Circulating Tumor Cells and Bevacizumab Pharmacokinetics during Neoadjuvant Treatment Combining Chemotherapy and Bevacizumab for Early Breast Cancer: Ancillary Analysis of the AVASTEM Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients’ Clinicopathological Features
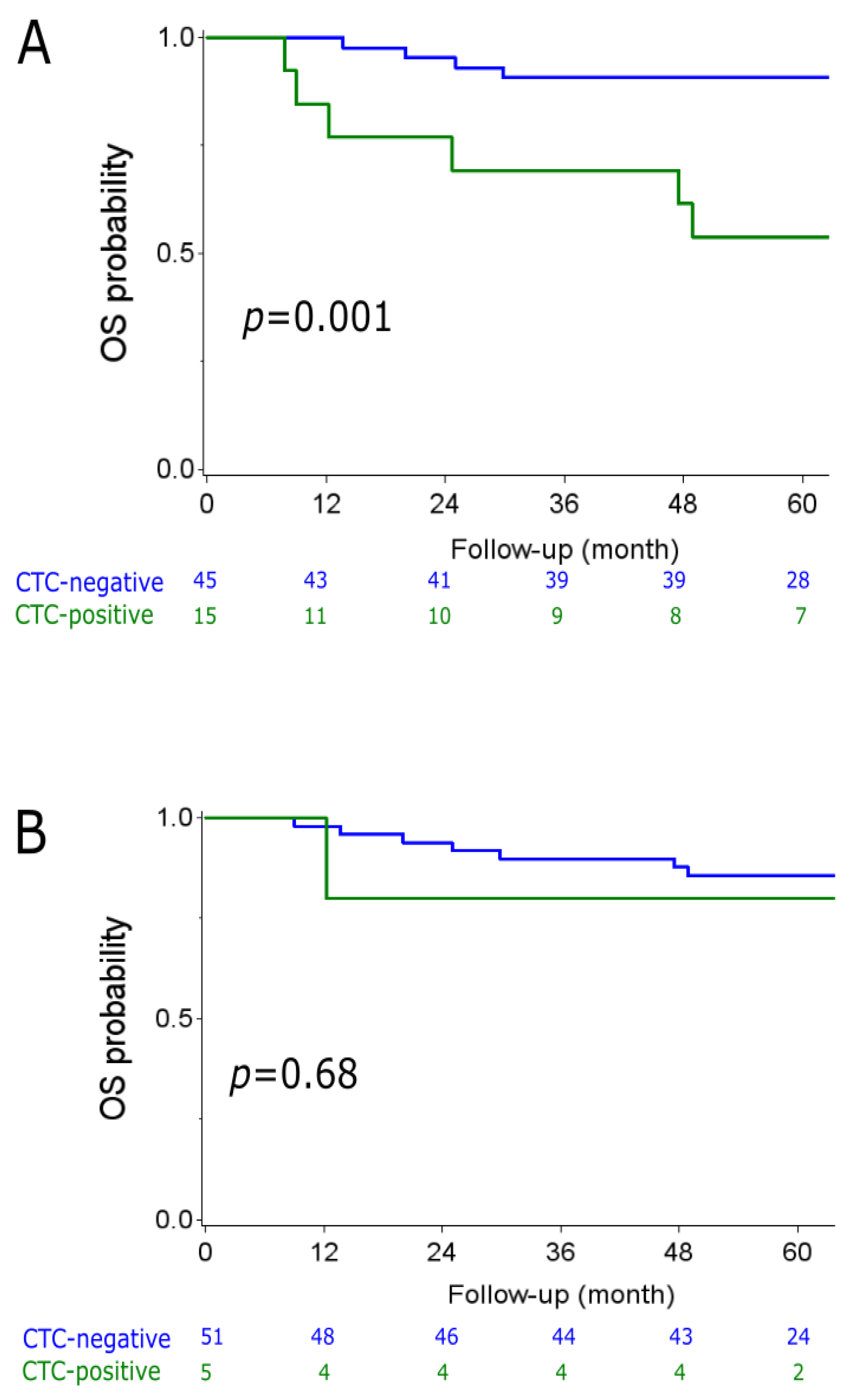
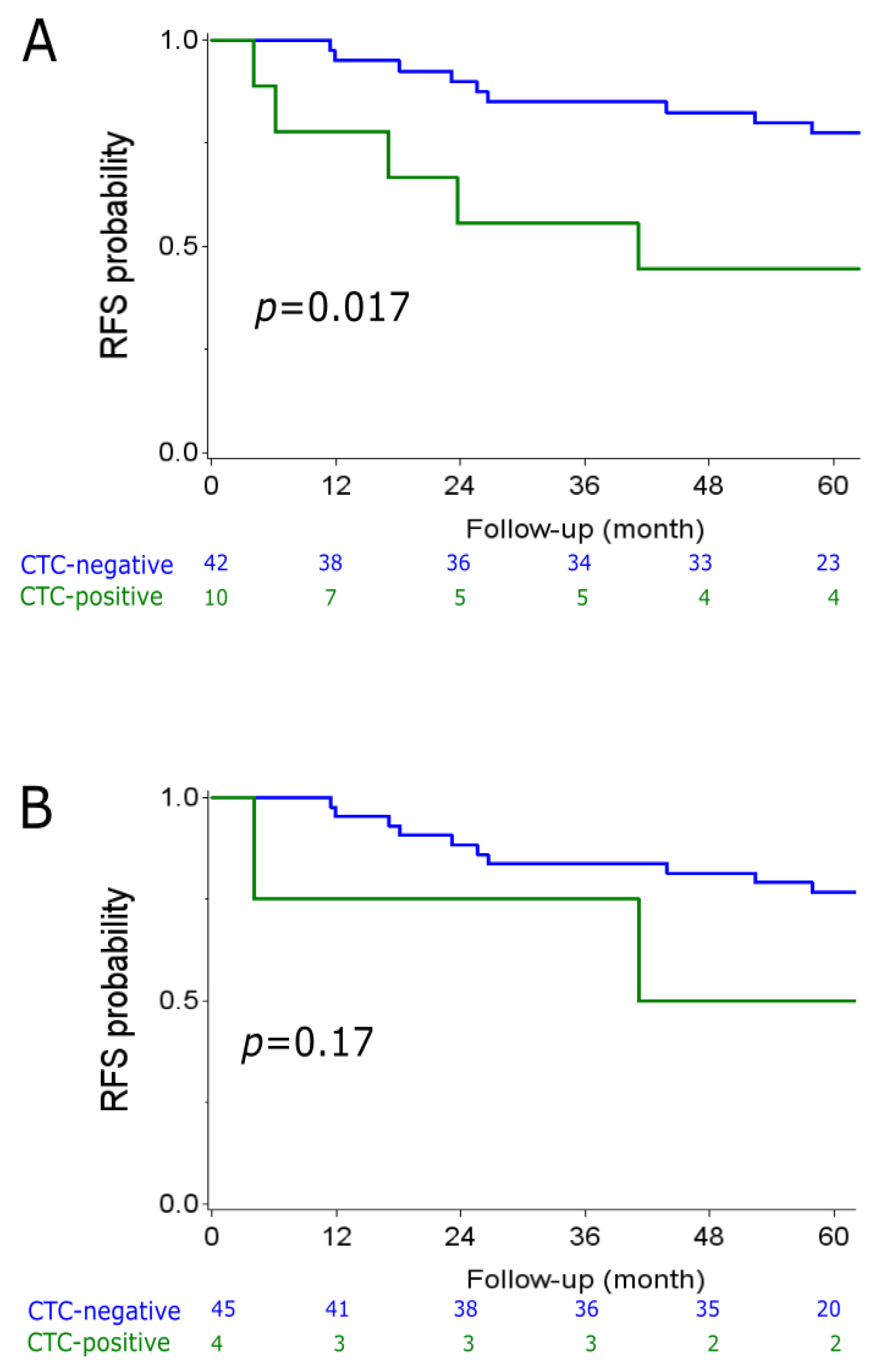
2.2. CTC Analyzes
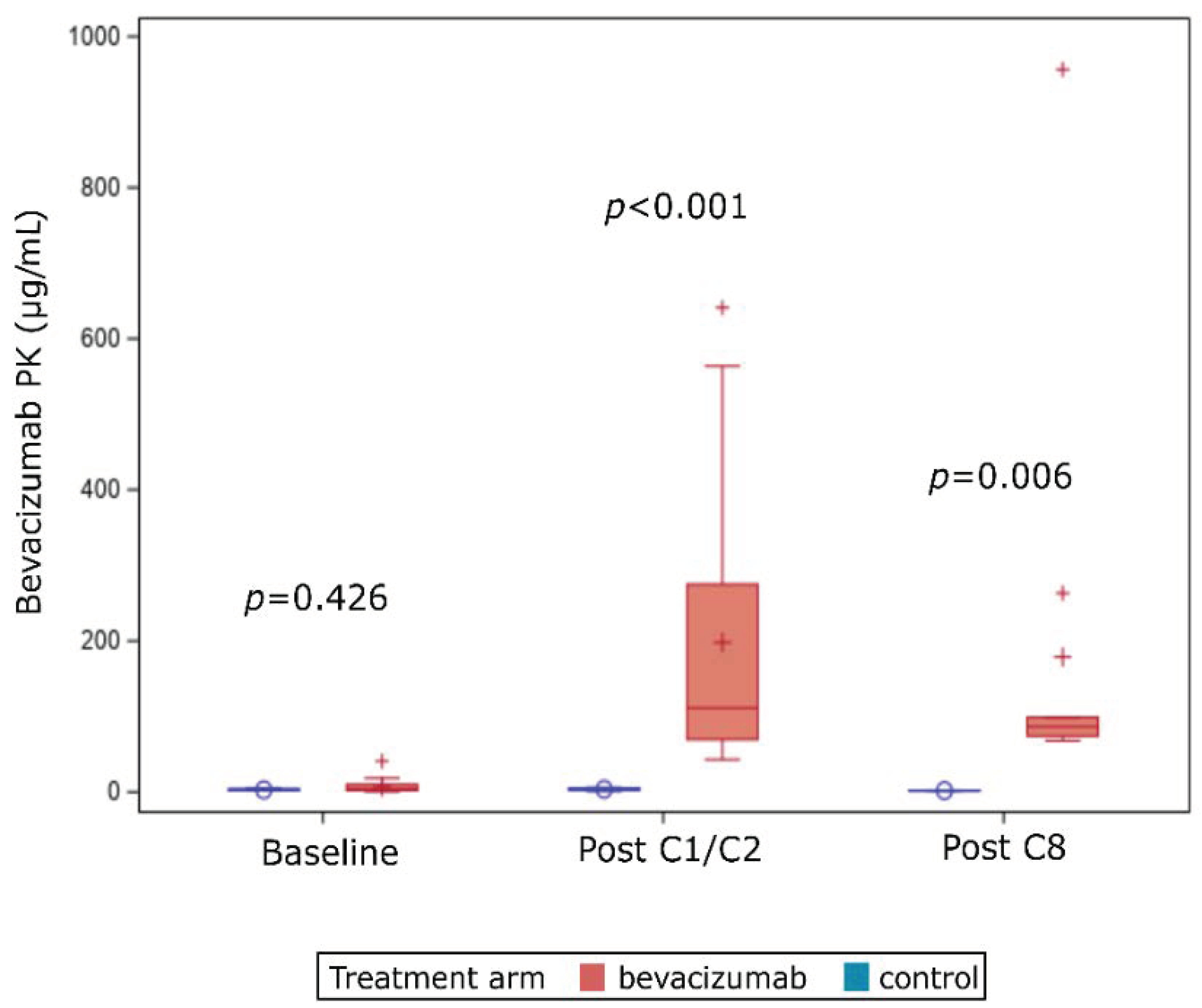
2.3. PK Analyzes
2.4. Correlation of PK to Bevacizumab-Related Adverse Events
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Blood Samples Collection Timeframe
4.3. CTC Analysis
4.4. Bevacizumab PK Analysis
4.5. Statistical Analyzes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, G.F.; Meltzer, A.J.; Lucarelli, E.A.; Cantor, J.P.; Curcillo, P.G. Breast conservation after neoadjuvant chemotherapy for stage II carcinoma of the breast. J. Am. Coll. Surg. 2005, 201, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Meric-Bernstam, F.; Hunt, K.K.; Thames, H.D.; Oswald, M.J.; Outlaw, E.D.; Strom, E.A.; McNeese, M.D.; Kuerer, H.M.; Ross, M.I.; et al. Breast Conservation After Neoadjuvant Chemotherapy: The M.D. Anderson Cancer Center Experience. JCO 2004, 22, 2303–2312. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Huang, J.; Chen, Y.; Zhang, J.; Zhang, B.; Shi, C.; Liu, L. Bevacizumab Addition in Neoadjuvant Treatment Increases the Pathological Complete Response Rates in Patients with HER-2 Negative Breast Cancer Especially Triple Negative Breast Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0160148. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Boucher, Y.; Duda, D.G.; Martin, J.D.; Seano, G.; Ancukiewicz, M.; Barry, W.T.; Goel, S.; Lahdenrata, J.; Isakoff, S.J.; et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl. Acad. Sci. USA 2015, 112, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, M.; Giordano, A.; Jackson, S.; De Giorgi, U.; Mego, M.; Cohen, E.N.; Gao, H.; Anfossi, S.; Handy, B.C.; Ueno, N.T.; et al. Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res. 2014, 16, 440. [Google Scholar] [CrossRef]
