Simple Summary
Immunotherapy has emerged as an attractive alternative to conventional therapies for the clinical management of prostate cancer (PCa); it not only specifically targets malignant cells while protecting healthy tissue, but it also appears to augment the therapeutic effect of existing treatments when used in combination. The identification of biomarkers with prognostic and preventive clinical significance has facilitated the incorporation of immune-targeted agents in clinical trials, with the aim to assess therapeutic efficacy in patient sub-populations that have been stratified on the basis of specific molecular traits and prognostic variables. This perfectly fits the rationale of precision medicine, which aims to match patients with targeted therapies so as to achieve the maximum clinical benefit. The numerous clinical trials currently evaluating multiple immunotherapeutic approaches in PCa patients, both alone and in combination with other treatments, offer much hope for achieving significant advances in the decision for precision treatment of the disease.
Abstract
Prostate cancer (PCa) is the most frequently diagnosed type of cancer among Caucasian males over the age of 60 and is characterized by remarkable heterogeneity and clinical behavior, ranging from decades of indolence to highly lethal disease. Despite the significant progress in PCa systemic therapy, therapeutic response is usually transient, and invasive disease is associated with high mortality rates. Immunotherapy has emerged as an efficacious and non-toxic treatment alternative that perfectly fits the rationale of precision medicine, as it aims to treat patients on the basis of patient-specific, immune-targeted molecular traits, so as to achieve the maximum clinical benefit. Antibodies acting as immune checkpoint inhibitors and vaccines entailing tumor-specific antigens seem to be the most promising immunotherapeutic strategies in offering a significant survival advantage. Even though patients with localized disease and favorable prognostic characteristics seem to be the ones that markedly benefit from such interventions, there is substantial evidence to suggest that the survival benefit may also be extended to patients with more advanced disease. The identification of biomarkers that can be immunologically targeted in patients with disease progression is potentially amenable in this process and in achieving significant advances in the decision for precision treatment of PCa.
1. Introduction
Prostate cancer (PCa), an age-related disease predominantly affecting men over the age of 60, is the most frequently diagnosed type of cancer and the second most common cause of cancer-related death, after skin cancer, among men worldwide [1,2]. The disease is characterized by remarkable heterogeneity, and patients with apparently similar histological features usually display a variety of clinical behavior and outcome, ranging from decades of indolence to highly lethal disease [3]. This is probably the reason behind the observed substantial mortality from aggressive disease, despite the majority of patients being diagnosed with slow-progressing or even inert PCa [2]. The disease has a greater prevalence in the West [4,5], yet considerable variability exists among certain populations; men of African ancestry appear more susceptible to developing PCa and have a worse prognosis than white men or men of Hispanic origin [6,7] whereas Hispanic men exhibit significantly lower incidence and mortality rates than non-Hispanic white men [8]. In addition to age and race, a family history also increases the risk of developing the disease by even two- to three-fold if the affected individual is a first-degree relative [9], thereby ranking PCa among the cancers with the highest heritability [10,11]. On the other hand, migrant studies have found that populations of the same race and origin may increase their risk of developing PCa over time by moving to countries with a higher incidence of the disease [12]; this suggests that, apart from genetic contributors, lifestyle, and environmental factors are also actively involved in the development of the disease. Such factors may include a diet high in red meat, milk products, processed food, fat content, and low in fruit and vegetables [9], as well as tobacco use, obesity, and lack of physical activity [12].
Therapeutic options range from active surveillance in cases of less aggressive disease, to radiation therapy for localized disease, and surgery in combination with cytotoxic therapy for more advanced disease. If the cancer is limited to the prostate, then it is described as localized disease and considered curable; if it has spread outside the prostate to the bones or other sites, then several targeted therapies can be used, including hormonal treatment, chemotherapy, radiotherapy, and immunotherapy [13,14]. Clinical outcome is significantly associated with age, underlying health conditions, cancer histology, and the extent of disease [15]. Suppression of androgen receptor (AR) signaling through androgen deprivation therapy (ADT) has been the primary therapeutic approach for metastatic PCa for more than 70 years, since its benefits were first reported by Charles Huggins in 1941 [16,17]. Nowadays, this translates to either surgical or medical castration, the latter including the use of luteinizing hormone-releasing hormone agonists or antagonists, regardless of whether anti-androgen drugs are being used or not [16]. Despite the high rate of progression-free survival (PFS) following ADT, with near-certain remissions usually lasting 1–2 years in the majority of cases, 30–50% of patients progress to castration-resistant prostate cancer (CRPC) and eventually relapse [18]. CRPC includes the spectrum of PCa ranging from asymptomatic disease to advanced CRPC (metastatic CRPC or mCRPC), characterized by an over-activation and over-expression of the AR, which results in the transcription of downstream target genes that promote carcinogenesis [19,20]. In patients with mCRPC the cancer cells usually spread to the bones and lymph nodes [21], ultimately developing therapeutic resistance regardless of the treatment modality applied, whether this is anti-androgen therapy, cytotoxic drugs, or radiopharmaceuticals. These patients have limited treatment options and a very bad prognosis [22]. Metastatic bone disease, in particular, is the main cause of PCa-related pain requiring palliative radiotherapy and of serious skeletal-related events such as bone fractures and spinal cord compression, often requiring orthopedic surgery, which greatly influence patient quality of life [23]. Considering that approximately one-fifth of the world’s population is estimated to be ≥ 60 years old by the year 2050 [22], this also highlights the profound socio-economic consequences of the disease and the urgency for devising new therapies.
Since the completion of the TAX327 trial in 2004, docetaxel plus prednisolone has been established as the first-line chemotherapeutic treatment for CRPC, offering a modest 2.5-month prolongation of median overall survival (OS) before the emergence of therapeutic resistance [16,24,25]. In the last decade or so, the development of new technologies for the characterization of PCa has led to significant progress in the field of systemic treatment and to the approval of additional drugs. These include the chemotherapeutic drug cabazitaxel [26], the androgen signaling inhibitors abiraterone, enzalutamide, apalutamide, and darolutamide [27,28,29], the alpha-emitter bone-seeking radioisotope radium-233 [30,31], and the immunotherapeutic drug Sipuleucel-T [32], all of which have demonstrated significant improvements in OS. However, despite the development of these relatively rapid therapeutic interventions, and the testing of many more such compounds in clinical trials, approved therapies are administered to relatively unselected patients, solely based on clinical characteristics such as performance status and tolerance [3]. Optimal sequencing and combinations of drugs are yet to be determined, as is also the selection of reliable biomarkers for predicting response to therapy. In this context, precision medicine aims to implement rational combination treatment schemes and to match patients with targeted therapies so as to optimize therapeutic effects and to prevent metastatic disease. Considering the remarkable heterogeneity of PCa, its molecular complexity, and its multifactorial nature, the incorporation of clinically valuable prognostic and predictive biomarker stratification for appropriate patient selection is potentially amenable in this process. In addition, the management of PCa is beginning to embrace the precision medicine approach with the use of new technologies, such as liquid tumor profiling, non-coding RNA diagnostics, genomic and proteomic analysis, gene editing, array-based technologies, and next-generation sequencing [2,3,33].
In the past few decades, significant advances have also been achieved in our understanding of the immune system and its relationship with cancer. As the immune system is able to recognize and eliminate newly developing cancer cells, and therefore capable of preventing the onset and progression of malignant disease [34], immunotherapy has emerged as a promising treatment modality for a number of cancer types, including melanoma, renal cell carcinoma, hematologic malignancies, breast cancer and PCa [35]. By targeting malignant cells while at the same time sparing healthy tissue from the damage that is usually induced by radiation and chemotherapy, immunotherapy offers the promise of a non-toxic and efficacious treatment alternative [36]. PCa has attracted a lot of interest as a suitable target for immunotherapeutic intervention, mainly because the tissue itself expresses multiple tumor-associated antigens (TAAs) which the adaptive immune system recognizes, and is also characterized by relatively slow growth kinetics which may provide a longer time frame for the development of effective anti-tumor immune responses [37]. Even though Sipuleucel-T is currently the only FDA-approved immunotherapy option for PCa, with demonstrated PFS (progression-free survival) or OS improvement in clinical trials, there is ample promise on the horizon, as a large number of clinical trials are evaluating various immunotherapeutic approaches in PCa patients; these include immune checkpoint inhibitors, tumor-specific antigen approaches in the form of vaccines, and immunomodulating agents such as antibodies and antibody-drug conjugates [38].
This review gives a detailed account of the immunologic platforms that have so far been associated with immunotherapeutic efficacy and which may constitute crucial targets for achieving significant advances in the decision for precision treatment of PCa. We discuss therapeutic efficacy in patient sub-populations that have been stratified on the basis of prognostic variables and highlight the patient groups most likely to benefit from immunotherapeutic interventions. Finally, we describe the clinical barriers associated with the application of immunotherapy in the management of the disease, as well as possible solutions to circumventing these problems.
2. Immunotherapy as a Precision Treatment Tool for PCa
Disease occurrence and progression in prostate cancer are regarded as a function of biomarkers, mainly in the form of tumor-specific antigens or genetic aberrations that can be used for diagnosis, risk assessment, and prognosis, as well as for precision-guided therapeutics [39]. Diagnostic biomarkers for PCa are essentially prostate-specific antigens with the potential to not only discriminate between indolent and advanced disease, but also to be targeted therapeutically; these include prostate-specific-antigen (PSA), prostate acid phosphatase (PAP), prostate-specific membrane antigen (PSMA), prostate stem cell antigen (PSCA), prostate cancer antigen 3 (PCA3), NY-ESO-1, mucin-1 (MUC1), GRB2-like endophilin B2 (SH3GLB2), T-cell receptor alternate reading frame protein (TARP) and the six transmembrane epithelial antigens of the prostate (STEAP), among many others [40,41]. During the last decade, there has been a significant increase in the number of identified prostate-specific genomic biomarkers that can be used to reliably estimate relative genetic risk, prognosis and tumor aggressiveness of the disease, and have therefore been the subject of intense investigation for their significance in the decision for therapy selection [42,43,44,45]. Importantly, PCa biomarkers can also become molecular targets for immunotherapy: diagnostic biomarkers because they constitute prostate-specific antigens that the immune system can be primed to recognize, and genomic biomarkers because they may include genes that are involved in the regulation of the immune response [43].
Immunotherapies for PCa include both passive treatment approaches, such as direct delivery of monoclonal antibodies (mAbs) with high tumor antigen specificity, and active approaches, such as vaccines. Adjuvants for cancer immunotherapy include organic molecules, inorganic compounds, nanoparticles, polymers, and colloids such as gels, sols, and emulsions, and can be combined with both active and passive forms of immunotherapy with the aim to enhance the immune response [46,47]. Notably, cytokines can be used as adjuvants in combination with other immunotherapeutic agents, as, for example, in tumor cell-based cancer vaccines [36]. Immunotherapeutic strategies employing monoclonal antibodies can be further divided into antibody-drug conjugates [48,49,50,51,52], artificial bi-specific T cell-engaging antibodies, or BiTEs [53,54,55,56,57,58], and immune checkpoint inhibitors [59]. Cell-based immunotherapy, on the other hand, is the adoptive cell transfer (ACT) into patients of T cells that have been genetically modified to contain a chimeric antigen receptor (hence the term CAR T cells) that targets a prostate-specific antigen [59,60,61]; nonetheless, some of these immunotherapeutic interventions are still in the pre-clinical or early clinical (phase I) stage and, as such, they do not yet provide enough evidence to support therapeutic efficacy (Figure 1). Below, we discuss the immunotherapeutic strategies that have been shown to confer clinical benefit to PCa patients, in terms of PFS or OS, and which may be further investigated as precision treatment options for PCa.
