Simple Summary
Little is known about whether residual axillary disease after neoadjuvant chemotherapy carries a different prognostic value by breast cancer subtype. We retrospectively evaluated the axillary involvement (0, 1 to 3 positive nodes, ≥4 positive nodes) on surgical specimens from a cohort of 1197 patients treated with neoadjuvant chemotherapy, and analyzed its association with survival outcomes. Relapse free survival was significantly associated with the number of positive nodes, but this effect was different by breast cancer subtype (Pinteraction = 0.004). High risk patients were those with 4 or more nodes involved in the luminal subgroup, whereas patients with 1 node or more involved had a decreased prognosis in triple negative and HER2 positive breast cancer subgroups. The prognostic value of residual axillary disease should be interpreted according to breast cancer subtype to accurately stratify patients with a high risk of recurrence after neoadjuvant chemotherapy who should be offered second line therapies.
Abstract
Introduction: The three different breast cancer subtypes (Luminal, HER2-positive, and triple negative (TNBCs) display different natural history and sensitivity to treatment, but little is known about whether residual axillary disease after neoadjuvant chemotherapy (NAC) carries a different prognostic value by BC subtype. Methods: We retrospectively evaluated the axillary involvement (0, 1 to 3 positive nodes, ≥4 positive nodes) on surgical specimens from a cohort of T1-T3NxM0 BC patients treated with NAC between 2002 and 2012. We analyzed the association between nodal involvement (ypN) binned into three classes (0; 1 to 3; 4 or more), relapse-free survival (RFS) and overall survival (OS) among the global population, and according to BC subtypes. Results: 1197 patients were included in the analysis (luminal (n = 526, 43.9%), TNBCs (n = 376, 31.4%), HER2-positive BCs (n = 295, 24.6%)). After a median follow-up of 110.5 months, ypN was significantly associated with RFS, but this effect was different by BC subtype (Pinteraction = 0.004), and this effect was nonlinear. In the luminal subgroup, RFS was impaired in patients with 4 or more nodes involved (HR 2.8; 95% CI [1.93; 4.06], p < 0.001) when compared with ypN0, while it was not in patients with 1 to 3 nodes (HR = 1.24, 95% CI = [0.86; 1.79]). In patients with TNBC, both 1-3N+ and ≥4 N+ classes were associated with a decreased RFS (HR = 3.19, 95% CI = [2.05; 4.98] and HR = 4.83, 95% CI = [3.06; 7.63], respectively versus ypN0, p < 0.001). Similar decreased prognosis were observed among patients with HER2-positive BC (1-3N +: HR = 2.7, 95% CI = [1.64; 4.43] and ≥4 N +: HR = 2.69, 95% CI = [1.24; 5.8] respectively, p = 0.003). Conclusion: The prognostic value of residual axillary disease should be considered differently in the 3 BC subtypes to accurately stratify patients with a high risk of recurrence after NAC who should be offered second line therapies.
1. Introduction
Neoadjuvant chemotherapy (NAC) has been for decades the cornerstone of treatment strategy for locally advanced breast cancers (BC) (T3-T4), and tumors not accessible to conservative treatment. Since the publication of the CREATE-X [1] and the KATHERINE trial [2], new post-neoadjuvant treatment options have emerged in triple negative (TNBCs) and HER2-positive BC. Beyond the increase of breast conservative surgery rates, NAC provides a way to assess the tumor chemosensitivity and evaluate the mechanisms of resistance to chemotherapy through the evaluation of residual tumor burden.
Axillary lymph node involvement is the most important prognostic factor in BC, and has long been proven to be correlated with poor survival outcomes [3,4,5,6]. In the neoadjuvant setting, several studies have established the critical role of nodal burden in the assessment of prognosis after NAC in large cohorts of patients [7,8,9,10,11].
Pathologic complete response (pCR) is defined as the absence of invasive cancer in the breast and axillary lymph nodes, and has been shown to be associated with a better long-term survival among BC patients treated with NAC. Although nodal axillary response has been described as a superior prognostic parameter after NAC [12,13], overall pCR is more frequently used and has been adopted by the Food and Drug Administration and the European Medicines Agency as an important endpoint in BC neoadjuvant studies [14].
The prognostic value of pCR to predict event-free survival varies among BC subtypes [15,16]. In 2014, a meta-analysis by Cortazar et al. [17] including 11,955 patients found a stronger association between pCR and long-term outcomes in patients with TNBCs (RFS: HR = 0.24, 95% CI [0.18–0.33]) and in those with HER2-positive hormone receptor negative BC (RFS: HR = 0.15, 95% CI [0.09–0.27]); whereas the association was less marked in HER2-positive hormone receptor positive BC (RFS: HR = 0.58, 95% CI [0.42–0.82]) and luminal BC (RFS: HR = 0.49, 95% CI [0.33–0.71]).
However, the evidence evaluating the prognostic impact of residual axillary burden after NAC according to BC subtypes is scarce. Most of the studies evaluating the prognostic impact of axillary response to NAC classified patients in a binary manner, depending on the presence or absence of residual nodal disease, without taking into account the number of axillary lymph nodes involved; fewer studies, if any, performed upfront comparison of the prognostic significance by the BC subtype.
The aim of our study is to evaluate the impact of the number of axillary nodes involved on survival outcomes according to BC subtype in a real-life cohort of breast cancer patients treated with NAC.
2. Results
2.1. Baseline Patients’ and Tumors’ Characteristics
A total of 1197 patients were included in the cohort. Patients’ baseline characteristics are summarized in Table 1. Median age was 48 years old. Patient’s repartition by subtype was as follows: luminal (n = 526, 43.9%), TNBC (n = 376, 31.4%), HER2-positive (n = 295; 24.6%). The nodal status of patients at diagnosis was as follows: 525 patients (44%) were node negative before neoadjuvant treatment (n = 235 luminal BC (45%); n = 171 TNBC (45.5%); n = 119 HER-2 positive BC (40.3%)). Out of the 295 HER-2 positive patients, 204 (69.2%) received HER-2 targeted therapy.

Table 1.
