Repeat Local Treatment of Recurrent Colorectal Liver Metastases, the Role of Neoadjuvant Chemotherapy: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Neoadjuvant Chemotherapy
2.3. Repeat Local Treatment Procedures
2.4. Follow-Up
2.5. Data Collection and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Characteristics
3.3. Complications
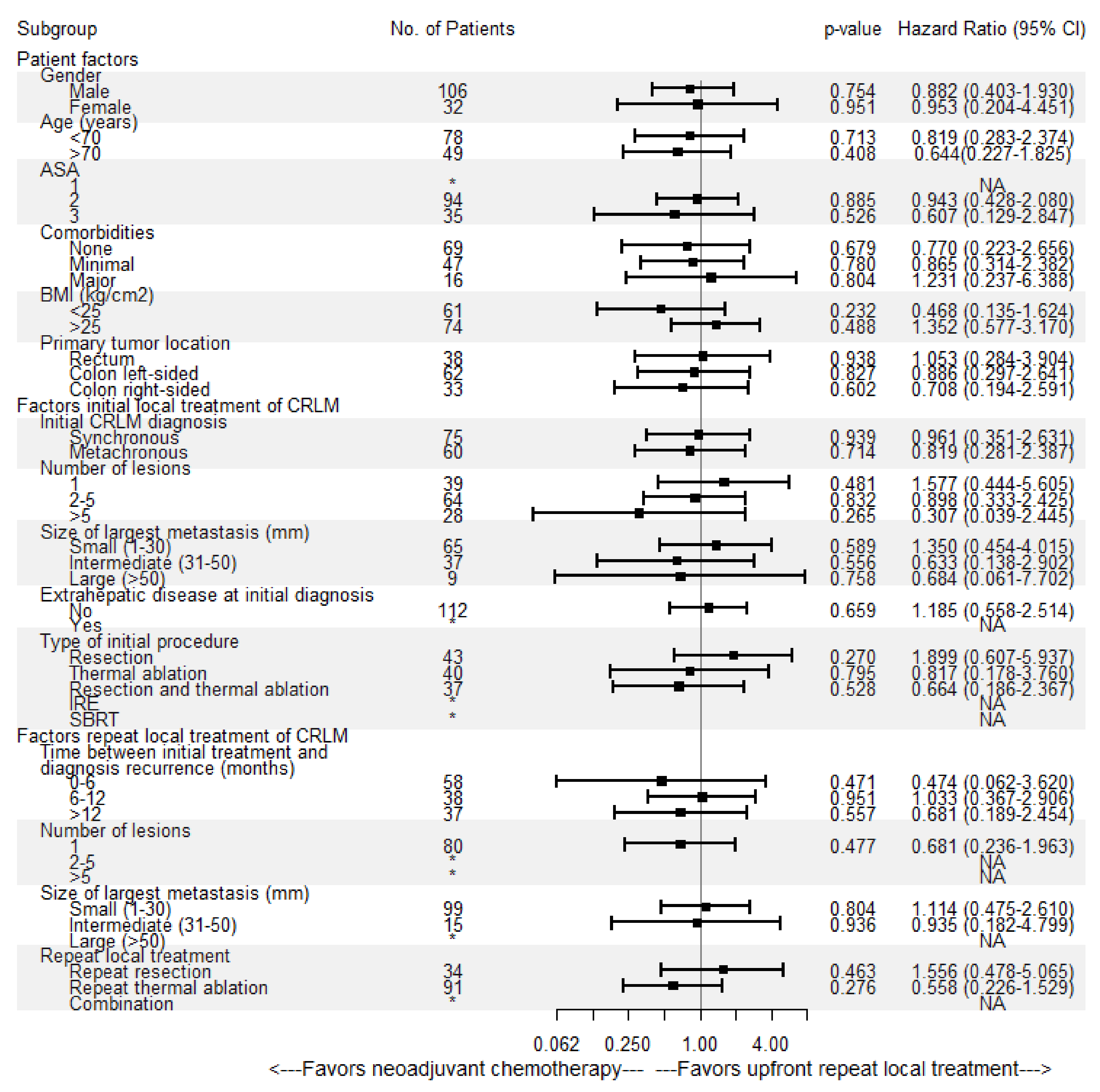
3.4. Local Tumor Progression-Free Survival
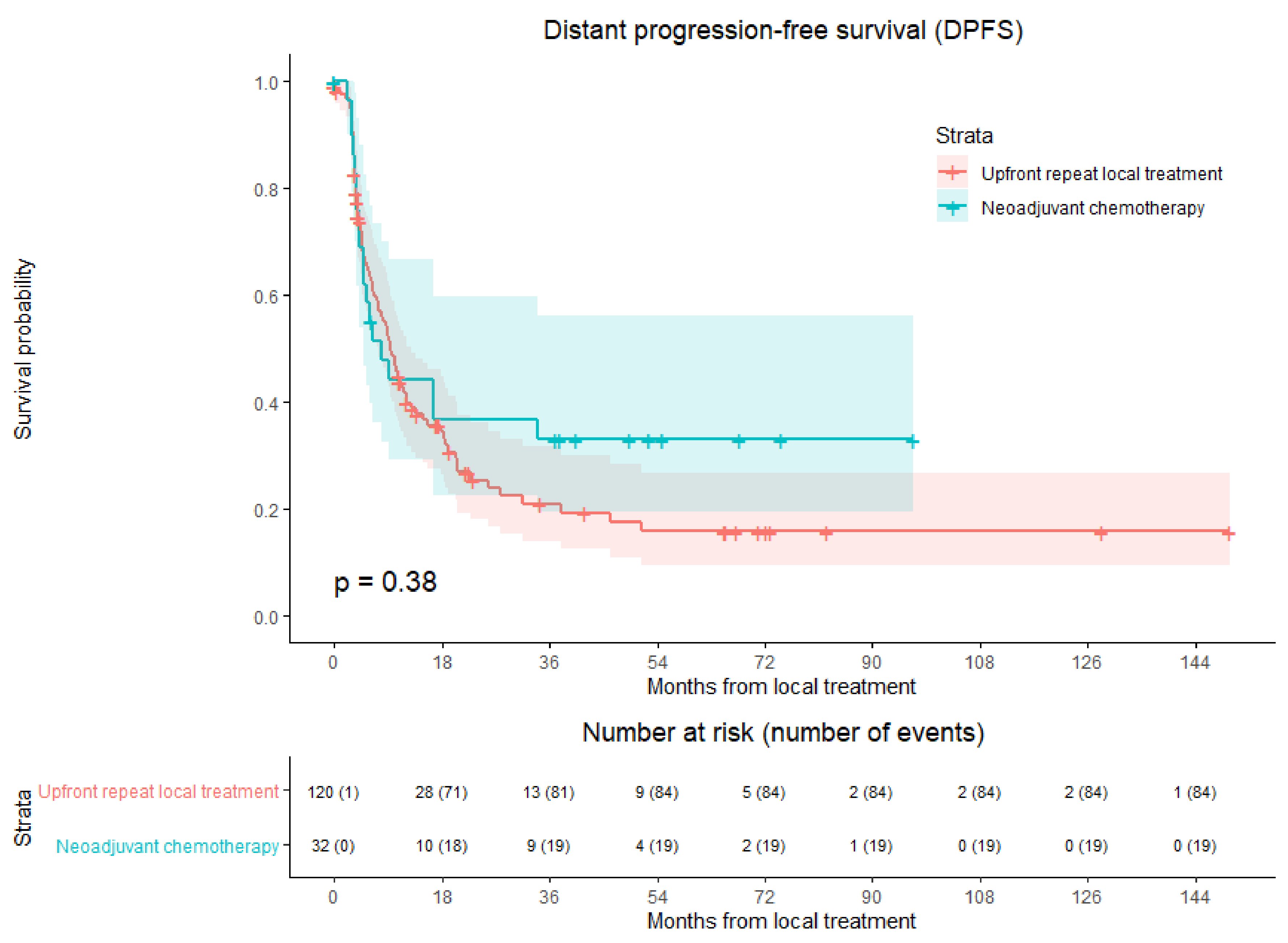
3.5. Distant Progression-Free survival
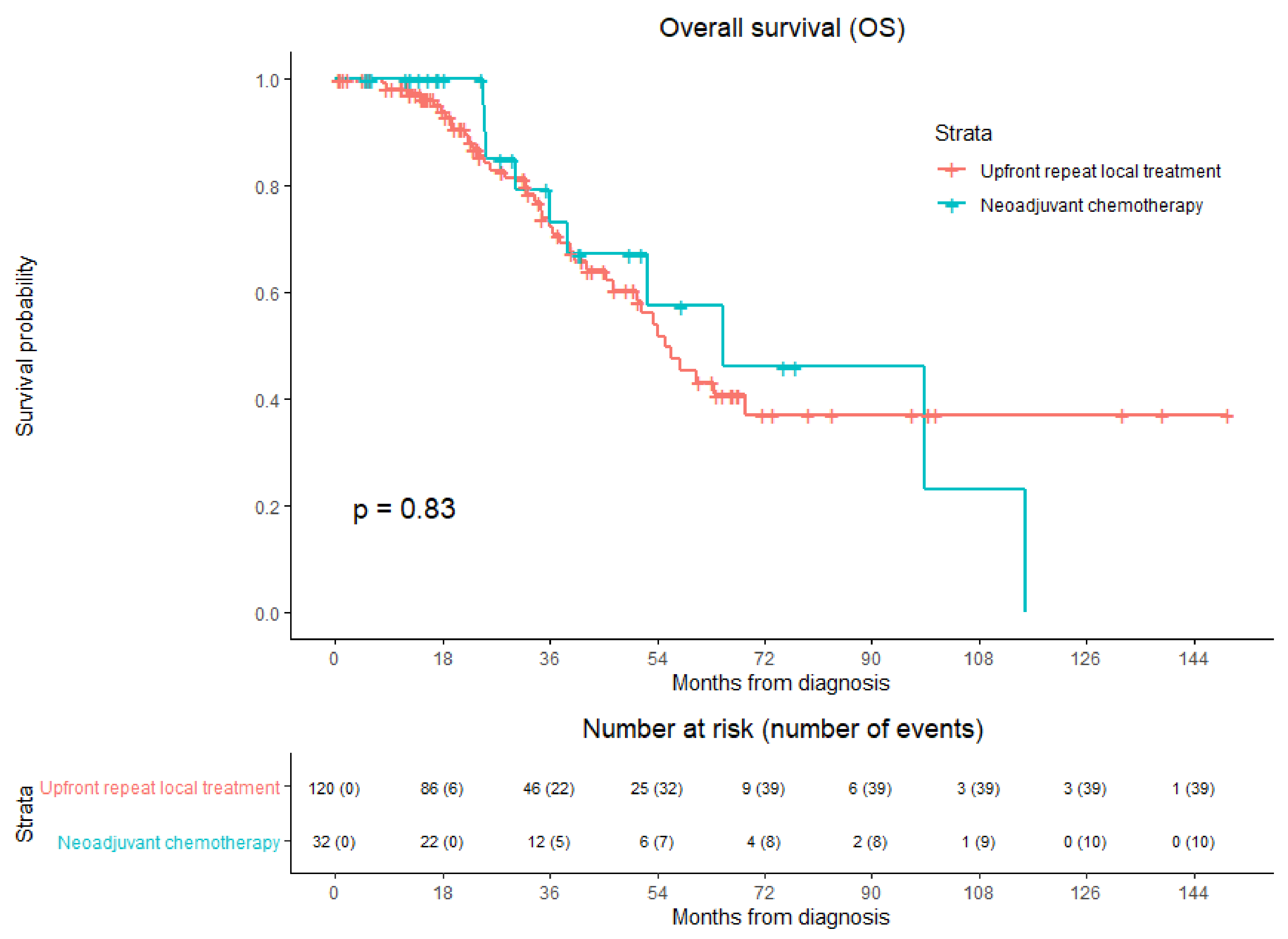
3.6. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18F-FDG | [18F]-Fluoro-2-deoxy-D-glucose |
| AmCORE | Amsterdam Colorectal Liver Met Registry |
| BRAF | V-raf murine sarcoma viral oncogene homolog B |
| COLLISION | Colorectal Liver Metastases: Surgery vs. Thermal Ablation |
| Ce | Contrast enhancement |
| CI | Confidence interval |
| CRC | Colorectal cancer |
| CRLM | Colorectal liver metastases |
| CT | Computed tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DPFS | Distant progression-free survival |
| HR | Hazard ratio |
| IKNL | The Dutch Comprehensive Cancer Centre; Integraal Kankercentrum Nederland |
| IRE | Irreversible electroporation |
| IQR | Interquartile range |
| JSCCR | Japanese Society for Cancer of the Colon and Rectum |
| LTP | Local tumor progression |
| LTPFS | Local tumor progression-free survival |
| MRI | Magnetic resonance imaging |
| MSI | Microsatellite instability |
| MWA | Microwave ablation |
| NAC | Neoadjuvant chemotherapy |
| NICE | UK National Institute for Health and Care Excellence |
| OS | Overall survival |
| PET | Positron emission tomography |
| RAS | Rat sarcoma viral oncogene homolog |
| RCT | Randomized controlled trial |
| RFA | Radiofrequency ablation |
| RR | Risk ratio |
| SBRT | Stereotactic body radiation therapy |
| SD | Standard deviation |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- WHO. Estimated Age-Standardized Incidence Rates (World) in 2020, All Cancers, Both Sexes, All Ages. Available online: http://gco.iarc.fr/today/online-analysis-map (accessed on 4 March 2021).
