.F.; Heldens, S.; Chen, H.; et al. ERK Inhibition Overcomes Acquired Resistance to MEK Inhibitors. Mol. Cancer Ther. 2012, 11, 1143–1154. [Google Scholar] [CrossRef]
- Ning, C.; Liang, M.; Liu, S.; Wang, G.; Edwards, H.; Xia, Y.; Polin, L.; Dyson, G.; Taub, J.W.; Mohammad, R.M.; et al. Targeting ERK enhances the cytotoxic effect of the novel PI3K and mTOR dual inhibitor VS-5584 in preclinical models of pancreatic cancer. Oncotarget 2017, 8, 44295–44311. [Google Scholar] [CrossRef]
- Shi, H.; Kong, X.; Ribas, A.; Lo, R.S. Combinatorial treatments that overcome PDGFRbeta-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res. 2011, 71, 5067–5074. [Google Scholar] [CrossRef]
- Bryant, K.L.; Stalnecker, C.A.; Zeitouni, D.; Klomp, J.E.; Peng, S.; Tikunov, A.P.; Gunda, V.; Pierobon, M.; Waters, A.M.; George, S.D.; et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 2019, 25, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Dudek-Perić, A.M.; Maes, H.; Garg, A.D.; Gabrysiak, M.; Demirsoy, S.; Swinnen, J.V.; Agostinis, P. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in Vemuraf-enib-resistant melanoma cells. Biochem. Pharmacol. 2015, 93, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, S.; Xia, H.; Yu, H.; Tang, Q.; Bi, F. MEK nuclear localization promotes YAP stability via sequestering beta-TrCP in KRAS mutant cancer cells. Cell Death Differ. 2019, 26, 2400–2415. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.H.; Gao, Y.; Soucheray, M.; Pulido, I.; Kikuchi, E.; Rodríguez, M.L.; Gandhi, R.; Lafuente-Sanchis, A.; Aupí, M.; Fernández-Coronado, J.A.; et al. CXCR7 Reactivates ERK Signaling to Promote Resistance to EGFR Kinase Inhibitors in NSCLC. Cancer Res. 2019, 79, 4439–4452. [Google Scholar] [CrossRef]