- Nakamura, S.; Yagata, H.; Ohno, S.; Yamaguchi, H.; Iwata, H.; Tsunoda, N.; Ito, Y.; Tokudome, N.; Toi, M.; Kuroi, K.; et al. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer 2010, 17, 199–204. [Google Scholar] [CrossRef]
- Thery, L.; Meddis, A.; Cabel, L.; Proudhon, C.; Latouche, A.; Pierga, J.-Y.; Bidard, F.-C. Circulating Tumor Cells in Early Breast Cancer. JNCI Cancer Spectr. 2019, 3, pkz026. [Google Scholar] [CrossRef]
- Wülfing, P.; Borchard, J.; Buerger, H.; Heidl, S.; Zänker, K.S.; Kiesel, L.; Brandt, B. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin. Cancer Res. 2006, 12, 1715–1720. [Google Scholar] [CrossRef]
- Janni, W.J.; Rack, B.; Terstappen, L.W.M.M.; Pierga, J.-Y.; Taran, F.-A.; Fehm, T.; Hall, C.; de Groot, M.R.; Bidard, F.-C.; Friedl, T.W.P.; et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin. Cancer Res. 2016, 22, 2583–2593. [Google Scholar] [CrossRef]
- Lucci, A.; Hall, C.S.; Lodhi, A.K.; Bhattacharyya, A.; Anderson, A.E.; Xiao, L.; Bedrosian, I.; Kuerer, H.M.; Krishnamurthy, S. Circulating tumour cells in non-metastatic breast cancer: A prospective study. Lancet Oncol. 2012, 13, 688–695. [Google Scholar] [CrossRef]
- Riethdorf, S.; Müller, V.; Loibl, S.; Nekljudova, V.; Weber, K.; Huober, J.; Fehm, T.; Schrader, I.; Hilfrich, J.; Holms, F.; et al. Prognostic Impact of Circulating Tumor Cells for Breast Cancer Patients Treated in the Neoadjuvant “Geparquattro” Trial. Clin. Cancer Res. 2017, 23, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Radovich, M.; Jiang, G.; Hancock, B.A.; Chitambar, C.; Nanda, R.; Falkson, C.; Lynce, F.C.; Gallagher, C.; Isaacs, C.; Blaya, M.; et al. Association of Circulating Tumor DNA and Circulating Tumor Cells After Neoadjuvant Chemotherapy With Disease Recurrence in Patients with Triple-Negative Breast Cancer: Preplanned Secondary Analysis of the BRE12-158 Randomized Clinical Trial. JAMA Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Caulet, M.; Lecomte, T.; Bouché, O.; Rollin, J.; Gouilleux-Gruart, V.; Azzopardi, N.; Léger, J.; Borg, C.; Douillard, J.