Patients and tumor characteristics by post- neoadjuvant chemotherapy (NAC) nodal involvement.
After NAC, 43% of the patients (515/1197) had a nodal involvement. Patients with bigger tumors, with clinical baseline nodal involvement, luminal BCs (versus TNBC or HER2- positive), low proliferative tumors (versus high proliferative), with lower immune infiltration (versus high TIL levels) were more likely to have a nodal involvement at NAC completion. Among HER-2 positive BC patients, those having been treated with trastuzumab were more likely to have no axillary disease after NAC (Table S1). Axillary staging technique was axillary lymph node dissection for the majority of patients (n = 1169, 97.7%).
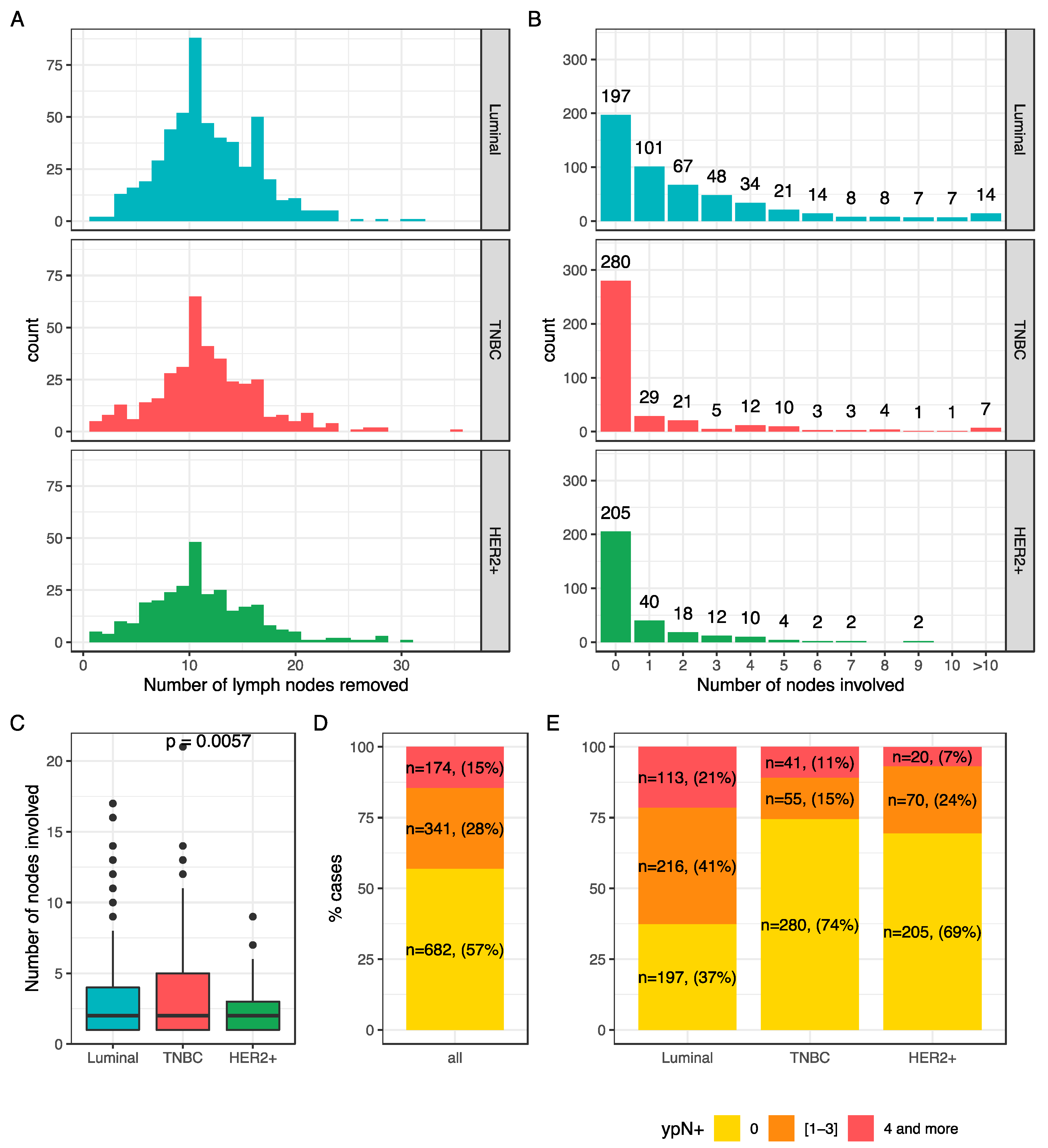
The number of nodes ranged from 1 to 35 (median: 11) (Figure 1A) and the number of lymph nodes involved varied from 0 to 21(Figure 1B). In case of nodal involvement, the median number of nodes involved was 2 (Figure 1C), and the repartition was significantly different among BC subtypes. Overall, 57% of the patients had no nodal involvement at axillar surgery (n = 682), 28% had a mild nodal involvement (n = 341), and 15% (n = 174) had a high nodal involvement (Figure 1D). This repartition was significantly different by BC subtype (p < 0.001) (Figure 1E).
Figure 1.
Nodal burden after NAC: number of lymph nodes removed according to BC subtype (A); number of involved nodes according to BC subtype (B); mean number of nodes involved after NAC according to BC subtype (C); node involvement repartition after NAC in the whole population (D) and according to BC subtype (E).
2.2. Association Between Post-NAC Involvement and Tumor Characteristics
Among post-NAC characteristics, node positivity was associated with RCB index (Table 2, Figure S1A), with the presence of lymphovascular invasion (Figure S1B), and with higher post-NAC tumor cellularity (Figure S1C). Neither post-NAC mitotic index (Figure S1D), stromal (Figure S1E) nor IT TILs (Figure S1F) were significantly associated with post-NAC nodal status. Similar patterns were observed within each BC subtype (Figure S2A–F), with the very exception of post-NAC tumor cellularity (all three BC subtypes), post-NAC mitotic index (luminal BC), and str TILs levels (HER2-positive BC) that were significantly higher with increasing number of nodes involved (Figure S2).

Table 2.