- Neto, J.M.F.; Nadal, E.; Bosdriesz, E.; Ooft, S.N.; Farre, L.; McLean, C.; Klarenbeek, S.; Jurgens, A.; Hagen, H.; Wang, L.; et al. Multiple low dose therapy as an effective strategy to treat EGFR inhibitor-resistant NSCLC tumours. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Ray, P.; Dutta, D.; Haque, I.; Nair, G.; Mohammed, J.; Parmer, M.; Kale, N.; Orr, M.; Jain, P.; Banerjee, S.; et al. pH-Sensitive Nanodrug Carriers for Codelivery of ERK Inhibitor and Gemcitabine Enhance the Inhibition of Tumor Growth in Pancreatic Cancer. Mol. Pharm. 2021, 18, 87–100. [Google Scholar] [CrossRef]
- Mao, J.; Yang, H.; Cui, T.; Pan, P.; Kabir, N.; Chen, D.; Ma, J.; Chen, X.; Chen, Y.; Yang, Y. Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT. Eur. J. Pharmacol. 2018, 832, 39–49. [Google Scholar] [CrossRef]
- Bian, Y.; Guo, D. Targeted Therapy for Hepatocellular Carcinoma: Co-Delivery of Sorafenib and Curcumin Using Lactosylated pH-Responsive Nanoparticles. Drug Des. Dev. Ther. 2020, 14, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, T.; Taniguchi, K. The Sprouty/Spred family as tumor suppressors: Coming of age. Cancer Sci. 2019, 110, 1525–1535. [Google Scholar] [CrossRef]
- Momeny, M.; Khorramizadeh, M.R.; Ghaffari, S.H.; Yousefi, M.; Yekaninejad, M.S.; Esmaeili, R.; Jahanshiri, Z.; Nooridaloii, M.R. Effects of silibinin on cell growth and invasive properties of a human hepatocellular carcinoma cell line, HepG-2, through inhibition of extracellular signal-regulated kinase 1/2 phosphorylation. Eur. J. Pharmacol. 2008, 591, 13–20. [Google Scholar] [CrossRef]