-Y.; Manfredi, S.; et al. Bevacizumab Pharmacokinetics Influence Overall and Progression-Free Survival in Metastatic Colorectal Cancer Patients. Clin. Pharm. 2016, 55, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Charafe-Jauffret, E.; Pierga, J.-Y.; Curé, H.; Lambaudie, E.; Genre, D.; Houvenaeghel, G.; Viens, P.; Ginestier, C.; Bertucci, F.; et al. Stem Cells Inhibition by Bevacizumab in Combination with Neoadjuvant Chemotherapy for Breast Cancer. J. Clin. Med. 2019, 8, 612. [Google Scholar] [CrossRef]
- Nguyen, D.; Yu, J.; Reinhold, W.C.; Yang, S.X. Association of Independent Prognostic Factors and Treatment Modality with Survival and Recurrence Outcomes in Breast Cancer. JAMA Netw. Open 2020, 3, e207213. [Google Scholar] [CrossRef]
- Ashworth, T. A Case of Cancer in Which Cells Similar to Those in the Tumours Were Seen in the Blood after Death. Med. J. Aust. 1869, 14, 146–147. [Google Scholar]
- Bidard, F.-C.; Proudhon, C.; Pierga, J.-Y. Circulating tumor cells in breast cancer. Mol. Oncol. 2016, 10, 418–430. [Google Scholar] [CrossRef]
- Pantel, K.; Schlimok, G.; Braun, S.; Kutter, D.; Lindemann, F.; Schaller, G.; Funke, I.; Izbicki, J.R.; Riethmüller, G. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J. Natl. Cancer Inst. 1993, 85, 1419–1424. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Wang, P.; Wang, X.; Peng, J.; Zhu, Y.-W.; Shen, N. The significant prognostic value of circulating tumor cells in triple-negative breast cancer: A meta-analysis. Oncotarget 2016, 7, 37361–37369. [Google Scholar] [CrossRef]
- Riethdorf, S.; Mueller, V.; Mauermann, O.; Rau, T.; Loibl, S.; Eidtmann, H.; Solbach, C.; Tesch, H.; Schrader, I.; Kittel, K.; et al. Abstract PD04-06: Changes in Circulating Tumor and Endothelial Cells in Peripheral Blood of Patients Treated in the Neoadjuvant Chemotherapy Plus Targeted Treatment Breast Cancer Study “GeparQuinto”. In Poster Discussion Abstracts; American Association for Cancer Research: Philadelphia, PA, USA, 2010; pp. PD04–PD06. [Google Scholar]
