Neoadjuvant and Adjuvant Immunotherapy in Non-Small Cell Lung Cancer—Clinical Trials Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Chemotherapy in Early Lung Cancer
3. Neoadjuvant Immunotherapy in Clinical Trials for NSCLC Patients
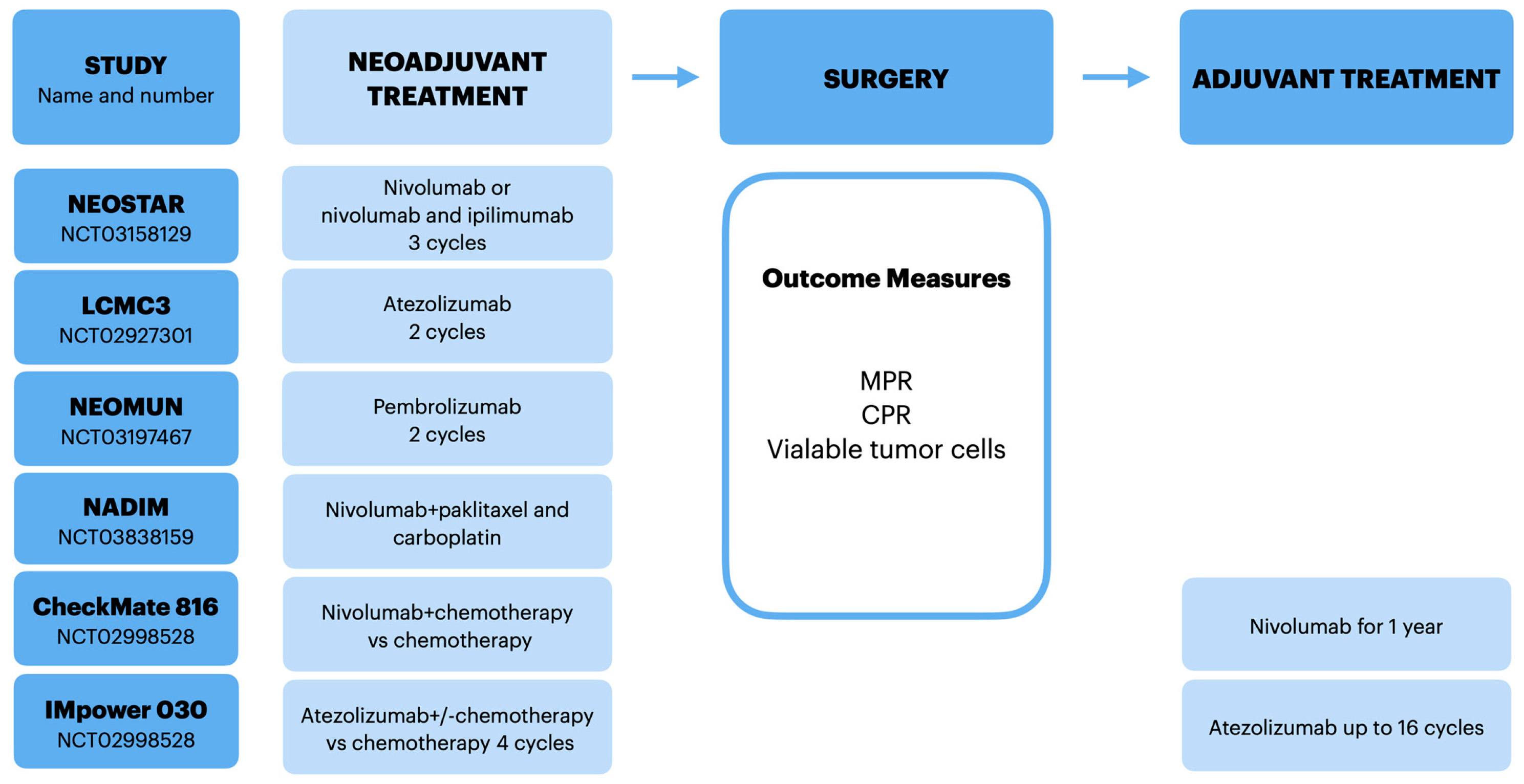
3.1. NEOSTAR
3.2. LCMC3
3.3. NEOMUN
4. Combination of Immunotherapy and Chemotherapy in Neoadjuvant Treatment in NSCLC Patients
4.1. NADIM
4.2. CheckMate 816
4.3. IMpower 030
4.4. CheckMate 77T
4.5. AEGAN
5. Adjuvant Immunotherapy in NSCLC Patients
5.1. IMpower010
5.2. NADIM-ADJUVANT
6. Predictive Biomarkers for Adjuvant and Neoadjuvant Immunotherapies
6.1. Pathological Outcomes
6.2. PD-L1 Expression
6.3. Circulating Tumor DNA
6.4. Radiological Response
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Goya, T.; Koshiishi, Y.; Sohara, Y.; Eguchi, K.; Mori, M.; Nakanishi, Y.; Tsuchiya, R.; Shimokata, K.; Inoue, H.; et al. A Japanese Lung Cancer Registry Study: Prognosis of 13,010 Resected Lung Cancers. J. Thorac. Oncol. 2008, 3, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Rad, R.; Li, R.; Paul, M.K.; Dubinett, S.M.; Liu, B. The Biology of Lung Cancer: Development of More Effective Methods for Prevention, Diagnosis, and Treatment. Clin. Chest Med. 2020, 41, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Li, L.; Chen, X.; Chen, N.; Song, W.; Cui, J. Neoadjuvant and Adjuvant Immunotherapy: Opening New Horizons for Patients with Early-Stage Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 575472. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.; Stephens, R.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Yendamuri, S.; Groman, A.; Miller, A.; Demmy, T.; Hennon, M.; Dexter, E.; Picone, A.; Nwogu, C.; Dy, G.-K. Risk and benefit of neoadju- vant therapy among patients undergoing resection for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2018, 53, 656–663. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Chaft, J.E.; William, W.N., Jr.; Rusch, V.; Pisters, K.; Kalhor, N.; Pataer, A.; Travis, W.D.; Swisher, S.; Kris, M.G.; et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014, 15, e42–e50. [Google Scholar] [CrossRef]
- Taiwo, E.O.; Yorio, J.T.; Yan, J.; Gerber, D.E. How have we diagnosed early-stage lung cancer without radiographic screening? A contemporary single-center experience. PLoS ONE 2012, 7, e52313. [Google Scholar] [CrossRef][Green Version]
