Thyroid Hormone Replacement Therapy Is Associated with Longer Overall Survival in Patients with Resectable Gastroesophageal Cancer: A Retrospective Single-Center Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients Recruitment
2.3. Data Recruitment
2.4. Data Safety
2.5. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Overall Survival in Regard to Patient and Tumor Characteristics
3.3. Thyroid Parameters
3.4. Thyroid Hormones and Their Correlation with the Overall Survival
3.5. Thyroid Diseases and Their Correlation with the Overall Survival
3.6. Multivariate Analysis
4. Discussion
4.1. Demographic and Tumor Characteristics
4.2. Thyroid Hormone Status
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| fT3 | Free triiodothyronine |
| fT4 | Free thyroxine |
| GEJ | Gastroesophageal junction |
| GI | Gastrointestinal |
| HR | Hazard ratio |
| NTIS | Nonthyroidal illness syndrome |
| OS | Overall survival |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TSH | Thyroid-stimulating hormone |
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; May, M.T.; Ingle, S.; Lu, M.; Yang, Z.; Tang, J. Prognostic Models for Predicting Overall Survival in Patients with Primary Gastric Cancer: A Systematic Review. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hercbergs, A.H.; Ashur-Fabian, O.; Garfield, D. Thyroid hormones and cancer: Clinical studies of hypothyroidism in oncology. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Li, Z.; Bai, S.; Zhang, H.; Tang, M.; Lei, Y.; Chen, L.; Liang, S.; Zhao, Y.L.; Wei, Y.; et al. Mechanism of cancer cell adaptation to metabolic stress: Proteomics identification of a novel thyroid hormone-mediated gastric carcinogenic signaling pathway. Mol Cell Proteom. 2009, 8, 70–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeller, L.C.; Führer, D. Thyroid hormone, thyroid hormone receptors, and cancer: A clinical perspective. Endocrine-Related Cancer. 2013, 20, R19–R29. [Google Scholar] [CrossRef] [Green Version]
- Puhr, H.C.; Wolf, P.; Berghoff, A.S.; Schoppmann, S.F.; Preusser, M.; Ilhan-Mutlu, A. Elevated Free Thyroxine Levels Are Associated with Poorer Overall Survival in Patients with Gastroesophageal Cancer: A Retrospective Single Center Analysis. Horm. Cancer 2019, 11, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Mousa, S.A.; Lin, H.-Y.; Tang, H.Y.; Hercbergs, A.; Luidens, M.K.; Davis, P.J. Modulation of angiogenesis by thyroid hormone and hormone analogues: Implications for cancer management. Angiogenesis 2014, 17, 463–469. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Edgren, G.; Adami, H.-O.; Weiderpass, E.; Nyrén, O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2012, 62, 1406–1414. [Google Scholar] [CrossRef]