- Azim, H.A.; Rothé, F.; Aura, C.M.; Bavington, M.; Maetens, M.; Rouas, G.; Gebhart, G.; Gamez, C.; Eidtmann, H.; Baselga, J.; et al. Circulating tumor cells and response to neoadjuvant paclitaxel and HER2-targeted therapy: A sub-study from the NeoALTTO phase III trial. Breast 2013, 22, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Belin, L.; Delaloge, S.; Lerebours, F.; Ngo, C.; Reyal, F.; Alran, S.; Giacchetti, S.; Marty, M.; Lebofsky, R.; et al. Time-Dependent Prognostic Impact of Circulating Tumor Cells Detection in Non-Metastatic Breast Cancer: 70-Month Analysis of the REMAGUS02 Study. Int. J. Breast Cancer 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pierga, J.-Y.; Bidard, F.-C.; Autret, A.; Petit, T.; Andre, F.; Dalenc, F.; Levy, C.; Ferrero, J.-M.; Romieu, G.; Bonneterre, J.; et al. Circulating tumour cells and pathological complete response: Independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann. Oncol. 2017, 28, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Michiels, S.; Riethdorf, S.; Mueller, V.; Esserman, L.J.; Lucci, A.; Naume, B.; Horiguchi, J.; Gisbert-Criado, R.; Sleijfer, S.; et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 560–567. [Google Scholar] [CrossRef]
- Kwan, T.T.; Bardia, A.; Spring, L.M.; Giobbie-Hurder, A.; Kalinich, M.; Dubash, T.; Sundaresan, T.; Hong, X.; LiCausi, J.A.; Ho, U.; et al. A Digital RNA Signature of Circulating Tumor Cells Predicting Early Therapeutic Response in Localized and Metastatic Breast Cancer. Cancer Discov. 2018, 8, 1286–1299. [Google Scholar] [CrossRef]
- Han, K.; Peyret, T.; Marchand, M.; Quartino, A.; Gosselin, N.H.; Girish, S.; Allison, D.E.; Jin, J. Population pharmacokinetics of bevacizumab in cancer patients with external validation. Cancer Chemother. Pharmacol. 2016, 78, 341–351. [Google Scholar] [CrossRef]