- Cascone, T.; William, W.N.; Weissferdt, A.; Lin, H.Y.; Leung, C.H.; Carter, B.W.; Fossella, F.V.; Mott, F.; Papadimitrakopoulou, V.; Blumenschein, G.R., Jr.; et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J. Clin. Oncol. 2019, 37, 8504. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Sepesi, B.; Leung, C.H.; Lin, H.Y.; William, W.N.; Weissferdt, A.; Pataer, A.; Godoy, M.; Fossella, F.V.; Blumenschein, G.; et al. Impact of genomic aberrations and additional therapies on survival outcomes of patients with operable non-small cell lung cancer (NSCLC) from the NEOSTAR study. J. Clin. Oncol. 2021, 39, 8542. [Google Scholar] [CrossRef]
- Kwiatkowski, D.J.; Rusch, V.W.; Chaft, J.E.; Johnson, B.E.; Nicholas, A.; Wistuba, I.I.; Merritt, R.; Lee, J.M.; Bunn, P.A.; Tang, Y.; et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J. Clin. Oncol. 2019, 37, 8503. [Google Scholar] [CrossRef]
- Eichhorn, F.; Klotz, L.V.; Bischoff, H.; Thomas, M.; Lasitschka, F.; Winter, H.; Hoffmann, H.; Eichhorn, M.E. Neoadjuvant anti-programmed Death-1 immunotherapy by Pembrolizumab in resectable nodal positive stage II/IIIa non-small-cell lung cancer (NSCLC): The NEOMUN trial. BMC Cancer 2019, 19, 413. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; Campelo, R.G.; Casal, J.; Domine, M.; Majem, M.; Rodriguez-Abreu, D.; Martinez-Marti, A.; De Cas- tro Carpeno, J.; et al. OA13.05 NADIM Study: Updated Clinical Research and Outcomes. J. Thorac. Oncol. 2019, 14, S241. [Google Scholar] [CrossRef]
- Provencio-Pulla, M.; Nadal-Alforja, E.; Cobo, M.; Insa, A.; Costa Rivas, M.; Majem, M.; Rodriguez-Abreu, D.; Lopez-Vivanco, G.; Domine, M.; Del Barco Morillo, E.; et al. Neoadjuvant chemo/immunotherapy for the treatment of stages IIIA resectable non-small cell lung cancer (NSCLC): A phase II multicenter exploratory study—NADIM study-SLCG. J. Clin. Oncol. 2018, 36, 8521. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Felip, E.; Broderick, S.; Girard, N.; Awad, M.M.; Kerr, K.; Blackwood-Chirchir, A.; Yang, R.; Geese, W.J.; et al. CheckMate 816: A Phase 3 Trial of Neoadjuvant Nivolumab Plus Ipilimumab or Chemotherapy vs Chemotherapy in Early-Stage NSCLC. J. Thorac. Oncol. 2017, 13, S831–S832. [Google Scholar]
- Spicer, J.; Wang, C.; Tanaka, F.; Saylors, G.B.; Chen, K.-N.; Liberman, M.; Vokes, E.E.; Girard, N.; Lu, S.; Provencio, M.; et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoad- juvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2021, 39 (Suppl. 15), 8503. [Google Scholar] [CrossRef]
- Shu, C.A.; Grigg, C.; Chiuzan, C.; Garofano, R.F.; Patel, V.; Hernandez, S.; Negri, T.; Sacher, A.G.; Smith-Marrone, S.; Stoopler, M.; et al. Neoadjuvant atezolizumab+ chemotherapy in resectable non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2018, 36, 8532. [Google Scholar] [CrossRef]
