Artificial Intelligence in Brain Tumour Surgery—An Emerging Paradigm
Abstract
:Simple Summary
Abstract
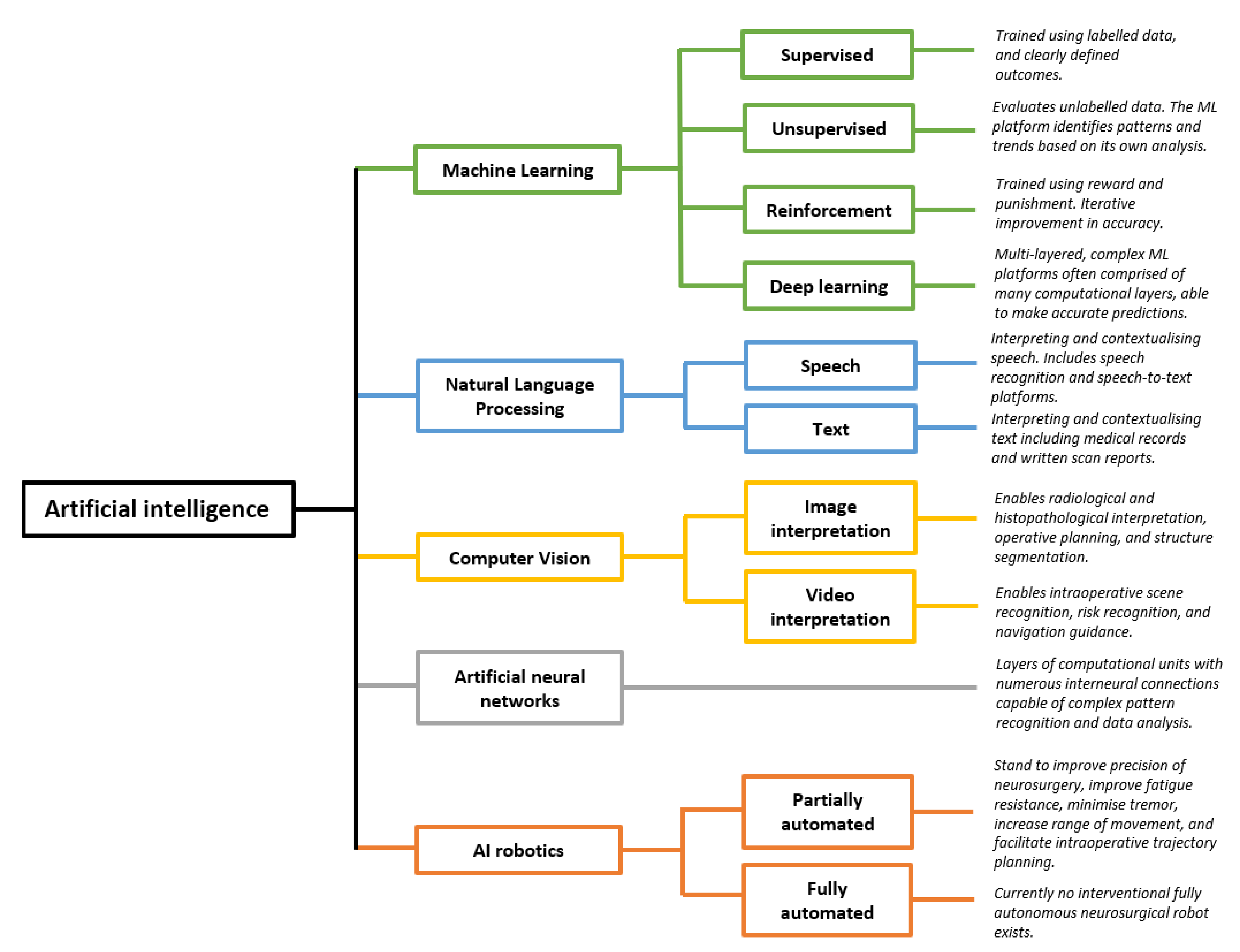
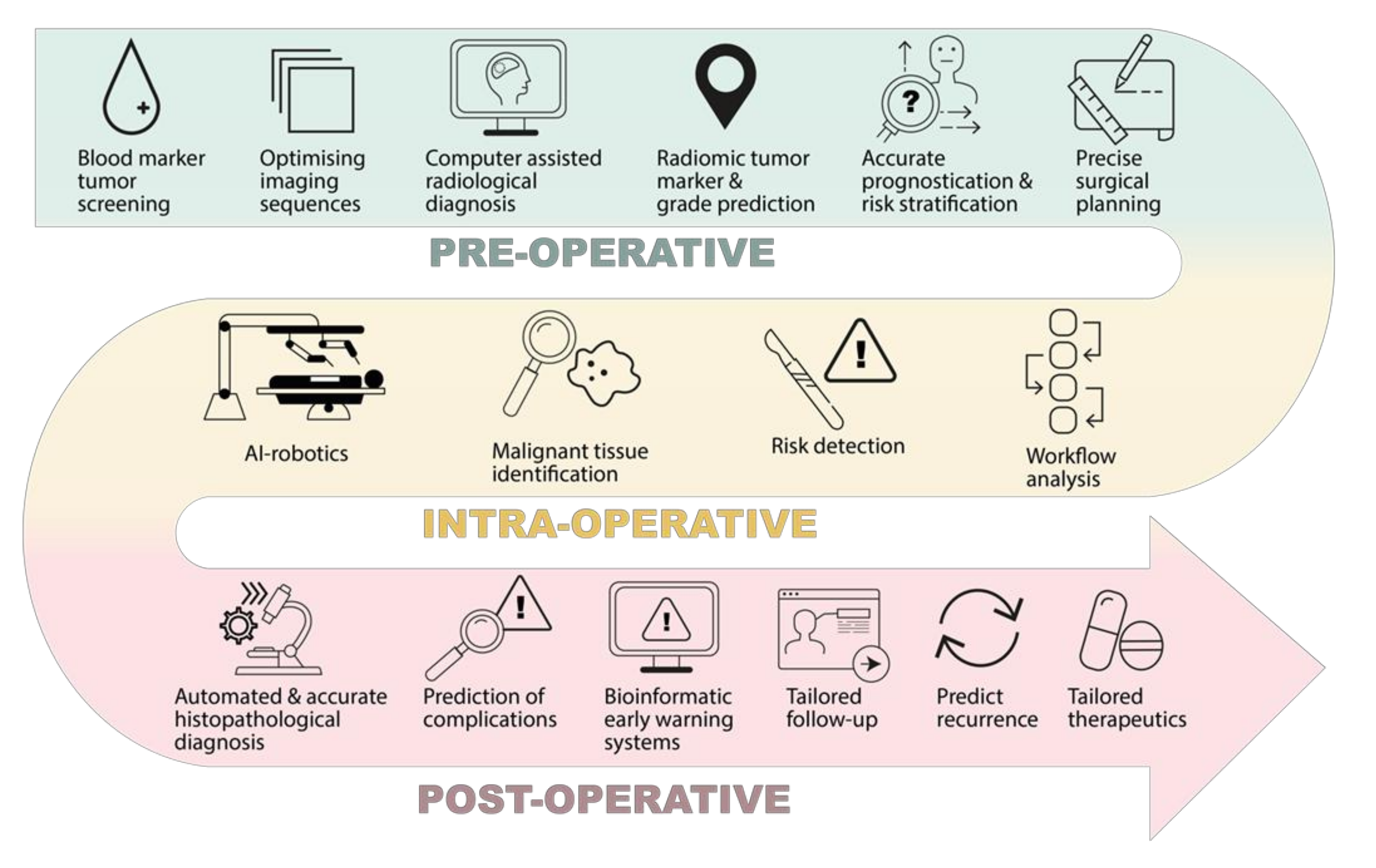
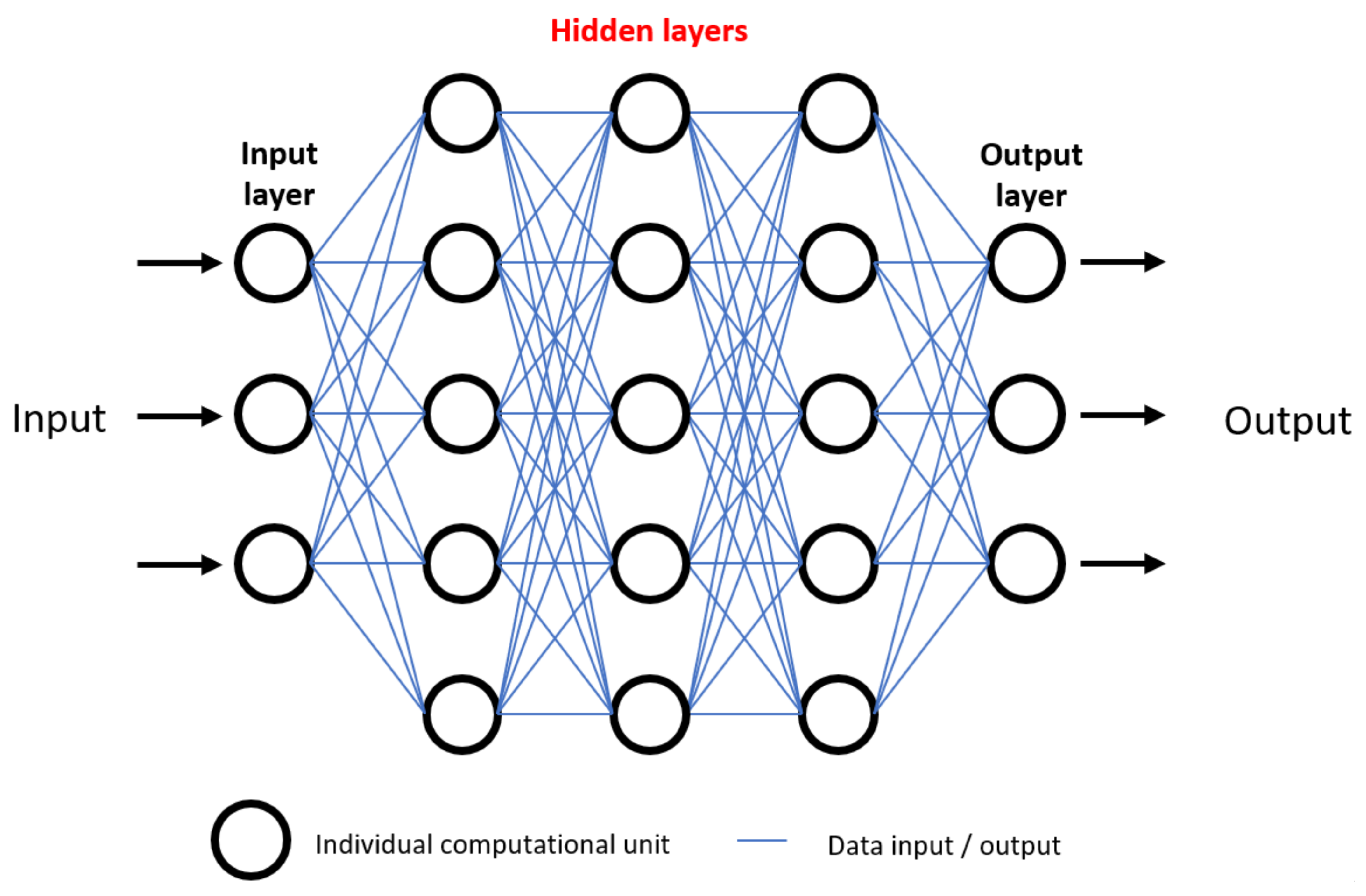
1. Introduction
2. Pre-Operative Phase
2.1. Screening and Diagnosis
2.2. Planning
3. Intra-Operative Phase
3.1. Tissue
3.2. Workflow
4. Post-Operative Phase
4.1. Inpatient and Acute Care
4.2. Outpatient and Oncological Care
5. Barriers, Evaluation, and Ethics
5.1. Barriers
5.2. Evaluation
5.3. Ethics
- (1)
- New AI systems must be introduced in gradual phases to the public, with emphasis placed on their role in assisting rather than performing autonomously.