- Nugue, G.; Bidart, M.; Arlotto, M.; Mousseau, M.; Berger, F.; Pelletier, L. Monitoring monoclonal antibody delivery in oncology: The example of bevacizumab. PLoS ONE 2013, 8, e72021. [Google Scholar] [CrossRef]
- Shih, T.; Lindley, C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin. Ther. 2006, 28, 1779–1802. [Google Scholar] [CrossRef]
- Kirschbrown, W.P.; Kågedal, M.; Wang, B.; Lindbom, L.; Knott, A.; Mack, R.; Monemi, S.; Nijem, I.; Girish, S.; Freeman, C.; et al. Pharmacokinetic and exploratory exposure-response analysis of pertuzumab in patients with operable HER2-positive early breast cancer in the APHINITY study. Cancer Chemother. Pharmacol. 2019, 83, 1147–1158. [Google Scholar] [CrossRef]
- Chen, S.-C.; Quartino, A.; Polhamus, D.; Riggs, M.; French, J.; Wang, X.; Vadhavkar, S.; Smitt, M.; Hoersch, S.; Strasak, A.; et al. Population pharmacokinetics and exposure-response of trastuzumab emtansine in advanced breast cancer previously treated with ≥2 HER2-targeted regimens. Br. J. Clin. Pharmacol. 2017, 83, 2767–2777. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Brown, J.; Dent, R.; Jackisch, C.; Mackey, J.; Pivot, X.; Steger, G.G.; Suter, T.M.; Toi, M.; Parmar, M.; et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): Primary results of a randomised, phase 3 trial. Lancet Oncol. 2013. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Eidtmann, H.; Rezai, M.; Fasching, P.A.; Tesch, H.; Eggemann, H.; Schrader, I.; Kittel, K.; Hanusch, C.; Kreienberg, R.; et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med. 2012, 366, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.W.; Chan, A.; Dirix, L.Y.; Cortés, J.; Pivot, X.; Tomczak, P.; Delozier, T.; Sohn, J.H.; Provencher, L.; Puglisi, F.; et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2010, 28, 3239–3247. [Google Scholar] [CrossRef]
