18F-FDG-PET/CT in Patients with Advanced, Radioiodine Refractory Thyroid Cancer Treated with Lenvatinib
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Evaluation of Treatment Response
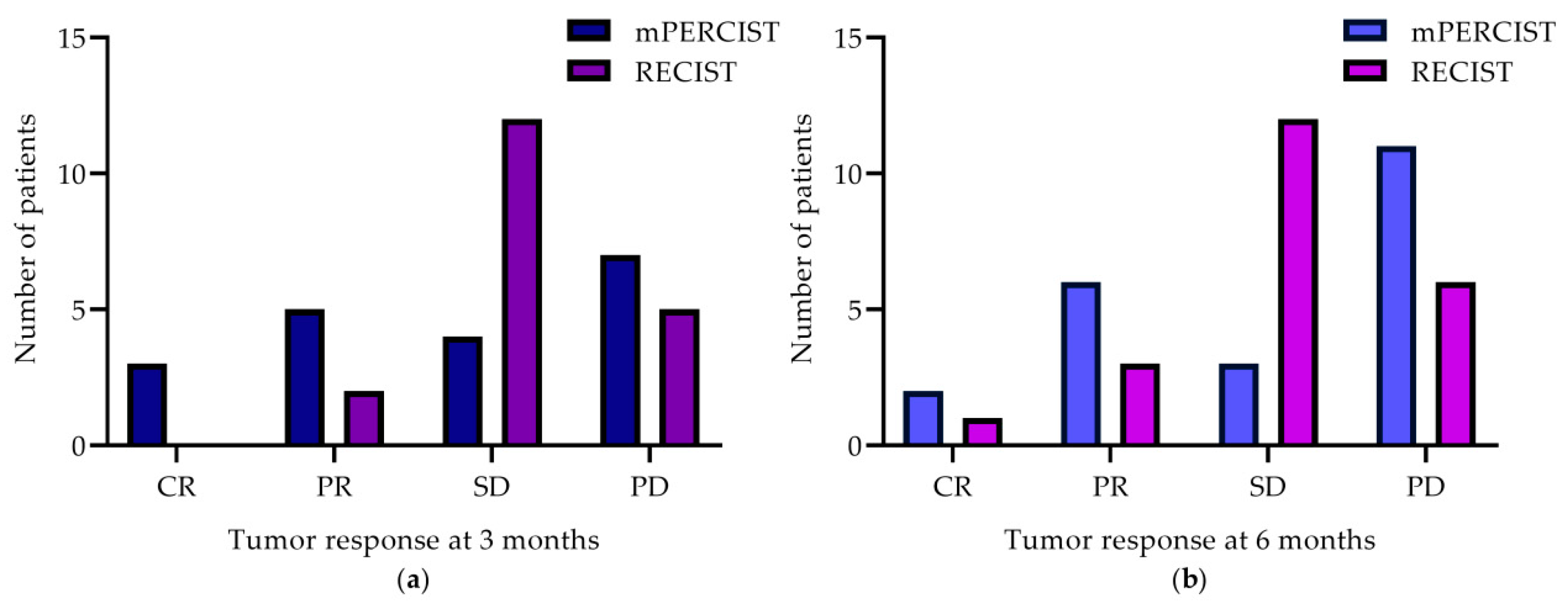
2.2.1. Treatment Response According to mPERCIST and RECIST
2.2.2. Additional Single PET-Parameters
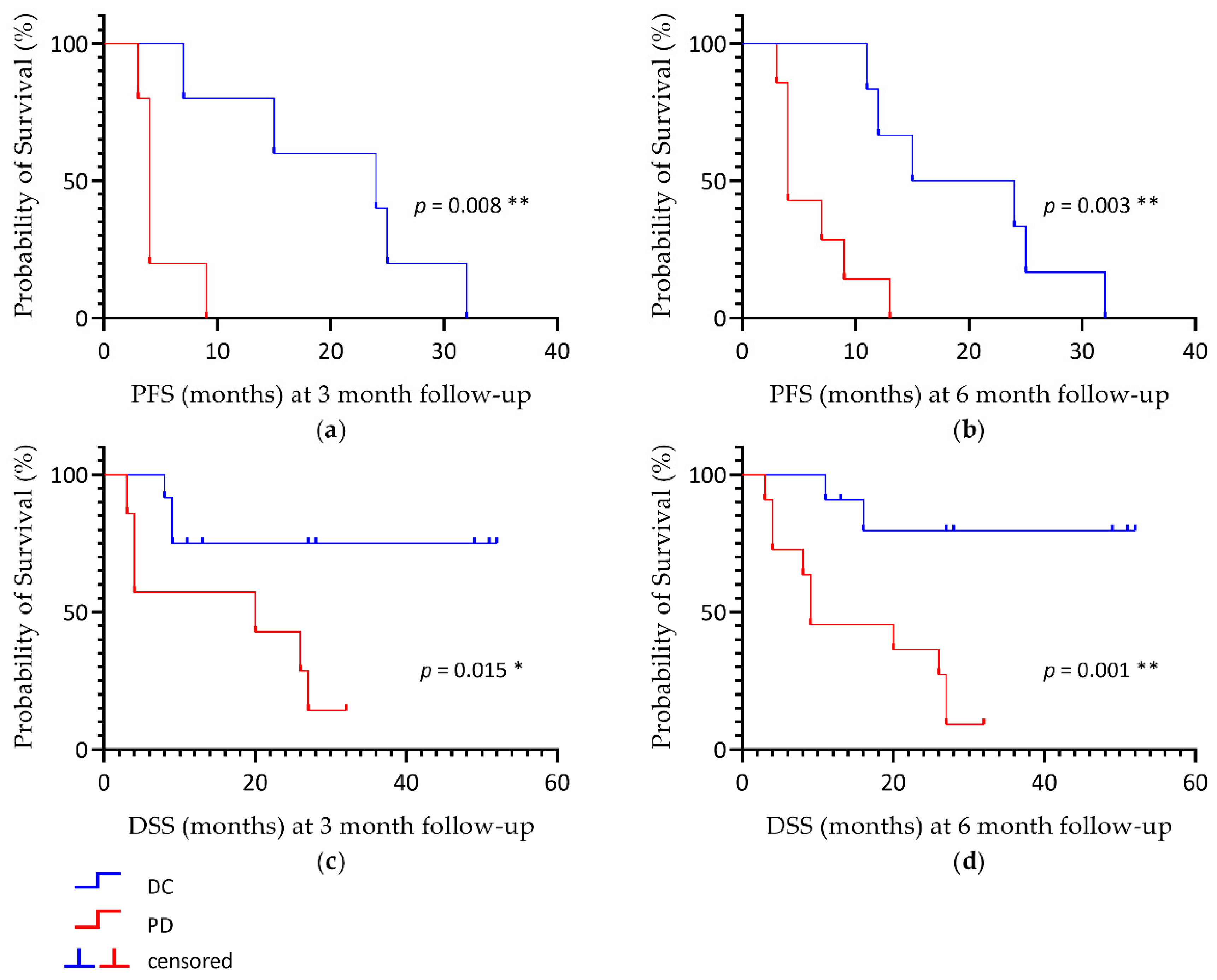
2.3. Outcome Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Ethics Statement
4.3. Imaging Techniques
4.4. Response Evaluation
4.4.1. RECIST 1.1
4.4.2. mPERCIST and Other PET-Parameters
4.5. Outcome Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlumberger, M.; Sherman, S.I. Approach to the patient with advanced differentiated thyroid cancer. Eur. J. Endocrinol. 2012, 166, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Links, T.P.; van Tol, K.M.; Jager, P.L.; Plukker, J.T.; Piers, D.A.; Boezen, H.M.; Dullaart, R.P.; de Vries, E.G.; Sluiter, W.J. Life expectancy in differentiated thyroid cancer: A novel approach to survival analysis. Endocr. Relat. Cancer 2005, 12, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Eustatia-Rutten, C.F.; Corssmit, E.P.; Biermasz, N.R.; Pereira, A.M.; Romijn, J.A.; Smit, J.W. Survival and death causes in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M. Management of refractory thyroid cancers. Ann. D’endocrinol. 2011, 72, 149–157. [Google Scholar] [CrossRef]
- Schlumberger, M.J. Diagnostic follow-up of well-differentiated thyroid carcinoma: Historical perspective and current status. J. Endocrinol. Investig. 1999, 22, 3–7. [Google Scholar]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Nair, A.; Lemery, S.J.; Yang, J.; Marathe, A.; Zhao, L.; Zhao, H.; Jiang, X.; He, K.; Ladouceur, G.; Mitra, A.K.; et al. FDA Approval Summary: Lenvatinib for Progressive, Radio-iodine-Refractory Differentiated Thyroid Cancer. Clin. Cancer Res. 2015, 21, 5205–5208. [Google Scholar] [CrossRef]