- (2)
- The scientific community should engage in a clear and transparent discussion with the wider public, highlighting the benefits and specific functions of AI.
- (3)
- Statistical data from prior testing should be provided to the public to support the case for the safety of AI [215].
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marcus, H.J.; Hughes-Hallett, A.; Kwasnicki, R.M.; Darzi, A.; Yang, G.-Z.; Nandi, D. Technological innovation in neurosurgery: A quantitative study. J. Neurosurg. 2015, 123, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senders, J.T.; Staples, P.C.; Karhade, A.V.; Zaki, M.M.; Gormley, W.B.; Broekman, M.L.; Smith, T.R.; Arnaout, O. Machine Learning and Neurosurgical Outcome Prediction: A Systematic Review. World Neurosurg. 2018, 109, 476–486. [Google Scholar] [CrossRef]
- Senders, J.T.; Zaki, M.M.; Karhade, A.V.; Chang, B.; Gormley, W.B.; Broekman, M.L.; Smith, T.R.; Arnaout, O. An introduction and overview of machine learning in neurosurgical care. Acta Neurochir. 2017, 160, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panesar, S.S.; Kliot, M.; Parrish, R.; Fernandez-Miranda, J.; Cagle, Y.; Britz, G.W. Promises and Perils of Artificial Intelligence in Neurosurgery. Neurosurgery 2019, 87, 33–44. [Google Scholar] [CrossRef]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Senders, B.J.T.; Arnaout, O.; Karhade, B.A.V.; Dasenbrock, H.H.; Gormley, W.B.; Broekman, M.L.; Smith, T.R. Natural and Artificial Intelligence in Neurosurgery: A Systematic Review. Neurosurgery 2017, 83, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Artificial Intelligence in Surgery: Promises and Perils. Ann. Surg. 2018, 268, 70–76. [Google Scholar] [CrossRef]
- Enchev, Y. Neuronavigation: Geneology, reality, and prospects. Neurosurg. Focus 2009, 27, E11. [Google Scholar] [CrossRef] [Green Version]
- D’Albis, T.; Haegelen, C.; Essert, C.; Fernández-Vidal, S.; Lalys, F.; Jannin, P. PyDBS: An automated image processing workflow for deep brain stimulation surgery. Int. J. Comput. Assist. Radiol. Surg. 2014, 10, 117–128. [Google Scholar] [CrossRef] [Green Version]
- De Momi, E.; Ferrigno, G. Robotic and artificial intelligence for keyhole neurosurgery: The ROBOCAST project, a multi-modal autonomous path planner. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 715–727. [Google Scholar] [CrossRef]
- Bonrath, E.M.; E Gordon, L.; Grantcharov, T.P. Characterising ‘near miss’ events in complex laparoscopic surgery through video analysis. BMJ Qual. Saf. 2015, 24, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q. Computer-assisted neurosurgery: Yesterday, today and tomorrow. J. Neurol. Clin. Neurosci. 2017, 1, 1–2. [Google Scholar]
- Pandya, S.; Motkoski, J.W.; Serrano-Almeida, C.; Greer, A.D.; Latour, I.; Sutherland, G.R. Advancing neurosurgery with image-guided robotics. J. Neurosurg. 2009, 111, 1141–1149. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, M.; Brennan, P.M.; Zienius, K.; Kurian, K.M.; Hollingworth, W.; Weller, D.; Hamilton, W.; Grant, R.; Ben-Shlomo, Y. Symptoms in primary care with time to diagnosis of brain tumours. Fam. Pr. 2018, 35, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Keeble, S.; Abel, G.A.; Saunders, C.L.; McPhail, S.; Walter, F.M.; Neal, R.D.; Rubin, G.P.; Lyratzopoulos, G. Variation in promptness of presentation among 10,297 patients subsequently diagnosed with one of 18 cancers: Evidence from a National Audit of Cancer Diagnosis in Primary Care. Int. J. Cancer 2014, 135, 1220–1228. [Google Scholar] [CrossRef] [Green Version]
- Lyratzopoulos, G.; Abel, G.; McPhail, S.; Neal, R.D.; Rubin, G.P. Measures of promptness of cancer diagnosis in primary care: Secondary analysis of national audit data on patients with 18 common and rarer cancers. Br. J. Cancer 2013, 108, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Podnar, S.; Kukar, M.; Gunčar, G.; Notar, M.; Gošnjak, N.; Notar, M. Diagnosing brain tumours by routine blood tests using machine learning. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Tsvetkov, P.O.; Devred, F. Plasmatic Signature of Disease by Differential Scanning Calorimetry (DSC). Breast Cancer 2019, 1964, 45–57. [Google Scholar]
- Tsvetkov, P.O.; Tabouret, E.; Roman, A.Y.; Romain, S.; Bequet, C.; Ishimbaeva, O.; Honoré, S.; Figarella-Branger, D.; Chinot, O.; Devred, F. Differential scanning calorimetry of plasma in glioblastoma: Toward a new prognostic / monitoring tool. Oncotarget 2018, 9, 9391–9399. [Google Scholar] [CrossRef] [Green Version]
- Gunčar, G.; Kukar, M.; Notar, M.; Brvar, M.; Černelč, P.; Notar, M.; Notar, M. An application of machine learning to haematological diagnosis. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Luo, Q.; Li, Y.; Luo, L.; Diao, W. Comparisons of the accuracy of radiation diagnostic modalities in brain tumor. Medicine 2018, 97, e11256. [Google Scholar] [CrossRef]
- Tsvetkov, P.O.; Eyraud, R.; Ayache, S.; Bougaev, A.; Malesinski, S.; Benazha, H.; Gorokhova, S.; Buffat, C.; Dehais, C.; Sanson, M.; et al. An AI-Powered Blood Test to Detect Cancer Using NanoDSF. Cancers 2021, 13, 1294. [Google Scholar] [CrossRef]
- Kan, L.K.; Drummond, K.; Hunn, M.; Williams, D.; O’Brien, T.J.; Monif, M. Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol. Open 2020, 2, e000069. [Google Scholar] [CrossRef]
- Brown, A.D.; Marotta, T.R. Using machine learning for sequence-level automated MRI protocol selection in neuroradiology. J. Am. Med. Inform. Assoc. 2018, 25, 568–571. [Google Scholar] [CrossRef]
- Boland, G.W.; Duszak, R.; Kalra, M. Protocol Design and Optimization. J. Am. Coll. Radiol. 2014, 11, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Schemmel, A.; Lee, M.; Hanley, T.; Pooler, B.D.; Kennedy, T.; Field, A.; Wiegmann, D.; Yu, J.-P.J. Radiology Workflow Disruptors: A Detailed Analysis. J. Am. Coll. Radiol. 2016, 13, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-P.J.; Kansagra, A.P.; Mongan, J. The Radiologist’s Workflow Environment: Evaluation of Disruptors and Potential Implications. J. Am. Coll. Radiol. 2014, 11, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.D.; Marotta, T.R. A Natural Language Processing-based Model to Automate MRI Brain Protocol Selection and Prioritization. Acad. Radiol. 2017, 24, 160–166. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and radiogenomics in gliomas: A contemporary update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef]
- Cameron, A.; Khalvati, F.; Haider, M.A.; Wong, A. MAPS: A Quantitative Radiomics Approach for Prostate Cancer Detection. IEEE Trans. Biomed. Eng. 2016, 63, 1145–1156. [Google Scholar] [CrossRef]
- Parmar, C.; Grossmann, P.; Rietveld, D.; Rietbergen, M.M.; Lambin, P.; Aerts, H.J.W.L. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer. Front. Oncol. 2015, 5, 272. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kato, F.; Oyama-Manabe, N.; Li, R.; Cui, Y.; Tha, K.; Yamashita, H.; Kudo, K.; Shirato, H. Identifying Triple-Negative Breast Cancer Using Background Parenchymal Enhancement Heterogeneity on Dynamic Contrast-Enhanced MRI: A Pilot Radiomics Study. PLoS ONE 2015, 10, e0143308. [Google Scholar] [CrossRef]
- Wu, W.; Parmar, C.; Grossmann, P.; Quackenbush, J.; Lambin, P.; Bussink, J.; Mak, R.; Aerts, H.J.W.L. Exploratory Study to Identify Radiomics Classifiers for Lung Cancer Histology. Front. Oncol. 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shofty, B.; Artzi, M.; Ben Bashat, D.; Liberman, G.; Haim, O.; Kashanian, A.; Bokstein, F.; Blumenthal, D.T.; Ram, Z.; Shahar, T. MRI radiomics analysis of molecular alterations in low-grade gliomas. Int. J. Comput. Assist. Radiol. Surg. 2017, 13, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Ahn, S.S.; Chang, J.H.; Kang, S.-G.; Kim, E.H.; Kim, S.H.; Jain, R.; Lee, S.-K. Machine learning and radiomic phenotyping of lower grade gliomas: Improving survival prediction. Eur. Radiol. 2020, 30, 3834–3842. [Google Scholar] [CrossRef] [PubMed]