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.R.; Puijk, R.S.; Van Tilborg, A.A.J.M.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; De Jong, K.P.; Richel, D.J.; et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Scheele, J.; Stangl, R.; Altendorf-Hofmann, A. Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. BJS 1990, 77, 1241–1246. [Google Scholar] [CrossRef]
- Stangl, R.; Altendorf-Hofmann, A.; Charnley, R.; Scheele, J. Factors influencing the natural history of colorectal liver metastases. Lancet 1994, 343, 1405–1410. [Google Scholar] [CrossRef]
- Wagner, J.S.; Adson, M.A.; VAN Heerden, J.A.; Ilstrup, D.M. The Natural History of Hepatic Metastases from Colorectal Cancer. Ann. Surg. 1984, 199, 502–508. [Google Scholar] [CrossRef]
- Yang, Q.; Liao, F.; Huang, Y.; Jiang, C.; Liu, S.; He, W.; Kong, P.; Zhang, B.; Xia, L. Longterm effects of palliative local treatment of incurable metastatic lesions in colorectal cancer patients. Oncotarget 2016, 7, 21034–21045. [Google Scholar] [CrossRef][Green Version]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef] [PubMed]
- Gleisner, A.L.; Choti, M.A.; Assumpcao, L.; Nathan, H.; Schulick, R.D.; Pawlik, T.M. Colorectal Liver Metastases. Arch. Surg. 2008, 143, 1204–1212. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Vauthey, J.-N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A. Recurrence and Outcomes Following Hepatic Resection, Radiofrequency Ablation, and Combined Resection/Ablation for Colorectal Liver Metastases. Ann. Surg. 2004, 239, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.; Timmer, F.; Geboers, B.; Schouten, E.; Opperman, J.; Scheffer, H.; de Vries, J.; Versteeg, K.; et al. Primary Tumor Sidedness, RAS and BRAF Mutations and MSI Status as Prognostic Factors in Patients with Colorectal Liver Metastases Treated with Surgery and Thermal Ablation: Results from the Amsterdam Colorectal Liver Met Registry (AmCORE). Biomedicines 2021, 9, 962. [Google Scholar] [CrossRef]
- Tomlinson, J.S.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kornprat, P.; Gonen, M.; Kemeny, N.; Brennan, M.F.; Blumgart, L.H.; D’Angelica, M. Actual 10-Year Survival After Resection of Colorectal Liver Metastases Defines Cure. J. Clin. Oncol. 2007, 25, 4575–4580. [Google Scholar] [CrossRef] [PubMed]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef]