Tumor characteristics by post-NAC nodal involvement.
2.3. Survival Analyses
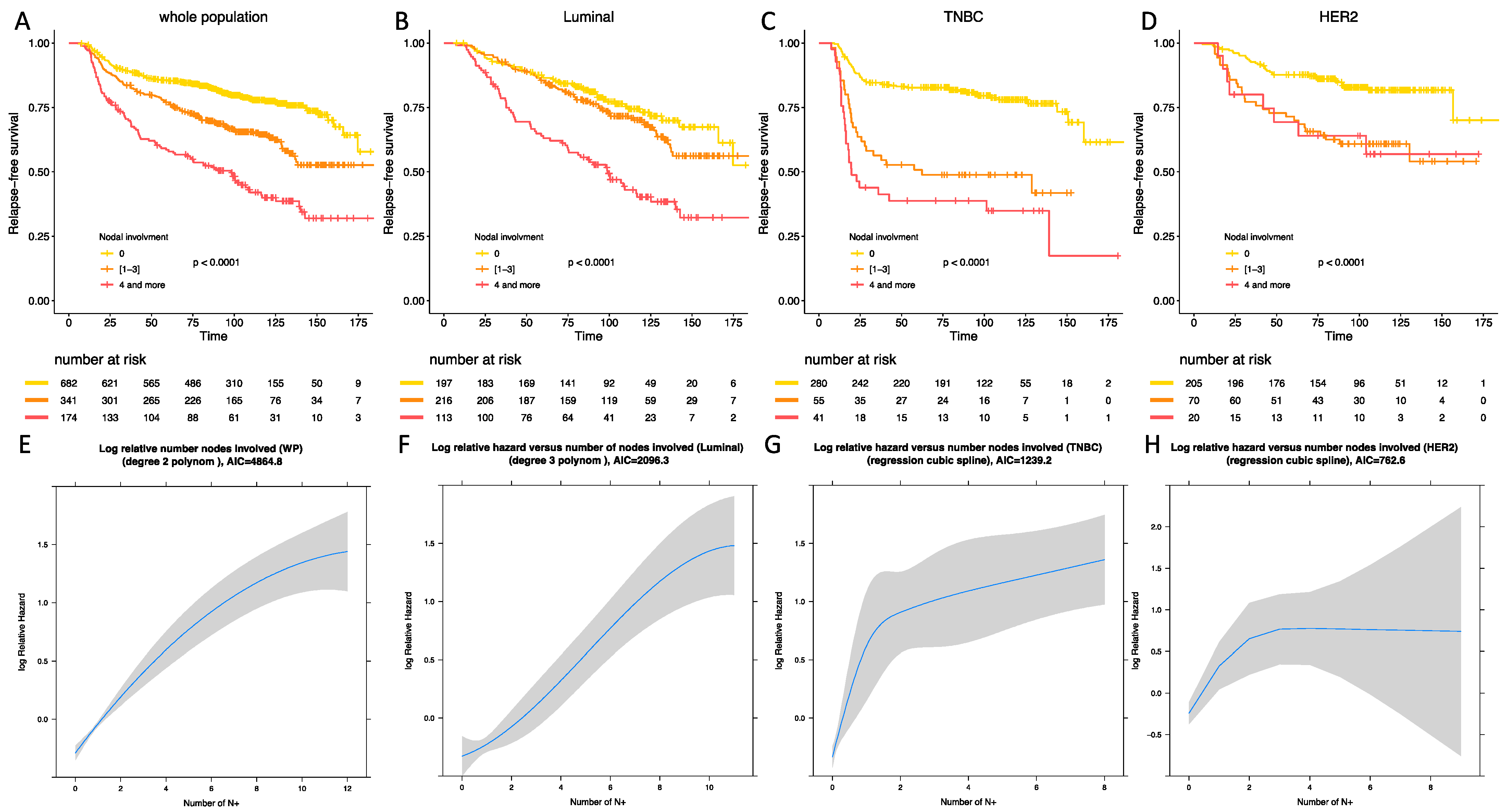
With a median follow-up of 110.5 months (118.6 months for luminal BC patients, 102.6 months for TNBC patients, 106.3 months for HER-2 positive BC patients), 371 patients experienced relapse, and 228 died. After univariate and multivariate analysis, post-NAC nodal involvement was significantly associated with RFS in the whole population (p < 0.001) (Table 3). After analyses by BC subtype, the association between nodal involvement binned by 3 classes and RFS was significant in all the BC subgroups, but this association was significantly different according to the BC subtype (Pinteraction = 0.004). In the whole population, mild post-NAC nodal involvement (1 to 3); and high nodal involvement were associated with an impaired RFS after univariate analysis (HR = 1.79, 95% CI [1.42–2.28] and HR = 3.3, 95% CI [2.59–4.32]) and after multivariate analysis (HR = 2.06, 95% CI [1.59–2.66] and HR = 3.6, 95% CI [2.73–4.75]) (Figure 2A). The association between RFS and axillary involvement compared with pCR was also studied (Figures S3 and S4). p values tended to be lower with nodal status and AIC were systematically lower with nodal involvement (Supplementary Material, Table S2).

Table 3.
Association of clinical and pathological pre and post-NAC parameters with relapse-free survival after univariate and multivariate analysis in the whole population.
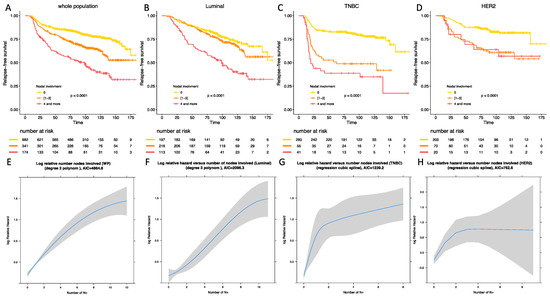
Figure 2.
Relapse-free survival according to BC subtype in the whole population (A), in luminal BC (B), in TNBC (C), in HER-2 positive BC (D). Statistical models reflecting the association between relapse free survival and nodal status in the whole population (E), in luminal BC (F), in TNBC (G), and in HER-2 positive BC (H).