- Schneider, T.C.; Abdulrahman, R.M.; Corssmit, E.P.; Morreau, H.; Smit, J.W.; Kapiteijn, E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: Final results of a phase II trial. Eur. J. Endocrinol. 2012, 167, 643–650. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Habra, M.A. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat. Rev. 2016, 42, 47–55. [Google Scholar] [CrossRef]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Yamamoto, Y.; Funahashi, Y.; Tsuruoka, A.; Watanabe, T.; Wakabayashi, T.; Uenaka, T.; Asada, M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer 2008, 122, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Ziai, D.; Wagner, T.; El Badaoui, A.; Hitzel, A.; Woillard, J.B.; Melloni, B.; Monteil, J. Therapy response evaluation with FDG-PET/CT in small cell lung cancer: A prognostic and comparison study of the PERCIST and EORTC criteria. Cancer Imaging 2013, 13, 73–80. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kameyama, R.; Murota, M.; Bandoh, S.; Ishii, T.; Nishiyama, Y. Early assessment of therapeutic response using FDG PET in small cell lung cancer. Mol. Imaging Biol. 2009, 11, 467–472. [Google Scholar] [CrossRef]
- Joo Hyun, O.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Jacene, H.A.; Leboulleux, S.; Baba, S.; Chatzifotiadis, D.; Goudarzi, B.; Teytelbaum, O.; Horton, K.M.; Kamel, I.; Macura, K.J.; Tsai, H.L.; et al. Assessment of interobserver reproducibility in quantitative 18F-FDG PET and CT measurements of tumor response to therapy. J. Nucl. Med. 2009, 50, 1760–1769. [Google Scholar] [CrossRef]
- Hildebrandt, M.G.; Gerke, O.; Baun, C.; Falch, K.; Hansen, J.A.; Farahani, Z.A.; Petersen, H.; Larsen, L.B.; Duvnjak, S.; Buskevica, I.; et al. [18F]Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET)/Computed Tomography (CT) in Suspected Recurrent Breast Cancer: A Prospective Comparative Study of Dual-Time-Point FDG-PET/CT, Contrast-Enhanced CT, and Bone Scintigraphy. J. Clin. Oncol. 2016, 34, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gonen, M.; Dickler, M.; Weber, W.A. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, C.M.; Choi, H.J.; Lee, W.J.; Song, S.Y.; Lee, J.H.; Lee, J.D. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative ¹⁸F-FDG PET/CT in Patients with Pancreatic Cancer. J. Nucl. Med. 2014, 55, 898–904. [Google Scholar] [CrossRef]