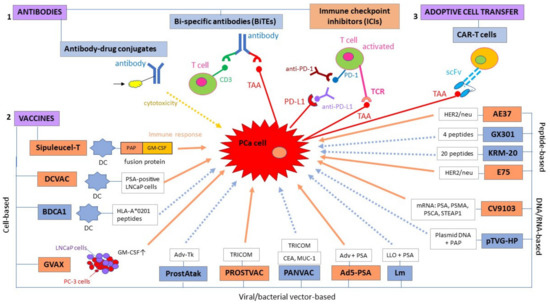
Figure 1.
Immunotherapeutic strategies for prostate cancer fall into three main categories: (1) antibodies, (2) vaccines, and (3) adoptive cell transfer; these can be subdivided into smaller categories depending on the mode of action. Immunotherapeutic modalities in orange boxes represent strategies that have been shown to confer a survival advantage to prostate cancer (PCa) patients, whereas immunotherapies in blue boxes are either in pre-clinical/early clinical development or they have so far failed to demonstrate a survival benefit in terms of progression-free survival (PFS) or overall survival (OS). Similarly, orange arrows represent an immune response, whereas dotted blue arrows represent a possible but not yet confirmed immune response. Ad5: adenovirus type 5; AdV-tk: adenoviral vector containing a herpes virus-derived thymidine-kinase; CEA: carcinoembryonic antigen; DC: dendritic cell; GM-CSF: granulocyte-macrophage colony-stimulating factor; HLA: human leukocyte antigen; Lm: listeria monocytogenes; LLO: listeria monocytogenes (Lm)-listeriolysin O; LNCaP: lymph node-derived human prostate adenocarcinoma cell line; MUC-1: mucin-1; PAP: prostatic acid phosphatase; PC-3: prostate cancer cell line derived from bone metastasis; PD-1: programmed death receptor-1; PD-L1: programmed death-ligand 1; PSCA: prostate stem cell antigen; PSMA: prostate-specific membrane antigen; scFv: single chain variable fragment; STEAP: six transmembrane epithelial antigen of the prostate; TRICOM: TRIad of Co-stimulatory Molecules.
2.1. Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) represent a class of mAbs that have the ability to inhibit immune checkpoint receptors and, therefore, to prevent the inactivation of T-cell function. Immune checkpoint receptors include cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1), and programmed death ligand 1 (PD-L1), and antibodies (ICIs) against them have been shown to induce potent anti-tumor immune responses in a variety of cancers [59].
CTLA-4 is a transmembrane protein that is expressed on T lymphocytes and competitively binds CD80 and CD86 on antigen-presenting cells (APCs), thereby creating a negative feedback loop that prevents T-cells from killing other cells, including cancer cells [62]. Ipilimumab, the ICI blocking the function of CTLA-4, started being tested in PCa clinical trials shortly after its FDA approval for the treatment of melanoma in 2011 [63,64]. Following encouraging results from phase I studies in patients with mCRPC, where it was shown that ipilimumab in combination with granulocyte macrophage-colony-stimulating factor (GM-CSF) in PCa vaccines induces significant PSA declines, an open-label phase I/II multicenter study (NCT00323882) investigating ipilimumab in patients with mCRPC, suggested clinical anti-tumor activity, as supported by manageable adverse events and substantial disease control [65,66,67]. A subsequent phase III clinical trial (NCT00861614) found a significantly higher OS in mCRPC patients with favorable prognostic characteristics that received ipilimumab as compared to a placebo drug [59,68], with OS rates following a two to three times higher trend at three years onwards in cases where ipilimumab was administered along with radiotherapy [69]. On the contrary, patients with asymptomatic or minimally symptomatic chemotherapy-naïve PCa have not been shown to gain any clinical benefit from ipilimumab monotherapy in terms of OS [70].
PD-1 is another transmembrane protein expressed on T cells; its receptor interacts with the PD-1 ligand (PD-L1) that is expressed on both normal and malignant cells, and their interaction constitutes an important checkpoint of T lymphocyte inhibition [62,71]. PD-1 binding to PD-L1 on tumor cells results in an inhibition of apoptosis, T-lymphocyte tolerance and an increase in tumor cell survival [71]. ICIs that act as inhibitors of PD-1 include nivolumab and pembrolizumab, whereas ICIs of PD-L1 include atezolizumab, avelumab, and durvalumab [62]. Even though primary prostate cancers are characterized by an infiltration of PD-1 expressing CD8+ T cells, mCRPC shows minimal expression of the PD-L1 ligand, which represents a significant obstacle when applying anti-PD-1/PD-L1 monotherapy [59,72,73]; only mCRPC patients who have developed resistance to the anti-androgen enzalutamide have been associated with an upregulation of PD-L1 [74]. Clinical trials testing nivolumab or pembrolizumab as monotherapy in unselected mCRPC patients have produced unsatisfactory results in terms of demonstrating a significant survival benefit, with the only exceptions being partial responses in enzalutamide-resistant patients and in patients with microsatellite instability (MSI) [72,75,76]. Indeed, there is enough evidence to suggest that patients with DNA mismatch repair mechanism (MRM) mutations demonstrate anecdotal sensitivity and may derive benefit from treatment with pembrolizumab, possibly due to the higher rate of TAAs and MSI, despite these being relatively uncommon in PCa [62,76,77,78]. Preliminary data from recent clinical trials seem to be more encouraging, as pembrolizumab monotherapy has been shown (i) to induce durable objective responses and minimal adverse events in a subset of patients with heavily pre-treated, advanced PD-L1-positive PCa (NCT02054806) [79] and (ii) to evoke anti-tumor activity with durable clinical responses and significant OS estimates in a subset of treatment-refractory (docetaxel and one or more targeted endocrine therapies) mCRPC patients (NCT02787005) [80]. Therefore, PD-L1 expression, high MSI and high tumor mutational burden (TMB) are currently regarded as biomarkers for pembrolizumab therapy selection for patients with advanced PCa [81].
However, despite the promising results of pre-clinical and clinical studies, immune checkpoint blockade-based immunotherapy appears to be less efficient in PCa as compared to other cancer types; this is mostly due to the fact that prostate cancer is a “cold” tumor, characterized by minimal T-cell infiltration, low TMB, low PD-1 expression and downregulated or non-existent MHC class I expression, a necessary prerequisite for successful immune checkpoint inhibition [62]. In order to overcome therapeutic limitations, to enhance the therapeutic potential of ICIs and to extend the survival benefit to larger subsets of patients, many studies are currently investigating combinations of two different types of ICIs, or ICIs with other treatment modalities. Examples include combination regimens of nivolumab with ipilimumab [82,83,84], nivolumab with docetaxel [85], pembrolizumab with enzalutamide [86,87], and ipilimumab with GM-CSF [65], among many others. A significant number of clinical trials are currently ongoing or recruiting with the aim to investigate the efficacies of ICIS, both in monotherapy and in various therapeutic combinations; a list of ongoing studies is given in Table 1.

Table 1.
Ongoing clinical trials evaluating immune checkpoint inhibitors (ICIs) as therapeutic regimens for PCa (adapted from Kim et al., 2020 and Rizzo et al., 2020) [59,88].
2.2. Vaccines
Therapeutic vaccines for PCa are designed with the aim to elicit an adaptive immune response via antigen presentation; because prostate cells express remarkably more TAAs than most other types of human tissue, TAAs constitute the most significant part, followed by immune adjuvants such as cytokines [62]. The major categories that have so far been shown to offer some clinical advantage in terms of prolonged PFS or OS are as follows:
2.2.1. Cell-Based Vaccines
Cell-based vaccines can be immune cell-based, such as dendritic cell vaccines, or whole tumor cell-based, hence the name tumor cell vaccines.
Sipuleucel-T is an autologous dendritic cell (DC) vaccine that elicits an immune response to the prostatic acid phosphatase (PAP) antigen; vaccine preparation includes leukapheresis of peripheral mononuclear cells from the patient, their ex-vivo exposure to a fusion protein containing the PAP antigen and GM-CSF for 36–44 h and then infusion back into the patient for a total of three treatments over a 6-week period [32,104]. It is currently the only FDA-approved therapeutic cancer vaccine and is specifically aimed at asymptomatic or minimally symptomatic mCRPC patients with no visceral metastases, following evidence of improved OS in the phase III setting of four clinical trials, namely D9901 (NCT00005947) [105], D9902A (NCT01133704) [106], IMPACT (NCT00065442) [32] and PROTECT (NCT00779402) [107]. The extended survival benefit was estimated at approximately 4 months, as compared to the placebo group, which, combined with no measurable change in PSA or tumor burden and no significant difference in disease progression, caused enough controversy regarding the utility of sipuleucel-T in the management of PCa [32,105,106,108]. However, it was later realized that the survival effect might have been underestimated, as patients in the placebo group with disease progression, who constituted over 50% of the total patients enrolled in the IMPACT study [32], were allowed to cross over to receive the vaccine; these patients had a significantly longer OS of 20 months, as compared to the 9.8 months in the group that never crossed over [105,109]. Consistent with these findings, a more recent trial, named PROCEED (NCT01306890), has provided further evidence of Sipuleucel-T’s safety and tolerability, and of a longer OS benefit in lower baseline PSA quartiles as opposed to higher baseline PSA quartiles [109,110,111]. Importantly, a substantial number of patients experienced a long treatment-free interval between Sipuleucel-T and subsequent therapeutic regimens, which may also reflect a clinical benefit [111]. On the contrary, sipuleucel-T is not supported for use in CRPC patients with only increased serum PSA levels as evidence of disseminated disease [107,109,112,113], but there is enough evidence to suggest that patients with localized PCa before radical prostatectomy might benefit from a systemic and local tumor response to vaccine treatment [62,114,115].
DCVAC/PCa is another dendritic cell vaccine involving leukapheresis and in vitro activation of autologous mature dendritic cells pulsed with killed PSA-positive LNCaP cells [116]. Phase II clinical trials testing the efficacy of the vaccine have produced conflicting results. For example, one study evaluating DCVAC/PCa in combination with prednisone and docetaxel chemotherapy in men with mCRPC has demonstrated a manageable safety profile, a 6–7 month OS advantage and the induction and maintenance of PSA-specific T cells [88,117]; another study did not find this combination regimen to be beneficial in the long term, despite the induction of an immune response [105,109,118]. As a result, a randomized, double-blind, placebo-controlled, phase III clinical trial (VIABLE, NCT02111577) is currently ongoing with the aim to determine the safety and efficacy of DCVAC/PCa in combination with docetaxel and prednisone (versus docetaxel and prednisone alone) in patients with mCRPC [119].