In luminal BCs, mild post-NAC nodal involvement was not associated with an impaired RFS when compared with ypN0 tumors (HR = 1.24, 95% CI [0.86–1.79] in univariate analysis and HR = 1.18, 95% CI [0.82–1.71] in multivariate analysis) (Table S3), whereas patients with a high nodal involvement were associated with an adverse prognosis (HR = 2.8, 95% CI [1.93–4.06] in univariate analysis and HR = 2.68, 95% CI [1.84–3.89] in multivariate analysis) (Figure 2B). In TNBCs, both mild (HR = 3.19, 95% CI [2.05–4.98] for univariate analysis, HR = 3.17, 95% CI [2.03–4.95] for multivariate analysis) (Table S4) and high post-NAC nodal involvement (HR = 4.83, 95% CI [3.06–7.63]; HR = 4.52, 95% CI [2.85–7.17]) were associated with an impaired RFS when compared with ypN0 tumors. The difference between [1,2,3] and 4 or more was statistically significant (p < 0.001) (Figure 2C). In HER2-positive BCs, patients who had tumors with a mild nodal involvement were at a higher risk of relapse (HR = 2.7, 95% CI [1.64–4.43]) (Table S5) when compared with node negative tumors, but the prognosis was not significantly different from patients with 4 nodes involved or more (HR = 2.69, 95% CI [1.24–5.8]) (Figure 2D).
There was a significant deviation to the linearity assumption of the association between RFS and post-NAC nodal involvement in the whole population and in the 3 BC subtypes. After statistical modelization, the statistical models best fitted a second-degree polynomial (whole population and luminal subgroup Figure 2E,F respectively), and a restricted cubic spline (TNBC and HER2-positive BCs, Figure 2G,H respectively).
After multivariate analysis (Tables S3–S5), post-NAC nodal involvement was significantly associated with RFS in luminal and TNBCs, but not in HER2-positive BC.
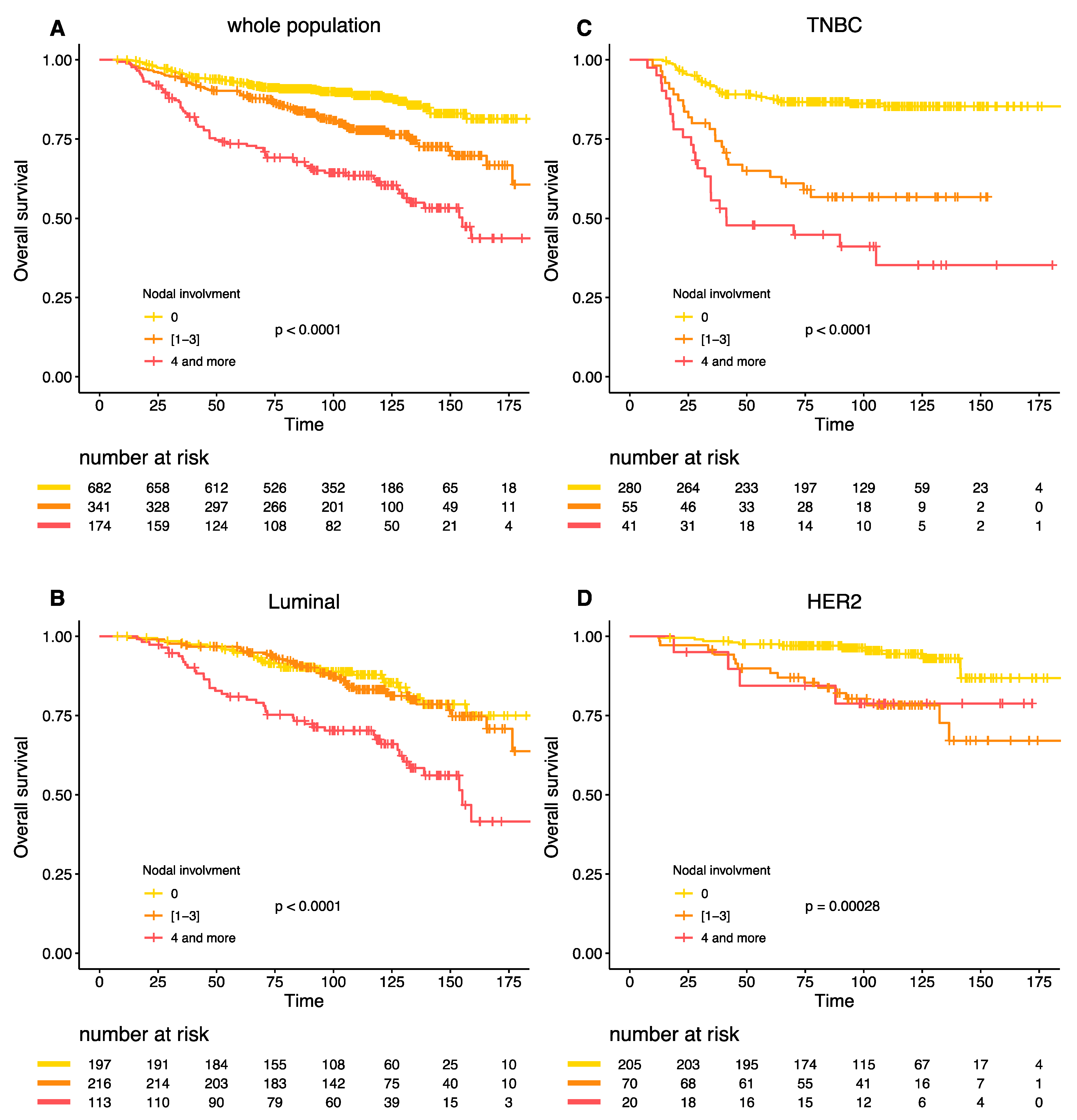
Similar results were obtained for the overall survival (Figure 3A–D). The interaction between BC subtype, post-NAC nodal involvement and survival was highly significant (Pinteraction = 0.005).