- Peters, S.; Kim, A.W.; Solomon, B.; Gandara, D.R.; Dziadziuszko, R.; Brunelli, A.; Garassino, M.C.; Reck, M.; Wang, L.; To, I.; et al. Impower030: Phase III study evaluating neoadjuvant treatment of resectable stage II-IIIB non-small cell lung cancer (nsclc) with ate- zolizumab (atezo) + chemotherapy. Ann. Oncol. 2019, 30 (Suppl. 2), ii26–ii30. [Google Scholar] [CrossRef]
- Cascone, T.; Provencio, M.; Sepesi, B.; Lu, S.; Aanur, N.; Li, S.; Spicer, J.; Checkmate, T. A phase III trial of neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) followed by adjuvant nivo in resectable early-stage NSCLC. J. Clin. Oncol. 2020, 38, TPS9076. [Google Scholar] [CrossRef]
- Heymach, J.; Taube, J.; Mitsudomi, T.; Harpole, D.; Aperghis, M.; Trani, L.; Powell, M.; Dennis, P.; Reck, M. P1.18-02 The AEGEAN Phase 3 Trial of Neoadjuvant/Adjuvant Durvalumab in Patients with Resectable Stage II/III NSCLC. J. Thorac. Oncol. 2019, 14, S625–S626. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Altorki, N.K.; Zhou, C.; Csoszi, T.; Vynnychenko, I.O.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. IMpower010: Primary results of a Phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2021, 39, 8500. [Google Scholar] [CrossRef]
- Calvo, V.; Domine, M.; Sullivan, I.; Gonzalez-Laribba, J.-L.; Ortega, A.L.; Bernabe, R.; Sala, M.A.; Campos, B.; De Castro, J.; Martil- Martorell, P.; et al. A phase III clinical trial of adjuvant chemotherapy versus chemoimmunotherapy for stage IB-IIIA completely resected non-small cell lung cancer (NSCLC) patients nadim-adjuvant: New adjuvant trial of chemotherapy versus chemoimmunotherapy. J. Clin. Oncol. 2021, 39, TPS8581. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Bunn, P.A., Jr.; Chaft, J.E.; McCoach, C.; Parez, E.A.; Scagliotti, G.V.; Carbone, D.P.; Aerts, H.; Aisner, D.; Bergh, J.; et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J. Thorac. Oncol. 2018, 13, 1818–1831. [Google Scholar] [CrossRef]
- Pataer, A.; Kalhor, N.; Correa, A.M.; Raso, M.G.; Erasmus, J.J.; Kim, E.S.; Behrens, C.; Lee, J.J.; Roth, J.A.; Stewart, D.J.; et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Weissferdt, A.; Pataer, A.; Vaporciyan, A.A.; Correa, A.M.; Sepesi, B.; Moran, C.A.; Wistuba, I.I.; Roth, J.A.; Shewale, J.B.; Heymach, J.V.; et al. Agreement on Major Pathological Response in NSCLC Patients Receiving Neoadjuvant Chemotherapy. Clin. Lung Cancer 2020, 21, 341–348. [Google Scholar] [CrossRef]