GVAX is a tumor cell-based vaccine which, as the name implies, consists of irradiated whole tumor cells that have been genetically modified to constitutively express GM-CSF; the prostate tumor cells are extracted from the hormone-sensitive cell line LNCaP and the hormone-refractory cell line PC-3, which derive from nodal and bone sites of metastasis, respectively [88,120]. This strategy has the advantage of inducing immune responses to multiple TAAs without the need for HLA matching [121]. So far, an enhanced median survival has only been observed in hormone-refractory PCa patients following high dose boost vaccinations as compared to low dose boosts and radiotherapy in phase I/II settings [122,123,124].
2.2.2. Vector-Based Vaccines
Vector-based vaccines may include vectors derived from oncolytic viruses, based on the rationale that these can infect tumor cells and cause them to self-destruct, or vectors derived from bacterial pathogens that are actively phagocytosed by APCs and are thereby able to generate TAAs and to enable specific T cell immune responses.
PROSTVAC-VF (PSA-TRICOM) is a viral vector vaccine that uses two recombinant poxvirus vectors, both of which include a plasmid carrying the transgenes that code for PSA: one that is derived from vaccinia virus (PROSTVAC-V) and contains a triad of T cell co-stimulatory molecules (TRICOM), namely LFA-3, B7.1 and ICAM-1, in conjunction with PSA, and one that is derived from fowlpox virus (PROSTVAC-F) which serves to deliver booster doses [20,62,109]. The scientific rationale behind this vaccine is that the vaccinia vector acts as a single dose immunogenic factor, eliciting a strong immune response both against PSA and the viral protein, leading to the destruction of PSA-positive tumor cells and subsequently to the release of a wider range of TAAs (antigen spreading) that stimulate additional pro-inflammatory signals and additional tumor-specific T cell immune responses [109,125,126]. Under the same rationale, the fowlpox vector, transduced to code for the same TAA (PSA), is used for subsequent booster vaccinations in order to bypass the problem of the vaccinia virus vector being neutralized by the host immune system, as the former (PROSTVAC-F) is able to penetrate APCs without invoking the production of high volumes of neutralizing antibodies [62,127,128].
PROSTVAC-VF clinical testing in patients with localized PCa and in patients with advanced PCa has produced inconclusive results in terms of demonstrating an improvement in PFS or OS [62,129,130,131,132,133,134,135,136]. However, when used in combination with other forms of PCa therapy, such as chemotherapy, ADT, radiotherapy, and ICIs, the immunotherapeutic efficacy of the vaccine seems to be endorsed [62,109]. For example, concurrent administration of PROSTVAC-VF with docetaxel in metastatic androgen-independent PCa patients has been shown to confer a longer PFS as compared to patients receiving chemotherapy alone [137]. Similarly, patients with non-metastatic CRPC who receive the vaccine prior to second-line anti-androgen therapy with nilutamide may derive a greater clinical benefit in terms of improved OS, as compared to patients who receive nilutamide alone or prior to immunotherapy [138,139]; preliminary evidence from a randomized phase II study investigating the efficacy of the anti-androgen flutamide with and without PROSTVAC-VF in patients with non-metastatic CRPC suggests an improvement in time to treatment failure in the combination arm [140]. Interestingly, the concurrent administration of PROSTVAC-VF and the radiopharmaceutical samarium-153-EDTMP in patients with non-visceral mCRPC has demonstrated a PSA response and longer PFS in the combination arm [62,141]. As a result, PROSTVAC-VF is currently being investigated in early phase trials as combination therapy with ICIs such as nivolumab (NCT02933255) in patients with localized PCa and CRPC, with nivolumab/ipilimumab (NCT03532217) in patients with metastatic, hormone-sensitive PCa, as well as in conjunction with other immunotherapeutic agents such as bi-functional fusion protein MSB011359C (targeting PD-L1 and TGF-β) in men with recurrent disease after localized radical treatment [109,142,143].
Ad5-PSA is another viral-vector vaccine derived from replication-deficient recombinant adenovirus type 5 (Ad5), based on pre-clinical evidence that the Ad5 vector has the ability to elicit durable cellular and humoral immune responses, especially when combined with a gelfoam collagen matrix that acts as an adjuvant, even in the presence of high titer anti-adenovirus antibodies [144,145]. Substantial anti-PSA immune responses and prolonged survival have been observed in patients with measurable mCRPC in a phase I clinical setting [146], whereas preliminary results from a phase II trial (NCT00583024) investigating Ad5-PSA diluted in gelfoam matrix in patients with non-metastatic and early metastatic CRPC suggest a prolonged metastasis-free survival benefit [147]. Interestingly, chimpanzee adenoviral (ChAd) vectors might represent an attractive alternative to human Ad5 vectors as vaccine candidates for PCa immunotherapy, as recent evidence not only highlights their safety and ability to induce antigen-specific humoral and cellular immunity, but also their ability to bypass the problem of pre-existing immunity that is associated with human Ad vectors [148].
2.2.3. DNA/mRNA-Based Vaccines
DNA- and RNA-based vaccines consist of plasmid DNA and mRNA, respectively, that is modified to encode for a tumor-specific antigen, resulting in an immune response when expressed by transfected cells via both the MHC class I and MHC class II pathways [149,150]. They represent a promising alternative to conventional vaccine strategies due to their high potency, developmental feasibility, low manufacturing cost, and acceptable safety profile [150].
CV9103 is an mRNA-based vaccine encoding for a number of different TAAs simultaneously: PSA, PSMA, PSCA, and STEAP1 [109,151]. A phase I/II study investigating CV9103 in patients with CRPC with rising PSA and predominantly existing metastases declared that the vaccine is safe, well-tolerated and displays a remarkably high level of cellular immunogenicity [152]; subsequent analysis revealed that a significant correlation exists between immunogenicity and a prolonged survival outcome, despite unfavorable patient characteristics, thereby suggesting a therapeutic benefit [153].
2.2.4. Antigen or Peptide-Based Vaccines
Based on the rationale that individual patients will elicit substantially different immune responses against TAAs, due to both their tumor and immune cells being diverse and heterogeneous, personalized selection and administration of HLA-matched peptides, based on pre-existing patient immunity status prior to vaccination, constitutes an attractive immunotherapeutic approach referred to as personalized peptide vaccination (PPV) [154]. Pre-existing host immunity, or immunological memory, to the vaccine antigen(s) is a necessary prerequisite in order to induce rapid and robust immune responses [155]. The advantage of peptide-based vaccines is that peptides induce robust and rapid cytotoxic T lymphocyte (CTL) activation without the costs and cell availability limitations associated with cell-based vaccines. In this respect, many vaccine TAA peptides have so far been identified, both for CTL (MHC class I) and for T helper cells (MHC class II), with numerous platforms investigating PPVs both as monotherapy and in combination with other forms of cancer therapy [156]. Below we discuss the most promising immunotherapeutic platforms employing PPV.
PPV plus chemotherapy: Significantly longer PFS and OS have been observed in HLA-A24-positive CRPC patients treated with 14 PPVs in combination with low-dose estramustine phosphate (EMP), a dual estrogen and chemotherapy medication (nornitrogen mustard linked to estradiol-1β-phosphate), as compared to standard dose EMP, with peptide-specific immune responses also being strongly associated with PSA doubling time [157,158,159].
PPV plus glucocorticoids: Following substantial evidence that low doses of dexamethasone can be beneficial in the treatment of hormone-refractory PCa, both as monotherapy and in combination with PPV [160,161,162,163,164], a clinical benefit has also been demonstrated in chemotherapy-naïve CRPC patients receiving PPV immunotherapy with low-dose dexamethasone (as compared to receiving dexamethasone alone), evidenced as both longer PSA-related PFS and OS [165].
HER2/neu peptides: these constitute TAAs that appear to be over-expressed in a variety of cancers, including PCa, and have therefore been used as targets of active immunotherapy [166,167]. Specifically, HER-2/neu has been shown to stimulate cell division and to activate the AR pathway in the absence of androgen, thereby increasing the malignant potential of prostate cancer cells and the development of CRPC [168,169,170,171]. One such example is E75 (HER2/neu 366–379, or nelipepimut-S), a nine amino acid peptide derived from the extracellular domain of the HER2 protein that has been shown to elicit prominent immunologic responses in both the pre-clinical and clinical settings [172]. Specifically, cell cultures from PCa patients at risk of recurrence stimulated with E75 appeared to activate E75-specific lymphocytes with tumor-specific cytolytic activity against the HER2/neu-positive cell lines [173]. Similarly, in a phase I/II trial investigating the safety and efficacy of E75 in preventing PSA recurrence in high-risk PCa patients, the vaccine appeared to prevent or delay recurrences if completed before PSA recurrence in HLA-A2 (+) patients, thereby warranting a larger phase II trial to confirm these findings [174,175].
Another example is the AE37 vaccine, which includes an Ii-key-modified HER-2/neu peptide (Ii-key/HER-2 (776–790) or AE37), an immunoregulatory segment of the Ii protein (the Ii-Key peptide) that has been specifically modified so as to loosen the epitope-binding groove of MHC class II molecules and to permit direct charging of MHC class II epitopes to the peptide-binding groove, thereby circumventing the need for intracellular epitope processing [156,171]. The AE37 peptide vaccine has been shown to elicit compelling T helper cell and CTL responses, as well as increased anti-tumor activity, in a series of pre-clinical studies, significantly more so than the native, non-modified HER-2 (776–790) (or AE36) peptide [156,176,177,178]. Following encouraging results from a phase I clinical trial of a hybrid AE37 vaccine (HER2/neu with recombinant GM-CSF as adjuvant) in breast cancer patients [179], the first phase I study testing the same hybrid vaccine in HER2/neu-positive PCa patients demonstrated safety and clinical efficacy in inducing antigen-specific immune responses in patients with castrate-sensitive and castrate-resistant PCa [171]. Immunologic 4-year follow-up assessment revealed vaccine-specific long-term immunity in most patients; notably, those who had received booster vaccination had a more favorable clinical outcome in terms of metastasis-free survival (MFS) or OS as compared to patients with similar clinical characteristics and/or histology at diagnosis who did not receive booster doses, thereby highlighting the need for administering booster shots in order to sustain immunological memory [180]. Furthermore, a retrospective analysis of biomarkers predicting the immunologic and clinical responses to AE37, concluded that patients expressing HLA-A24 and/or HLA-DR11 alleles demonstrate increased vaccination-specific immunity and prolonged OS, as opposed to patients expressing the HLA-A2 allele, who are characterized by high frequencies of circulating Tregs, which is in turn associated with a negative immunological response and decreased OS [181,182]; in addition, lower pre-existing TGF-β plasma levels appear to correlate with a better immunological response to the vaccine and prolonged OS, whereas higher levels of pre-existing IFN-γ-producing T cells are significantly associated with higher delayed-type hypersensitivity (DTH) immune responses and improved OS, as compared to patients with compromised pre-existing immunity to AE36 [183]. Larger cohort studies are warranted in order validate the identified biomarkers and to establish their clinical utility.