- Beiko, J.; Suki, D.; Hess, K.R.; Fox, B.D.; Cheung, V.; Cabral, M.; Shonka, N.; Gilbert, M.R.; Sawaya, R.; Prabhu, S.S.; et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro-Oncology 2014, 16, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chang, K.; Ramkissoon, S.; Tanguturi, S.; Bi, W.L.; Reardon, D.A.; Ligon, K.L.; Alexander, B.M.; Wen, P.Y.; Huang, R.Y. Multimodal MRI features predict isocitrate dehydrogenase genotype in high-grade gliomas. Neuro-Oncology 2017, 19, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Kamdar, M.R. MRI to MGMT: Predicting methylation status in glioblastoma patients using convolutional recurrent neural networks. Biocomputing 2018 2018, 23, 331–342. [Google Scholar]
- Ambrosini, R.D.; Wang, P.; O’Dell, W.G. Computer-aided detection of metastatic brain tumors using automated three-dimensional template matching. J. Magn. Reson. Imaging 2010, 31, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjam, R.; Parmar, H.A.; Noll, D.; Tsien, C.I.; Cao, Y. An approach for computer-aided detection of brain metastases in post-Gd T1-W MRI. Magn. Reson. Imaging 2012, 30, 824–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Ramírez, Ú.; Arana, E.; Moratal, D. Brain metastases detection on MR by means of three-dimensional tumor-appearance template matching. J. Magn. Reson. Imaging 2016, 44, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, L.; Kim, Y.J.; Choi, S.H.; Kim, K.-G.; Kang, J.H.; Kang, Y.; Bae, Y.J.; Yoo, R.-E.; Kim, J.; Lee, K.J.; et al. Computer-aided detection of brain metastasis on 3D MR imaging: Observer performance study. PLoS ONE 2017, 12, e0178265. [Google Scholar]
- Bousabarah, K.; Ruge, M.; Brand, J.-S.; Hoevels, M.; Rueß, D.; Borggrefe, J.; Hokamp, N.G.; Visser-Vandewalle, V.; Maintz, D.; Treuer, H.; et al. Deep convolutional neural networks for automated segmentation of brain metastases trained on clinical data. Radiat. Oncol. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Charron, O.; Lallement, A.; Jarnet, D.; Noblet, V.; Clavier, J.-B.; Meyer, P. Automatic detection and segmentation of brain metastases on multimodal MR images with a deep convolutional neural network. Comput. Biol. Med. 2018, 95, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dikici, E.; Ryu, J.L.; Demirer, M.; Bigelow, M.; White, R.D.; Slone, W.; Erdal, B.S.; Prevedello, L.M. Automated Brain Metastases Detection Framework for T1-Weighted Contrast-Enhanced 3D MRI. IEEE J. Biomed. Heal. Inform. 2020, 24, 2883–2893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grøvik, E.; Yi, D.; Iv, M.; Tong, E.; Rubin, D.; Zaharchuk, G. Deep learning enables automatic detection and segmentation of brain metastases on multisequence MRI. J. Magn. Reson. Imaging 2020, 51, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Wang, B.; Ming, Y.; Liu, X.; Jiang, Z.; Wang, C.; Liu, X.; Chen, L.; Qu, J.; Xu, S.; et al. Deep learning–based detection and segmentation-assisted management of brain metastases. Neuro-Oncology 2020, 22, 505–514. [Google Scholar] [CrossRef]
- Zhang, M.; Young, G.S.; Chen, H.; Li, J.; Qin, L.; McFaline-Figueroa, J.R.; Reardon, D.A.; Cao, X.; Wu, X.; Xu, X. Deep-Learning Detection of Cancer Metastases to the Brain on MRI. J. Magn. Reson. Imaging 2020, 52, 1227–1236. [Google Scholar] [CrossRef]
- Zhou, Z.; Sanders, J.W.; Johnson, J.M.; Gule-Monroe, M.K.; Chen, M.M.; Briere, T.M.; Wang, Y.; Son, J.B.; Pagel, M.D.; Li, J.; et al. Computer-aided Detection of Brain Metastases in T1-weighted MRI for Stereotactic Radiosurgery Using Deep Learning Single-Shot Detectors. Radiol. 2020, 295, 407–415. [Google Scholar] [CrossRef]
- Georgiadis, P.; Cavouras, D.; Kalatzis, I.; Daskalakis, A.; Kagadis, G.; Sifaki, K.; Malamas, M.; Nikiforidis, G.; Solomou, E. Improving brain tumor characterization on MRI by probabilistic neural networks and non-linear transformation of textural features. Comput. Methods Programs Biomed. 2008, 89, 24–32. [Google Scholar] [CrossRef]
- Zarandi, M.F.; Zarinbal, M.; Izadi, M. Systematic image processing for diagnosing brain tumors: A Type-II fuzzy expert system approach. Appl. Soft Comput. 2011, 11, 285–294. [Google Scholar] [CrossRef]
- Hsieh, K.L.-C.; Chen, C.-Y.; Lo, C.-M. Quantitative glioma grading using transformed gray-scale invariant textures of MRI. Comput. Biol. Med. 2017, 83, 102–108. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, L.-F.; Zhang, X.; Hu, Y.-C.; Han, Y.; Liu, Z.-C.; Nan, H.-Y.; Sun, Q.; Sun, Y.-Z.; Yang, Y.; et al. Radiomics strategy for glioma grading using texture features from multiparametric MRI. J. Magn. Reson. Imaging 2018, 48, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liao, W.; Cao, D.; Zhao, L.; Wu, X.; Kong, L.; Zhou, G.; Zhao, Y.; Wang, D. [An artificial neural network model for glioma grading using image information]. Zhong Nan Da Xue Xue Bao Yi Xue Ban = J. Cent. South Univ. Med. Sci. 2018, 43, 1315–1322. [Google Scholar] [CrossRef]
- Ranjith, G.; Parvathy, R.; Vikas, V.; Chandrasekharan, K.; Nair, S. Machine learning methods for the classification of gliomas: Initial results using features extracted from MR spectroscopy. Neuroradiol. J. 2015, 28, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yan, L.-F.; Hu, Y.-C.; Li, G.; Yang, Y.; Han, Y.; Sun, Y.-Z.; Liu, Z.-C.; Tian, Q.; Han, Z.-Y.; et al. Optimizing a machine learning based glioma grading system using multi-parametric MRI histogram and texture features. Oncotarget 2017, 8, 47816–47830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Man, C.; Gong, L.; Dong, D.; Yu, X.; Wang, S.; Fang, M.; Wang, S.; Fang, X.; Chen, X.; et al. A deep learning radiomics model for preoperative grading in meningioma. Eur. J. Radiol. 2019, 116, 128–134. [Google Scholar] [CrossRef]
- Park, Y.W.; Oh, J.; You, S.C.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kim, S.H.; Lee, S.-K. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur. Radiol. 2018, 29, 4068–4076. [Google Scholar] [CrossRef]
- Wu, S.; Meng, J.; Yu, Q.; Li, P.; Fu, S. Radiomics-based machine learning methods for isocitrate dehydrogenase genotype prediction of diffuse gliomas. J. Cancer Res. Clin. Oncol. 2019, 145, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Akkus, Z.; Ali, I.; Sedlář, J.; Agrawal, J.P.; Parney, I.; Giannini, C.; Erickson, B.J. Predicting Deletion of Chromosomal Arms 1p/19q in Low-Grade Gliomas from MR Images Using Machine Intelligence. J. Digit. Imaging 2017, 30, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.; Grinband, J.; Weinberg, B.D.; Bardis, M.; Khy, M.; Cadena, G.; Su, M.-Y.; Cha, S.; Filippi, C.G.; Bota, D.; et al. Deep-Learning Convolutional Neural Networks Accurately Classify Genetic Mutations in Gliomas. Am. J. Neuroradiol. 2018, 39, 1201–1207. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, F.; Pan, C.; Zhu, M.; Zhang, X.; Zhang, L.; Liao, H. A Cascaded Deep Convolutional Neural Network for Joint Segmentation and Genotype Prediction of Brainstem Gliomas. IEEE Trans. Biomed. Eng. 2018, 65, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellah, M.K.; Awad, A.I.; Khalaf, A.A.; Hamed, H.F. A review on brain tumor diagnosis from MRI images: Practical implications, key achievements, and lessons learned. Magn. Reson. Imaging 2019, 61, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, E.J.; Post, M.; Mannil, M.; Kusters, B.; ter Laan, M.; Meijer, F.; Henssen, D. Accuracy of Machine Learning Algorithms for the Classification of Molecular Features of Gliomas on MRI: A Systematic Literature Review and Meta-Analysis. Cancers 2021, 13, 2606. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shi, Z.; Lian, Y.; Li, Z.; Liu, T.; Gao, Y.; Wang, Y.; Chen, L.; Mao, Y. Noninvasive IDH1 mutation estimation based on a quantitative radiomics approach for grade II glioma. Eur. Radiol. 2016, 27, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Wijethilake, N.; Islam, M.; Ren, H. Radiogenomics model for overall survival prediction of glioblastoma. Med. Biol. Eng. Comput. 2020, 58, 1767–1777. [Google Scholar] [CrossRef]