- Ling, Y.; Li, N.; Li, L.; Guo, C.; Wei, J.; Yuan, P.; Tan, F.; Tao, X.; Wang, S.; Wang, Z.; et al. Different pathologic responses to neoadjuvant anti-PD-1 in primary squamous lung cancer and regional lymph nodes. NPJ Precis. Oncol. 2020, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, C.; Zhong, W.-Z. Neoadjuvant immunotherapy for non–small cell lung cancer: State of the art. Cancer Commun. 2021, 41, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.S.; Le Teuff, G.; Shepherd, F.A.; Landais, C.; Hainaut, P.; Filipits, M.; Pirker, R.; Chevalier, T.L.; Graziano, S.; Kratze, R.; et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in re-sected non-small cell lung cancer. Ann. Oncol. 2017, 28, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Zaric, B.; Brcic, L.; Buder, A.; Brandstetter, A.; Buresch, J.O.; Traint, S.; Kovacevic, T.; Stojsic, V.; Perin, B.; Pirker, R.; et al. PD-1 and PD-L1 Protein Expression Predict Survival in Completely Resected Lung Adenocarcinoma. Clin. Lung Cancer 2018, 19, e957–e963. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, M.; D’Incecco, A.; Ligorio, C.; Damiani, S.; Puccetti, M.; Bravaccini, S.; Terracciano, L.; Bennati, C.; Minuti, G.; Vecchiarelli, S.; et al. Programmed death ligand 1 expression in early stage, resectable non-small cell lung cancer. Oncotarget 2019, 10, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Su, L.; Qian, C. Circulating tumor DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef]
- Nagasaka, M.; Uddin, M.H.; Al-Hallak, M.N.; Rahman, S.; Balasubramanian, S.; Sukari, A.; Azmi, A.S. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer. Mol. Cancer 2021, 20, 82. [Google Scholar] [CrossRef]
- Czaplicka, M.; Niciński, K.; Nowicka, A.; Szymborski, T.; Chmielewska, I.; Trzcińska-Danielewicz, J.; Girstun, A.; Kamińska, A. Effect of Varying Expression of EpCAM on the Efficiency of CTCs Detection by SERS-Based Immunomagnetic Optofluidic Device. Cancers 2020, 12, 3315. [Google Scholar] [CrossRef]
- Osman, A.M.; Korashi, H.I. PET/CT implication on bronchogenic carcinoma TNM staging and follow-up using RECIST/PERCIST criteria: A comparative study with CT. Egypt. J. Radiol. Nucl. Med. 2020, 51, 16. [Google Scholar] [CrossRef]
- Lang, D.; Wahl, G.; Poier, N.; Graf, S.; Kiesl, D.; Lamprecht, B.; Gabriel, M. Impact of PET/CT for Assessing Response to Immunotherapy—A Clinical Perspective. J. Clin. Med. 2020, 9, 3483. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Leal, J.P.; Fu, W.; Wang, H.; Chaft, J.E.; Hellmann, M.D.; Pomper, M.; Forde, P.M. CT and PET radiomic features associated with major pathologic response to neoadjuvant immunotherapy in early-stage non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2020, 38, 9031. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.; Ardizzoni, A.; Barlesi, F.; Cho, B.; De Marchi, P.; Goto, Y.; Lu, S.; Paz-Ares, L.; Spigel, D.; Thomas, M.; et al. P2.01-02 CANOPY-A: A Phase 3 Study of Canakinumab as Adjuvant Therapy in Patients with Surgically Resected NSCLC. J. Thorac. Oncol. 2019, 14, S638–S639. [Google Scholar] [CrossRef]