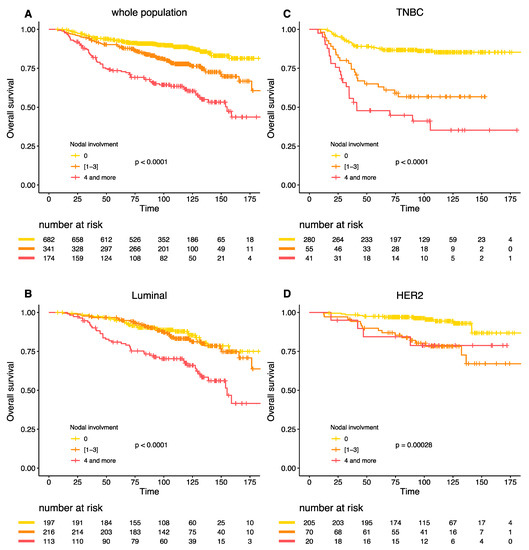
Figure 3.
Overall survival according to post NAC nodal involvement in the whole population (A), Luminal BC (B), TNBC (C), and HER-2 positive BC (D).
3. Discussion
In this retrospective study of 1197 BC patients treated with NAC, we confirmed the strong prognostic value of nodal involvement after NAC, and we identified a marked difference in the prognostic impact of the axillar burden among the 3 BC subtypes.
Our study provides several new insights. First, it is in line with the previous reports showing that the prognostic value of the axillary burden outperformed the value of the widely used binary endpoint pathological complete response. Rouzier et al. [12] found a higher correlation between RFS and axillary response to primary chemotherapy than with tumoral breast response in 152 BC patients. Hennessy et al. [13] found no impact of residual breast disease on survival outcomes among patients having achieved axillary pCR in a cohort of 403 BC patients with initial nodal involvement treated with NAC. This was confirmed by Dominici et al. [18] in 2010 in a retrospective study of 102 HER-2 positive patients.
Second, along with previous studies (Table 4), we found a higher rate of post-NAC negative nodal status in case of TNBC, HER2 positive BC, small tumor size, high-grade tumors [10,19,20,21,22,23,24,25], and high Ki67 [26,27]. In 2014, Boughey et al. [20] studied 694 BC patients treated with NAC with clinical nodal involvement at diagnosis. They found significantly higher rates of post-NAC ypN0 status among TNBC and HER-2 positive BC subgroups (49.4% and 64.7% respectively) than in luminal BC patients (21.1%). In 2016, Mougalian et al. [10] found similar results in a cohort of 1600 stage II/III N+ BC patients: post-NAC negative nodal status rates repartition was 16.4% for luminal BC versus 40.8% for TNBCs and 47.3% for HER-2 positive BC.

Table 4.
Summary of previous studies comparing prognosis according to nodal involvement after neoadjuvant chemotherapy (NAC) according to breast cancer subtype.
Our study supports the previous findings that pathological response has different prognostic implications across BC subtypes. It also explores the survival impact of the number of involved nodes after NAC according to BC subtype which has rarely been explored. So far, most studies evaluating the prognostic impact of post-NAC nodal involvement used the binary endpoint ypN0 versus ypN+ [10,12]. Four studies [9,19,20,25] used binned classes approaching the TNM classification (N0; N1: 1 to 3 nodes involved; N2: 4 to 9 nodes involved; N3: 10 or more nodes involved) (Table 4). However, to our knowledge, no study compared upfront the prognostic impact of nodal involvement according to BC subtypes nor performed linearity tests. Our results show that the prognostic value of the number of post-NAC positive nodes differs according to BC subtype. It has been demonstrated that achieving ypT0 and ypN0 in luminal BC is not as predictive of relapse free survival as it is in TNBC or HER-2 positive BC [17]. Results from our study show that patients with luminal BC presenting post-NAC axillary residual disease up to 3 positive nodes had a similar prognosis to those with no axillary residual disease, while we evidenced a negative impact on survival outcomes when the number of nodes involved was 4 or above. Our data support the argument that a reporting system incorporating this information should be routinely used following NAC. The prognostic impact of low-to-intermediate nodal involvement (1 to 3 nodes involved after chemotherapy) has also been studied in the adjuvant setting. Retrospective analyses from randomized trials have suggested that the recurrence score of a 21 gene assay [28,29] could identify a subset of ER + /HER-2 negative BC patients with positive nodes who did not derive a significant benefit from chemotherapy: Albain et al. [30] and Dowsett et al. [31] found low risks of distant metastases in luminal low recurrence score N+ disease and luminal low recurrence score disease with 1 to 3 nodes involved respectively. The withholding of adjuvant chemotherapy for this category of BC patients is currently being evaluated in an ongoing trial [32]. In the TNBC subgroup, as previously identified by our team [33], a positive nodal status after NAC was a poor prognostic factor, and the prognosis was worsened as soon as one lymph node was involved. However, as shown by the cubic spline statistical model best fitting the data, the slope of the increase of the risk was maximal between 0 and 2 lymph nodes, and the slope decreased thereafter. Finally, in the HER-2 positive BC subgroup, the existence of residual axillary disease was a poor prognostic factor and the magnitude of the risk was similar for patients with 1 to 3 nodes involved and those with 4 or more nodes involved (RFS HR 2.68 95% CI [1.63–4.41] vs. 2.67 95% CI [1.24–5.77]), though the interpretation might be limited by the weak effective of the latter category (n = 20 out of 295 HER-2 positive BC patients, 6.8%).
Regarding post-NAC parameters, our study found that node positivity was associated with lymphovascular invasion, which is in line with a previous study describing LVI as a strong prognostic marker [34].
To the best of our knowledge, we report here the first upfront comparison of the prognostic value of residual axillary disease among each BC subtype, while taking into account the number of positive nodes after NAC. In addition, we evidenced that the relationship between nodal involvement and relapse free survival was nonlinear, and this was true in every BC subtype. The main strengths of our study include its large statistical power, its long-term follow-up. Limits of our study include its retrospective design and the absence of external independent validation. It should also be precise that the incidence of missing data was high for several variables (notably LVI, TIL levels, RCB index, Ki 67 and BRCA status), which may have had an impact on the presence or absence of statistically significant associations, although they were removed from multivariate analyses if variables had missing data in more than 30% of the cases. RCB classification data were missing for 40% of the patients, which can be explained by the fact that this study’s cohort predates Fraser Symman’s 2007 paper describing the RCB classification [35]. Pathological response data were extracted from pathology reports and retrospective pathological review of the slides was performed when possible in case of missing data, but was not systematic.