- Miles, D.W.; Diéras, V.; Cortés, J.; Duenne, A.-A.; Yi, J.; O’Shaughnessy, J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: Pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 2013. [Google Scholar] [CrossRef]
- Leyland-Jones, B.; Colomer, R.; Trudeau, M.E.; Wardley, A.; Latreille, J.; Cameron, D.; Cubedo, R.; Al-Sakaff, N.; Feyereislova, A.; Catalani, O.; et al. Intensive loading dose of trastuzumab achieves higher-than-steady-state serum concentrations and is well tolerated. J. Clin. Oncol. 2010, 28, 960–966. [Google Scholar] [CrossRef]
- Luo, Y.; Li, W.; Jiang, Z.; Zhang, Q.; Wang, L.; Mao, Y.; Tjan-Heijnen, V.C.G.; Im, S.-A.; McConnell, R.; Bejarano, S.; et al. Pharmacokinetics of pertuzumab administered concurrently with trastuzumab in Chinese patients with HER2-positive early breast cancer. Anticancer Drugs 2019, 30, 866–872. [Google Scholar] [CrossRef]
- Madic, J.; Kiialainen, A.; Bidard, F.-C.; Birzele, F.; Ramey, G.; Leroy, Q.; Frio, T.R.; Vaucher, I.; Raynal, V.; Bernard, V.; et al. Circulating tumor DNA and circulating tumor cells in metastatic triple negative breast cancer patients. Int. J. Cancer 2015, 136, 2158–2165. [Google Scholar] [CrossRef]


| Clinical Features | Baseline | p-Value | Post-C1 | p-Value | ||
|---|---|---|---|---|---|---|
| CTC-Negative (N = 45) | CTC-Positive (N = 15) | CTC-Negative (N = 51) | CTC-Positive (N = 5) | |||
| Age, years (median, min–max) | 50.4 (24.3–68.5) | 56.6 (28.3–66.3) | 0.83 | 50.6 (24.3–68.5) | 48.7 (28.4–62.3) | 0.46 |
| Menopausal | 9 (20) | 4 (27) | 0.72 | 10 (20) | 1 (20) | 1 |
| Tumor size T2 | 20 (44) | 3 (20) | 0.16 | 21 (41) | 1 (20) | 0.61 |
| Tumor size T3 | 14 (31) | 5 (33) | 17 (33) | 2 (40) | ||
| Tumor size T4 | 11 (24) | 7 (47) | 13 (25) | 2 (40) | ||
| Positive axillary lymph node | 33 (75) | 15 (100) | 0.05 | 39 (78) | 5 (100) | 0.57 |
| De novo metastatic | 3 (7) | 5 (33) | 0.02 | 6 (12) | 1 (20) | 0.50 |
| CTC Status | Bevacizumab Arm | Control Arm | p-Value * | |
|---|---|---|---|---|
| CTC-baseline | negative | 29 (76.32) | 16 (72.73) | 0.77 |
| positive | 9 (23.68) | 6 (27.27) | ||
