Patterns and Relevance of Langerhans Islet Invasion in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Histological Specimens
2.2. Cells Lines
2.3. Immunofluorescence
2.4. Islet Invasion Severity Score
2.5. 3D Migration Assay
2.6. Islet Cell Mass Quantification
2.7. Statistical Analysis
3. Results
3.1. Langerhans Islet Invasion in Human PDAC Exhibits Four Different Morphological Patterns of Severity
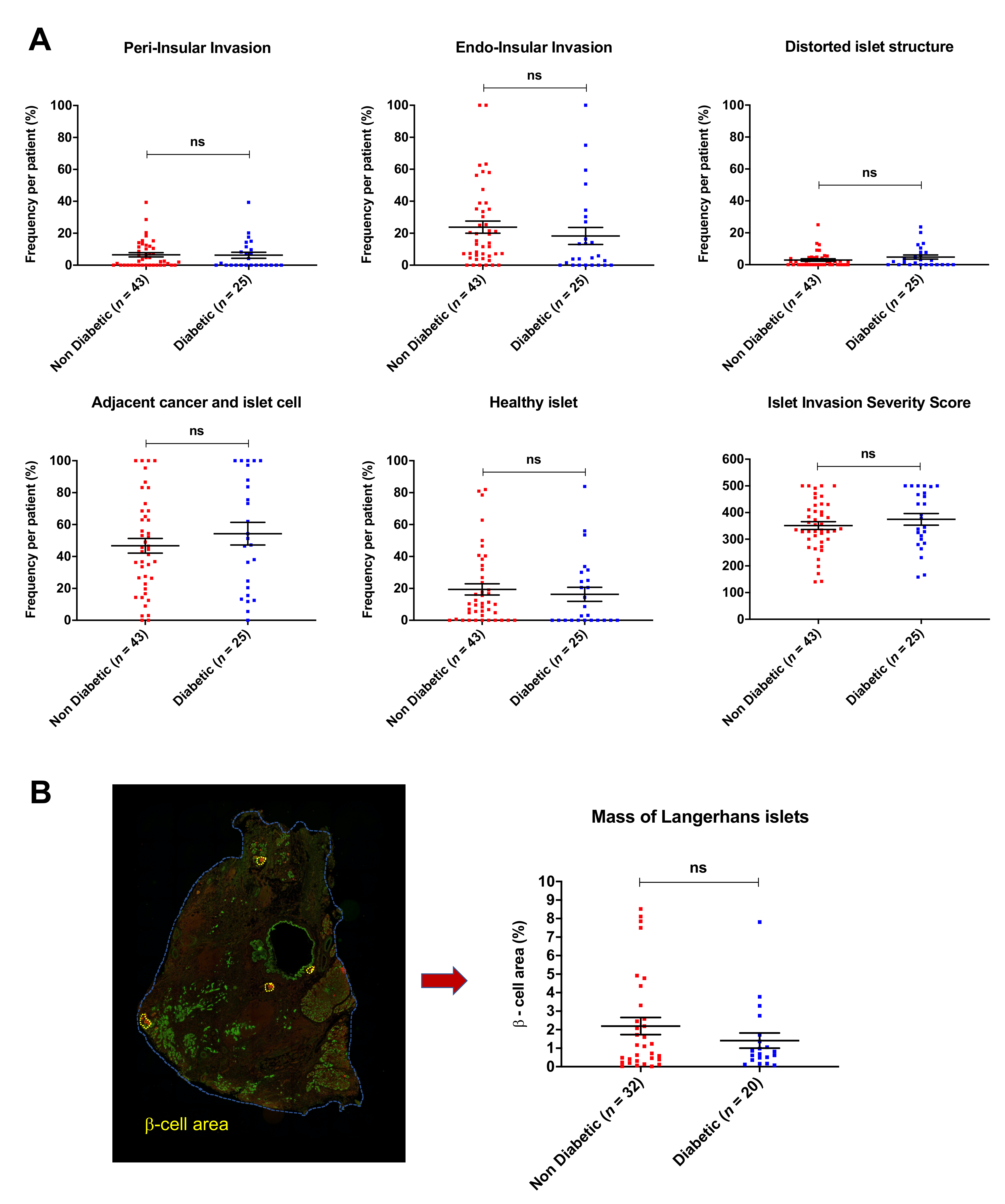
3.2. Islet Invasion Is Not Associated with Diabetes Mellitus
3.3. Impact of Islet Invasion Pattern on Patient Survival
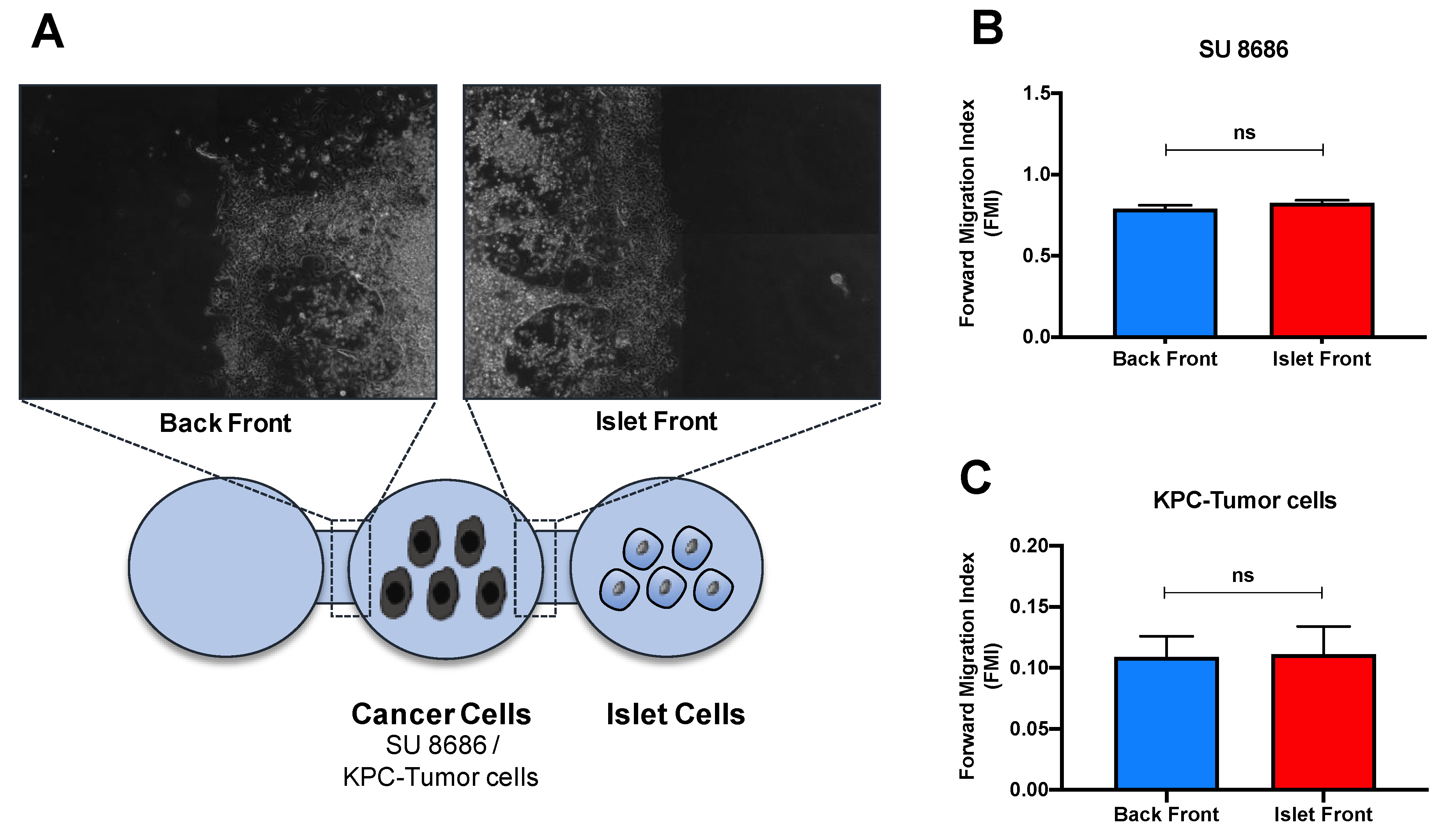
3.4. Islet Invasion Is Not Caused by the Endocrine/Exocrine Crosstalk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Timmons, L.J.; Ransom, J.; de Andrade, M.; Petersen, G.M. Pancreatic cancer-associated diabetes mellitus: Prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008, 134, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Pannala, R.; Basu, A.; Petersen, G.M.; Chari, S.T. New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009, 10, 88–95. [Google Scholar] [CrossRef]
- Permert, J.; Ihse, I.; Jorfeldt, L.; von Schenck, H.; Arnqvist, H.J.; Larsson, J. Pancreatic cancer is associated with impaired glucose metabolism. Eur. J. Surg. 1993, 159, 101–107. [Google Scholar] [PubMed]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, P.; Vander Hoorn, S.; Christophi, C.; Nikfarjam, M. Association of diabetes mellitus and pancreatic adenocarcinoma: A meta-analysis of 88 studies. Ann. Surg. Oncol. 2014, 21, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Magruder, J.T.; Elahi, D.; Andersen, D.K. Diabetes and pancreatic cancer: Chicken or egg? Pancreas 2011, 40, 339–351. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, G.; Ling, Y.; Chen, G.; Zhou, T. The early diagnosis of pancreatic cancer and diabetes: What’s the relationship? J. Gastrointest Oncol. 2014, 5, 481–488. [Google Scholar] [CrossRef]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef]