- Gillams, A.; Goldberg, N.; Ahmed, M.; Bale, R.; Breen, D.; Callstrom, M.; Chen, M.H.; Choi, B.I.; De Baere, T.; Dupuy, D.; et al. Thermal ablation of colorectal liver metastases: A position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur. Radiol. 2015, 25, 3438–3454. [Google Scholar] [CrossRef]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Nieuwenhuizen, S.; Puijk, R.; Bemd, B.V.D.; Aldrighetti, L.; Arntz, M.; Boezem, P.V.D.; Bruynzeel, A.; Burgmans, M.; De Cobelli, F.; Coolsen, M.; et al. Resectability and Ablatability Criteria for the Treatment of Liver Only Colorectal Metastases: Multidisciplinary Consensus Document from the COLLISION Trial Group. Cancers 2020, 12, 1779. [Google Scholar] [CrossRef]
- Puijk, R.S.; COLLISION Trial Group; Ruarus, A.H.; Vroomen, L.G.P.H.; Van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; De Jong, M.C.; De Vries, J.J.J.; Zonderhuis, B.M.; et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer 2018, 18, 821. [Google Scholar] [CrossRef]
- Kamarinos, N.V.; Kaye, E.; Sofocleous, C. Image-Guided Thermal Ablation for Colorectal Liver Metastases. Tech. Vasc. Interv. Radiol. 2020, 23, 100672. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; De Baère, T.; Dodd, G.D.; et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Solbiati, L.; Ahmed, M.; Cova, L.; Ierace, T.; Brioschi, M.; Goldberg, S.N. Small Liver Colorectal Metastases Treated with Percutaneous Radiofrequency Ablation: Local Response Rate and Long-term Survival with Up to 10-year Follow-up. Radiology 2012, 265, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Gonen, M.; Erinjeri, J.P.; Brown, K.T.; Covey, A.M.; Alago, W.; Durack, J.; Maybody, M.; Brody, L.A.; et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes—A 10-year Experience at a Single Center. Radiology 2016, 278, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, J.; Chen, H.; Hu, C.; Sun, B.; Wang, H.; Shi, Q.; Long, J.; Zhang, H.; Li, W. A prognostic nomogram for colorectal cancer liver metastases after percutaneous thermal ablation. Int. J. Hyperth. 2017, 34, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Garg, S.; Petrovic, L.M.; Gonen, M.; Petre, E.N.; Klimstra, D.S.; Solomon, S.B.; Brown, K.T.; Brody, L.A.; Covey, A.M.; et al. Ki-67 is a Prognostic Biomarker of Survival after Radiofrequency Ablation of Liver Malignancies. Ann. Surg. Oncol. 2012, 19, 4262–4269. [Google Scholar] [CrossRef]