Our study has pragmatic implications. If confirmed in independent studies, it suggests that the cut-off to consider high-risk patients after NAC completion should be different according to BC subtypes: 4 or more nodes involved for luminal BC patients, and 1 for TNBC and HER-2 positive BC patients. With the widespread routine use of NAC for TNBC and HER2-positive BC patients 1 2, second-line trials in the post neoadjuvant setting for high risk patients are increasing testing the addition of chemotherapy, PARP inhibitors [36], immunotherapy [37], cyclin-dependent kinase inhibitors [38], or vaccines [39]. We previously demonstrated that TILs-enriched luminal BRCA tumors [40] and TILs-enriched HER-positive BC tumors are at a high risk of relapse [41] and could benefit from additional therapies. In the light of these current scientific developments, residual axillary disease is not a predictive factor of the efficacy of such specific therapies, but our findings are of particular importance since they may help to identify more accurately the high-risk patients who might benefit from such treatments by considering the number of residual positive nodes after NAC as a cornerstone of prognostication, provided that it is interpreted according to histological BC subtype.
4. Materials and Methods
4.1. Patients
We analyzed a previously described retrospective cohort of patients [42,43] with invasive breast carcinoma stage T1-T3NxM0 and treated with NAC at Institut Curie, Paris, between 2002 and 2012 (NEOREP Cohort, CNIL declaration number). We included unilateral, non-recurrent, non-inflammatory, non-metastatic tumors, excluding T4 tumors. All patients received NAC, followed by surgery and radiotherapy. NAC regimens changed over our recruitment period (anthracycline-based regimen or sequential anthracycline-taxanes regimen), with trastuzumab used in an adjuvant and/or neoadjuvant setting since 2005. Endocrine therapy (tamoxifen or aromatase inhibitor) was prescribed when indicated. The study was approved by the Breast Cancer Study Group of Institut Curie and was conducted according to institutional and ethical rules concerning research on tissue specimens and patients. Informed consent from patients was not required by French regulations.
4.2. Tumor Samples and Pathological Review
4.2.1. BC Subtypes
Cases were considered estrogen receptor (ER) or progesterone receptor (PR) positive (+) if at least 10% of the tumor cells expressed estrogen and/or progesterone receptors (ER/PR), in accordance with the guidelines used in France [44]. HER2 expression was determined by immunohistochemistry with scoring in accordance with the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines [45]. Scores 3+ were reported as positive, score 1+/0 as negative (-). Tumors with scores 2+ were further tested by FISH. HER2 gene amplification was defined in accordance with ASCO/CAP guidelines. We evaluated a mean of 40 tumor cells per sample and the mean HER2 signals per nuclei was calculated: a HER2/CEN17 ratio ≥ 2 was considered positive, and a ratio < 2 negative. BC subtypes were defined as follows: tumors positive for either ER or PR, and negative for HER2 were classified as luminal; tumors positive for HER2 were considered to be HER2-positive BC; tumors negative for ER, PR, and HER2 were considered to be triple-negative breast cancers (TNBC). Tumor cellularity was defined as the percentage of tumor cells (in situ and invasive) on the specimen (biopsy or surgical specimen). Mitotic index was reported per 10 high power fields (HPF) (1 HPF = 0.301 mm2).
4.2.2. Post-NAC Nodal Involvement (ypN)
Post-NAC nodal involvement (ypN) was divided into three categories: no axillary involvement (ypN = 0), intermediate involvement (1 to 3 nodes involved, 1 ≤ ypN ≤ 3), and high axillary involvement (4 or more nodes involved, ypN ≥ 4). Nodal extent was also analyzed as a continuous variable.
4.2.3. Residual Cancer Burden Index (RCB)
Histological components of the “Residual Cancer Burden” were retrieved for calculating the score as described in 2007 by Symmans [35]. RCB index enables the classification of residual disease into four categories: RCB-0 (complete pathologic response = pCR), RCB-I (minimal residual disease), RCB-II (moderate residual disease), and RCB-III (extensive residual disease). RCB was calculated through the web-based calculator that is freely available on the Internet (www.mdanderson.org/breastcancer_RCB).
4.2.4. TILs and LVI
Lymphovascular invasion (LVI) was defined as the presence of carcinoma cells within a finite endothelial-lined space (a lymphatic or blood vessel). Tumor infiltrating lymphocytes (TILs) were defined as the presence of mononuclear cells infiltrate (including lymphocytes and plasma cells, excluding polymorphonuclear leukocytes), and were also evaluated retrospectively for research purposes, according to the recommendations of the international TILs Working Group [46,47].
4.3. Study Endpoints
Relapse-free survival (RFS) was defined as the time from surgery to death, loco-regional recurrence or distant recurrence, whichever occurred first, and overall survival (OS) was defined as the time from surgery to death. Patients for whom none of these events were recorded were censored at the date of their last known contact. Survival cutoff date analysis was 1 February 2019.
4.4. Statistical Analysis
The study population was described in terms of frequencies for qualitative variables, or medians and associated ranges for quantitative variables. Chi-square tests were performed to search for differences between subgroups for each variable (considered significant for p-values ≤ 0.05). Survival probabilities were estimated by the Kaplan–Meier method, and survival curves were compared in log-rank tests. Hazard ratios and their 95% confidence intervals were calculated with the Cox proportional hazards model. Variables with a p-value for the likelihood ratio test equal to 0.05 or lower in univariate analysis were selected for inclusion in the multivariate analysis. A forward stepwise selection procedure was used to establish the final multivariate model and the significance threshold was 5%. For variables that were significantly correlated, collinearity was avoided by retaining only one variable, based on its clinical relevance or likelihood ratio. Variables with missing data in more than 30% of the cases were removed from the multivariate analyses.