- Basso, D.; Brigato, L.; Veronesi, A.; Panozzo, M.P.; Amadori, A.; Plebani, M. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res. 1995, 15, 2585–2588. [Google Scholar]
- Girelli, C.M.; Reguzzoni, G.; Limido, E.; Savastano, A.; Rocca, F. Pancreatic carcinoma: Differences between patients with or without diabetes mellitus. Recenti Prog. Med. 1995, 86, 143–146. [Google Scholar] [PubMed]
- Pannala, R.; Leibson, C.L.; Rabe, K.G.; Timmons, L.J.; Ransom, J.; de Andrade, M.; Petersen, G.M.; Chari, S.T. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am. J. Gastroenterol. 2009, 104, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Permert, J.; Herrington, M.; Kazakoff, K.; Pour, P.M.; Adrian, T.E. Early changes in islet hormone secretion in the hamster pancreatic cancer model. Teratog. Carcinog. Mutagen. 2001, 21, 59–67. [Google Scholar] [CrossRef]
- Sah, R.P.; Nagpal, S.J.; Mukhopadhyay, D.; Chari, S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Fangmann, L.; Teller, S.; Stupakov, P.; Friess, H.; Ceyhan, G.O.; Demir, I.E. 3D Cancer Migration Assay with Schwann Cells. Methods Mol. Biol. 2018, 1739, 317–325. [Google Scholar] [CrossRef]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- Permert, J.; Ihse, I.; Jorfeldt, L.; von Schenck, H.; Arnquist, H.J.; Larsson, J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br. J. Surg. 1993, 80, 1047–1050. [Google Scholar] [CrossRef]
- Basso, D.; Plebani, M.; Fogar, P.; Del Favero, G.; Briani, G.; Meggiato, T.; Panozzo, M.P.; Ferrara, C.; D’Angeli, F.; Burlina, A. Beta-cell function in pancreatic adenocarcinoma. Pancreas 1994, 9, 332–335. [Google Scholar] [CrossRef]