- Adam, R.; Delvart, V.; Pascal, G.; Valeanu, A.; Castaing, D.; Azoulay, D.; Giacchetti, S.; Paule, B.; Kunstlinger, F.; Ghémard, O.; et al. Rescue Surgery for Unresectable Colorectal Liver Metastases Downstaged by Chemotherapy. Ann. Surg. 2004, 240, 644–658. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shimizu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone for liver metastasis from colorectal cancer: JCOG0603 study. J. Clin. Oncol. 2020, 38, 4005. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Zhu, D.; Zhong, Y.; Wei, Y.; Ye, L.; Lin, Q.; Ren, L.; Ye, Q.; Liu, T.; Xu, J.; Qin, X. Effect of Neoadjuvant Chemotherapy in Patients with Resectable Colorectal Liver Metastases. PLoS ONE 2014, 9, e86543. [Google Scholar] [CrossRef]
- Nordlinger, B.; Van Cutsem, E.; Rougier, P.; Köhne, C.-H.; Ychou, M.; Sobrero, A.; Adam, R.; Arvidsson, D.; Carrato, A.; Georgoulias, V.; et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur. J. Cancer 2007, 43, 2037–2045. [Google Scholar] [CrossRef]
- Benoist, S.; Nordlinger, B. The Role of Preoperative Chemotherapy in Patients with Resectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2009, 16, 2385–2390. [Google Scholar] [CrossRef]
- Tanaka, K.; Adam, R.; Shimada, H.; Azoulay, D.; Lévi, F.; Bismuth, H. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. BJS 2003, 90, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Kemeny, N.; Jarnagin, W.; DeMatteo, R.; Blumgart, L.; Fong, Y. Importance of Response to Neoadjuvant Chemotherapy in Patients Undergoing Resection of Synchronous Colorectal Liver Metastases. J. Gastrointest. Surg. 2003, 7, 109–117. [Google Scholar] [CrossRef]
- Adam, R.; Wicherts, D.A.; De Haas, R.J.; Aloia, T.; Lévi, F.; Paule, B.; Guettier, C.; Kunstlinger, F.; Delvart, V.; Azoulay, D.; et al. Complete Pathologic Response After Preoperative Chemotherapy for Colorectal Liver Metastases: Myth or Reality? J. Clin. Oncol. 2008, 26, 1635–1641. [Google Scholar] [CrossRef]
- Adam, R.; Pascal, G.; Azoulay, D.; Tanaka, K.; Castaing, D.; Bismuth, H. Liver Resection for Colorectal Metastases. Ann. Surg. 2003, 238, 871–884. [Google Scholar] [CrossRef]