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef]
- Cho, S.J.; Sunwoo, L.; Baik, S.H.; Bae, Y.J.; Choi, B.S.; Kim, J.H. Brain metastasis detection using machine learning: A systematic review and meta-analysis. Neuro-Oncology 2021, 23, 214–225. [Google Scholar] [CrossRef]
- Glare, P.; Sinclair, C. Palliative Medicine Review: Prognostication. J. Palliat. Med. 2008, 11, 84–103. [Google Scholar] [CrossRef]
- Oermann, E.K.; Kress, M.-A.S.; Collins, B.T.; Collins, S.P.; Morris, D.; Ahalt, S.C.; Ewend, M.G. Predicting Survival in Patients With Brain Metastases Treated With Radiosurgery Using Artificial Neural Networks. Neurosurgery 2013, 72, 944–952. [Google Scholar] [CrossRef]
- Panesar, S.S.; D’Souza, R.N.; Yeh, F.-C.; Fernandez-Miranda, J.C. Machine Learning Versus Logistic Regression Methods for 2-Year Mortality Prognostication in a Small, Heterogeneous Glioma Database. World Neurosurg. X 2019, 2, 100012. [Google Scholar] [CrossRef]
- Zhao, R.; Krauze, A.V. Survival Prediction in Gliomas: Current State and Novel Approaches. In Gliomas; Debinski, W., Ed.; Exon Publications: Brisbane, Australia, 2021; pp. 151–170. [Google Scholar]
- Malhotra, K.; Navathe, S.B.; Chau, D.H.; Hadjipanayis, C.; Sun, J. Constraint based temporal event sequence mining for Glioblastoma survival prediction. J. Biomed. Inform. 2016, 61, 267–275. [Google Scholar] [CrossRef]
- Macyszyn, L.; Akbari, H.; Pisapia, J.M.; Da, X.; A Attiah, M.; Pigrish, V.; Bi, Y.; Pal, S.; Davuluri, R.V.; Roccograndi, L.; et al. Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neuro-Oncology 2016, 18, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emblem, K.; Pinho, M.; Zöllner, F.G.; Due-Tønnessen, P.; Hald, J.K.; Schad, L.R.; Meling, T.R.; Rapalino, O.; Bjornerud, A. A Generic Support Vector Machine Model for Preoperative Glioma Survival Associations. Radiology 2015, 275, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Gennatas, E.D.; Wu, A.; Braunstein, S.E.; Morin, O.; Chen, W.; Magill, S.T.; Gopinath, C.; Villaneueva-Meyer, J.E.; Perry, A.; McDermott, M.W.; et al. Preoperative and postoperative prediction of long-term meningioma outcomes. PLoS ONE 2018, 13, e0204161. [Google Scholar]
- Ko, C.-C.; Zhang, Y.; Chen, J.-H.; Chang, K.-T.; Chen, T.-Y.; Lim, S.-W.; Wu, T.-C.; Su, M.-Y. Pre-operative MRI Radiomics for the Prediction of Progression and Recurrence in Meningiomas. Front. Neurol. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Morin, O.; Chen, W.; Nassiri, F.; Susko, M.; Magill, S.T.; Vasudevan, H.N.; Wu, A.; Vallières, M.; Gennatas, E.D.; Valdes, G.; et al. Integrated models incorporating radiologic and radiomic features predict meningioma grade, local failure, and overall survival. Neuro-Oncology Adv. 2019, 1, vdz011. [Google Scholar] [CrossRef] [Green Version]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.-M.; WiIdrick, D.M. Neurosurgical Outcomes in a Modern Series of 400 Craniotomies for Treatment of Parenchymal Tumors. Neurosurgery 1998, 42, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Orringer, D.; Lau, D.; Khatri, S.; Zamora-Berridi, G.J.; Zhang, K.; Wu, C.; Chaudhary, N.; Sagher, O. Extent of resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect on survival. J. Neurosurg. 2012, 117, 851–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paw, I.; Carpenter, R.; Watabe, K.; Debinski, W.; Lo, H.-W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, H.J.; Williams, S.; Hughes-Hallett, A.; Camp, S.J.; Nandi, D.; Thorne, L. Predicting surgical outcome in patients with glioblastoma multiforme using pre-operative magnetic resonance imaging: Development and preliminary validation of a grading system. Neurosurg. Rev. 2017, 40, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, A.P.; Marcus, H.J.; Camp, S.J.; Nandi, D.; Kitchen, N.; Thorne, L. Improved Prediction of Surgical Resectability in Patients with Glioblastoma using an Artificial Neural Network. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Moisi, M.D.; Page, J.; Gahramanov, S.; Oskouian, R.J. Bullet Fragment of the Lumbar Spine: The Decision is More Important than the Incision. Glob. Spine J. 2015, 5, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Deeley, A.M.; Chen, A.; Datteri, R.; Noble, J.H.; Cmelak, A.J.; Donnelly, E.F.; Malcolm, A.W.; Moretti, L.; Jaboin, J.; Niermann, K.; et al. Comparison of manual and automatic segmentation methods for brain structures in the presence of space-occupying lesions: A multi-expert study. Phys. Med. Biol. 2011, 56, 4557–4577. [Google Scholar] [CrossRef]
- Bondiau, P.-Y.; Malandain, G.; Chanalet, S.; Marcy, P.-Y.; Habrand, J.-L.; Fauchon, F.; Paquis, P.; Courdi, A.; Commowick, O.; Rutten, I.; et al. Atlas-based automatic segmentation of MR images: Validation study on the brainstem in radiotherapy context. Int. J. Radiat. Oncol. 2005, 61, 289–298. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Thiele, F.; Shakirin, G.; Zopfs, D.; Faymonville, A.; Timmer, M.; Maintz, D.; Perkuhn, M.; Borggrefe, J. Fully automated detection and segmentation of meningiomas using deep learning on routine multiparametric MRI. Eur. Radiol. 2019, 29, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Dolz, J.; Betrouni, N.; Quidet, M.; Kharroubi, D.; Leroy, H.A.; Reyns, N.; Massoptier, L.; Vermandel, M. Stacking denoising auto-encoders in a deep network to segment the brainstem on MRI in brain cancer patients: A clinical study. Comput. Med. Imaging Graph. 2016, 52, 8–18. [Google Scholar] [CrossRef]
- Havaei, M.; Davy, A.; Warde-Farley, D.; Biard, A.; Courville, A.; Bengio, Y.; Pal, C.; Jodoin, P.-M.; Larochelle, H. Brain tumor segmentation with Deep Neural Networks. Med. Image Anal. 2017, 35, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wu, G.; Commander, L.A.; Szary, S.; Jewells, V.; Lin, W.; Shen, D. Segmenting Hippocampus from Infant Brains by Sparse Patch Matching with Deep-Learned Features. Med. Image Comput. Comput. Assist. Interv. 2014, 17, 308–315. [Google Scholar]
- Zhang, W.; Li, R.; Deng, H.; Wang, L.; Lin, W.; Ji, S.; Shen, D. Deep convolutional neural networks for multi-modality isointense infant brain image segmentation. NeuroImage 2015, 108, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Tustison, N.J.; Shrinidhi, K.L.; Wintermark, M.; Durst, C.; Kandel, B.M.; Gee, J.C.; Grossman, M.C.; Avants, B.B. Optimal Symmetric Multimodal Templates and Concatenated Random Forests for Supervised Brain Tumor Segmentation (Simplified) with ANTsR. Neuroinformatics 2015, 13, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Pinto, A.; Alves, V.; Silva, C. Brain Tumor Segmentation Using Convolutional Neural Networks in MRI Images. IEEE Trans. Med. Imaging 2016, 35, 1240–1251. [Google Scholar] [CrossRef]
- Tonutti, M.; Gras, G.; Yang, G.-Z. A machine learning approach for real-time modelling of tissue deformation in image-guided neurosurgery. Artif. Intell. Med. 2017, 80, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrobala, A.; Malicki, J. Beam orientation in stereotactic radiosurgery using an artificial neural network. Radiother. Oncol. 2014, 111, 296–300. [Google Scholar] [CrossRef]
- Marcus, H.J.; Vakharia, V.N.; Sparks, R.; Rodionov, R.; Kitchen, N.; McEvoy, A.W.; Miserocchi, A.; Thorne, L.; Ourselin, S.; Duncan, J.S. Computer-Assisted Versus Manual Planning for Stereotactic Brain Biopsy: A Retrospective Comparative Pilot Study. Oper Neurosurg. (Hagerstown) 2020, 18, 417–422. [Google Scholar] [CrossRef]