- Cersosimo, E.; Pisters, P.W.; Pesola, G.; McDermott, K.; Bajorunas, D.; Brennan, M.F. Insulin secretion and action in patients with pancreatic cancer. Cancer 1991, 67, 486–493. [Google Scholar] [CrossRef]
- Fox, J.N.; Frier, B.M.; Armitage, M.; Ashby, J.P. Abnormal insulin secretion in carcinoma of the pancreas: Response to glucagon stimulation. Diabet Med. 1985, 2, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duell, E.J.; Yu, K.; Risch, H.A.; Olson, S.H.; Kooperberg, C.; Wolpin, B.M.; Jiao, L.; Dong, X.; Wheeler, B.; et al. Pathway analysis of genome-wide association study data highlights pancreatic development genes as susceptibility factors for pancreatic cancer. Carcinogenesis 2012, 33, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Ahsan, H. Genome-wide “pleiotropy scan” identifies HNF1A region as a novel pancreatic cancer susceptibility locus. Cancer Res. 2011, 71, 4352–4358. [Google Scholar] [CrossRef]
- Schrader, H.; Menge, B.A.; Schneider, S.; Belyaev, O.; Tannapfel, A.; Uhl, W.; Schmidt, W.E.; Meier, J.J. Reduced pancreatic volume and beta-cell area in patients with chronic pancreatitis. Gastroenterology 2009, 136, 513–522. [Google Scholar] [CrossRef]
- Bateman, A.C.; Turner, S.M.; Thomas, K.S.; McCrudden, P.R.; Fine, D.R.; Johnson, P.A.; Johnson, C.D.; Iredale, J.P. Apoptosis and proliferation of acinar and islet cells in chronic pancreatitis: Evidence for differential cell loss mediating preservation of islet function. Gut 2002, 50, 542–548. [Google Scholar] [CrossRef]
- Van Roessel, S.; Kasumova, G.G.; Verheij, J.; Najarian, R.M.; Maggino, L.; de Pastena, M.; Malleo, G.; Marchegiani, G.; Salvia, R.; Ng, S.C.; et al. International Validation of the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients With Resected Pancreatic Cancer. JAMA Surg. 2018, 153, e183617. [Google Scholar] [CrossRef]