- Ismaili, N. Treatment of colorectal liver metastases. World J. Surg. Oncol. 2011, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Van Tilborg, A.A.; Scheffer, H.J.; van der Meijs, B.B.; van Werkum, M.H.; Melenhorst, M.C.; Tol, P.M.V.D.; Meijerink, M.R. Transcatheter CT Hepatic Arteriography–Guided Percutaneous Ablation to Treat Ablation Site Recurrences of Colorectal Liver Metastases: The Incomplete Ring Sign. J. Vasc. Interv. Radiol. 2015, 26, 583–587.e1. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ruarus, A.H.; Scheffer, H.J.; Vroomen, L.G.; Van Tilborg, A.A.; De Vries, J.J.; Berger, F.H.; Tol, P.M.V.D.; Meijerink, M.R. Percutaneous Liver Tumour Ablation: Image Guidance, Endpoint Assessment, and Quality Control. Can. Assoc. Radiol. J. 2018, 69, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sofocleous, C.T.; Erinjeri, J.; Petre, E.N.; Gonen, M.; Do, R.; Brown, K.; Covey, A.M.; Brody, L.A.; Alago, W.; et al. Margin Size is an Independent Predictor of Local Tumor Progression After Ablation of Colon Cancer Liver Metastases. Cardiovasc. Interv. Radiol. 2012, 36, 166–175. [Google Scholar] [CrossRef]
- Shady, W.; Petre, E.N.; Do, K.G.; Gonen, M.; Yarmohammadi, H.; Brown, K.T.; Kemeny, N.E.; D’Angelica, M.; Kingham, P.T.; Solomon, S.B.; et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 2017, 29, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Solbiati, M.; Muglia, R.; Goldberg, S.N.; Ierace, T.; Rotilio, A.; Passera, K.M.; Marre, I.; Solbiati, L. A novel software platform for volumetric assessment of ablation completeness. Int. J. Hyperth. 2019, 36, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Kaye, E.A.; Cornelis, F.H.; Petre, E.N.; Tyagi, N.; Shady, W.; Shi, W.; Zhang, Z.; Solomon, S.B.; Sofocleous, C.T.; Durack, J.C. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur. Radiol. 2018, 29, 2698–2705. [Google Scholar] [CrossRef]
- Laimer, G.; Jaschke, N.; Schullian, P.; Putzer, D.; Eberle, G.; Solbiati, M.; Solbiati, L.; Goldberg, S.N.; Bale, R. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur. Radiol. 2021, 31, 6489–64991. [Google Scholar] [CrossRef] [PubMed]