- Fabelo, H.; Halicek, M.; Ortega, S.; Shahedi, M.; Szolna, A.; Piñeiro, J.F.; Sosa, C.; O’Shanahan, A.J.; Bisshopp, S.; Espino, C.; et al. Deep Learning-Based Framework for In Vivo Identification of Glioblastoma Tumor using Hyperspectral Images of Human Brain. Sensors 2019, 19, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Berger, M.S. Glioma Extent of Resection and its Impact on Patient Outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain shift in neuronavigation of brain tumors: A review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef]
- Reinges, M.H.T.; Nguyen, H.-H.; Krings, T.; Rohde, V.; Gilsbach, J.M.; Hütter, B.-O. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: Limits of conventional neuronavigation. Acta Neurochir. 2004, 146, 369–377. [Google Scholar] [CrossRef]
- Floeth, F.W.; Sabel, M.; Ewelt, C.; Stummer, W.; Felsberg, J.; Reifenberger, G.; Steiger, H.J.; Stoffels, G.; Coenen, H.H.; Langen, K.-J. Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur. J. Nucl. Med. Mol. Imaging 2010, 38, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Fabelo, H.; Ortega, S.; Lazcano, R.; Madroñal, D.; Callicó, G.M.; Juárez, E.; Salvador, R.; Bulters, D.; Bulstrode, H.; Szolna, A.; et al. An Intraoperative Visualization System Using Hyperspectral Imaging to Aid in Brain Tumor Delineation. Sensors 2018, 18, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabelo, H.; Ortega, S.; Ravi, D.; Kiran, B.R.; Sosa, C.; Bulters, D.; Callico, G.; Bulstrode, H.; Szolna, A.; Piñeiro, J.F.; et al. Spatio-spectral classification of hyperspectral images for brain cancer detection during surgical operations. PLoS ONE 2018, 13, e0193721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, H.; Halig, L.V.; Schuster, D.; Osunkoya, A.; Master, V.; Nieh, P.T.; Chen, G.Z.; Fei, B. Hyperspectral imaging and quantitative analysis for prostate cancer detection. J. Biomed. Opt. 2012, 17, 0760051–07600510. [Google Scholar] [CrossRef]
- Kim, B.; Kehtarnavaz, N.; LeBoulluec, P.; Liu, H.; Peng, Y.; Euhus, D. Automation of ROI extraction in hyperspectral breast images. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Melbourne, Australia, 15–18 September 2013; Volume 2013, pp. 3658–3661. [Google Scholar]
- Han, Z.; Zhang, A.; Wang, X.; Sun, Z.; Wang, M.D.; Xie, T. In vivouse of hyperspectral imaging to develop a noncontact endoscopic diagnosis support system for malignant colorectal tumors. J. Biomed. Opt. 2016, 21, 016001. [Google Scholar] [CrossRef] [Green Version]
- Dicker, D.T.; Lerner, J.; Van Belle, P.; Barth, S.F.; Guerry, D.; Herlyn, M.; E Elder, D.; El-Deiry, W.S. Differentiation of normal skin and melanoma using high resolution hyperspectral imaging. Cancer Biol. Ther. 2006, 5, 1033–1038. [Google Scholar] [CrossRef]
- Manni, F.; Van Der Sommen, F.; Fabelo, H.; Zinger, S.; Shan, C.; Edström, E.; Elmi-Terander, A.; Ortega, S.; Callicó, G.M.; De With, P.H.N. Hyperspectral Imaging for Glioblastoma Surgery: Improving Tumor Identification Using a Deep Spectral-Spatial Approach. Sensors 2020, 20, 6955. [Google Scholar] [CrossRef]
- Urbanos, G.; Martín, A.; Vázquez, G.; Villanueva, M.; Villa, M.; Jimenez-Roldan, L.; Chavarrías, M.; Lagares, A.; Juárez, E.; Sanz, C. Supervised Machine Learning Methods and Hyperspectral Imaging Techniques Jointly Applied for Brain Cancer Classification. Sensors 2021, 21, 3827. [Google Scholar] [CrossRef]
- Ji, M.; Lewis, S.; Camelo-Piragua, S.; Ramkissoon, S.H.; Snuderl, M.; Venneti, S.; Fisher-Hubbard, A.; Garrard, M.; Fu, D.; Wang, A.C.; et al. Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci. Transl. Med. 2015, 7, 309ra163. [Google Scholar] [CrossRef] [Green Version]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Rosman, G.; Witkowski, E.R.; Stafford, C.; Navarrete-Welton, A.; Rattner, D.W.; Lillemoe, K.D.; Rus, D.L.; Meireles, O.R. Computer Vision Analysis of Intraoperative Video. Ann. Surg. 2019, 270, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Grenda, T.R.; Pradarelli, J.C.; Dimick, J.B. Using Surgical Video to Improve Technique and Skill. Ann. Surg. 2016, 264, 32–33. [Google Scholar] [CrossRef]
- Krauss, A.; Muensterer, O.J.; Neumuth, T.; Wachowiak, R.; Donaubauer, B.; Korb, W.; Burgert, O. Workflow Analysis of Laparoscopic Nissen Fundoplication in Infant Pigs—A Model for Surgical Feedback and Training. J. Laparoendosc. Adv. Surg. Tech. 2009, 19, s117–s122. [Google Scholar] [CrossRef] [PubMed]
- Strauß, G.; Fischer, M.; Meixensberger, J.; Falk, V.; Trantakis, C.; Winkler, D.; Bootz, F.; Burgert, O.; Dietz, A.; Lemke, H.U. Bestimmung der Effizienz von intraoperativer Technologie. HNO 2006, 54, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.K.; Chang, A.; Albrani, T.; Vincent, C. Constructing hierarchical task analysis in surgery. Surg. Endosc. 2007, 22, 107–111. [Google Scholar] [CrossRef]
- Maktabi, M.; Neumuth, T. Online time and resource management based on surgical workflow time series analysis. Int. J. Comput. Assist. Radiol. Surg. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Marcus, H.J.; Khan, D.Z.; Borg, A.; Buchfelder, M.; Cetas, J.S.; Collins, J.W.; Dorward, N.L.; Fleseriu, M.; Gurnell, M.; Javadpour, M.; et al. Pituitary society expert Delphi consensus: Operative workflow in endoscopic transsphenoidal pituitary adenoma resection. Pituitary. 2021, 1–15. [Google Scholar] [CrossRef]
- Khan, D.Z.; Luengo, I.; Barbarisi, S.; Addis, C.; Culshaw, L.; Dorward, N.L.; Haikka, P.; Jain, A.; Kerr, K.; Koh, C.H.; et al. Automated operative workflow analysis of endoscopic pituitary surgery using machine learning: Development and preclinical evaluation. JNS 2021, in press. (IDEAL stage 0). [Google Scholar]
- Adler, J.R., Jr.; Chang, S.D.; Murphy, M.J.; Doty, J.; Geis, P.; Hancock, S.L. The Cyberknife: A Frameless Robotic System for Radiosurgery. Ster. Funct. Neurosurg. 1997, 69, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ohnmeiss, D.D.; Lieberman, I.H. Robotic-assisted pedicle screw placement: Lessons learned from the first 102 patients. Eur. Spine J. 2013, 22, 661–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, J.J.; Shao, J.; Neifert, S.; Gibbs, W.N.; Habboub, G.; Steinmetz, M.P.; Benzel, E.; Mroz, T.E. Artificial Intelligence and Robotics in Spine Surgery. Glob. Spine J. 2021, 11, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shademan, A.; Decker, R.S.; Opfermann, J.D.; Leonard, S.; Krieger, A.; Kim, P.C.W. Supervised autonomous robotic soft tissue surgery. Sci. Transl. Med. 2016, 8, 337ra64. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A.; Salehi, A.; Limbrick, D.D.; Smyth, M.D. Applications of a robotic stereotactic arm for pediatric epilepsy and neurooncology surgery. J. Neurosurg. Pediatr. 2017, 20, 364–370. [Google Scholar] [CrossRef]
- Kwoh, Y.S.; Hou, J.; Jonckheere, E.A.; Hayati, S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans. Biomed. Eng. 1988, 35, 153–160. [Google Scholar] [CrossRef]
- Marcus, H.J.; Hughes-Hallett, A.; Cundy, T.P.; Yang, G.-Z.; Darzi, A.; Nandi, D. da Vinci robot-assisted keyhole neurosurgery: A cadaver study on feasibility and safety. Neurosurg. Rev. 2015, 38, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Marcus, H.; Nandi, D.; Darzi, A.; Yang, G.-Z. Surgical Robotics through a Keyhole: From Today’s Translational Barriers to Tomorrow’s “Disappearing” Robots. IEEE Trans. Biomed. Eng. 2013, 60, 674–681. [Google Scholar] [CrossRef]
- Panesar, S.; Cagle, Y.; Chander, D.; Morey, J.; Fernandez-Miranda, J.; Kliot, M. Artificial Intelligence and the Future of Surgical Robotics. Ann. Surg. 2019, 270, 223–226. [Google Scholar] [CrossRef]
- Kaji, D.; Arvind, V.; Kim, J.; Caridi, J.M.; Cho, S.K. Artificial Intelligence (AI) Can Predict Complications Better than Traditional Statistical Testing Following Fusion for Anterior Lumbar Fusion (ALF). Spine J. 2017, 17, S146. [Google Scholar] [CrossRef]