- Ceyhan, G.O.; Liebl, F.; Maak, M.; Schuster, T.; Becker, K.; Langer, R.; Demir, I.E.; Hartel, M.; Friess, H.; Rosenberg, R. The severity of neural invasion is a crucial prognostic factor in rectal cancer independent of neoadjuvant radiochemotherapy. Ann. Surg. 2010, 252, 797–804. [Google Scholar] [CrossRef]
- Liebl, F.; Demir, I.E.; Rosenberg, R.; Boldis, A.; Yildiz, E.; Kujundzic, K.; Kehl, T.; Dischl, D.; Schuster, T.; Maak, M.; et al. The severity of neural invasion is associated with shortened survival in colon cancer. Clin. Cancer Res. 2013, 19, 50–61. [Google Scholar] [CrossRef]


| All Patients | ||
|---|---|---|
| n = 68 | ||
| n | % | |
| Age (median, years) | 66.0 | |
| Sex | ||
| Male | 43 | 63.2 |
| Female | 25 | 36.8 |
| Neoadjuvant Therapy | 2 | 2.9 |
| Type of surgery | ||
| Head resection | 42 | 61.8 |
| Distal pancreatectomy | 14 | 20.7 |
| Total pancreatectomy | 12 | 17.6 |
| Tumor stage | ||
| T1 | 0 | 0 |
| T2 | 4 | 5.9 |
| T3 | 54 | 79.4 |
| T4 | 10 | 14.7 |
| Lymph node | ||
| N0 | 27 | 39.7 |
| N1 | 20 | 29.4 |
| N2 | 21 | 30.9 |
| Grading | ||
| 1 | 4 | 5.9 |
| 2 | 36 | 52.9 |
| 3 | 28 | 41.2 |
| Resection margin | ||
| R0 | 40 | 58.8 |
| R1 | 22 | 32.4 |
| RX | 6 | 8.8 |
| UICC Classification (8th) | ||
| I | 2 | 2.9 |
| IIA | 24 | 35.3 |
| IIB | 25 | 36.8 |
| III | 9 | 13.2 |
| IV ** | 8 | 11.8 |
| Median survival (months) | 20.7 | (n = 57) |
| Diabetes mellitus | ||
| Yes | 25 | 36.8 |
| No | 43 | 63.2 |
| UICC | n | Diabetes (%) | No Diabetes (%) | IISS (Mean) | Low IISS | High IISS |
|---|---|---|---|---|---|---|
| IB | 19 | 6 (32) | 13 (68) | 360 | 10 | 9 |
| IIA | 7 | 3 (43) | 4 (57) | 363 | 3 | 4 |
| IIB | 15 | 8 (53) | 7 (47) | 332 | 10 | 5 |
| III | 19 | 3 (16) | 16 (84) | 365 | 10 | 9 |