- Are, C.; Gonen, M.; Zazzali, K.; DeMatteo, R.P.; Jarnagin, W.R.; Fong, Y.; Blumgart, L.H.; D’Angelica, M. The Impact of Margins on Outcome After Hepatic Resection for Colorectal Metastasis. Ann. Surg. 2007, 246, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kurilova, I.; Bendet, A.; Petre, E.N.; Boas, F.E.; Kaye, E.; Gonen, M.; Covey, A.; Brody, L.A.; Brown, K.T.; Kemeny, N.E.; et al. Factors Associated With Local Tumor Control and Complications After Thermal Ablation of Colorectal Cancer Liver Metastases: A 15-year Retrospective Cohort Study. Clin. Color. Cancer 2020, 20, e82–e95. [Google Scholar] [CrossRef]
- Han, K.; Kim, J.H.; Yang, S.G.; Park, S.H.; Choi, H.-K.; Chun, S.-Y.; Kim, P.N.; Park, J.; Lee, M. A Single-Center Retrospective Analysis of Periprocedural Variables Affecting Local Tumor Progression after Radiofrequency Ablation of Colorectal Cancer Liver Metastases. Radiology 2021, 298, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sotirchos, V.S.; Petrovic, L.M.; Gonen, M.; Klimstra, D.S.; Do, R.K.G.; Petre, E.N.; Garcia, A.R.; Barlas, A.; Erinjeri, J.P.; Brown, K.T.; et al. Colorectal Cancer Liver Metastases: Biopsy of the Ablation Zone and Margins Can Be Used to Predict Oncologic Outcome. Radiology 2016, 280, 949–959. [Google Scholar] [CrossRef]
- Viganò, L.; Costa, G.; Cimino, M.; Procopio, F.; Donadon, M.; Del Fabbro, D.; Belghiti, J.; Kokudo, N.; Makuuchi, M.; Vauthey, J.-N.; et al. R1 Resection for Colorectal Liver Metastases: A Survey Questioning Surgeons about Its Incidence, Clinical Impact, and Management. J. Gastrointest. Surg. 2018, 22, 1752–1763. [Google Scholar] [CrossRef]
- Achterberg, F.B.; Mulder, B.G.S.; Meijer, R.P.J.; Bonsing, B.A.; Hartgrink, H.H.; Mieog, J.S.D.; Zlitni, A.; Park, S.-M.; Sarasqueta, A.F.; Vahrmeijer, A.L.; et al. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann. Transl. Med. 2020, 8, 1448. [Google Scholar] [CrossRef]
- Keil, S.; Bruners, P.; Schiffl, K.; Sedlmair, M.; Mühlenbruch, G.; Günther, R.W.; Das, M.; Mahnken, A.H. Radiofrequency Ablation of Liver Metastases—Software-Assisted Evaluation of the Ablation Zone in MDCT: Tumor-Free Follow-Up Versus Local Recurrent Disease. Cardiovasc. Interv. Radiol. 2009, 33, 297–306. [Google Scholar] [CrossRef]
- Sotirchos, V.S.; Fujisawa, S.; Vakiani, E.; Solomon, S.B.; Manova-Todorova, K.O.; Sofocleous, C.T. Fluorescent Tissue Assessment of Colorectal Cancer Liver Metastases Ablation Zone: A Potential Real-Time Biomarker of Complete Tumor Ablation. Ann. Surg. Oncol. 2019, 26, 1833–1840. [Google Scholar] [CrossRef]