3. The Main Challenges Associated with the Implementation of Immunotherapy into Clinical Practice and Possible Ways to Circumvent Them
The implementation of PCa immunotherapy into clinical practice is associated with a number of challenges. First and foremost, the efficacy of immunotherapeutic applications is largely unpredictable; optimal evaluation and timing of vaccine-specific T cell responses remains unclear, despite being considered critical for the selection of therapeutic agents and therefore for the development of precision-tailored therapeutics [50]. Specifically, there is a lack of change in short-term PFS, contrary to what is usually observed with multiple agents [184]. Also, in contrast to cytotoxic chemotherapy agents, tumor burden may not present with significant changes within a short period of time following immunotherapy and seems to rely on immunologic memory [62]. In other words, there is a great need to properly evaluate the correlation of immune response with anti-tumor activity; the incorporation of extended endpoints and additional immune-related criteria in the design of clinical trials, as well as the standardization of immune response methodologies in multicenter trials, may contribute towards a better understanding of its relationship with clinical outcomes [50,185].
Tumor heterogeneity among different patients adds even more complexity to the picture, as it may be driven by far more complex mutational heterogeneity, providing subclonal cell populations with inherent plasticity and the ability to re-differentiate as new clones, thereby contributing to immune resistance and therapeutic failure [185]. This increasing number of genetic mutations detected across tumor types further increases the difficulty to identify clinically significant biomarkers and, subsequently, the patients that are most likely to respond to biomarker-specific treatments. A growing volume of data from clinical trials has demonstrated that only a subset of patients derive clinical benefit from PCa immunotherapy, markedly patients with smaller tumor volumes and less aggressive, or indolent, disease [184,186]. In addition, there is enough evidence to suggest that PCa immunotherapy is more likely to result in improved outcomes if administered as first-line treatment, as for example in the case of Sipuleucel-T or ipilimumab, whereas patients with advanced disease, such as those with lung and liver metastases, may actually fare worse [68,186,187]. It has been suggested that in patients with advanced disease, vaccines may induce an immune reaction in both the normal and tumor prostate tissue and thereby to cause a temporary rise in PSA and in measurable lesions [50]. The latter may be further complicated by the fact that the most common site of metastasis in PCa is the bone which, not only is regarded as a non-measurable site, but it also produces many growth factors and interleukins capable of further stimulating metastatic growth and causing therapeutic resistance [50,188]. In this case, the latter takes place both due to the fact that the oxygen-poor bone microenvironment is rich in Tregs and other tumor suppressor cells, as well as because it releases growth factors, such as TGF-β, that also act to abrogate the immune response [62]. Therefore, immune resistance that occurs before the application of immunotherapy constitutes a major challenge.
The identification of tumor-resistant clones by performing pre- and post-treatment tumor biopsies could aid in identifying the patients most likely to present with acquired immune resistance and in developing biomarker-specific combination treatments to either prevent or bypass therapeutic resistance [185]. Numerous platforms are currently evaluating combinations of treatments, where immunotherapeutic agents are tested both in conjunction with more traditional therapies such as ADT, chemotherapy, and radiotherapy, as well as with other types of immunotherapy; a significant number of these combinations have already been found to augment the effect of immunotherapy, to enhance the anti-tumor immune response and to diminish immune tolerance, leading to improved outcomes [62,184]. In addition, the lack of toxicity that is associated with the majority of the currently investigated immunotherapies, especially vaccines, makes them very attractive candidates for use in both early and adjuvant settings of PCa clinical management; such therapeutic modalities offer the possibility of a longer and unremitting therapeutic response without suffering the unwanted side-effects of toxic treatments, at least so in cases where there is no significant disease progression and toxic treatments can be substantially delayed [116]. In this process, it is imperative to identify additional genetic mutations, biomarkers, and cancer pathways that characterize the pathogenesis of PCa, with the aim to further unravel tumor heterogeneity, to identify clinically significant and targetable tumor antigens and in this way to introduce therapeutic combinations that target multiple mutations at the same time [185]. Liquid and solid tumor biopsies could offer significant advantage in the characterization of tumor heterogeneity and in the identification of the targetable mutations in patients with disease progression; this could lead to more precision-orientated therapeutic decisions and hopefully extend clinical benefit to patient subtypes with more advanced disease [185]. Last but not least, even though the use of immunotherapy for cancer prevention has been largely experimental, the FDA approval of adjuvant immunotherapy for patients with a high risk of melanoma recurrence, offers much hope for the development of similar immunopreventive strategies for other highly immunogenic cancer types, such as PCa [185,189,190].
4. Conclusions
Immunotherapy has emerged as an attainable and potent weapon in the quiver of precision medicine for the treatment of PCa. Despite the numerous challenges associated with its clinical implementation, immunotherapy constitutes a viable and promising treatment modality, a shift from conventional treatment approaches, that perfectly fits the rationale of precision oncology. The development of new technologies has accelerated the identification of immune-targeted biomarkers with prognostic and predictive significance, largely contributing to the rational, appropriately sequenced combination of treatment schemes and in the matching of patients with targeted therapies so as to achieve the maximum clinical benefit. Even though the majority of patients that seem to derive clinical advantage from the currently available immunotherapeutic strategies markedly fall into localized and/or non-metastatic disease subtypes, there is substantial evidence to suggest that patients with unfavorable characteristics such as predominantly existing metastases may also experience improved outcomes, as seen with cancer vaccines Ad5-PSA and CV9103 and through combinations of ICIs with other treatment modalities. Data from the numerous ongoing clinical trials are expected to shed more light into this rapidly evolving picture of biomarker-tailored immune-mediated therapies, to help us apply immunotherapy to a wider range of PCa patients and to achieve successful treatment even in cases of high-risk or persistent disease.
Author Contributions
M.A. performed the literature search, drafted the main structure of the manuscript, prepared the manuscript, tables and figures, performed all revision modifications; V.Z. conceived the initial idea and was actively involved in the design, formatting and extensive reviewing of the manuscript. Both authors have read and agree to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was co-funded by the European Regional Development Fund and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (project code: T1EDK-01404, project acronym and title: “NEOVIOPRO-Identification of new predictive biomarkers for prostate cancer”).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AA | African Americans |
| A2AR | adenosine 2A receptor |
| Ad5 | adenovirus type 5 |
| ADT | androgen deprivation therapy |
| AdV-tk | adenoviral vector containing a herpes virus-derived thymidine-kinase gene |
| AE | adverse event |
| AR | androgen receptor |
| AR-V7 | androgen receptor variant 7 |
| APC | antigen-presenting cell |
| CA | Caucasian Americans |
| CEA | carcinoembryonic antigen |
| ChAd | chimpanzee adenovirus |
| CRPC | castrate-resistant prostate cancer |
| CT | clinical trial |
| CTLA-4 | cytotoxic T-lymphocyte associated antigen 4 |
| CTL | cytotoxic T-lymphocyte |
| DC | dendritic cell |
| DDR | DNA damage repair |
| DTH | delayed-type hypersensitivity |
| EMP | estramustine phosphate |
| GM-CSF | granulocyte macrophage-colony-stimulating factor |
| HER2 | human epidermal growth factor receptor 2 |
| HLA | human leukocyte antigen |
| IC | immune checkpoint |
| ICAM-1 | intracellular adhesion molecule-1 |
| ICI | immune checkpoint inhibitors |
| IFN | interferon |
| LLO | listeria monocytogenes (Lm)-listeriolysin O |
| LNCaP | lymph node-derived human prostate adenocarcinoma cell line |
| mAbs | monoclonal antibodies |
| mCRPC | metastatic castration-resistant prostate cancer |
| MHC | major histocompatibility complex |
| MFS | metastasis-free survival |
| MRM | mismatch repair mechanism |
| MSI | microsatellite instability |
| MUC1 | mucin-1 |
| ORR | objective response rate |
| OS | overall survival |
| PAP | prostatic acid phosphatase |
| PARP | poly(ADP-ribose) polymerase |
| PCa | prostate cancer |
| PCA3 | prostate cancer antigen 3 |
| PC-3 | prostate cancer cell line derived from bone metastasis |
| PD-1 | programmed death receptor-1 |
| PD-L1 | programmed death-ligand 1 |
| PPV | personalized peptide vaccination |
| PSA | prostate-specific antigen |
| PSCA | prostate stem cell antigen |
| PSMA | prostate-specific membrane antigen |
| scFv | single chain variable fragment |
| STEAP | six transmembrane epithelial antigen of the prostate |
| TCR | T cell receptor |
| TAAs | tumor-associated antigens |
| TARP | T-cell receptor alternate reading frame protein |
| TCR | T cell receptor |
| TGF-β | tumor growth factor β |
| TILs | tumor infiltrating lymphocytes |
| TMB | tumor mutation burden |