- Varghese, L.; Agarwal, C.; Tyagi, A.; Singh, R.P.; Agarwal, R. Silibinin Efficacy against Human Hepatocellular Carcinoma. Clin. Cancer Res. 2005, 11, 8441–8448. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Litscher, G. Curcumin and Photobiomodulation in Chronic Viral Hepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 7150. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoaty, A.A.A.; Zhang, P.; Lin, W.; Fan, Y.J.; Ye, S.N.; Xu, J.H. C0818, a novel curcumin derivative, induces ROS-dependent cytotoxicity in human hepatocellular car-cinoma cells in vitro via disruption of Hsp90 function. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef]
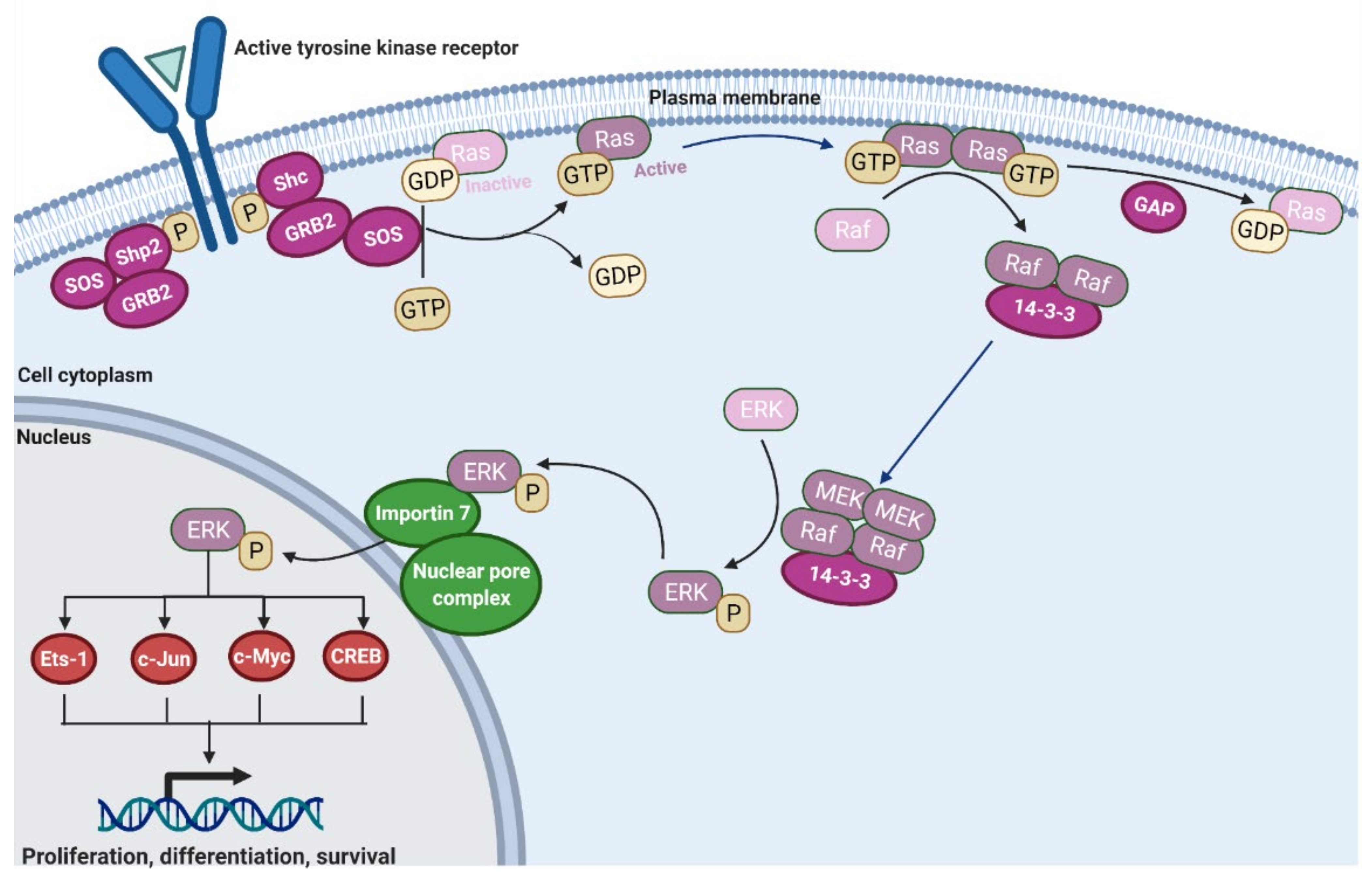

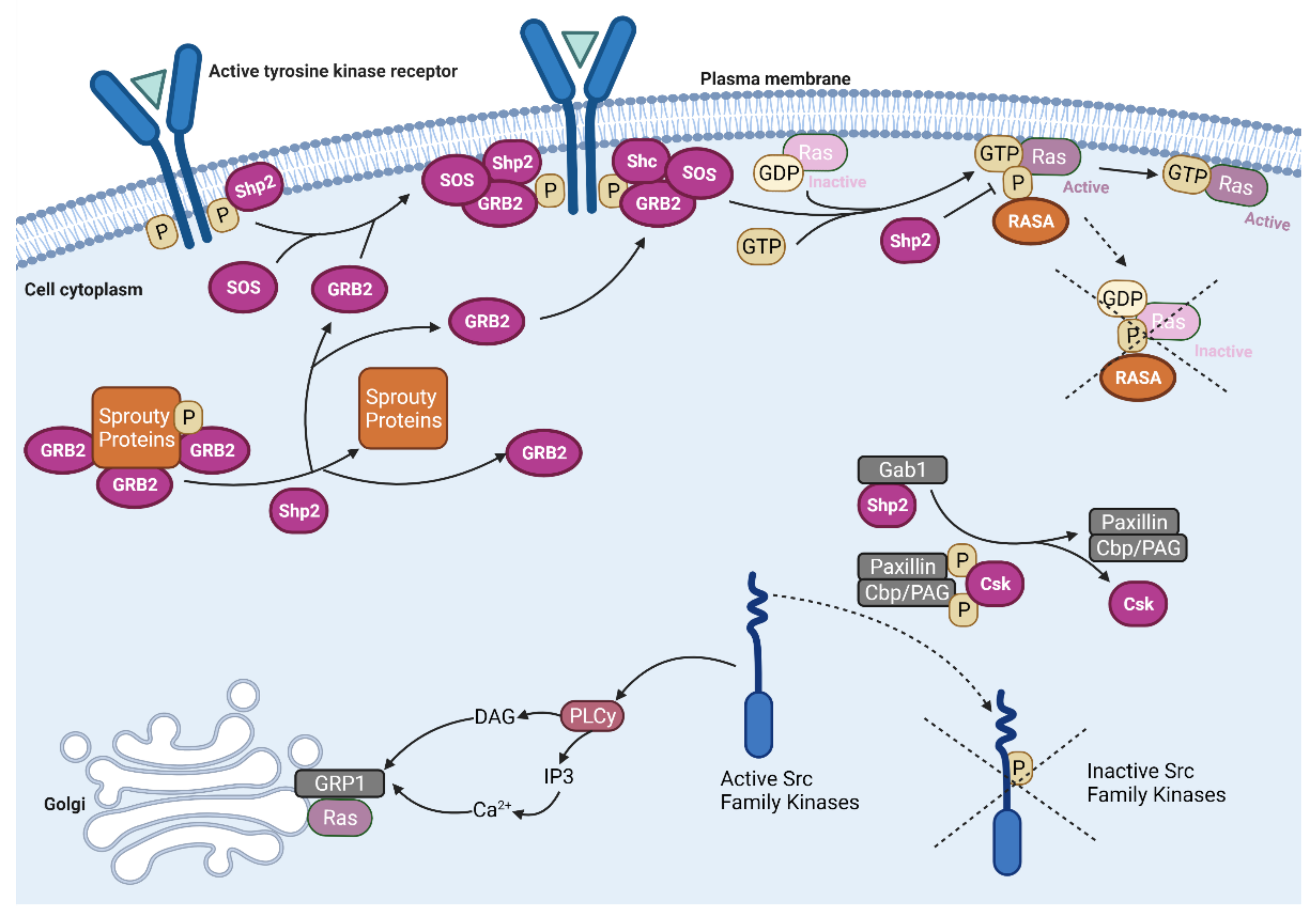
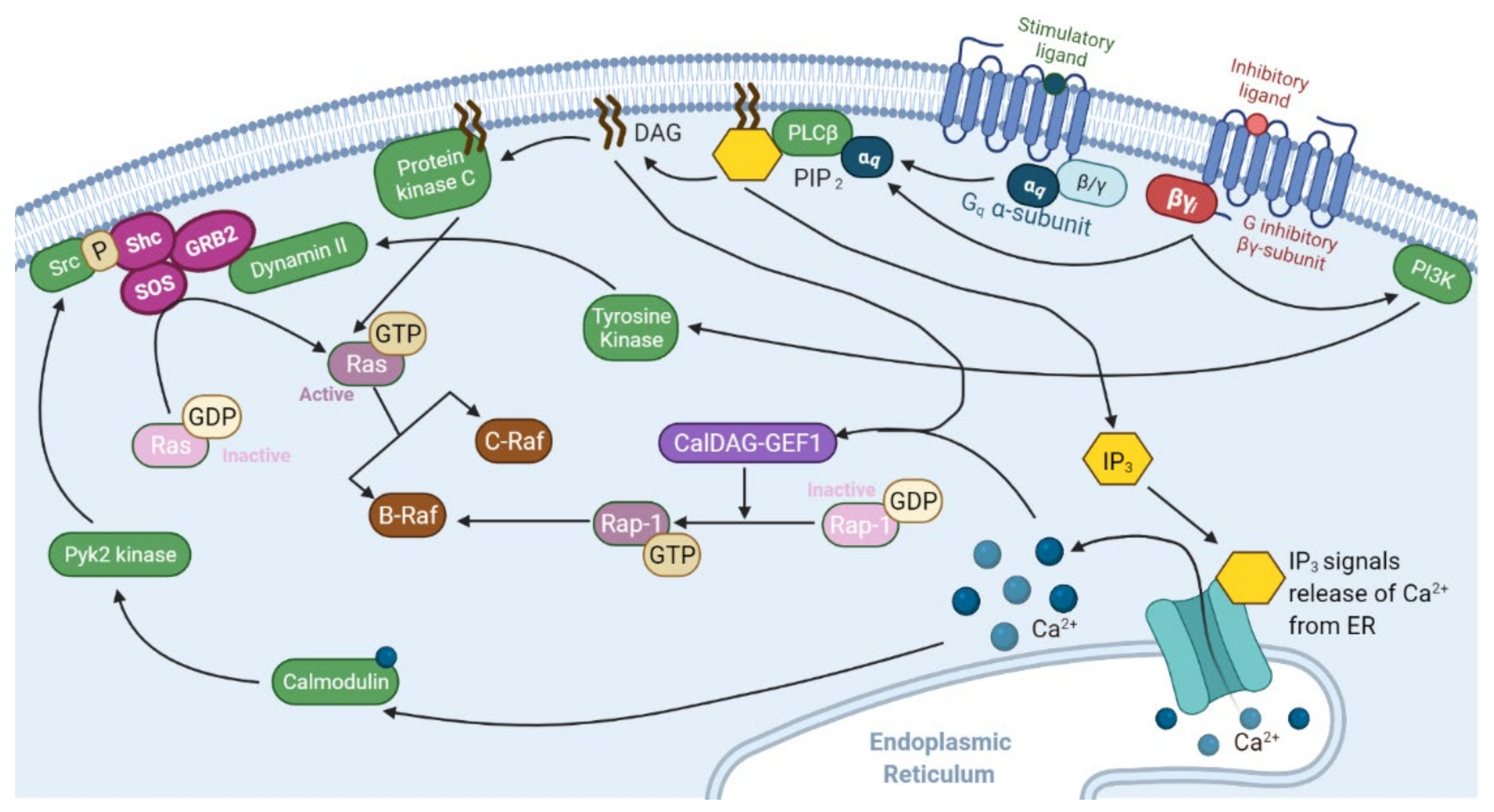
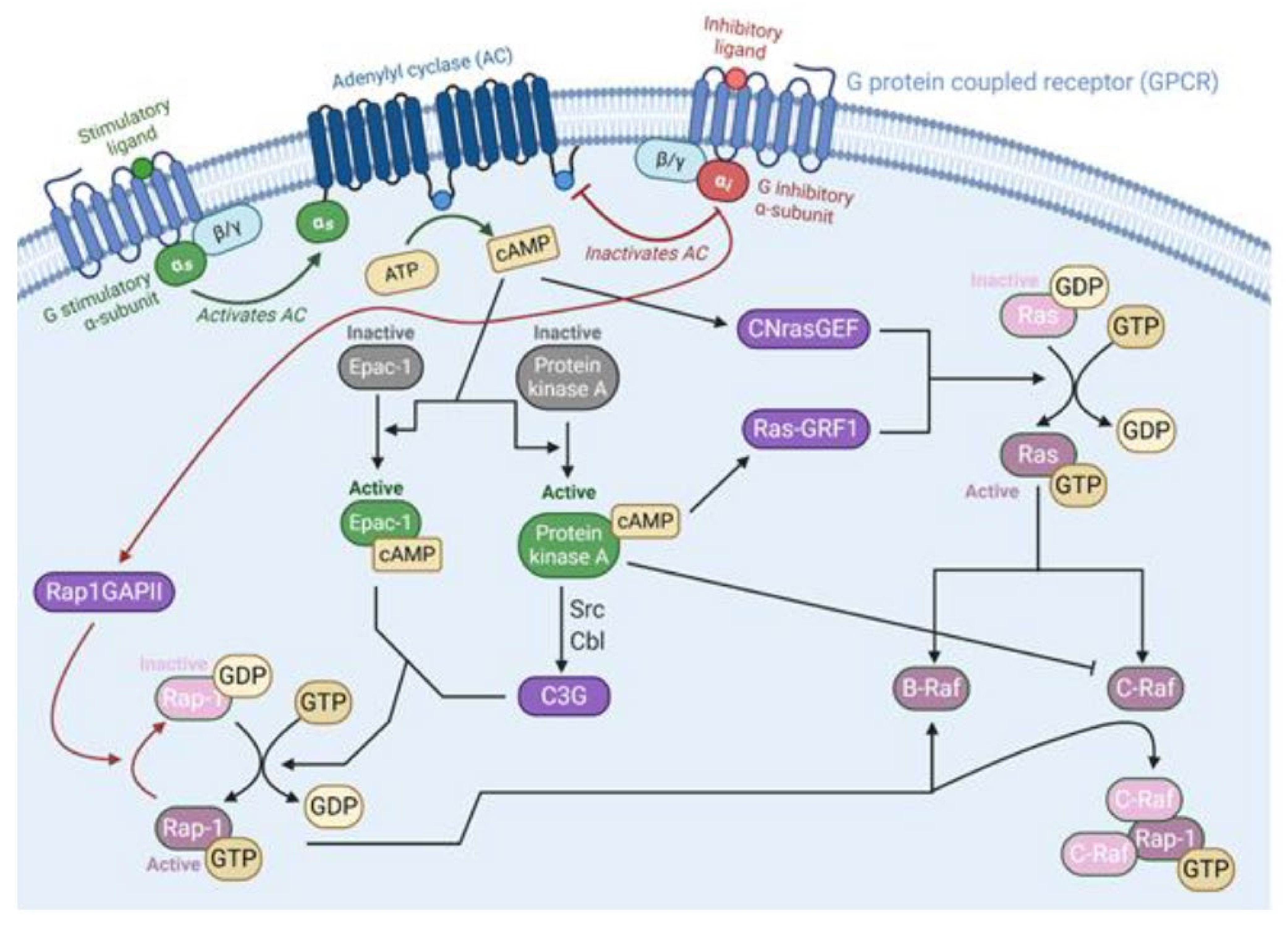
| Blood Cancer | Ras-Raf Pathology | General Treatment Protocol | 5-Year Survival | References |
|---|---|---|---|---|
| Myelodysplastic Syndromes | NRas, KRas mutation in 7–48% of patients. | Use of erythropoietin stimulating agents to mitigate symptoms. Allogeneic stem cell transplant for higher risk patients. | 29% | [163,164,165,166,167] |
| Acute Myeloid Leukemia | NRas, KRas mutations in 10–27% of de novo patients. | Induction via cytarabine and the addition of an anthracycline for patients followed by consolidation. | 24% | [167,168,169,170] |
| Acute Lymphoblastic Leukemia | NRas, KRas mutations in 5–22% of patients. BRaf mutations have been found in infants and children with acute B and T-cell lymphoblastic leukemia. Small sample found ~21% of ALL patients with BRaf mutations. | Induction using vincristine, corticosteroids, L asparaginase, and an anthracycline for patients followed by consolidation. | Between 30 and 45% | [171,172,173,174] |