- Lu, S.; Yan, M.; Li, C.; Yan, C.; Zhu, Z.; Lu, W. Machine-learning-assisted prediction of surgical outcomes in patients undergoing gastrectomy. Chin. J. Cancer Res. 2019, 31, 797–805. [Google Scholar] [CrossRef]
- Harris, A.H.S.; Kuo, A.C.; Weng, Y.; Trickey, A.W.; Bowe, T.; Giori, N.J. Can Machine Learning Methods Produce Accurate and Easy-to-use Prediction Models of 30-day Complications and Mortality after Knee or Hip Arthroplasty? Clin. Orthop. Relat. Res. 2019, 477, 452–460. [Google Scholar] [CrossRef]
- Merath, K.; Hyer, J.M.; Mehta, R.; Farooq, A.; Bagante, F.; Sahara, K.; Tsilimigras, D.I.; Beal, E.; Paredes, A.Z.; Wu, L.; et al. Use of Machine Learning for Prediction of Patient Risk of Postoperative Complications After Liver, Pancreatic, and Colorectal Surgery. J. Gastrointest. Surg. 2020, 24, 1843–1851. [Google Scholar] [CrossRef]
- Campillo-Gimenez, B.; Garcelon, N.; Jarno, P.; Chapplain, J.M.; Cuggia, M. Full-text automated detection of surgical site infections secondary to neurosurgery in Rennes, France. Stud. Heal. Technol. Inform. 2013, 192, 572–575. [Google Scholar]
- Hopkins, B.S.; Mazmudar, A.; Driscoll, C.; Svet, M.; Goergen, J.; Kelsten, M.; Shlobin, N.A.; Kesavabhotla, K.; Smith, Z.A.; Dahdaleh, N.S. Using artificial intelligence (AI) to predict postoperative surgical site infection: A retrospective cohort of 4046 posterior spinal fusions. Clin. Neurol. Neurosurg. 2020, 192, 105718. [Google Scholar] [CrossRef]
- Collins, I.; Wilson-MacDonald, J.; Chami, G.; Burgoyne, W.; Vineyakam, P.; Berendt, T.; Fairbank, J. The diagnosis and management of infection following instrumented spinal fusion. Eur. Spine J. 2008, 17, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Zitnik, M.; Agrawal, M.; Leskovec, J. Modeling polypharmacy side effects with graph convolutional networks. Bioinformatics 2018, 34, i457–i466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferroni, P.; Zanzotto, F.M.; Scarpato, N.; Riondino, S.; Nanni, U.; Roselli, M.; Guadagni, F. Risk Assessment for Venous Thromboembolism in Chemotherapy-Treated Ambulatory Cancer Patients. Med. Decis. Mak. 2017, 37, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, R.-S.; Mi, Z.; Yang, B.-R.; Kau, L.-J.; Bitew, M.A.; Li, T.-Y. Body posture recognition and turning recording system for the care of bed bound patients. Technol. Health Care 2015, 24, S307–S312. [Google Scholar] [CrossRef]
- Luboz, V.; Bailet, M.; Grivot, C.B.; Rochette, M.; Diot, B.; Bucki, M.; Payan, Y. Personalized modeling for real-time pressure ulcer prevention in sitting posture. J. Tissue Viability 2018, 27, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Howcroft, J.; Kofman, J.; Lemaire, E. Prospective Fall-Risk Prediction Models for Older Adults Based on Wearable Sensors. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1812–1820. [Google Scholar] [CrossRef]
- Bates, D.W.; Levine, D.; Syrowatka, A.; Kuznetsova, M.; Craig, K.J.T.; Rui, A.; Jackson, G.P.; Rhee, K. The potential of artificial intelligence to improve patient safety: A scoping review. Digit. Med. 2021, 4, 1–8. [Google Scholar]
- Vu, L.; Kefayati, S.; Idé, T.; Pavuluri, V.; Jackson, G.; Latts, L.; Zhong, Y.; Agrawal, P.; Chang, Y.-C. Predicting Nocturnal Hypoglycemia from Continuous Glucose Monitoring Data with Extended Prediction Horizon. AMIA. Annu. Symp. Proc. 2019 2020, 2019, 874–882. [Google Scholar]
- Johnson, A.E.W.; Ghassemi, M.M.; Nemati, S.; Niehaus, K.E.; Clifton, D.A.; Clifford, G. Machine Learning and Decision Support in Critical Care. Proc. IEEE Inst. Electr. Electron. Eng. 2016, 104, 444–466. [Google Scholar] [CrossRef] [Green Version]
- Ker, J.; Bai, Y.; Lee, H.Y.; Rao, J.; Wang, L. Automated brain histology classification using machine learning. J. Clin. Neurosci. 2019, 66, 239–245. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Katabi, N.; Chen, H.-S.; Cheng, Y.-S.L. Interobserver Variation among Pathologists in Evaluating Perineural Invasion for Oral Squamous Cell Carcinoma. Head Neck Pathol. 2016, 10, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daisy, P.S.; Anitha, T.S. Can artificial intelligence overtake human intelligence on the bumpy road towards glioma therapy? Med. Oncol. 2021, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Matos, J.; Ataky, S.; Britto, A.D.S.; de Oliveira, L.S.; Koerich, A.L. Machine Learning Methods for Histopathological Image Analysis: A Review. Electronics 2021, 10, 562. [Google Scholar] [CrossRef]
- Malon, C.; Brachtel, E.; Cosatto, E.; Graf, H.P.; Kurata, A.; Kuroda, M.; Meyer, J.S.; Saito, A.; Wu, S.; Yagi, Y. Mitotic figure recognition: Agreement among pathologists and computerized detector. Anal. Cell. Pathol. 2012, 35, 97–100. [Google Scholar] [CrossRef]
- Bejnordi, B.E.; Veta, M.; Van Diest, P.J.; Van Ginneken, B.; Karssemeijer, N.; Litjens, G.; Van Der Laak, J.A.W.M.; Hermsen, M.; Manson, Q.F.; Balkenhol, M.; et al. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA 2017, 318, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, D.; Linder, N.; Turkki, R.; Nordling, S.; Kovanen, P.E.; Verrill, C.; Walliander, M.; Lundin, M.; Haglund, C.; Lundin, J. Deep learning based tissue analysis predicts outcome in colorectal cancer. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Feldman, M.D.; Abels, E.; Ashfaq, R.; Beltaifa, S.; Cacciabeve, N.G.; Cathro, H.P.; Cheng, L.; Cooper, K.; Dickey, G.E.; et al. Whole Slide Imaging Versus Microscopy for Primary Diagnosis in Surgical Pathology. Am. J. Surg. Pathol. 2018, 42, 39–52. [Google Scholar] [CrossRef]
- Nagpal, K.; Foote, D.; Tan, F.; Liu, Y.; Chen, P.-H.C.; Steiner, D.F.; Manoj, N.; Olson, N.; Smith, J.L.; Mohtashamian, A.; et al. Development and Validation of a Deep Learning Algorithm for Gleason Grading of Prostate Cancer From Biopsy Specimens. JAMA Oncol. 2020, 6, 1372–1380. [Google Scholar] [CrossRef]
- Wang, X.; Barrera, C.; Velu, P.; Bera, K.; Prasanna, P.; Khunger, M.; Khunger, A.; Velcheti, V.; Madabhushi, A. Computer extracted features of cancer nuclei from H&E stained tissues of tumor predicts response to nivolumab in non-small cell lung cancer. J. Clin. Oncol. 2018, 36, 12061. [Google Scholar]
- Wang, X.; Janowczyk, A.; Zhou, Y.; Thawani, R.; Fu, P.; Schalper, K.; Velcheti, V.; Madabhushi, A. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci. Rep. 2017, 7, 13543. [Google Scholar]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 2018, 23, 181–193.e7. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.H.; Sangoi, A.R.; Leung, S.; Marinelli, R.J.; Nielsen, T.O.; van de Vijver, M.J.; West, R.B.; van de Rijn, M.; Koller, D. Systematic Analysis of Breast Cancer Morphology Uncovers Stromal Features Associated with Survival. Sci. Transl. Med. 2011, 3, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Mobadersany, P.; Yousefi, S.; Amgad, M.; Gutman, D.A.; Barnholtz-Sloan, J.S.; Vega, J.E.V.; Brat, D.J.; Cooper, L.A.D. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc. Natl. Acad. Sci. USA 2018, 115, E2970–E2979. [Google Scholar] [CrossRef] [Green Version]
- Snead, D.R.J.; Tsang, Y.; Meskiri, A.; Kimani, P.; Crossman, R.J.; Rajpoot, N.M.; Blessing, E.; Chen, K.; Gopalakrishnan, K.; Matthews, P.; et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology 2016, 68, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, W.S.; Lele, S.M.; West, W.W.; Lazenby, A.J.; Smith, L.M.; Hinrichs, S.H. Concordance between whole-slide imaging and light microscopy for routine surgical pathology. Hum. Pathol. 2012, 43, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Schoenfield, L.; Slaw, R.J.; Yerian, L.; Sun, Z.; Henricks, W.H. Validation of Whole Slide Imaging for Primary Diagnosis in Surgical Pathology. Arch. Pathol. Lab. Med. 2013, 137, 518–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, J.; Hoogi, A.; Depeursinge, A.; Rubin, D.L. Automated classification of brain tumor type in whole-slide digital pathology images using local representative tiles. Med. Image Anal. 2016, 30, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, S.; Fabelo, H.; Camacho, R.; Plaza, M.D.L.L.; Callicó, G.M.; Sarmiento, R. Detecting brain tumor in pathological slides using hyperspectral imaging. Biomed. Opt. Express 2018, 9, 818–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertosun, M.G.; Rubin, D.L. Automated Grading of Gliomas using Deep Learning in Digital Pathology Images: A modular approach with ensemble of convolutional neural networks. AMIA Annu. Symp. Proc. 2015, 2015, 1899–1908. [Google Scholar] [PubMed]