- Zoller, M.; Kohlfuerst, S.; Igerc, I.; Kresnik, E.; Gallowitsch, H.J.; Gomez, I.; Lind, P. Combined PET/CT in the follow-up of differentiated thyroid carcinoma: What is the impact of each modality? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Vural, G.U.; Akkas, B.E.; Ercakmak, N.; Basu, S.; Alavi, A. Prognostic significance of FDG PET/CT on the follow-up of patients of differentiated thyroid carcinoma with negative 131I whole-body scan and elevated thyroglobulin levels: Correlation with clinical and histopathologic characteristics and long-term follow-up data. Clin. Nucl. Med. 2012, 37, 953–959. [Google Scholar] [CrossRef]
- Dong, M.J.; Liu, Z.F.; Zhao, K.; Ruan, L.X.; Wang, G.L.; Yang, S.Y.; Sun, F.; Luo, X.G. Value of 18F-FDG-PET/PET-CT in differentiated thyroid carcinoma with radioiodine-negative whole-body scan: A meta-analysis. Nucl. Med. Commun. 2009, 30, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.L.; Mankoff, D.A.; Goulart, B.H.; Eaton, K.D.; Capell, P.T.; Kell, E.M.; Bauman, J.E.; Martins, R.G. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin. Cancer Res. 2010, 16, 5260–5268. [Google Scholar] [CrossRef]
- Marotta, V.; Ramundo, V.; Camera, L.; Del Prete, M.; Fonti, R.; Esposito, R.; Palmieri, G.; Salvatore, M.; Vitale, M.; Colao, A.; et al. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: Efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin. Endocrinol. 2013, 78, 760–767. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl 1), 122s–150s. [Google Scholar] [CrossRef]
- Pinker, K.; Riedl, C.C.; Ong, L.; Jochelson, M.; Ulaner, G.A.; McArthur, H.; Dickler, M.; Gönen, M.; Weber, W.A. The Impact That Number of Analyzed Metastatic Breast Cancer Lesions Has on Response Assessment by 18F-FDG PET/CT Using PERCIST. J. Nucl. Med. 2016, 57, 1102–1104. [Google Scholar] [CrossRef]
- Michl, M.; Lehner, S.; Paprottka, P.M.; Ilhan, H.; Bartenstein, P.; Heinemann, V.; Boeck, S.; Albert, N.L.; Fendler, W.P. Use of PERCIST for Prediction of Progression-Free and Overall Survival After Radioembolization for Liver Metastases from Pancreatic Cancer. J. Nucl. Med. 2016, 57, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Devriese, J.; Beels, L.; Maes, A.; Van de Wiele, C.; Pottel, H. Impact of PET reconstruction protocols on quantification of lesions that fulfil the PERCIST lesion inclusion criteria. EJNMMI Phys. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Choi, H. Response evaluation of gastrointestinal stromal tumors. Oncologist 2008, 13 (Suppl 2), 4–7. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.M.; Chuang, H.H.; Madewell, J.E.; Ueno, N.T. Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST. J. Cancer 2010, 1, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Bénard, F.; Sterman, D.; Smith, R.J.; Kaiser, L.R.; Albelda, S.M.; Alavi, A. Prognostic value of FDG PET imaging in malignant pleural mesothelioma. J. Nucl. Med. 1999, 40, 1241–1245. [Google Scholar]
- Bruzzi, P.; Del Mastro, L.; Sormani, M.P.; Bastholt, L.; Danova, M.; Focan, C.; Fountzilas, G.; Paul, J.; Rosso, R.; Venturini, M. Objective response to chemotherapy as a potential surrogate end point of survival in metastatic breast cancer patients. J. Clin. Oncol. 2005, 23, 5117–5125. [Google Scholar] [CrossRef]
- Leboulleux, S.; Bastholt, L.; Krause, T.; de la Fouchardiere, C.; Tennvall, J.; Awada, A.; Gómez, J.M.; Bonichon, F.; Leenhardt, L.; Soufflet, C.; et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 2 trial. Lancet Oncol. 2012, 13, 897–905. [Google Scholar] [CrossRef]
- Sher, A.; Lacoeuille, F.; Fosse, P.; Vervueren, L.; Cahouet-Vannier, A.; Dabli, D.; Bouchet, F.; Couturier, O. For avid glucose tumors, the SUV peak is the most reliable parameter for [(18)F]FDG-PET/CT quantification, regardless of acquisition time. EJNMMI Res. 2016, 6, 21. [Google Scholar] [CrossRef]
- Manohar, P.M.; Beesley, L.J.; Bellile, E.L.; Worden, F.P.; Avram, A.M. Prognostic Value of FDG-PET/CT Metabolic Parameters in Metastatic Radioiodine-Refractory Differentiated Thyroid Cancer. Clin. Nucl. Med. 2018, 43, 641–647. [Google Scholar] [CrossRef]
- Benz, M.R.; Allen-Auerbach, M.S.; Eilber, F.C.; Chen, H.J.; Dry, S.; Phelps, M.E.; Czernin, J.; Weber, W.A. Combined assessment of metabolic and volumetric changes for assessment of tumor response in patients with soft-tissue sarcomas. J. Nucl. Med. 2008, 49, 1579–1584. [Google Scholar] [CrossRef]