| Chronic Myelomonocytic Leukemia | NRas, KRas mutations in 30–50% with CMML. Subset of patients with CMML-1 presented BRaf mutations (~7%). | Lack of consensus about a treatment that markedly expands overall survival rate. Hypomethylating agents have shown some promise and are approved by the FDA for CMML. Allogeneic stem cell transplantation regarded as only curative treatment. | 18.5% | [175,176,177,178,179] |
| Chronic Myeloid Leukemia | NRas mutations in up to 1/3 of atypical CML. Chronic CML patients present up to 17% of Ras mutations, up to 58% of acute CML patients have Ras mutations. | Standard treatment involves the use of tyrosine kinase inhibitors in sequence. | 61% | [166,169,180,181] |
| Solid Cancer | Ras-Raf Pathology | General Treatment Protocol | 5-Year Survival Rate | References |
|---|---|---|---|---|
| Pancreatic Adenocarcinoma | KRas mutations in up to 90% of patients. BRaf mutation in 14% of patients. | Surgical resection and/or chemotherapy. | less than 5%. | [166,182,183,184,185] |
| Melanoma | Ras mutations in up to 36% of patients. BRaf mutations in 27–70% of patients. | Surgical resection. Chemotherapy and novel targeted therapies, including BRaf inhibitors, may be used when surgical resection is not possible. | 91%. | [169,186,187,188,189] |
| Non-Small Cell Lung Cancer | KRas mutations in 22–36% of patients. BRaf in ~2 to 5% of patients. | Surgical resection and/or chemotherapy. | 25% | [190,191,192,193,194,195] |
| Colorectal Cancer | KRas mutations in 40–60% of patients. BRaf mutation in 18% of patients. | Surgical resection and/or chemotherapy. | 65% | [169,187,196,197] |
| Seminoma | KRas, NRas mutations in 7–40% of patients. | Radical orchiectomy with subsequent chemotherapy. | 86.4% | [166,198,199,200] |