- Pandeya, N.; Webb, P.; Sadeghi, S.; Green, A.C.; Whiteman, D. Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: Are the effects modified by smoking, NSAIDs or acid suppressants? Gut 2009, 59, 31–38. [Google Scholar] [CrossRef]
- Wheeler, J.B.; Reed, C.E. Epidemiology of Esophageal Cancer. Surg. Clin. N. Am. 2012, 92, 1077–1087. [Google Scholar] [CrossRef]
- Vaughan, T.L.; Davis, S.; Kristal, A.; Thomas, D.B. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: Adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 1995, 4, 85–92. [Google Scholar]
- Beatson, G.T. On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Trans. Medico-Chirurgical Soc. Edinb. 1896, 15, 153–179. [Google Scholar]
- Cristofanilli, M.; Yamamura, Y.; Kau, S.W.; Bevers, T.; Strom, S.; Patangan, M.; Hsu, L.; Krishnamurthy, S.; Theriault, R.L.; Hortobagyi, G.N. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer 2005, 103, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Wippel, C.; Starzer, A.M.; Ballarini, N.; Wolpert, F.; Bergen, E.; Wolf, P.; Steindl, A.; Widhalm, G.; Gatterbauer, B.; et al. Hypothyroidism correlates with favourable survival prognosis in patients with brain metastatic cancer. Eur. J. Cancer 2020, 135, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Iishi, H.; Tatsuta, M.; Baba, M.; Yamamoto, R.; Taniguchi, H. Enhancement by thyroxine of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Br. J. Cancer 1993, 68, 515–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmohl, K.A.; Mueller, A.M.; Dohmann, M.; Spellerberg, R.; Urnauer, S.; Schwenk, N.; Ziegler, S.I.; Bartenstein, P.; Nelson, P.J.; Spitzweg, C.; et al. Integrin αvβ3-Mediated Effects of Thyroid Hormones on Mesenchymal Stem Cells in Tumor Angiogenesis. Thyroid 2019, 29, 1843–1857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Yeh, C.-T.; Lin, K.-H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef] [Green Version]
- Al-Batran, S.E.; Hofheinz, R.D.; Schmalenberg, H.; Strumberg, D.; Goekkurt, E.; Angermeier, S.; Jia, Y.; Miao, R.; Xue, K.; Li, Z.; et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7): Results of the phase II-portion—A multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. J. Clin. Oncol. 2020, 38, 4501. [Google Scholar] [CrossRef]