| TRICOM | TRIad of CO-stimulatory Molecules |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Malik, A.; Srinivasan, S.; Batra, J. A New Era of Prostate Cancer Precision Medicine. Front. Oncol. 2019, 9, 1263. [Google Scholar] [CrossRef]
- Galazi, M.; Rodriguez-Vida, A.; Ng, T.; Mason, M.; Chowdhury, S. Precision medicine for prostate cancer. Expert Rev. Anticancer Ther. 2014, 14, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Marugame, T.; Katanoda, K. International comparisons of cumulative risk of breast and prostate cancer, from cancer incidence in five continents Vol. VIII. Jpn. J. Clin. Oncol. 2006, 36, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Gilliland, F.D.; Eley, J.W.; Harlan, L.C.; Stephenson, R.A.; Stanford, J.L.; Albertson, P.C.; Hamilton, A.S.; Hunt, W.C.; Potosky, A.L. Racial and ethnic differences in advanced-stage prostate cancer: The Prostate Cancer Outcomes Study. J. Natl. Cancer Inst. 2001, 93, 388–395. [Google Scholar] [CrossRef]
- Jones, R.A.; Wenzel, J. Prostate cancer among African-American males: Understanding the current issues. J. Natl. Black Nurses Assoc. 2005, 16, 55–62. [Google Scholar]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer—Analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Humphrey, P.A. Cancers of the male reproductive organs. In World Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; International Agency for Research on Cancer: Lyon, France, 2014; p. 453. [Google Scholar]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Moller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef]
- Jansson, F.; Drevin, L.; Frisell, T.; Stattin, P.; Bratt, O.; Akre, O. Concordance of Non-Low-Risk Disease Among Pairs of Brothers With Prostate Cancer. J. Clin. Oncol. 2018, 36, 1847–1852. [Google Scholar] [CrossRef]
- Wilson, K.M.; Mucci, L.A. Diet and Lifestyle in Prostate Cancer. In Prostate Cancer: Cellular and Genetic Mechanisms of Disease Development and Progression, 2nd ed.; Dehm, S., Tindall, D.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 1210, pp. 1–27. [Google Scholar]
- Leslie, S.W.; Soon-Sutton, T.L.; Sajjad, H.; Siref, L.E. Prostate Cancer; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef]
- Loriot, Y.; Massard, C.; Fizazi, K. Recent developments in treatments targeting castration-resistant prostate cancer bone metastases. Ann. Oncol. 2012, 23, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2018, 25, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Cheng, H.H.; Lin, D.W.; Yu, E.Y. Advanced clinical states in prostate cancer. Urol. Clin. N. Am. 2012, 39, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Bryce, A.H.; Antonarakis, E.S. Androgen receptor splice variant 7 in castration-resistant prostate cancer: Clinical considerations. Int. J. Urol. 2016, 23, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, I.; Tortorella, E.; Giantulli, S.; Scarpa, S.; Sciarra, A. Immunotherapy in Prostate Cancer: Recent Advances and Future Directions. EM J. Urol. 2019, 7, 51–61. [Google Scholar]
- Lobo-Martins, S.; Ferreira, A.R.; Mansinho, A.; Casimiro, S.; Leitzel, K.; Ali, S.; Lipton, A.; Costa, L. Impact of Extraskeletal Metastases on Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer with Bone Metastases. Cancers 2020, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, W.R. The evolving role of immunotherapy in prostate cancer. Ann. Oncol. 2012, 23 (Suppl. 8), viii22–viii27. [Google Scholar] [CrossRef]
- Xylinas, E.; Kluth, L.; Shariat, S.F. Words of wisdom. Re: Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: Exploratory analysis of data from the COU-AA-301 randomised trial. Eur. Urol. 2013, 63, 1132–1133. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Soban, F.; de Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Brave, M.; Weinstock, C.; Brewer, J.R.; Chi, D.C.; Suzman, D.L.; Cheng, J.; Zhang, L.; Sridhara, R.; Ibrahim, A.; Kluetz, P.G.; et al. An FDA Review of Drug Development in Nonmetastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2020, 26, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Parker, C. The safety and efficacy of radium-223 dichloride for the treatment of advanced prostate cancer. Expert Rev. Anticancer Ther. 2016, 16, 911–918. [Google Scholar] [CrossRef][Green Version]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Nelson, P.; Fang, M. Genomic profiling defines subtypes of prostate cancer with the potential for therapeutic stratification. Clin. Cancer Res. 2013, 19, 4058–4066. [Google Scholar] [CrossRef]
- Pardoll, D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin. Oncol. 2015, 42, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Cancer Immunotherapy, Part 2: Efficacy, Safety, and Other Clinical Considerations. Pharm. Ther. 2017, 42, 452–463. [Google Scholar]
- Alatrash, G.; Jakher, H.; Stafford, P.D.; Mittendorf, E.A. Cancer immunotherapies, their safety and toxicity. Expert Opin. Drug Saf. 2013, 12, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Fong, L. Immunotherapy for Prostate Cancer: Where Do We Go from here?—Part 1: Prostate Cancer Vaccines. Oncology 2018, 32, 112–120. [Google Scholar]
- Comiskey, M.C.; Dallos, M.C.; Drake, C.G. Immunotherapy in Prostate Cancer: Teaching an Old Dog New Tricks. Curr. Oncol. Rep. 2018, 20, 75. [Google Scholar] [CrossRef]
- Renfro, L.A.; An, M.W.; Mandrekar, S.J. Precision oncology: A new era of cancer clinical trials. Cancer Lett. 2017, 387, 121–126. [Google Scholar] [CrossRef]
- Prokhnevska, N.; Emerson, D.A.; Kissick, H.T.; Redmond, W.L. Immunological Complexity of the Prostate Cancer Microenvironment Influences the Response to Immunotherapy. In Prostate Cancer: Cellular and Genetic Mechanisms of Disease Development and Progression, 2nd ed.; Dehm, S., Tindall, D.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–147. [Google Scholar] [CrossRef]
- Hall, W.A.; Lawton, C.A.; Jani, A.B.; Pollack, A.; Feng, F.Y. Biomarkers of Outcome in Patients with Localized Prostate Cancer Treated With Radiotherapy. Semin. Radiat. Oncol. 2017, 27, 11–20. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Chinnaiyan, A.M. Advancing precision medicine for prostate cancer through genomics. J. Clin. Oncol. 2013, 31, 1866–1873. [Google Scholar] [CrossRef][Green Version]
- Velonas, V.M.; Woo, H.H.; dos Remedios, C.G.; Assinder, S.J. Current status of biomarkers for prostate cancer. Int. J. Mol. Sci. 2013, 14, 11034–11060. [Google Scholar] [CrossRef]
- Smits, M.; Mehra, N.; Sedelaar, M.; Gerritsen, W.; Schalken, J.A. Molecular biomarkers to guide precision medicine in localized prostate cancer. Expert Rev. Mol. Diagn. 2017, 17, 791–804. [Google Scholar] [CrossRef]
- Counago, F.; Lopez-Campos, F.; Diaz-Gavela, A.A.; Almagro, E.; Fenandez-Pascual, E.; Henriquez, I.; Lozano, R.; Linares Espinos, E.; Gomez-Iturriaga, A.; de Velasco, G.; et al. Clinical Applications of Molecular Biomarkers in Prostate Cancer. Cancers 2020, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.G.; Li, Y.M. Emerging Adjuvants for Cancer Immunotherapy. Front. Chem. 2020, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Nikolopoulou, A.; Jhanwar, Y.S.; Kaur, G.; Tagawa, S.T.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Radioimmunotherapy of Metastatic Prostate Cancer with (1)(7)(7)Lu-DOTAhuJ591 Anti Prostate Specific Membrane Antigen Specific Monoclonal Antibody. Curr. Radiopharm. 2016, 9, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Niaz, M.O.; Sun, M.; Ramirez-Fort, M.K.; Niaz, M.J. Review of Lutetium-177-labeled Anti-prostate-specific Membrane Antigen Monoclonal Antibody J591 for the Treatment of Metastatic Castration-resistant Prostate Cancer. Cureus 2020, 12, e7107. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Agarwal, N.; Choueiri, T.K.; Kantoff, P.W. Recent advances in immunotherapy for the treatment of prostate cancer. Expert Opin. Biol. Ther. 2011, 11, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Lutje, S.; Gerrits, D.; Molkenboer-Kuenen, J.D.; Herrmann, K.; Fracasso, G.; Colombatti, M.; Boerman, O.C.; Heskamp, S. Characterization of Site-Specifically Conjugated Monomethyl Auristatin E- and Duocarmycin-Based Anti-PSMA Antibody-Drug Conjugates for Treatment of PSMA-Expressing Tumors. J. Nucl. Med. 2018, 59, 494–501. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Kantoff, P.; Vogelzang, N.J.; Mega, A.; Fleming, M.T.; Stephenson, J.J., Jr.; Frank, R.; Shore, N.D.; Dreicer, R.; McClay, E.F.; et al. Phase 1 study of PSMA ADC, an antibody-drug conjugate targeting prostate-specific membrane antigen, in chemotherapy-refractory prostate cancer. Prostate 2019, 79, 604–613. [Google Scholar] [CrossRef]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Buhler, P.; Wolf, P.; Gierschner, D.; Schaber, I.; Katzenwadel, A.; Schultze-Seemann, W.; Wetterauer, U.; Tacke, M.; Swamy, M.; Schamel, W.W.; et al. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunol. Immunother. 2008, 57, 43–52. [Google Scholar] [CrossRef]
- Feldmann, A.; Arndt, C.; Topfer, K.; Stamova, S.; Krone, F.; Cartellieri, M.; Koristka, S.; Michalk, I.; Lindemann, D.; Schmitz, M.; et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J. Immunol. 2012, 189, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Raum, T.; Lutterbuese, R.; Voelkel, M.; Deegen, P.; Rau, D.; Kischel, R.; Hoffmann, P.; Brandl, C.; Schuhmacher, J.; et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol. Cancer Ther. 2012, 11, 2664–2673. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Trad, A.; Baumgart, A.; Huske, L.; Lorenzen, I.; Chalaris, A.; Grotzinger, J.; Dechow, T.; Scheller, J.; Rose-John, S. A novel bispecific single-chain antibody for ADAM17 and CD3 induces T-cell-mediated lysis of prostate cancer cells. Biochem. J. 2012, 445, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, J.; Lei, T.; Ma, W.; Zhang, M. The bispecific anti-CD3 x anti-CD155 antibody mediates T cell immunotherapy for human prostate cancer. Investig. New Drugs 2019, 37, 810–817. [Google Scholar] [CrossRef]
- Kim, T.J.; Koo, K.C. Current Status and Future Perspectives of Checkpoint Inhibitor Immunotherapy for Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5484. [Google Scholar] [CrossRef]
- Al-Khami, A.A.; Mehrotra, S.; Nishimura, M.I. Adoptive immunotherapy of cancer: Gene transfer of T cell specificity. Self Nonself 2011, 2, 80–84. [Google Scholar] [CrossRef][Green Version]