| Bladder Cancer | HRas, NRas mutations shown in up to 80% of patients, although Ras mutations generally considered present in 10% of patients. | Immunotherapy and/or chemotherapy, with radical cystectomy after invasion of muscle. | 80.8% | [201,202,203,204] |
| Hepatocellular Carcinoma | NRas mutations in 30%, KRas mutations in 1.6% of patients. BRaf mutations in 14% of patients. | Surgical resection, liver transplantation, and/or chemotherapy. | 15% | [156,166,187,205,206] |
| Ovarian Cancer | KRas mutation in 13.7% of patients. BRaf mutations in 4–30% of patients | Cytoreductive surgery followed by chemotherapy. | 40% | [187,188,207,208] |
| Renal Cell Carcinoma | Ras mutations in up to 13% of patients. | Tyrosine kinase, m-TOR, and VEGF inhibitors. Other targeting therapies including immunotherapy are used as well. | Between 92.5 and 12% depending on localization. | [166,209,210,211] |
| Inhibitor | Notes | Clinical Trials | Targeted Cancers |
|---|---|---|---|
| BI 1701963 | Binds SOS1 protein, inhibiting its activation of KRas | NCT04111458, NCT04975256, NCT04835714 | Lung, colon, and lung cancer |
| RMC-4630 | Selective inhibitor of Shp2, indirectly inhibiting KRas | NCT03634982, NCT03989115, NCT04916236 | Pancreatic, colorectal, and non-small cell lung cancer |
| TNO155 | Selective inhibitor of Shp2, indirectly inhibiting KRas | NCT04330664, NCT04292119, NCT04000529 | Lung, head and neck, esophageal, gastrointestinal and colorectal cancer |
| BBP-398 | Selective inhibitor of Shp2, indirectly inhibiting KRas | NCT04528836 | Advanced solid tumors |
| Sorafenib | Inhibitor of multiple intracellular and cell surface kinases such as CRaf and BRaf that are involved in tumor cell signaling, angiogenesis, and apoptosis | NCT01730937, NCT03518502, NCT01371981 | Liver and thyroid cancer, leukemias |