- Mousavi, H.S.; Rao, G.; Rao, A.U.K.; Monga, V. Automated discrimination of lower and higher grade gliomas based on histopathological image analysis. J. Pathol. Inform. 2015, 6, 15. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Yao, Z.; Xin, B.; Wang, B.; Lan, C.; Qin, Y.; Xu, S.; He, D.; Liu, Y. Machine Learning Models for Multiparametric Glioma Grading With Quantitative Result Interpretations. Front. Neurosci. 2019, 12, 1046. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Cooper, L.A.D.; Wang, F.; Gao, J.; Teodoro, G.; Scarpace, L.; Mikkelsen, T.; Schniederjan, M.J.; Moreno, C.S.; Saltz, J.H.; et al. Machine-Based Morphologic Analysis of Glioblastoma Using Whole-Slide Pathology Images Uncovers Clinically Relevant Molecular Correlates. PLoS ONE 2013, 8, e81049. [Google Scholar]
- Yonekura, A.; Kawanaka, H.; Prasath, V.B.S.; Aronow, B.J.; Takase, H. Automatic disease stage classification of glioblastoma multiforme histopathological images using deep convolutional neural network. Biomed. Eng. Lett. 2018, 8, 321–327. [Google Scholar] [CrossRef]
- Gvozdanovic, A.; Mangiapelo, R.; Patel, R.; Kirby, G.; Kitchen, N.; Miserocchi, A.; McEvoy, A.; Grover, P.; Thorne, L.; Fersht, N.; et al. Implementation of the Vinehealth application, a digital health tool, into the care of patients living with brain cancer. J. Clin. Oncol. 2021, 39, e13582. [Google Scholar] [CrossRef]
- Meskó, B.; Görög, M. A short guide for medical professionals in the era of artificial intelligence. Digit. Med. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Ohri, N.; Kabarriti, R.; Bodner, W.R.; Mehta, K.J.; Shankar, V.; Halmos, B.; Haigentz, M.; Rapkin, B.; Guha, C.; Kalnicki, S.; et al. Continuous Activity Monitoring During Concurrent Chemoradiotherapy. Int. J. Radiat. Oncol. Bio. Phys. 2017, 97, 1061–1065. [Google Scholar] [CrossRef] [Green Version]
- Izmailova, E.S.; Wood, W.A. Biometric Monitoring Technologies in Cancer: The Past, Present, and Future. JCO Clin. Cancer Inform. 2021, 5, 728–733. [Google Scholar] [CrossRef]
- Wright, A.A.; Raman, N.; Staples, P.; Schonholz, S.; Cronin, A.; Carlson, K.; Keating, N.L.; Onnela, J.-P. The HOPE Pilot Study: Harnessing Patient-Reported Outcomes and Biometric Data to Enhance Cancer Care. JCO Clin. Cancer Inform. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Gresham, G.; Hendifar, A.E.; Spiegel, B.; Neeman, E.; Tuli, R.; Rimel, B.J.; Figlin, R.A.; Meinert, C.L.; Piantadosi, S.; Shinde, A. Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. Digit. Med. 2018, 1, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Stewart, T.; Bhulani, N.; Dong, Y.; Rahimi, Z.; Crane, K.; Rethorst, C.; Beg, M.S. Feasibility of Wearable Physical Activity Monitors in Patients With Cancer. JCO Clin. Cancer Inform. 2018, 2, 1–10. [Google Scholar] [PubMed]
- Kalagara, S.; Eltorai, A.E.M.; Durand, W.M.; DePasse, J.M.; Daniels, A.H. Machine learning modeling for predicting hospital readmission following lumbar laminectomy. J. Neurosurg. Spine 2019, 30, 344–352. [Google Scholar]
- Parker, S.L.; Sivaganesan, A.; Chotai, S.; McGirt, M.J.; Asher, A.L.; Devin, C.J. Development and validation of a predictive model for 90-day readmission following elective spine surgery. J. Neurosurg. Spine 2018, 29, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Sivaganesan, A.; Zuckerman, S.; Khan, I.; Nian, H.; Harrell, F.E.; Pennings, J.S.; Harbaugh, R.; Foley, K.T.; Bydon, M.; Asher, A.L.; et al. Predictive Model for Medical and Surgical Readmissions Following Elective Lumbar Spine Surgery. Spine 2019, 44, 588–600. [Google Scholar] [CrossRef]
- Khurana, T.; Kumar, S.; Mondal, S. PNS139 Value Assessment Frameworks: Have They Reached Their Destination? Value Heal. 2020, 23, S310. [Google Scholar] [CrossRef]
- Yauney, G.; Shah, P. Reinforcement Learning with Action-Derived Rewards for Chemotherapy and Clinical Trial Dosing Regimen Selection. In Proceedings of the 3rd Machine Learning for Healthcare Conference Anonymous: PMLR, Palo Alto, CA, USA, 17–18 August 2018; Volume 85, pp. 161–226. [Google Scholar]
- Chen, G.; Tsoi, A.; Xu, H.; Zheng, W.J. Predict effective drug combination by deep belief network and ontology fingerprints. J. Biomed. Inform. 2018, 85, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lin, M.C.M.; Fan, W.; Tian, L.; Wang, J.; Ng, S.S.; Wang, M.; Kung, H.; Li, D. An Intronic Polymorphism in GRP78 Improves Chemotherapeutic Prediction in Non-small Cell Lung Cancer. Chest 2012, 141, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Fan, W.; Luo, H.; Zhu, X. The emerging roles of artificial intelligence in cancer drug development and precision therapy. Biomed. Pharmacother. 2020, 128, 110255. [Google Scholar] [CrossRef]
- Pantuck, A.J.; Lee, D.-K.; Kee, T.; Wang, P.; Lakhotia, S.; Silverman, M.H.; Mathis, C.; Drakaki, A.; Belldegrun, A.S.; Ho, C.-M.; et al. Modulating BET Bromodomain Inhibitor ZEN-3694 and Enzalutamide Combination Dosing in a Metastatic Prostate Cancer Patient Using CURATE.AI, an Artificial Intelligence Platform. Adv. Ther. 2018, 1, 1800104. [Google Scholar] [CrossRef]
- Jabbari, P.; Rezaei, N. Artificial intelligence and immunotherapy. Expert Rev. Clin. Immunol. 2019, 15, 689–691. [Google Scholar] [CrossRef]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef]
- Van den Bent, M.J.; Wefel, J.S.; Schiff, D.; Taphoorn, M.J.; Jaeckle, K.; Junck, L.; Armstrong, T.; Choucair, A.; Waldman, A.D.; Gorlia, T.; et al. Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011, 12, 583–593. [Google Scholar] [CrossRef]
- Kickingereder, P.; Isensee, F.; Tursunova, I.; Petersen, J.; Neuberger, U.; Bonekamp, D.; Brugnara, G.; Schell, M.; Kessler, T.; Foltyn, M.; et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: A multicentre, retrospective study. Lancet Oncol. 2019, 20, 728–740. [Google Scholar] [CrossRef] [Green Version]
- Luke Oakden-Rayner. Exploring the ChestXray14 Dataset: Problems. Available online: https://lukeoakdenrayner.wordpress.com/2017/12/18/the-chestxray14-dataset-problems/ (accessed on 17 July 2021).
- Zhou, H.; Vallières, M.; Bai, H.X.; Su, C.; Tang, H.; Oldridge, D.; Zhang, Z.; Xiao, B.; Liao, W.; Tao, Y.; et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro-Oncology 2017, 19, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Surgical Video in the Age of Big Data. Ann. Surg. 2018, 268, e47–e48. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Tenenholtz, N.A.; Rogers, J.K.; Schwarz, C.G.; Senjem, M.L.; Gunter, J.L.; Andriole, K.P.; Michalski, M. Medical Image Synthesis for Data Augmentation and Anonymization Using Generative Adversarial Networks. In International Workshop on Simulation and Synthesis in Medical Imaging; Springer: Cham, Switzerland, 2018; pp. 1–11. [Google Scholar]
- Chartsias, A.; Joyce, T.; Giuffrida, M.V.; Tsaftaris, S.A. Multimodal MR Synthesis via Modality-Invariant Latent Representation. IEEE Trans. Med. Imaging 2017, 37, 803–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.; Swetlitz, I. IBM’s Watson Recommended ‘Unsafe and Incorrect’ Cancer Treatments. Available online: https://www.statnews.com/2018/07/25/ibm-watson-recommended-unsafe-incorrect-treatments/ (accessed on 17 July 2021).