- Davis, Z.B.; Felices, M.; Verneris, M.R.; Miller, J.S. Natural Killer Cell Adoptive Transfer Therapy: Exploiting the First Line of Defense against Cancer. Cancer J. 2015, 21, 486–491. [Google Scholar] [CrossRef]
- Patel, D.; McKay, R.; Parsons, J.K. Immunotherapy for Localized Prostate Cancer: The Next Frontier? Urol. Clin. N. Am. 2020, 47, 443–456. [Google Scholar] [CrossRef]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An anti-CTLA-4 antibody for metastatic melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, W.; Gotlieb, V. The rapidly evolving therapies for advanced melanoma--Towards immunotherapy, molecular targeted therapy, and beyond. Crit. Rev. Oncol. Hematol. 2016, 99, 91–99. [Google Scholar] [CrossRef]
- Van den Eertwegh, A.J.; Versluis, J.; van den Berg, H.P.; Santegoets, S.J.; van Moorselaar, R.J.; van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I.; et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 509–517. [Google Scholar] [CrossRef]
- Madan, R.A.; Mohebtash, M.; Arlen, P.M.; Vergati, M.; Rauckhorst, M.; Steinberg, S.M.; Tsang, K.Y.; Poole, D.J.; Parnes, H.L.; Wright, J.J.; et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 501–508. [Google Scholar] [CrossRef]
- Slovin, S.F.; Higano, C.S.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.J.; Scher, H.I.; Chin, K.; Gagnier, P.; McHenry, M.B.; et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann. Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; den Eertwegh, A.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Martin, A.M.; Nirschl, T.R.; Nirschl, C.J.; Francica, B.J.; Kochel, C.M.; van Bokhoven, A.; Meeker, A.K.; Lucia, M.S.; Anders, R.A.; DeMarzo, A.M.; et al. Paucity of PD-L1 expression in prostate cancer: Innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015, 18, 325–332. [Google Scholar] [CrossRef]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar] [CrossRef]
- Graff, J.N.; Alumkal, J.J.; Drake, C.G.; Thomas, G.V.; Redmond, W.L.; Farhad, M.; Cetnar, J.P.; Ey, F.S.; Bergan, R.C.; Slottke, R.; et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016, 7, 52810–52817. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Shaukat, F.; Isaacsson Velho, P.; Kaur, H.; Shenderov, E.; Pardoll, D.M.; Lotan, T.L. Clinical Features and Therapeutic Outcomes in Men with Advanced Prostate Cancer and DNA Mismatch Repair Gene Mutations. Eur. Urol. 2019, 75, 378–382. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Flechon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499 e483. [Google Scholar] [CrossRef]
- Boudadi, K.; Suzman, D.L.; Anagnostou, V.; Fu, W.; Luber, B.; Wang, H.; Niknafs, N.; White, J.R.; Silberstein, J.L.; Sullivan, R.; et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 2018, 9, 28561–28571. [Google Scholar] [CrossRef]
- Wong, Y.N.S.; Sankey, P.; Josephs, D.H.; Jones, R.J.; Crabb, S.J.; Beare, S.; Duggan, M.; White, L.; Charlaftis, N.; Wheeler, G.; et al. Nivolumab and ipilimumab treatment in prostate cancer with an immunogenic signature (NEPTUNES). J. Clin. Oncol. 2019, 37, TPS5090. [Google Scholar] [CrossRef]
- Fizazi, K.; Gonzalez Mella, P.; Castellano, D.; Minatta, J.N.; Rezazadeh Kalebasty, A.; Shaffer, D.; Vazquez Limon, J.C.; Armstrong, A.J.; Sanchez Lopez, H.M.; Sharkey, B.; et al. Efficacy and safety of nivolumab in combination with docetaxel in men with metastatic castration-resistant prostate cancer in CheckMate 9KD. Ann. Oncol. 2019, 30, v851–v866. [Google Scholar] [CrossRef]
- Berry, W.R.; Fong, P.C.C.; Piulats, J.M.; Appleman, L.J.; Conter, H.J.; Feyerabend, S.; Shore, N.D.; Gravis, G.; Laguerre, B.; Gurney, H.; et al. KEYNOTE-365 cohort C updated results: Pembrolizumab (pembro) plus enzalutamide (enza) in abiraterone (abi)-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 102. [Google Scholar] [CrossRef]
- Graff, J.N.; Burgents, J.; Liang, L.W.; Stenzl, A. Phase III study of pembrolizumab (pembro) plus enzalutamide (enza) versus placebo plus enza for metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-641. J. Clin. Oncol. 2020, 38, TPS258. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Giunchi, F.; Cheng, L.; Lopez-Beltran, A.; Fiorentino, M.; Montironi, R.; et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells 2020, 9, 2051. [Google Scholar] [CrossRef] [PubMed]
- Rosser, C.J.; Hirasawa, Y.; Acoba, J.D.; Tamura, D.J.; Pal, S.K.; Huang, J.; Scholz, M.C.; Dorff, T.B. Phase Ib study assessing different sequencing regimens of atezolizumab (anti-PD-L1) and sipuleucel-T (SipT)in patients who have asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer. J. Clin. Oncol. 2020, 38, e17564. [Google Scholar] [CrossRef]
- Lopez, J.S.; Biondo, A.; Tiu, C.; Scaranti, M.; Ameratunga, M.; Zachariou, A.; Turner, A.; Tunariu, N.; Prout, T.; Parmar, M.; et al. Abstract CT140: Proof-of-concept evidence of immune modulation by blockade of the phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathway in the phase I dose escalation study of Ipatasertib (Ipa) in combination with atezolizumab (A) in patients (pts) with advanced solid tumors (Ice-CAP). In Proceedings of the AACR Annual Meeting 2020, Philadelphia, PA, USA, 6–11 November 2020. [Google Scholar]
- Bryce, A.H.; Dronca, R.S.; Costello, B.A.; Infante, J.R.; Ames, T.D.; Jimeno, J.; Karp, D.D. PT-112 in advanced metastatic castrate-resistant prostate cancer (mCRPC), as monotherapy or in combination with PD-L1 inhibitor avelumab: Findings from two phase I studies. J. Clin. Oncol. 2020, 38, 83. [Google Scholar] [CrossRef]
- Yap, T.A.; Konstantinopoulos, P.; Telli, M.L.; Saraykar, S.; Beck, J.T.; Galsky, M.D.; Abraham, J.; Wise, D.R.; Khasraw, M.; Rubovszky, G.; et al. Abstract P1-19-03: JAVELIN PARP Medley, a phase 1b/2 study of avelumab plus talazoparib: Results from advanced breast cancer cohorts. Cancer Res. 2020, 80. [Google Scholar] [CrossRef]
- Lim, E.A.; Bauer, T.M.; Patel, M.R.; Falchook, G.S.; Karlix, J.L.; Choe, J.H.; George, D.J.; Mugundu, G.M.; Pilling, E.; Chen, H.; et al. A phase I, open-label, multicenter study to assess the safety, pharmacokinetics, and preliminary antitumor activity of AZD4635 both as monotherapy and in combination in patients with advanced solid malignancies: Results from prostate cancer patients (NCT02740985). J. Clin. Oncol. 2020, 38, 5518. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Marhold, M.; Tomasich, E.; Udovica, S.; Merchant, A.; Krainer, M. Immune checkpoint inhibitors in Mcrpc—rationales, challenges and perspectives. Oncoimmunology 2019, 8, e1644109. [Google Scholar] [CrossRef]
- Hotte, S.J.; Winquist, E.; Chi, K.N.; Ellard, S.L.; Sridhar, S.; Emmenegger, U.; Salim, M.I.; Canil, C.; Kollmannsberger, C.K.; Hansen, A.R.; et al. CCTG IND 232: A Phase II Study of Durvalumab With or Without Tremelimumab in Patients with Metastatic Castration Resistant Prostate Cancer (mCRPC). In Proceedings of the ESMO 2019 Congress, Barcelona, Spain, 27 September–1 October 2019. [Google Scholar]
- Chi, K.N.; Mukherjee, S.; Saad, F.; Winquist, E.; Ong, M.; Kolinsky, M.P.; Sacher, A.G.; Ferrario, C.; Salim, M.; Macfarlane, R.J.; et al. Prostate cancer biomarker enrichment and treatment selection (PC-BETS) study: A Canadian cancer trials group phase II umbrella trial for metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 5551. [Google Scholar] [CrossRef]
- Scholz, M.; Yep, S.; Chancey, M.; Kelly, C.; Chau, K.; Turner, J.; Lam, R.; Drake, C.G. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. Immunotargets Ther. 2017, 6, 11–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alter, R.; Fleming, G.F.; Stadler, W.M.; Patnaik, A. A phase Ib/IIa study of rucaparib (PARP inhibitor) combined with nivolumab in metastatic castrate-resistant prostate cancer and advanced/recurrent endometrial cancer. J. Clin. Oncol. 2019, 37, TPS2663. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Papadatos-Pastos, D.; Pal, A.; Akay, M.; Ameratunga, M.; Mithra, S.; Ang, J.-E.; Levva, S.; Caldwell, R.; Riisnaes, R.; Crespo, M.; et al. HyPeR: A phase 1, dose escalation and expansion trial of guadecitabine (SGI-110), a second-generation hypomethylating agent in combination with pembrolizumab (MK3475) in patients with refractory solid tumors. In Proceedings of the Annual Meeting of the American Association for Cancer Research (AACR), Philadelphia, PA, USA, 6–11 November 2020. [Google Scholar]
- McNeel, D.G.; Eickhoff, J.C.; Jeraj, R.; Staab, M.J.; Straus, J.; Rekoske, B.; Liu, G. DNA vaccine with pembrolizumab to elicit antitumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 168. [Google Scholar] [CrossRef]
- Fong, P.C.C.; Retz, M.; Drakaki, A.; Massard, C.; Berry, W.R.; Romano, E.; Bono, J.S.D.; Feyerabend, S.; Appleman, L.J.; Conter, H.J.; et al. Keynote-365 cohort C: Pembrolizumab (pembro) plus enzalutamide (enza) in abiraterone (abi)-pretreated patients (pts) with metastatic castrate resistant prostate cancer (mCRPC). J. Clin. Oncol. 2019, 37, 171. [Google Scholar] [CrossRef]
- Patel, M.; Lum, L.G.; Deol, A.; Thakur, A.; Heath, E.I.; Chen, W.; Dobson, K.; Fontana, J.A.; Vaishampayan, U.N. Phase II trial of a novel immunotherapy combination of pembrolizumab and HER2 bi-armed activated T cells (BATs) in metastatic castrate resistant prostate cancer. J. Clin. Oncol. 2020, 38, 97. [Google Scholar] [CrossRef]
- Drake, C.G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 2010, 10, 580–593. [Google Scholar] [CrossRef]
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006, 24, 3089–3094. [Google Scholar] [CrossRef]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009, 115, 3670–3679. [Google Scholar] [CrossRef]
- Beer, T.M.; Bernstein, G.T.; Corman, J.M.; Glode, L.M.; Hall, S.J.; Poll, W.L.; Schellhammer, P.F.; Jones, L.A.; Xu, Y.; Kylstra, J.W.; et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin. Cancer Res. 2011, 17, 4558–4567. [Google Scholar] [CrossRef]