- Klempner, S.J.; Wainberg, Z.A.; Muro, K.; Chao, J.; Catenacci, D.V.; Ajani, J.A.; Al-Batran, S.-E.; Toms, N.; Knoderer, H.; Wei, R.; et al. Impact of frontline doublet versus triplet therapy on clinical outcomes: Exploratory analysis from the RAINBOW study. J. Clin. Oncol. 2020, 38, 4543. [Google Scholar] [CrossRef]
- Pinto, M.; Soares, P.; Ribatti, M. Thyroid hormone as a regulator of tumor induced angiogenesis. Cancer Lett. 2011, 301, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ko, P.J.; Pan, Y.S.; Lin, H.Y.; Whang-Peng, J.; Davis, P.J.; Wang, K. Role of thyroid hormone-integrin αvβ3-signal and therapeutic strategies in colorectal cancers. J. Biomed. Sci. 2021, 28, 24. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J. The epidemiology of thyroid disease. Br. Med Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Piekiełko-Witkowska, A. Molecular mechanism of thyroid hormone action in carcinogenesis. Thyroid. Res. 2013, 6, A48. [Google Scholar] [CrossRef] [Green Version]
- Kandemir, E.G.; Yonem, A.; Narin, Y. Gastric Carcinoma and Thyroid Status. J. Int. Med. Res. 2005, 33, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.L.; Hickey, B.; Hickey, J.L.; Pearce, S.H. Trends in thyroid hormone prescribing and consumption in the UK. BMC Public Health 2009, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Biondi, B.; Wartofsky, L. Treatment With Thyroid Hormone. Endocr. Rev. 2014, 35, 433–512. [Google Scholar] [CrossRef]
- Mortoglou, A.; Candiloros, H. The serum triiodothyronine to thyroxine (T3/T4) ratio in various thyroid disorders and after Levothyroxine replacement therapy. Hormones 2004, 3, 120–126. [Google Scholar] [CrossRef]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, A.; De Stefano, M.A.; Dentice, M.; Salvatore, D. Deiodinases and Cancer. Endocrinology 2021, 162. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.-Y.; Tang, H.-Y.; Leinung, M.; Mousa, S.; Hercbergs, A.; Davis, P.J. Action of Reverse T3 on Cancer Cells. Endocr. Res. 2019, 44, 148–152. [Google Scholar] [CrossRef]
- Rennert, G.; Rennert, H.S.; Pinchev, M.; Gruber, S.B. A Case–Control Study of Levothyroxine and the Risk of Colorectal Cancer. J. Natl. Cancer Inst. 2010, 102, 568–572. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-C.; Yu, Y.-Y.; Yang, H.-C.; Nguyen, P.A.; Poly, T.N.; Islam, M.; Iqbal, U.; Khan, H.A.A.; Wang, Y.-C.; Cheng, Y.-T.; et al. Levothyroxine use and the risk of breast cancer: A nation-wide population-based case–control study. Arch. Gynecol. Obstet. 2018, 298, 389–396. [Google Scholar] [CrossRef]