4.5. Linearity and Interaction Tests
We investigated the linearity of the association between nodal involvement and RFS/OS by comparing the model in which nodal involvement was considered to vary linearly with models based on restricted cubic spline fits and fractional polynomials, as previously described [48]. If significant deviation from the assumption of linearity was observed, based on the lowest AIC of the model, the variable was modeled with ypN binned into classes.
We tested the hypothesis of potentially different effects of ypN in different BC subtypes, by including interaction terms in the Cox model. A p-value of 0.10 was selected to determine the statistical significance of the interaction term, as it has been suggested because of a low power of the test in the interaction setting [49].
Data were processed and statistical analyses were carried out with R software version 3.1.2 (www.cran.r-project.org, R Foundation for Statistical Computing, 2009).
5. Conclusions
The prognostic value of residual axillary burden differs according to BC subtype. The number of residual positive nodes after NAC should be interpreted according to histological BC subtype to accurately stratify patients with a high risk of recurrence after NAC who should be offered second line therapies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/2/171/s1, Figure S1: Association between post-NAC involvement and tumor characteristics in the whole population: RCB index (A); Lymphovascular invasion (B); Tumor cellularity (C); Post-NAC mitotic index (D); Stromal TIL levels (E). Intra tumoral (IT) TIL levels (F), Figure S2: Association between post-NAC involvement and tumor characteristics according to BC subtype: RCB index (A); Lymphovascular invasion (B); Tumor cellularity (C); Post-NAC mitotic index (D); Stromal TIL levels (E). Intra tumoral (IT) TIL levels (F), Figure S3: Relapse Free Survival (RFS) according to pCR after neoadjuvant chemotherapy in the whole population and according to breast cancer subtype, Figure S4: Relapse Free Survival (RFS) according to nodal status after neoadjuvant chemotherapy in the whole population and according to breast cancer subtype, Table S1: Post-NAC nodal involvement according to NAC regimen among HER-2 positive BC patients (n=295), Table S2: Association between relapse free survival and pCR versus nodal status after neoadjuvant chemotherapy (Whole population, Luminal BC, TNBC and HER-2 positive BC), Table S3: Association between clinical and pathological pre and post-NAC parameters with relapse-free survival (Luminal BC population, univariate and multivariate analysis), Table S4: Association between clinical and pathological pre and post-NAC parameters with relapse-free survival (TNBC population, univariate and multivariate analysis), Table S5: Association between clinical and pathological pre and post-NAC parameters with relapse-free survival (HER-2 BC population, univariate and multivariate analysis).
Author Contributions
Conceptualization, E.L., F.R. and A.-S.H.; methodology, E.L., F.R. and A.-S.H.; software, A.-S.H., E.L., E.D. (Elise Dumas), E.D. (Eric Daoud) and B.G.; validation, E.L., N.G., J.-Y.P., F.C., Y.K., E.E.-A., G.B., M.L.; formal analysis, N.G., E.D. (Elise Dumas), E.D. (Eric Daoud), B.G., E.E.-A., G.B., M.L. and A.-S.H.; investigation, A.-S.H.; resources, A.-S.H.; data curation, E.D. (Elise Dumas), E.D. (Eric Daoud), B.G.; writing—original draft preparation, L.L.; writing—review and editing, L.L. and F.L.; visualization, A.-S.H.; supervision, A.-S.H. and F.R.; project administration, F.R.; funding acquisition, A.-S.H. All authors have read and agreed to the published version of the manuscript.
Funding
We thank Roche France for financial support for the construction of the Institut Curie neoadjuvant database (NEOREP). The funders had no role in study design, data collection and 374 analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Breast Cancer Study Group of Institut Curie (September 2020, CNIL declaration number 1547270).
Informed Consent Statement
Informed consent from patients was not required by French regulations.
Data Availability Statement
The data presented in this study are available in the present manuscript or in the Supplementary Material section.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Masuda, N.; Lee, S.J. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.S. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, P.L.; Page, D.L. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 966–978. [Google Scholar] [PubMed]
- Shek, L.L.; Godolphin, W. Model for breast cancer survival: Relative prognostic roles of axillary nodal status, TNM stage, estrogen receptor concentration, and tumor necrosis. Cancer Res. 1988, 48, 5565–5569. [Google Scholar] [PubMed]
- Saez, R.A.; McGuire, W.L.; Clark, G.M. Prognostic factors in breast cancer. Semin. Surg. Oncol. 1989, 5, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.L.; Allen, C.; Henson, D.E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989, 63, 181–187. [Google Scholar] [CrossRef]
- McCready, D.R.; Hortobagyi, G.N. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch. Surg. 1989, 124, 21–25. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Sahin, A.A. Incidence and Impact of Documented Eradication of Breast Cancer Axillary Lymph Node Metastases Before Surgery in Patients Treated with Neoadjuvant Chemotherapy. Ann. Surg. 1999, 230, 72. [Google Scholar] [CrossRef]
- Pierga, J.Y.; Mouret, E. Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br. J. Cancer 2000, 83, 1480–1487. [Google Scholar] [CrossRef]
- Mougalian, S.S.; Hernandez, M. Ten-Year Outcomes of Patients with Breast Cancer with Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy. JAMA Oncol. 2016, 2, 508–516. [Google Scholar] [CrossRef]
- Symmans, W.F.; Wei, C. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated with Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Extra, J.M. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J. Clin. Oncol. 2002, 20, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Hortobagyi, G.N. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J. Clin. Oncol. 2005, 23, 9304–9311. [Google Scholar] [CrossRef] [PubMed]
- Federal Register: Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. Guidance for Industry. Available online: https://www.federalregister.gov/documents/2014/10/07/2014-23845/pathological-complete-response-in-neoadjuvant-treatment-of-high-risk-early-stage-breast-cancer-use (accessed on 1 November 2020).