- Robbins, R.J.; Wan, Q.; Grewal, R.K.; Reibke, R.; Gonen, M.; Strauss, H.W.; Tuttle, R.M.; Drucker, W.; Larson, S.M. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J. Clin. Endocrinol. Metab. 2006, 91, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Guillem, J.G.; Moore, H.G.; Akhurst, T.; Klimstra, D.S.; Ruo, L.; Mazumdar, M.; Minsky, B.D.; Saltz, L.; Wong, W.D.; Larson, S. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: A means for determining longterm outcomes of rectal cancer. J. Am. Coll. Surg. 2004, 199, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Erdi, Y.E.; Macapinlac, H.; Rosenzweig, K.E.; Humm, J.L.; Larson, S.M.; Erdi, A.K.; Yorke, E.D. Use of PET to monitor the response of lung cancer to radiation treatment. Eur. J. Nucl. Med. 2000, 27, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mohan, A.; Bhalla, A.S.; Sharma, M.C.; Vishnubhatla, S.; Das, C.J.; Pandey, A.K.; Sekhar Bal, C.; Patel, C.D.; Sharma, P.; et al. Role of Various Metabolic Parameters Derived From Baseline 18F-FDG PET/CT as Prognostic Markers in Non-Small Cell Lung Cancer Patients Undergoing Platinum-Based Chemotherapy. Clin. Nucl. Med. 2018, 43, e8–e17. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.L.; Morris, L.G.T.; Ho, A.S. Selective use of radioactive iodine (RAI) in thyroid cancer: No longer “one size fits all”. Eur. journal Surg. Oncol. 2018, 44, 348–356. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.; Wan, H.; Fang, Y.; Zheng, R.; Zhang, W.; Liu, Y.; Chen, C.; Wu, N. Prognostic Value of Volume-Based Metabolic Parameters Obtained by 18F-FDG-PET/CT in Patients With Locally Advanced Squamous Cell Cervical Carcinoma. J. Comput. Assist. Tomogr. 2018, 42, 429–434. [Google Scholar] [CrossRef]
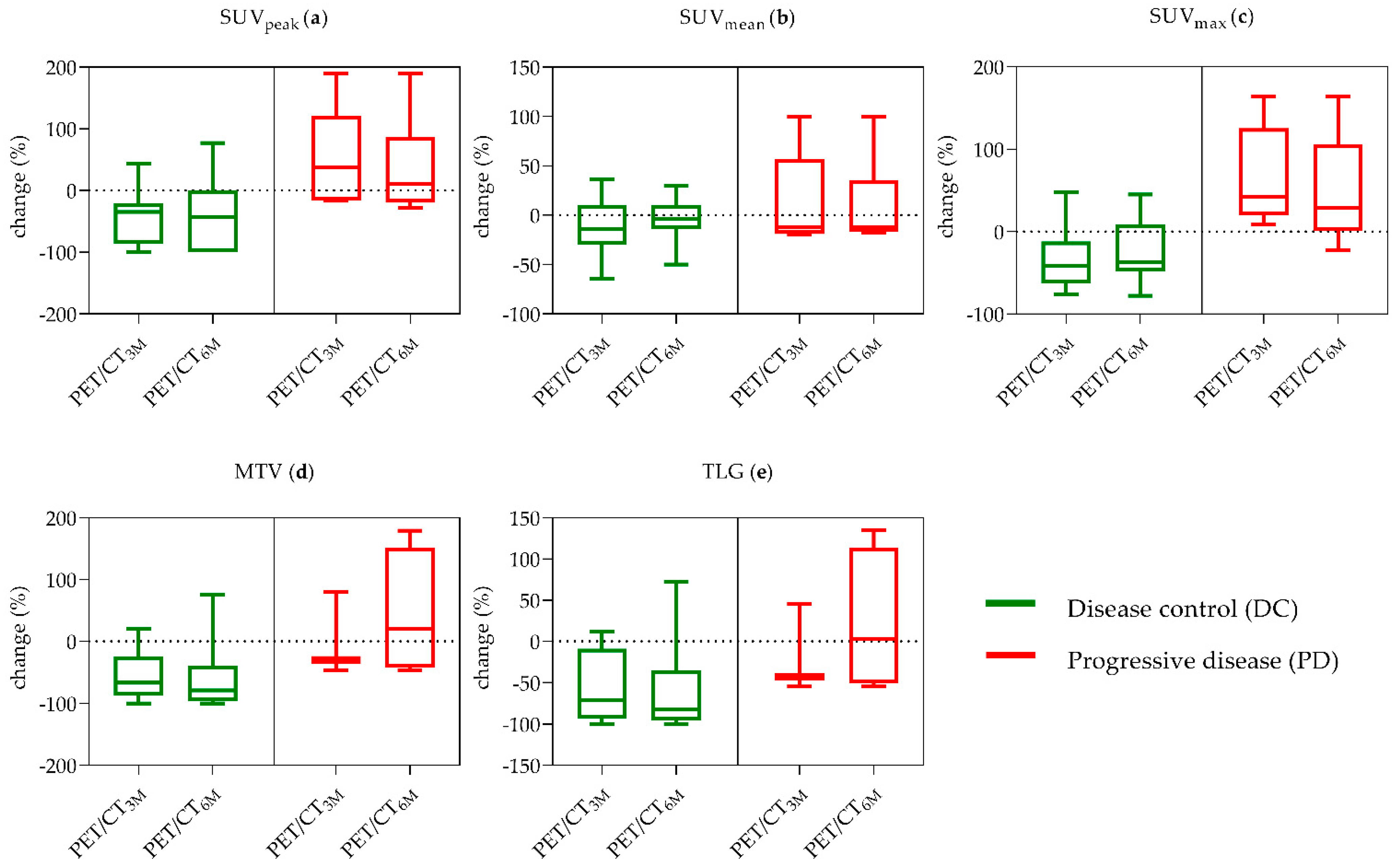
| Mean Change ±SD (%) | SUVpeak | SUVmean | SUVmax | MTV | TLG |
|---|---|---|---|---|---|
| Baseline to 3 Month Follow-Up | |||||
| DC (n = 13/18) | −43% ± 45% | −12% ± 28% | −33% ± 35% | −56% ± 38% | −56% ± 42% |
| PD (n = 5/19) | 49% ± 84% | 12% ± 51% | 66% ± 62% | 439% ± 718% | 797% ± 1489% |
| p-value | 0.004 ** | 0.336 | 0.003 ** | 0.010 * | 0.019 * |
| Baseline to 6 Month Follow-Up | |||||
| DC (n = 15/21) | −42% ± 57% | −2% ± 21% | −24% ± 35% | −23% ± 152% | −10% ± 189% |