- Tosovic, A.; Bondeson, A.-G.; Bondeson, L.; Ericsson, U.-B.; Manjer, J. T3 levels in relation to prognostic factors in breast cancer: A population-based prospective cohort study. BMC Cancer 2014, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Czarnecka, A.M.; Matak, D.; Szymański, L.; Czarnecka, K.; Lewicki, S.; Zdanowski, R.; Brzezianska-Lasota, E.; Szczylik, C. Triiodothyronine regulates cell growth and survival in renal cell cancer. Int. J. Oncol. 2016, 49, 1666–1678. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Chen, R.-Z.; Xia, Y.; Liang, J.-H.; Wang, L.; Zhu, H.-Y.; Wu, J.Z.; Fan, L.; Li, J.-Y.; Yang, T.; et al. Low T3 syndrome as a predictor of poor prognosis in chronic lymphocytic leukemia. Int. J. Cancer 2018, 143, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Hercbergs, A.; Mousa, S.A.; Davis, P.J. Nonthyroidal Illness Syndrome and Thyroid Hormone Actions at Integrin αvβ3. J. Clin. Endocrinol. Metab. 2018, 103, 1291–1295. [Google Scholar] [CrossRef] [Green Version]
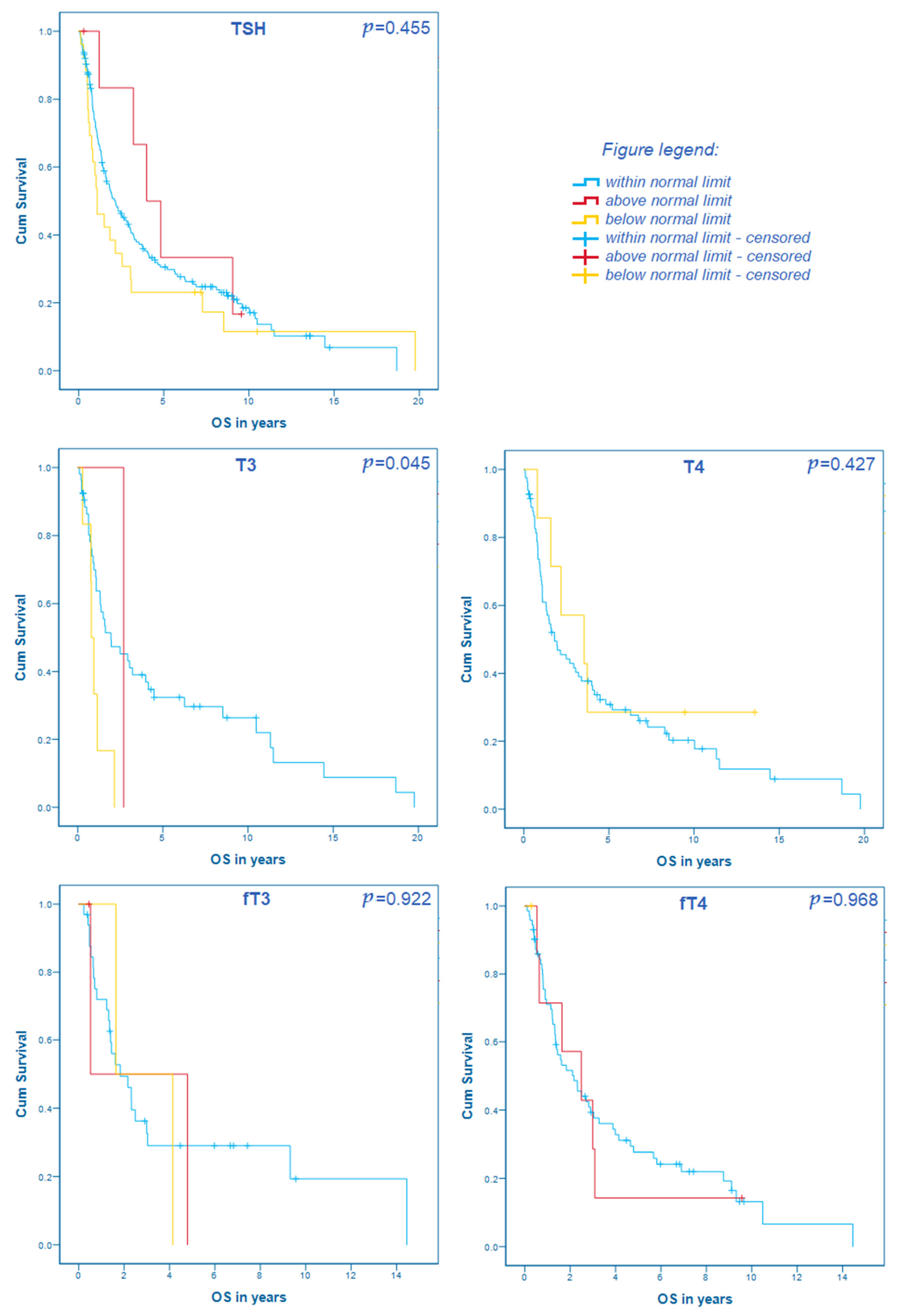
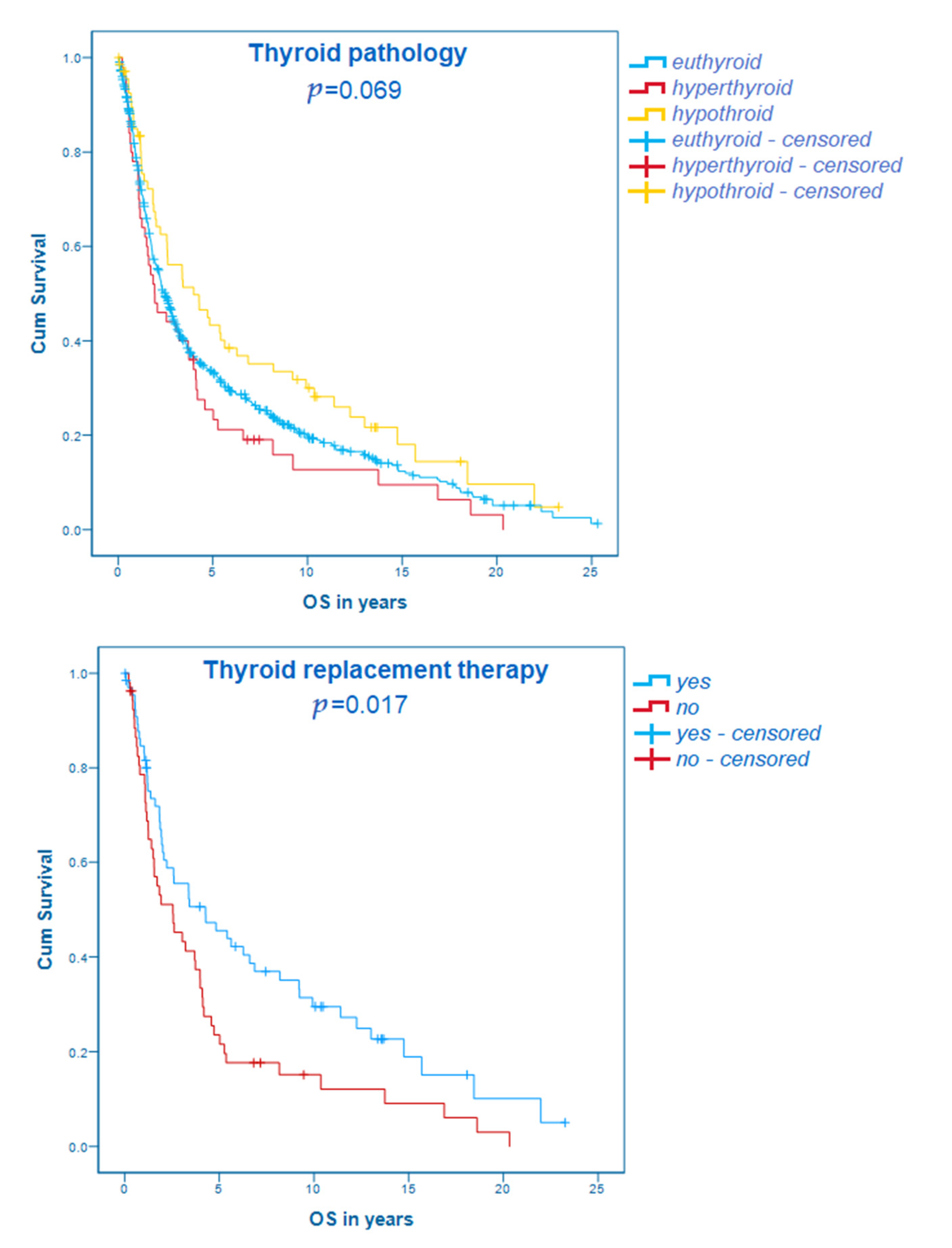
| Characteristic | Value | p | OS in Months (95% CI)/HR |
|---|---|---|---|
| Gender [n (%)] | 0.766 | ||
| male | 607 (70.2%) | 31.4 (27.0–35.8) | |
| female | 258 (29.8%) | 27.9 (23.0–32.8) | |
| Age (years) | <0.001 | 1.013 (1.006–1.020) | |
| median (min, max) | 64 (28; 92) | ||
| Body mass index (BMI) | 0.247 | 0.985 (0.961–1.010) | |
| median (min, max) | 24.8 (13.6; 50.0) | ||
| Localization of cancer [n (%)] | 0.005 | ||
| Gastric | 345 (39.9%) | 32.4 (25.5–39.3) | |
| Gastroesophageal junction | 281 (32.5%) | 26.7 (18.6–34.8) | |
| Esophageal | 239 (27.6%) | 27.3 (20.9–33.7) | |
| Histological type [n (%)] | 0.001 | ||
| Adenocarcinoma | 681 (78.7%) | 31.7 (27.1–36.3) | |
| Squamous cell carcinoma | 184 (21.3%) | 24.2 (18.2–30.2) | |
| Stage [n (%)] | <0.001 | ||
| Stage I | 155 (17.9%) | 87.6 (65.4–109.8) | |
| Stage II | 276 (31.9%) | 38.6 (30.2–47.0) | |
| Stage III | 422 (48.8%) | 20.1 (18.0–22.2) | |
| Resectable, but unknown stage | 12 (1.4%) |
| TSH. | T3 | T4 | fT3 | fT4 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | n | Median n (SD) | p | HR | n | Median n (SD) | p | HR | n | Median n (SD) | p | HR | n | Median n (SD) | p | HR | n | Median n (SD) | p | HR |