| CTC-post C1 | negative | 33 (94.29) | 18 (85.71) | 0.35 |
| positive | 2 (5.71) | 3 (14.29) | ||
| Pathological Response | CTC-Baseline | CTC-Post C1 | ||
|---|---|---|---|---|
| positive | negative | positive | negative | |
| pCR | 3 (23.08) | 13 (30.23) | 1 (20) | 14 (28.57) |
| RD | 10 (76.92) | 30 (69.77) | 4 (80) | 35 (71.42) |
| p-value * | 0.74 | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatier, R.; Pierga, J.-Y.; Curé, H.; Abulnaja, R.; Lambaudie, E.; Bidard, F.-C.; Extra, J.-M.; Sfumato, P.; Gonçalves, A. Circulating Tumor Cells and Bevacizumab Pharmacokinetics during Neoadjuvant Treatment Combining Chemotherapy and Bevacizumab for Early Breast Cancer: Ancillary Analysis of the AVASTEM Trial. Cancers 2021, 13, 140. https://doi.org/10.3390/cancers13010140
Sabatier R, Pierga J-Y, Curé H, Abulnaja R, Lambaudie E, Bidard F-C, Extra J-M, Sfumato P, Gonçalves A. Circulating Tumor Cells and Bevacizumab Pharmacokinetics during Neoadjuvant Treatment Combining Chemotherapy and Bevacizumab for Early Breast Cancer: Ancillary Analysis of the AVASTEM Trial. Cancers. 2021; 13(1):140. https://doi.org/10.3390/cancers13010140
Chicago/Turabian StyleSabatier, Renaud, Jean-Yves Pierga, Hervé Curé, Rakan Abulnaja, Eric Lambaudie, François-Clément Bidard, Jean-Marc Extra, Patrick Sfumato, and Anthony Gonçalves. 2021. "Circulating Tumor Cells and Bevacizumab Pharmacokinetics during Neoadjuvant Treatment Combining Chemotherapy and Bevacizumab for Early Breast Cancer: Ancillary Analysis of the AVASTEM Trial" Cancers 13, no. 1: 140. https://doi.org/10.3390/cancers13010140
APA StyleSabatier, R., Pierga, J.-Y., Curé, H., Abulnaja, R., Lambaudie, E., Bidard, F.-C., Extra, J.-M., Sfumato, P., & Gonçalves, A. (2021). Circulating Tumor Cells and Bevacizumab Pharmacokinetics during Neoadjuvant Treatment Combining Chemotherapy and Bevacizumab for Early Breast Cancer: Ancillary Analysis of the AVASTEM Trial. Cancers, 13(1), 140. https://doi.org/10.3390/cancers13010140