| IV | 8 | 5 (62) | 3 (38) | 397 | 3 | 5 |
| total | 68 | 25 (37) | 43 (63) | 360 | 36 | 32 |
| ns | ns | ns | ns | ns |
| Co-Expression Insulin/CK-19 | n (%) | IISS (Mean) | mOS (Months) |
|---|---|---|---|
| Yes | 19 (28) | 385 | 19 |
| No | 49 (72) | 350 | 22 |
| p = 0.20 | log rank = 0.537 |
| Type of Resection | n (%) | IISS (Mean) | Low IISS | High IISS | mOS |
|---|---|---|---|---|---|
| Head resection | 42 (61.8) | 354 | 24 | 18 | 19 |
| Total pancreatectomy | 12 (17.6) | 419 * | 2 | 10 | 18 ** |
| Distal pancreatectomy | 14 (20.6) | 327 | 10 | 4 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goess, R.; Mutgan, A.C.; Çalışan, U.; Erdoğan, Y.C.; Ren, L.; Jäger, C.; Safak, O.; Stupakov, P.; Istvanffy, R.; Friess, H.; et al. Patterns and Relevance of Langerhans Islet Invasion in Pancreatic Cancer. Cancers 2021, 13, 249. https://doi.org/10.3390/cancers13020249
Goess R, Mutgan AC, Çalışan U, Erdoğan YC, Ren L, Jäger C, Safak O, Stupakov P, Istvanffy R, Friess H, et al. Patterns and Relevance of Langerhans Islet Invasion in Pancreatic Cancer. Cancers. 2021; 13(2):249. https://doi.org/10.3390/cancers13020249
Chicago/Turabian StyleGoess, Ruediger, Ayse Ceren Mutgan, Umut Çalışan, Yusuf Ceyhun Erdoğan, Lei Ren, Carsten Jäger, Okan Safak, Pavel Stupakov, Rouzanna Istvanffy, Helmut Friess, and et al. 2021. "Patterns and Relevance of Langerhans Islet Invasion in Pancreatic Cancer" Cancers 13, no. 2: 249. https://doi.org/10.3390/cancers13020249
APA StyleGoess, R., Mutgan, A. C., Çalışan, U., Erdoğan, Y. C., Ren, L., Jäger, C., Safak, O., Stupakov, P., Istvanffy, R., Friess, H., Ceyhan, G. O., & Demir, I. E. (2021). Patterns and Relevance of Langerhans Islet Invasion in Pancreatic Cancer. Cancers, 13(2), 249. https://doi.org/10.3390/cancers13020249