| Overall cohort | 209 | 1.24 (1.22) | 0.642 | 0.970 | 60 | 1.08 (0.27) | 0.252 | 0.493 | 89 | 82.0 (16.93) | 0.647 | 1.003 | 38 | 3.04 (0.76) | 0.917 | 0.975 | 80 | 1.31 (0.31) | 0.631 | 0.887 |
| Stage I | 35 | 1.14 (0.78) | 0.299 | 1.367 | 6 | 1.00 (0.16) | 0.321 | n.m. | 11 | 78.0 (16.54) | 0.569 | 0.983 | 5 | 2.9 (1.45) | 0.766 | 0.269 | 15 | 1.22 (0.24) | 0.860 | 0.742 |
| Stage II | 55 | 1.32 (1.42) | 0.588 | 0.924 | 19 | 1.02 (0.36) | 0.203 | 0.360 | 30 | 82.0 (17.84) | 0.970 | 1.000 | 10 | 3.07 (0.26) | 0.564 | n.m. | 16 | 1.23 (0.27) | 0.157 | n.m. |
| Stage III | 117 | 1.30 (1.24) | 0.396 | 0.936 | 35 | 1.10 (0.24) | 0.243 | 0.288 | 47 | 83.0 (16.03) | 0.915 | 0.999 | 23 | 3.03 (0.66) | 0.956 | 0.977 | 49 | 1.38 (0.53) | 0.407 | 0.781 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puhr, H.C.; Reiter, T.J.; El-Mahrouk, M.; Saliternig, L.; Wolf, P.; Mair, M.J.; Steindl, A.; Paireder, M.; Asari, R.; Schoppmann, S.F.; et al. Thyroid Hormone Replacement Therapy Is Associated with Longer Overall Survival in Patients with Resectable Gastroesophageal Cancer: A Retrospective Single-Center Analysis. Cancers 2021, 13, 5050. https://doi.org/10.3390/cancers13205050
Puhr HC, Reiter TJ, El-Mahrouk M, Saliternig L, Wolf P, Mair MJ, Steindl A, Paireder M, Asari R, Schoppmann SF, et al. Thyroid Hormone Replacement Therapy Is Associated with Longer Overall Survival in Patients with Resectable Gastroesophageal Cancer: A Retrospective Single-Center Analysis. Cancers. 2021; 13(20):5050. https://doi.org/10.3390/cancers13205050
Chicago/Turabian StylePuhr, Hannah C., Thorsten J. Reiter, Mohamed El-Mahrouk, Lena Saliternig, Peter Wolf, Maximilian J. Mair, Ariane Steindl, Matthias Paireder, Reza Asari, Sebastian F. Schoppmann, and et al. 2021. "Thyroid Hormone Replacement Therapy Is Associated with Longer Overall Survival in Patients with Resectable Gastroesophageal Cancer: A Retrospective Single-Center Analysis" Cancers 13, no. 20: 5050. https://doi.org/10.3390/cancers13205050
APA StylePuhr, H. C., Reiter, T. J., El-Mahrouk, M., Saliternig, L., Wolf, P., Mair, M. J., Steindl, A., Paireder, M., Asari, R., Schoppmann, S. F., Berghoff, A. S., Preusser, M., & Ilhan-Mutlu, A. (2021). Thyroid Hormone Replacement Therapy Is Associated with Longer Overall Survival in Patients with Resectable Gastroesophageal Cancer: A Retrospective Single-Center Analysis. Cancers, 13(20), 5050. https://doi.org/10.3390/cancers13205050