| Sotorasib (AMG 510) | KRas inhibition specific to G12C mutation | NCT03600883, NCT04303780, NCT04933695 | Non-small cell lung cancer |
| MRTX849 | KRas inhibition selective to the G12C mutation | NCT04793958, NCT04685135 | Non-small cell lung and colorectal cancer |
| JAB-21822 | KRas inhibition selective to the G12C mutation | NCT05009329, NCT05002270 | Non-small cell lung and colorectal cancer |
| GFH925 | KRas inhibition selective to the G12C mutation | NCT05005234 | Advanced solid tumors |
| LY3537982 | KRas inhibition selective to the G12C mutation | NCT04956640 | Non-small cell lung, colorectal, endometrial, ovarian, and pancreatic cancer |
| Tipifarnib (R115777) | Farnesyltransferase inhibitor that prevents post-translational processing of Ras proteins. Prevents Ras from membrane binding | NCT03496766, NCT04997902, NCT03155620 | Non-small cell lung and head and neck cancer, non-Hodgkin lymphoma |
| Rigosertib | Targets mutated Ras pathway in leukemia by interacting with effectors proteins containing Ras binding domains | NCT04263090, NCT04263090, NCT03786237 | Myelodysplastic syndrome, non-small cell lung cancer, and squamous cell carcinoma |
| Trametinib (GSK1120212) | Non-ATP-competitive inhibitor of MEK1/2. FDA approved, suggested to use in combination with BRaf inhibitors due to resistance. | NCT03340506, NCT04940052, NCT02101788 | Non-small cell lung, thyroid, ovarian and peritoneal cancer, melanoma |
| Mirdametinib (PD0325901) | MEK1/2 inhibitor (derivative of CI-1040) | NCT02022982, NCT03905148, NCT04923126 | Ovarian, endometrial, pancreatic, thyroid, and non-small cell lung cancer, melanoma, glioma |