| PD (n = 6/22) | 36% ± 81% | 9% ± 46% | 49% ± 68% | 394% ± 652% | 687% ± 1358% |
| p-value | 0.023 * | 0.677 | 0.008 ** | 0.006 ** | 0.011 * |
| p-Value | mPERCIST | RECIST | ||
|---|---|---|---|---|
| 3 Months | 6 Months | 3 Months | 6 Months | |
| PFS | 0.008 ** | 0.003 ** | 0.196 | 0.114 |
| DSS | 0.015 * | 0.001 ** | 0.046 * | 0.039 * |
| Response Criteria | |
|---|---|
| Complete response (CR) | Normalization of all lesions to SUVpeak less than mean liver SUV and indistinguishable from surrounding background |
| Partial response (PR) | >30% decrease in SUVpeak; minimum 0.8 unit decrease in SUVpeak |
| Stable disease (SD) | Does not meet other criteria |
| Progressive disease (PD) | >30% increase in SUVpeak; minimum 0.8 unit increase in SUVpeak>75% increase in TLG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmaddy, F.; Burgard, C.; Beyer, L.; Koehler, V.F.; Bartenstein, P.; Fabritius, M.P.; Geyer, T.; Wenter, V.; Ilhan, H.; Spitzweg, C.; et al. 18F-FDG-PET/CT in Patients with Advanced, Radioiodine Refractory Thyroid Cancer Treated with Lenvatinib. Cancers 2021, 13, 317. https://doi.org/10.3390/cancers13020317
Ahmaddy F, Burgard C, Beyer L, Koehler VF, Bartenstein P, Fabritius MP, Geyer T, Wenter V, Ilhan H, Spitzweg C, et al. 18F-FDG-PET/CT in Patients with Advanced, Radioiodine Refractory Thyroid Cancer Treated with Lenvatinib. Cancers. 2021; 13(2):317. https://doi.org/10.3390/cancers13020317
Chicago/Turabian StyleAhmaddy, Freba, Caroline Burgard, Leonie Beyer, Viktoria Florentine Koehler, Peter Bartenstein, Matthias P. Fabritius, Thomas Geyer, Vera Wenter, Harun Ilhan, Christine Spitzweg, and et al. 2021. "18F-FDG-PET/CT in Patients with Advanced, Radioiodine Refractory Thyroid Cancer Treated with Lenvatinib" Cancers 13, no. 2: 317. https://doi.org/10.3390/cancers13020317
APA StyleAhmaddy, F., Burgard, C., Beyer, L., Koehler, V. F., Bartenstein, P., Fabritius, M. P., Geyer, T., Wenter, V., Ilhan, H., Spitzweg, C., & Todica, A. (2021). 18F-FDG-PET/CT in Patients with Advanced, Radioiodine Refractory Thyroid Cancer Treated with Lenvatinib. Cancers, 13(2), 317. https://doi.org/10.3390/cancers13020317