- Sobol, I.; Thompson, R.H.; Dong, H.; Krco, C.; Kwon, E.D. Immunotherapy in prostate cancer. Curr. Urol. Rep. 2015, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Hans, B.; Goel, S.; Bashorun, H.O.; Dovey, Z.; Tewari, A. Immunotherapy in prostate cancer: Current state and future perspectives. Ther. Adv. Urol. 2020, 12, 1756287220951404. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Drake, C.G. Immunotherapy for prostate cancer: Recent developments and future challenges. Cancer Metastasis Rev. 2014, 33, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Armstrong, A.J.; Sartor, A.O.; Vogelzang, N.J.; Kantoff, P.W.; McLeod, D.G.; Pieczonka, C.M.; Penson, D.F.; Shore, N.D.; Vacirca, J.; et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer 2019, 125, 4172–4180. [Google Scholar] [CrossRef] [PubMed]
- Beinart, G.; Rini, B.I.; Weinberg, V.; Small, E.J. Antigen-presenting cells 8015 (Provenge) in patients with androgen-dependent, biochemically relapsed prostate cancer. Clin. Prostate Cancer 2005, 4, 55–60. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Kibel, A.S.; Yu, E.Y.; Karsh, L.I.; Elfiky, A.; Shore, N.D.; Vogelzang, N.J.; Corman, J.M.; Millard, F.E.; Maher, J.C.; et al. Sequencing of Sipuleucel-T and Androgen Deprivation Therapy in Men with Hormone-Sensitive Biochemically Recurrent Prostate Cancer: A Phase II Randomized Trial. Clin. Cancer Res. 2017, 23, 2451–2459. [Google Scholar] [CrossRef]
- Ross, A.; Armstrong, A.J.; Pieczonka, C.M.; Bailen, J.L.; Tutrone, R.F.; Cooperberg, M.R.; Pavlovich, C.P.; Renzulli, J.F.; Haynes, H.; Sheikh, N.A.; et al. A comparison of sipuleucel-T (sip-T) product parameters from two phase III studies: PROVENT in active surveillance prostate cancer and IMPACT in metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 321. [Google Scholar] [CrossRef]
- Fong, L.; Cham, J.; Zhang, L.; DeVries, T.; Pufnock, J.; GuhaThakurta, D.; Letarte, S.; Hamm, D.; Trager, J.B.; Sheikh, N.A. Changes in circulating and intratumoral T cell clonotypes in sipuleucel-T-treated prostate cancer patients. J. Clin. Oncol. 2015, 33, e16008. [Google Scholar] [CrossRef]
- Cordes, L.M.; Gulley, J.L.; Madan, R.A. The evolving role of immunotherapy in prostate cancer. Curr. Opin. Oncol. 2016, 28, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Podrazil, M.; Horvath, R.; Becht, E.; Rozkova, D.; Bilkova, P.; Sochorova, K.; Hromadkova, H.; Kayserova, J.; Vavrova, K.; Lastovicka, J.; et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015, 6, 18192–18205. [Google Scholar] [CrossRef]
- Kongsted, P.; Borch, T.H.; Ellebaek, E.; Iversen, T.Z.; Andersen, R.; Met, O.; Hansen, M.; Lindberg, H.; Sengelov, L.; Svane, I.M. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study. Cytotherapy 2017, 19, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Beer, T.M.; Bartunkova, J.; Kuklík, R.; Miller, K.; Oh, W.K.; Oudard, S.; Pandha, H.S.; Sartor, A.O.; Spisek, R.; et al. Autologous dendritic cell vaccination (DCVAC/PCa) added to docetaxel chemotherapy in a double-blind, randomized phase III trial (VIABLE) in men with advanced (mCRPC) prostate cancer. J. Clin. Oncol. 2015, 33, TPS5070. [Google Scholar] [CrossRef]
- Simons, J.W.; Carducci, M.A.; Mikhak, B.; Lim, M.; Biedrzycki, B.; Borellini, F.; Clift, S.M.; Hege, K.M.; Ando, D.G.; Piantadosi, S.; et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin. Cancer Res. 2006, 12, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.D.; Li, B.; Gonzalez-Edick, M.; Lin, C.; Moskalenko, M.; Du, T.; Creson, J.; VanRoey, M.J.; Jooss, K. GM-CSF-secreting cancer immunotherapies: Preclinical analysis of the mechanism of action. Cancer Immunol. Immunother. 2007, 56, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Sacks, N.; Nemunaitis, J.; Urba, W.J.; Dula, E.; Centeno, A.S.; Nelson, W.G.; Ando, D.; Howard, C.; Borellini, F.; et al. Granulocyte macrophage colony-stimulating factor--secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 2007, 13, 3883–3891. [Google Scholar] [CrossRef]
- Higano, C.S.; Corman, J.M.; Smith, D.C.; Centeno, A.S.; Steidle, C.P.; Gittleman, M.; Simons, J.W.; Sacks, N.; Aimi, J.; Small, E.J. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 2008, 113, 975–984. [Google Scholar] [CrossRef]
- Geary, S.M.; Salem, A.K. Prostate cancer vaccines: Update on clinical development. Oncoimmunology 2013, 2, e24523. [Google Scholar] [CrossRef]
- Gulley, J.L.; Madan, R.A.; Tsang, K.Y.; Jochems, C.; Marte, J.L.; Farsaci, B.; Tucker, J.A.; Hodge, J.W.; Liewehr, D.J.; Steinberg, S.M.; et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol. Res. 2014, 2, 133–141. [Google Scholar] [CrossRef]
- Madan, R.A.; Arlen, P.M.; Mohebtash, M.; Hodge, J.W.; Gulley, J.L. Prostvac-VF: A vector-based vaccine targeting PSA in prostate cancer. Expert Opin. Investig. Drugs 2009, 18, 1001–1011. [Google Scholar] [CrossRef]
- Hodge, J.W.; McLaughlin, J.P.; Kantor, J.A.; Schlom, J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine 1997, 15, 759–768. [Google Scholar] [CrossRef]
- Mandl, S.J.; Rountree, R.B.; Dela Cruz, T.B.; Foy, S.P.; Cote, J.J.; Gordon, E.J.; Trent, E.; Delcayre, A.; Franzusoff, A. Elucidating immunologic mechanisms of PROSTVAC cancer immunotherapy. J. Immunother. Cancer 2014, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.P.; Kantoff, P.W.; Roper, K.; Xu, G.X.; Bubley, G.J.; Boyden, J.; Gritz, L.; Mazzara, G.; Oh, W.K.; Arlen, P.; et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin. Cancer Res. 2000, 6, 1632–1638. [Google Scholar] [PubMed]
- Arlen, P.M.; Skarupa, L.; Pazdur, M.; Seetharam, M.; Tsang, K.Y.; Grosenbach, D.W.; Feldman, J.; Poole, D.J.; Litzinger, M.; Steinberg, S.M.; et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J. Urol. 2007, 178, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.; Chen, A.P.; Dahut, W.; Arlen, P.M.; Bastian, A.; Steinberg, S.M.; Tsang, K.; Panicali, D.; Poole, D.; Schlom, J.; et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 2002, 53, 109–117. [Google Scholar] [CrossRef]
- Parsons, J.K.; Pinto, P.A.; Pavlovich, C.P.; Uchio, E.; Kim, H.L.; Nguyen, M.N.; Gulley, J.L.; Jamieson, C.; Hsu, P.; Wojtowicz, M.; et al. A Randomized, Double-blind, Phase II Trial of PSA-TRICOM (PROSTVAC) in Patients with Localized Prostate Cancer: The Immunotherapy to Prevent Progression on Active Surveillance Study. Eur. Urol. Focus 2018, 4, 636–638. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Wang, W.; Manola, J.; DiPaola, R.S.; Ko, Y.J.; Sweeney, C.; Whiteside, T.L.; Schlom, J.; Wilding, G.; Weiner, L.M. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): A trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2004, 22, 2122–2132. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Schuetz, T.J.; Blumenstein, B.A.; Glode, L.M.; Bilhartz, D.L.; Wyand, M.; Manson, K.; Panicali, D.L.; Laus, R.; Schlom, J.; et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010, 28, 1099–1105. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Gulley, J.L.; Pico-Navarro, C. Revised Overall Survival Analysis of a Phase II, Randomized, Double-Blind, Controlled Study of PROSTVAC in Men With Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 124–125. [Google Scholar] [CrossRef]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Ng, S.; Agarwal, N.; Parker, C.C.; Pook, D.W.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef]
- Arlen, P.M.; Gulley, J.L.; Parker, C.; Skarupa, L.; Pazdur, M.; Panicali, D.; Beetham, P.; Tsang, K.Y.; Grosenbach, D.W.; Feldman, J.; et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1260–1269. [Google Scholar] [CrossRef]
- Arlen, P.M.; Gulley, J.L.; Todd, N.; Lieberman, R.; Steinberg, S.M.; Morin, S.; Bastian, A.; Marte, J.; Tsang, K.Y.; Beetham, P.; et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J. Urol. 2005, 174, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Gulley, J.L.; Schlom, J.; Steinberg, S.M.; Liewehr, D.J.; Dahut, W.L.; Arlen, P.M. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin. Cancer Res. 2008, 14, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, M.; Gulley, J.L.; Heery, C.; Apolo, A.B.; Arlen, P.M.; Rauckhorst, M.; McMahon, S.; Dahut, W.L.; Schlom, J.; Madan, R.A. A randomized phase II study of flutamide with or without PSA-TRICOM in nonmetastatic castration-resistant prostate cancer (CRPC). J. Clin. Oncol. 2011, 29, 163. [Google Scholar] [CrossRef]
- Heery, C.R.; Madan, R.A.; Stein, M.N.; Stadler, W.M.; Di Paola, R.S.; Rauckhorst, M.; Steinberg, S.M.; Marte, J.L.; Chen, C.C.; Grenga, I.; et al. Samarium-153-EDTMP (Quadramet(R)) with or without vaccine in metastatic castration-resistant prostate cancer: A randomized Phase 2 trial. Oncotarget 2016, 7, 69014–69023. [Google Scholar] [CrossRef] [PubMed]
- Picardo, S.L.; Hansen, A.R. The PD-1/PD-L1 pathway in advanced prostate cancer-have we milked this cow? Ann. Transl. Med. 2019, 7, 346. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marte, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFbeta, in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef]
- Santra, S.; Seaman, M.S.; Xu, L.; Barouch, D.H.; Lord, C.I.; Lifton, M.A.; Gorgone, D.A.; Beaudry, K.R.; Svehla, K.; Welcher, B.; et al. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 2005, 79, 6516–6522. [Google Scholar] [CrossRef] [PubMed]
- Siemens, D.R.; Elzey, B.D.; Lubaroff, D.M.; Bohlken, C.; Jensen, R.J.; Swanson, A.K.; Ratliff, T.L. Cutting edge: Restoration of the ability to generate CTL in mice immune to adenovirus by delivery of virus in a collagen-based matrix. J. Immunol. 2001, 166, 731–735. [Google Scholar] [CrossRef]
- Lubaroff, D.M.; Konety, B.R.; Link, B.; Gerstbrein, J.; Madsen, T.; Shannon, M.; Howard, J.; Paisley, J.; Boeglin, D.; Ratliff, T.L.; et al. Phase I clinical trial of an adenovirus/prostate-specific antigen vaccine for prostate cancer: Safety and immunologic results. Clin. Cancer Res. 2009, 15, 7375–7380. [Google Scholar] [CrossRef]
- Vaena, D.A.; Nepple, K.G.; Brown, J.A.; Griffith, K.; Zehr, P.; Lubaroff, D. Time to biochemical and radiographic progression in patients treated with adenovirus-PSA vaccine for non-metastatic/early metastatic castrate-resistant prostate cancer: Phase 2 trial results. J. Clin. Oncol. 2015, 33, e16132. [Google Scholar] [CrossRef]
- Ewer, K.; Sebastian, S.; Spencer, A.J.; Gilbert, S.; Hill, A.V.S.; Lambe, T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum. Vaccines Immunother. 2017, 13, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Rausch, S.; Schwentner, C.; Stenzl, A.; Bedke, J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum. Vaccines Immunother. 2014, 10, 3146–3152. [Google Scholar] [CrossRef]