| Selumetinib (AZD6244) | Highly selective, non-ATP-competitive MEK1 inhibitor. | NCT04576117, NCT03705507, NCT03705507 | Non-small cell lung cancer, glioma, leukemia |
| Binimetinib (MEKTOVI; MEK162) | Highly selective, non-ATP-competitive MEK1/2 inhibitor. | NCT04657991, NCT02928224, NCT03843775 | Colorectal cancer, melanoma, and other BRaf mutant malignancies |
| RO5126766 (CH5127566) | Selective Raf/MEK1/2 inhibitor | NCT02407509, NCT03875820, NCT04720417 | Non-small cell lung, ovarian, and colorectal cancer, multiple myeloma, melanoma |
| HL-085 | Selective MEK1 inhibitor | NCT03973151, NCT03990077, NCT03781219 | Non-small cell lung cancer, melanoma, and BRaf mutant solid cancers |
| Ulixertinib (BVD-523) | ATP-competitive, reversible inhibitor of ERK1/2 | NCT04145297, NCT03417739, NCT04488003 | Gastrointestinal cancers, melanoma, and BRaf mutant solid cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dillon, M.; Lopez, A.; Lin, E.; Sales, D.; Perets, R.; Jain, P. Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers. Cancers 2021, 13, 5059. https://doi.org/10.3390/cancers13205059
Dillon M, Lopez A, Lin E, Sales D, Perets R, Jain P. Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers. Cancers. 2021; 13(20):5059. https://doi.org/10.3390/cancers13205059
Chicago/Turabian StyleDillon, Martha, Antonio Lopez, Edward Lin, Dominic Sales, Ron Perets, and Pooja Jain. 2021. "Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers" Cancers 13, no. 20: 5059. https://doi.org/10.3390/cancers13205059
APA StyleDillon, M., Lopez, A., Lin, E., Sales, D., Perets, R., & Jain, P. (2021). Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers. Cancers, 13(20), 5059. https://doi.org/10.3390/cancers13205059