- Houssami, N.; Macaskill, P.; von Minckwitz, G.; Marinovich, M.L.; Mamounas, E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur. J. Cancer 2012, 48, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Untch, M. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Dominici, L.S.; Negron, G. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer 2010, 116, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.C.; Zhang, Y.F. Axillary lymph node status, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr. Oncol. 2013, 20, e180–e192. [Google Scholar] [CrossRef]
- Boughey, J.C.; McCall, L.M. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: Findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann. Surg. 2014, 260, 608–616. [Google Scholar] [CrossRef]
- Mamtani, A.; Barrio, A.V. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients with Histologically Confirmed Nodal Metastases: Results of a Prospective Study. Ann. Surg. Oncol. 2016, 23, 3467–3474. [Google Scholar] [CrossRef]
- Fayanju, O.M.; Ren, Y. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) after Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Ann. Surg. 2018, 268, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, H.S. Prognostic Nomogram for Prediction of Axillary Pathologic Complete Response After Neoadjuvant Chemotherapy in Cytologically Proven Node-Positive Breast Cancer. Medicine 2015, 94, e1720. [Google Scholar] [CrossRef] [PubMed]
- AL-Tweigeri, T.; AlSayed, A. A multicenter prospective phase II trial of neoadjuvant epirubicin, cyclophosphamide, and 5-fluorouracil (FEC100) followed by cisplatin–docetaxel with or without trastuzumab in locally advanced breast cancer. Cancer Chemother. Pharmacol. 2016, 77, 147–153. [Google Scholar] [CrossRef]
- Boland, M.R.; McVeigh, T.P. Impact of receptor phenotype on nodal burden in patients with breast cancer who have undergone neoadjuvant chemotherapy. BJS Open 2017, 1, 39–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sueta, A.; Yamamoto, Y. Clinical significance of pretherapeutic Ki67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer: Is it equally useful across tumor subtypes? Surgery 2014, 155, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Chow, L.W.C. Randomized trial of preoperative docetaxel with or without capecitabine after 4 cycles of 5-fluorouracil– epirubicin–cyclophosphamide (FEC) in early-stage breast cancer: Exploratory analyses identify Ki67 as a predictive biomarker for response to neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2013, 142, 69–80. [Google Scholar] [PubMed]
- Andre, F.; Arnedos, M.; Goubar, A.; Ghouadni, A.; Delaloge, S. Ki67-no evidence for its use in node-positive breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 296–301. [Google Scholar] [CrossRef]
- Sotiriou, C.; Pusztai, L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009, 360, 790–800. [Google Scholar] [CrossRef]
- Albain, K.S.; Barlow, W.E. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010, 11, 55–65. [Google Scholar] [CrossRef]
- Dowsett, M.; Cuzick, J. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J. Clin. Oncol. 2010, 28, 1829–1834. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov: National Cancer Institute (NCI). A Phase III, Randomized Clinical Trial of Standard Adjuvant Endocrine Therapy +/− Chemotherapy in Patients with 1-3 Positive Nodes, Hormone Receptor-Positive and HER2-Negative Breast Cancer with Recurrence Score (RS) of 25 or Less. RxPONDER: A Clinical Trial Rx for Positive Node, Endocrine Responsive Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01272037 (accessed on 4 November 2020).
- Bonsang-Kitzis, H.; Chaltier, E. Beyond Axillary Lymph Node Metastasis, BMI and Menopausal Status Are Prognostic Determinants for Triple-Negative Breast Cancer Treated by Neoadjuvant Chemotherapy. PLoS ONE 2015, 10, e0144359. [Google Scholar] [CrossRef] [PubMed]
- Hamy, A.-S.; Lam, G.T.; Laas, E. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res. Treat. 2018, 169, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.Gov: AstraZeneca. A Randomised, Double-blind, Parallel Group, Placebo-controlled Multi-centre Phase III Study to Assess the Efficacy and Safety of Olaparib Versus Placebo as Adjuvant Treatment in Patients with gBRCA1/2 Mutations and High Risk HER2 Negative Primary Breast Cancer Who Have Completed Definitive Local Treatment and Neoadjuvant or Adjuvant Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT02032823 (accessed on 16 November 2020).
- ClinicalTrials.Gov: M.D. Anderson Cancer Center. Triple-Negative First-Line Study: Neoadjuvant Trial of Nab-Paclitaxel and MPDL3280A, a PDL-1 Inhibitor in Patients with Triple Negative Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02530489 (accessed on 16 November 2020).
- ClinicalTrials.Gov: German Breast Group. Phase III Study Evaluating Palbociclib (PD-0332991), a Cyclin-Dependent Kinase (CDK) 4/6 Inhibitor in Patients with Hormone-receptor-positive, HER2-normal Primary Breast Cancer with High Relapse Risk After Neoadjuvant Chemotherapy ‘PENELOPEB’. Available online: https://clinicaltrials.gov/ct2/show/NCT01864746 (accessed on 16 November 2020).
- ClinicalTrials.Gov: Cancer Insight, LLC. Phase II Trial of Combination Immunotherapy with Nelipepimut-S + GM-CSF (NeuVaxTM) and Trastuzumab in High-risk HER2+ Breast Cancer Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT02297698 (accessed on 16 November 2020).
- Grandal, B.; Evrevin, C. Impact of BRCA Mutation Status on Tumor Infiltrating Lymphocytes (TILs), Response to Treatment, and Prognosis in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Cancers 2020, 12, 3681. [Google Scholar] [CrossRef]
- Hamy, A.S.; Bonsang-Kitzis, H. Interaction between Molecular Subtypes and Stromal Immune Infiltration before and after Treatment in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Clin. Cancer Res. 2019, 25, 6731–6741. [Google Scholar] [CrossRef]
- Hamy, A.S.; Pierga, J.Y. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann. Oncol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef]
- Hamy, A.S.; Darrigues, L. Prognostic value of the Residual Cancer Burden index according to breast cancer subtype: Validation on a cohort of BC patients treated by neoadjuvant chemotherapy. PLoS ONE 2020, 15, e0234191. [Google Scholar] [CrossRef]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [PubMed]
- Harrell, F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2001. [Google Scholar]
- Selvin, S. Statistical Analysis of Epidemiologic Data. Statistical Analysis of Epidemiologic Data; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).