- Garrido, P.; Pujol, L.J.; Kim, E.S.; Lee, M.J.; Tsuboi, M.; Gómez-Rueda, A.; Benito, A.; Moreno, N.; Gorospe, L.; Dong, T.; et al. Canakinumab with and without pembrolizumab in patients with resectable non-small-cell lung cancer: CANOPY-N study design. Future Oncol. 2021, 17, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
| Neoadjuvant Immunotherapy Clinical Trials | |||
|---|---|---|---|
| Study | Active Treatment | MPR Rates | CPR Rates |
| NEOSTAR | Nivolumab vs. nivolumab + ipilimumab | 24% vs. 50% | 10% vs. 38% |
| LCMC3 | Atezolizumab 2 cycles before surgery and 1 year after surgery | 19% | 5% |
| Neoadjuvant Chemoimmunotherapy Clinical Trials | |||
| NADIM | Nivolumab + paclitaxel and carboplatin every 3 weeks, followed by adjuvant nivolumab for 1 year | 83% | 71% |
| CHECKMATE 816 | Nivolumab + 3 cycles of chemotherapy vs. 3 cycles of chemotherapy | 36.9% vs. 8.9% | 24% vs. 2.2% |
| MPR | major pathologic response is currently defined as an “estimated size of viable tumor divided by the size of the tumor bed” of 10% or less |
| CPR or pCR | complete pathologic response was defined as the absence of tumor cells in all resected specimens |
| Tumor bed | applies to the location of the pretreated tumor and includes viable tumor, necrotic areas, and stroma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewska, I.; Stencel, K.; Kalinka, E.; Ramlau, R.; Krawczyk, P. Neoadjuvant and Adjuvant Immunotherapy in Non-Small Cell Lung Cancer—Clinical Trials Experience. Cancers 2021, 13, 5048. https://doi.org/10.3390/cancers13205048
Chmielewska I, Stencel K, Kalinka E, Ramlau R, Krawczyk P. Neoadjuvant and Adjuvant Immunotherapy in Non-Small Cell Lung Cancer—Clinical Trials Experience. Cancers. 2021; 13(20):5048. https://doi.org/10.3390/cancers13205048
Chicago/Turabian StyleChmielewska, Izabela, Katarzyna Stencel, Ewa Kalinka, Rodryg Ramlau, and Paweł Krawczyk. 2021. "Neoadjuvant and Adjuvant Immunotherapy in Non-Small Cell Lung Cancer—Clinical Trials Experience" Cancers 13, no. 20: 5048. https://doi.org/10.3390/cancers13205048
APA StyleChmielewska, I., Stencel, K., Kalinka, E., Ramlau, R., & Krawczyk, P. (2021). Neoadjuvant and Adjuvant Immunotherapy in Non-Small Cell Lung Cancer—Clinical Trials Experience. Cancers, 13(20), 5048. https://doi.org/10.3390/cancers13205048