- Kübler, H.; Maurer, T.; Stenzl, A.; Feyerabend, S.; Steiner, U.; Schostak, M.; Schultze-Seemann, W.; Dorp, F.v.; Pilla, L.; Viatali, G.; et al. Final analysis of a phase I/IIa study with CV9103, an intradermally administered prostate cancer immunotherapy based on self-adjuvanted mRNA. J. Clin. Oncol. 2011, 29, 4535. [Google Scholar] [CrossRef]
- Kallen, K.-J.; Gnad-Vogt, U.; Scheel, B.; Rippin, G.; Stenzl, A. A phase I/IIa study of the mRNA based cancer vaccine CV9103 prepared with the RNActive technology results in distinctly longer survival than predicted by the Halabi Nomogram which correlates with the induction of antigen-specific immune responses. J. Immun. Ther. Cancer 2013, 1, P219. [Google Scholar] [CrossRef]
- Sasada, T.; Noguchi, M.; Yamada, A.; Itoh, K. Personalized peptide vaccination: A novel immunotherapeutic approach for advanced cancer. Hum. Vaccines Immunother. 2012, 8, 1309–1313. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Perez, S.A. Cancer Dormancy: A Regulatory Role for Endogenous Immunity in Establishing and Maintaining the Tumor Dormant State. Vaccines 2015, 3, 597–619. [Google Scholar] [CrossRef]
- Sotiriadou, N.N.; Kallinteris, N.L.; Gritzapis, A.D.; Voutsas, I.F.; Papamichail, M.; von Hofe, E.; Humphreys, R.E.; Pavlis, T.; Perez, S.A.; Baxevanis, C.N. Ii-Key/HER-2/neu(776-790) hybrid peptides induce more effective immunological responses over the native peptide in lymphocyte cultures from patients with HER-2/neu+ tumors. Cancer Immunol. Immunother. 2007, 56, 601–613. [Google Scholar] [CrossRef]
- Qin, Z.; Li, X.; Zhang, J.; Tang, J.; Han, P.; Xu, Z.; Yu, Y.; Yang, C.; Wang, C.; Xu, T.; et al. Chemotherapy with or without estramustine for treatment of castration-resistant prostate cancer: A systematic review and meta-analysis. Medicine 2016, 95, e4801. [Google Scholar] [CrossRef]
- Noguchi, M.; Kakuma, T.; Uemura, H.; Nasu, Y.; Kumon, H.; Hirao, Y.; Moriya, F.; Suekane, S.; Matsuoka, K.; Komatsu, N.; et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol. Immunother. 2010, 59, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Uemura, H.; Naito, S.; Akaza, H.; Yamada, A.; Itoh, K. A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low-dose estramustine in HLA-A24-positive patients with castration-resistant prostate cancer. Prostate 2011, 71, 470–479. [Google Scholar] [CrossRef]
- Storlie, J.A.; Buckner, J.C.; Wiseman, G.A.; Burch, P.A.; Hartmann, L.C.; Richardson, R.L. Prostate specific antigen levels and clinical response to low dose dexamethasone for hormone-refractory metastatic prostate carcinoma. Cancer 1995, 76, 96–100. [Google Scholar] [CrossRef]
- Nishimura, K.; Nonomura, N.; Yasunaga, Y.; Takaha, N.; Inoue, H.; Sugao, H.; Yamaguchi, S.; Ukimura, O.; Miki, T.; Okuyama, A. Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer 2000, 89, 2570–2576. [Google Scholar] [CrossRef]
- Nishimura, K.; Nonomura, N.; Satoh, E.; Harada, Y.; Nakayama, M.; Tokizane, T.; Fukui, T.; Ono, Y.; Inoue, H.; Shin, M.; et al. Potential mechanism for the effects of dexamethasone on growth of androgen-independent prostate cancer. J. Natl. Cancer Inst. 2001, 93, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Fujii, Y.; Iwai, A.; Kageyama, Y.; Kihara, K. Glucocorticoids suppress tumor angiogenesis and in vivo growth of prostate cancer cells. Clin. Cancer Res. 2006, 12, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Itoh, K.; Komatsu, N.; Yamashita, Y.; Shirakusa, T.; Yamada, A.; Moriya, F.; Ayatuka, H.; Mohamed, E.R.; Matsuoka, K.; et al. Dexamethasone did not suppress immune boosting by personalized peptide vaccination for advanced prostate cancer patients. Prostate 2008, 68, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Minami, T.; Nozawa, M.; Kimura, T.; Egawa, S.; Fujimoto, H.; Yamada, A.; Itoh, K.; Uemura, H. A Phase 2 Randomized Controlled Trial of Personalized Peptide Vaccine Immunotherapy with Low-dose Dexamethasone Versus Dexamethasone Alone in Chemotherapy-naive Castration-resistant Prostate Cancer. Eur. Urol. 2016, 70, 35–41. [Google Scholar] [CrossRef]
- Sharifi, N.; Salmaninejad, A.; Ferdosi, S.; Bajestani, A.N.; Khaleghiyan, M.; Estiar, M.A.; Jamali, M.; Nowroozi, M.R.; Shakoori, A. HER2 gene amplification in patients with prostate cancer: Evaluating a CISH-based method. Oncol. Lett. 2016, 12, 4651–4658. [Google Scholar] [CrossRef]
- Day, K.C.; Lorenzatti Hiles, G.; Kozminsky, M.; Dawsey, S.J.; Paul, A.; Broses, L.J.; Shah, R.; Kunja, L.P.; Hall, C.; Palanisamy, N.; et al. HER2 and EGFR Overexpression Support Metastatic Progression of Prostate Cancer to Bone. Cancer Res. 2017, 77, 74–85. [Google Scholar] [CrossRef]
- Osman, I.; Scher, H.I.; Drobnjak, M.; Verbel, D.; Morris, M.; Agus, D.; Ross, J.S.; Cordon-Cardo, C. HER-2/neu (p185neu) protein expression in the natural or treated history of prostate cancer. Clin. Cancer Res. 2001, 7, 2643–2647. [Google Scholar] [PubMed]
- Mellinghoff, I.K.; Vivanco, I.; Kwon, A.; Tran, C.; Wongvipat, J.; Sawyers, C.L. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 2004, 6, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, C.; Jackson, M.W.; Choong, C.S.; Stahl, J.; Marshall, V.R.; Horsfall, D.J.; Tilley, W.D. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate 2008, 68, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.A.; Kallinteris, N.L.; Bisias, S.; Tzonis, P.K.; Georgakopoulou, K.; Varla-Leftherioti, M.; Papamichail, M.; Thanos, A.; von Hofe, E.; Baxevanis, C.N. Results from a phase I clinical study of the novel Ii-Key/HER-2/neu(776-790) hybrid peptide vaccine in patients with prostate cancer. Clin. Cancer Res. 2010, 16, 3495–3506. [Google Scholar] [CrossRef] [PubMed]
- Clifton, G.T.; Peoples, G.E.; Mittendorf, E.A. The development and use of the E75 (HER2 369-377) peptide vaccine. Future Oncol. 2016, 12, 1321–1329. [Google Scholar] [CrossRef]
- Woll, M.M.; Hueman, M.T.; Ryan, G.B.; Ioannides, C.G.; Henderson, C.G.; Sesterhan, I.A.; Shrivasta, S.; McLeod, D.G.; Moul, J.W.; Peoples, G.E. Preclinical testing of a peptide-based, HER2/neu vaccine for prostate cancer. Int. J. Oncol. 2004, 25, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Hueman, M.T.; Dehqanzada, Z.A.; Novak, T.E.; Gurney, J.M.; Woll, M.M.; Ryan, G.B.; Storrer, C.E.; Fisher, C.; McLeod, D.G.; Ioannides, C.G.; et al. Phase I clinical trial of a HER-2/neu peptide (E75) vaccine for the prevention of prostate-specific antigen recurrence in high-risk prostate cancer patients. Clin. Cancer Res. 2005, 11, 7470–7479. [Google Scholar] [CrossRef]
- Gates, J.D.; Carmichael, M.G.; Benavides, L.C.; Holmes, J.P.; Hueman, M.T.; Woll, M.M.; Ioannides, C.G.; Robson, C.H.; McLeod, D.G.; Ponniah, S.; et al. Longterm followup assessment of a HER2/neu peptide (E75) vaccine for prevention of recurrence in high-risk prostate cancer patients. J. Am. Coll. Surg. 2009, 208, 193–201. [Google Scholar] [CrossRef]
- Sotiriadou, R.; Perez, S.A.; Gritzapis, A.D.; Sotiropoulou, P.A.; Echner, H.; Heinzel, S.; Mamalaki, A.; Pawelec, G.; Voelter, W.; Baxevanis, C.N.; et al. Peptide HER2(776-788) represents a naturally processed broad MHC class II-restricted T cell epitope. Br. J. Cancer 2001, 85, 1527–1534. [Google Scholar] [CrossRef][Green Version]
- Voutsas, I.F.; Gritzapis, A.D.; Mahaira, L.G.; Salagianni, M.; von Hofe, E.; Kallinteris, N.L.; Baxevanis, C.N. Induction of potent CD4+ T cell-mediated antitumor responses by a helper HER-2/neu peptide linked to the Ii-Key moiety of the invariant chain. Int. J. Cancer 2007, 121, 2031–2041. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Holmes, J.P.; Murray, J.L.; von Hofe, E.; Peoples, G.E. CD4+ T cells in antitumor immunity: Utility of an li-key HER2/neu hybrid peptide vaccine (AE37). Expert Opin. Biol. Ther. 2009, 9, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.P.; Benavides, L.C.; Gates, J.D.; Carmichael, M.G.; Hueman, M.T.; Mittendorf, E.A.; Murray, J.L.; Amin, A.; Craig, D.; von Hofe, E.; et al. Results of the first phase I clinical trial of the novel II-key hybrid preventive HER-2/neu peptide (AE37) vaccine. J. Clin. Oncol. 2008, 26, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.A.; Anastasopoulou, E.A.; Tzonis, P.; Gouttefangeas, C.; Kalbacher, H.; Papamichail, M.; Baxevanis, C.N. AE37 peptide vaccination in prostate cancer: A 4-year immunological assessment updates on a phase I trial. Cancer Immunol. Immunother. 2013, 62, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, C.N.; Papamichail, M.; Perez, S.A. Immunologic biomarkers in prostate cancer: The AE37 paradigm. Hum. Vaccines Immunother. 2014, 10, 1244–1247. [Google Scholar] [CrossRef]
- Anastasopoulou, E.A.; Voutsas, I.F.; Keramitsoglou, T.; Gouttefangeas, C.; Kalbacher, H.; Thanos, A.; Papamichail, M.; Perez, S.A.; Baxevanis, C.N. A pilot study in prostate cancer patients treated with the AE37 Ii-key-HER-2/neu polypeptide vaccine suggests that HLA-A*24 and HLA-DRB1*11 alleles may be prognostic and predictive biomarkers for clinical benefit. Cancer Immunol. Immunother. 2015, 64, 1123–1136. [Google Scholar] [CrossRef]
- Perez, S.A.; Anastasopoulou, E.A.; Papamichail, M.; Baxevanis, C.N. AE37 peptide vaccination in prostate cancer: Identification of biomarkers in the context of prognosis and prediction. Cancer Immunol. Immunother. 2014, 63, 1141–1150. [Google Scholar] [CrossRef]
- Strauss, J.; Madan, R.A. Integrating Immunotherapies in Prostate Cancer. Curr. Oncol. Rep. 2015, 17, 45. [Google Scholar] [CrossRef]
- Ventola, C.L. Cancer Immunotherapy, Part 3: Challenges and Future Trends. Pharm. Ther. 2017, 42, 514–521. [Google Scholar]
- Schellhammer, P.F.; Chodak, G.; Whitmore, J.B.; Sims, R.; Frohlich, M.W.; Kantoff, P.W. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 2013, 81, 1297–1302. [Google Scholar] [CrossRef]
- Gulley, J.L.; Madan, R.A.; Schlom, J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr. Oncol. 2011, 18, e150–e157. [Google Scholar] [CrossRef]
- Xiang, L.; Gilkes, D.M. The Contribution of the Immune System in Bone Metastasis Pathogenesis. Int. J. Mol. Sci. 2019, 20, 999. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.A.; Jaffee, E.M.; Lutz, E.R. Cancer immunoprevention--the next frontier. Cancer Prev. Res. 2014, 7, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J.; Beatty, P.L. Cancer immunoprevention. Curr. Opin. Immunol. 2016, 39, 52–58. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).