- Ribeiro, M.T.; Singh, S.; Guestrin, C. "Why Should I Trust You?" Explaining the predictions of any classifier. In Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar]
- Cabitza, F.; Rasoini, R.; Gensini, G.F. Unintended Consequences of Machine Learning in Medicine. JAMA 2017, 318, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Biundo, E.; Pease, A.; Segers, K.; de Groote, M.; d’Argent, T.; Schaetzen, E. The Socio-Economic Impact of AI in Healthcare. Available online: https://www.medtecheurope.org/resource-library/the-socio-economic-impact-of-ai-in-healthcare-addressing-barriers-to-adoption-for-new-healthcare-technologies-in-europe/ (accessed on 30 September 2021).
- Horsfall, H.L.; Palmisciano, P.; Khan, D.Z.; Muirhead, W.; Koh, C.H.; Stoyanov, D.; Marcus, H.J. Attitudes of the Surgical Team toward Artificial Intelligence in Neurosurgery: International 2-Stage Cross-Sectional Survey. World Neurosurg. 2021, 146, e724–e730. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Budhdeo, S.; Sparks, R.; Barone, D.G.; Marcus, H.J.; Pereira, E.A.; Tisdall, M.M. Brain–Machine Interfaces: The Role of the Neurosurgeon. World Neurosurg. 2021, 146, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Marcus, H.J.; Payne, C.; Hughes-Hallett, A.; Marcus, A.P.; Yang, G.-Z.; Darzi, A.; Nandi, D. Regulatory approval of new medical devices: Cross sectional study. BMJ 2016, 353, i2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedrakyan, A.; Campbell, B.; Merino, J.G.; Kuntz, R.; Hirst, A.; McCulloch, P. IDEAL-D: A rational framework for evaluating and regulating the use of medical devices. BMJ 2016, 353, i2372. [Google Scholar] [CrossRef]
- Marcus, H.J.; Payne, C.J.; Hughes-Hallett, A.; Gras, G.; Leibrandt, K.; Nandi, D.; Yang, G.-Z. Making the Leap. Ann. Surg. 2016, 263, 1077–1078. [Google Scholar] [CrossRef] [Green Version]
- Vasey, B.; Clifton, A.D.; Collins, S.G.; Denniston, K.A.; Faes, L.; Geerts, F.B.; Liu, X.; Morgan, L.; Watkinson, P.; McCulloch, P.; et al. The DECIDE-AI Steering Group DECIDE-AI: New reporting guidelines to bridge the development-to-implementation gap in clinical artificial intelligence. Nat. Med. 2021, 27, 186–187. [Google Scholar]
- Ibrahim, H.; Liu, X.; Rivera, S.C.; Moher, D.; Chan, A.-W.; Sydes, M.R.; Calvert, M.J.; Denniston, A.K. Reporting guidelines for clinical trials of artificial intelligence interventions: The SPIRIT-AI and CONSORT-AI guidelines. Trials 2021, 22, 1–5. [Google Scholar] [CrossRef]
- Rivera, S.C.; Liu, X.; Chan, A.-W.; Denniston, A.K.; Calvert, M.J. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI Extension. BMJ 2020, 370, 3210. [Google Scholar] [CrossRef]
- Kilkenny, M.F.; Robinson, K.M. Data quality: “Garbage in—garbage out”. Health Inf. Manag. J. 2018, 47, 103–105. [Google Scholar] [CrossRef] [Green Version]
- Piao, J.; McDonald, M.; Hounsell, N.; Graindorge, M.; Graindorge, T.; Malhene, N. Public Views towards Implementation of Automated Vehicles in Urban Areas. Transp. Res. Procedia 2016, 14, 2168–2177. [Google Scholar] [CrossRef] [Green Version]
- Jamjoom, A.A.B.; Jamjoom, A.M.A.; Marcus, H.J. Exploring public opinion about liability and responsibility in surgical robotics. Nat. Mach. Intell. 2020, 2, 194–196. [Google Scholar] [CrossRef]
- Awad, E.; Dsouza, S.; Kim, R.; Schulz, J.; Henrich, J.; Shariff, A.; Bonnefon, J.-F.; Rahwan, I. The Moral Machine experiment. Nature 2018, 563, 59–64. [Google Scholar] [CrossRef]
- Palmisciano, P.; Jamjoom, A.A.; Taylor, D.; Stoyanov, D.; Marcus, H.J. Attitudes of Patients and Their Relatives Toward Artificial Intelligence in Neurosurgery. World Neurosurg. 2020, 138, e627–e633. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.; Nevejans, N.; Allen, C.; Blyth, A.; Leonard, S.; Pagallo, U.; Holzinger, K.; Holzinger, A.; Sajid, M.I.; Ashrafian, H. Legal, regulatory, and ethical frameworks for development of standards in artificial intelligence (AI) and autonomous robotic surgery. Int. J. Med. Robot. 2019, 15, e1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, V.-T.; Riveros, C.; Ravaud, P. Patients’ views of wearable devices and AI in healthcare: Findings from the ComPaRe e-cohort. Digit. Med. 2019, 2, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengstler, M.; Enkel, E.; Duelli, S. Technological forecasting and social change. Technol. Forecast. Soc. Chang. 1970. [Google Scholar] [CrossRef]
- MacSween, S. A Public Opinion Survey- Unmanned Aerial Vehicles for Cargo, Commercial, and Passenger Transportation. In Proceedings of the 2nd AIAA “Unmanned Unlimited” Systems, Technologies, and Operations—Aerospac, San Diego, CA, USA, 15–18 September 2003; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2003. [Google Scholar] [CrossRef]
- Fast, E.; Horvitz, E. Long-Term Trends in the Public Perception of Artificial Intelligence. In Proceedings of the of the Thirty-First AAAI Conference on Artificial Intelligence, San Francisco, CA, USA, 4–9 February 2017; Volume 31, pp. 963–969. [Google Scholar]
- The European Parliament. A Comprehensive European Industrial Policy on Artificial Intelligence and Robotics. Available online: https://www.europarl.europa.eu/doceo/document/TA-8-2019-0081_EN.html (accessed on 18 July 2021).
- Brennen, S.; Howard, P.; Nielsen, K.R. An Industry-Led Debate: How UK Media Cover Artificial Intelligence. Available online: https://www.oxfordmartin.ox.ac.uk/publications/an-industry-led-debate-how-uk-media-cover-artificial-intelligence/ (accessed on 18 July 2021).
- Tanno, S. Killer Robots Must Have Human Control, Experts Warn. Available online: https://www.dailymail.co.uk/news/article-7899479/Killer-robots-human-control-experts-warn.html (accessed on 18 July 2021).
- Siau, K.; Wang, W. Building Trust in Artificial Intelligence, Machine Learning, and Robotics. Cut. Bus. Technol. J. 2018, 31, 47–53. [Google Scholar]
- Longoni, C.; Bonezzi, A.; Morewedge, C.K. Resistance to Medical Artificial Intelligence. J. Consum. Res. 2019, 46, 629–650. [Google Scholar] [CrossRef]
| Barrier | Proposed solution |
|---|---|
| Requirement of large datasets to train existing ML programs |
|
| Selection bias of training data |
|
| Patient confidentiality concerns when sharing patient data between units to train ML platforms |
|
| Slow progress in advancing ML programming |
|
| “Black box” conundrum |
|
| Poor contextualisation of uncertainty by ML programs |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, S.; Layard Horsfall, H.; Funnell, J.P.; Hanrahan, J.G.; Khan, D.Z.; Muirhead, W.; Stoyanov, D.; Marcus, H.J. Artificial Intelligence in Brain Tumour Surgery—An Emerging Paradigm. Cancers 2021, 13, 5010. https://doi.org/10.3390/cancers13195010
Williams S, Layard Horsfall H, Funnell JP, Hanrahan JG, Khan DZ, Muirhead W, Stoyanov D, Marcus HJ. Artificial Intelligence in Brain Tumour Surgery—An Emerging Paradigm. Cancers. 2021; 13(19):5010. https://doi.org/10.3390/cancers13195010
Chicago/Turabian StyleWilliams, Simon, Hugo Layard Horsfall, Jonathan P. Funnell, John G. Hanrahan, Danyal Z. Khan, William Muirhead, Danail Stoyanov, and Hani J. Marcus. 2021. "Artificial Intelligence in Brain Tumour Surgery—An Emerging Paradigm" Cancers 13, no. 19: 5010. https://doi.org/10.3390/cancers13195010
APA StyleWilliams, S., Layard Horsfall, H., Funnell, J. P., Hanrahan, J. G., Khan, D. Z., Muirhead, W., Stoyanov, D., & Marcus, H. J. (2021). Artificial Intelligence in Brain Tumour Surgery—An Emerging Paradigm. Cancers, 13(19), 5010. https://doi.org/10.3390/cancers13195010