- Saiura, A.; Yamamoto, J.; Hasegawa, K.; Koga, R.; Sakamoto, Y.; Hata, S.; Makuuchi, M.; Kokudo, N. Liver Resection for Multiple Colorectal Liver Metastases with Surgery Up-front Approach: Bi-institutional Analysis of 736 Consecutive Cases. World J. Surg. 2012, 36, 2171–2178. [Google Scholar] [CrossRef]
- Viganò, L.; Pedicini, V.; Comito, T.; Carnaghi, C.; Costa, G.; Poretti, D.; Franzese, C.; Personeni, N.; Del Fabbro, D.; Rimassa, L.; et al. Aggressive and Multidisciplinary Local Approach to Iterative Recurrences of Colorectal Liver Metastases. World J. Surg. 2018, 42, 2651–2659. [Google Scholar] [CrossRef]
- Viganò, L.; Ferrero, A.; Tesoriere, R.L.; Capussotti, L. Liver Surgery for Colorectal Metastases: Results after 10 Years of Follow-Up. Long-Term Survivors, Late Recurrences, and Prognostic Role of Morbidity. Ann. Surg. Oncol. 2008, 15, 2458–2464. [Google Scholar] [CrossRef]
- Odisio, B.C.; Yamashita, S.; Huang, S.Y.; Kopetz, S.; Ahrar, K.; Mizuno, T.; Conrad, C.; Aloia, T.A.; Chun, Y.S.; Gupta, S.; et al. Impact of Prior Hepatectomy History on Local Tumor Progression after Percutaneous Ablation of Colorectal Liver Metastases. J. Vasc. Interv. Radiol. 2018, 29, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Petrowsky, H.; Gonen, M.; Jarnagin, W.; Lorenz, M.; DeMatteo, R.; Heinrich, S.; Encke, A.; Blumgart, L.; Fong, Y. Second Liver Resections Are Safe and Effective Treatment for Recurrent Hepatic Metastases from Colorectal Cancer. Ann. Surg. 2002, 235, 863–871. [Google Scholar] [CrossRef]
- Adam, R.; Bismuth, H.; Castaing, D.; Waechter, F.; Navarro, F.; Abascal, A.; Majno, P.; Engerran, L. Repeat Hepatectomy for Colorectal Liver Metastases. Ann. Surg. 1997, 225, 51–62. [Google Scholar] [CrossRef]
- Takamoto, T.; Hashimoto, T.; Miyata, A.; Shimada, K.; Maruyama, Y.; Makuuchi, M. Repeat Hepatectomy After Major Hepatectomy for Colorectal Liver Metastases. J. Gastrointest. Surg. 2019, 24, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.; Timmer, F.; Geboers, B.; Schouten, E.; Opperman, J.; Scheffer, H.; Vries, J.; Swijnenburg, R.-J.; et al. Thermal Ablation Compared to Partial Hepatectomy for Recurrent Colorectal Liver Metastases: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers 2021, 13, 2769. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Petre, E.N.; Gonen, M.; Brown, K.; Solomon, S.B.; Covey, A.M.; Alago, W.; Brody, L.A.; Thornton, R.H.; D’Angelica, M.; et al. CT-guided Radiofrequency Ablation as a Salvage Treatment of Colorectal Cancer Hepatic Metastases Developing after Hepatectomy. J. Vasc. Interv. Radiol. 2011, 22, 755–761. [Google Scholar] [CrossRef]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.; Geboers, B.; Timmer, F.; Schouten, E.; Scheffer, H.; de Vries, J.; Ket, J.; Versteeg, K.; et al. The Role of Neoadjuvant Chemotherapy in Repeat Local Treatment of Recurrent Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.P.; Nana, G.R.; Jones, M.; Cairns, V.; Ngu, W.; Isherwood, J.; Dennison, A.R.; Garcea, G. Repeat hepatectomy is independently associated with favorable long-term outcome in patients with colorectal liver metastases. Cancer Med. 2017, 6, 331–338. [Google Scholar] [CrossRef]
- Neeff, H.P.; Drognitz, O.; Holzner, P.; Klock, A.; Bronsert, P.; Hopt, U.T.; Makowiec, F. Outcome after repeat resection of liver metastases from colorectal cancer. Int. J. Color. Dis. 2013, 28, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Pessaux, P.; Lermite, E.; Brehant, O.; Tuech, J.-J.; Lorimier, G.; Arnaud, J.-P. Repeat hepatectomy for recurrent colorectal liver metastases. J. Surg. Oncol. 2005, 93, 1–7. [Google Scholar] [CrossRef]
- Wicherts, D.A.; de Haas, R.J.; Salloum, C.; Andreani, P.; Pascal, G.; Sotirov, D.; Adam, R.; Castaing, D.; Azoulay, D. Repeat hepatectomy for recurrent colorectal metastases. BJS 2013, 100, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Capussotti, L.; Lapointe, R.; Barroso, E.; Hubert, C.; Giuliante, F.; Ijzermans, J.N.M.; Mirza, D.F.; Elias, D.; Adam, R. Early Recurrence After Liver Resection for Colorectal Metastases: Risk Factors, Prognosis, and Treatment. A LiverMetSurvey-Based Study of 6025 Patients. Ann. Surg. Oncol. 2013, 21, 1276–1286. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Nieuwenhuizen, S.; Dijkstra, M.; Puijk, R.S.; Timmer, F.E.F.; Nota, I.M.; Opperman, J.; Bemd, B.V.D.; Geboers, B.; Ruarus, A.H.; Schouten, E.A.C.; et al. Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry. Cancers 2021, 13, 4303. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Ruarus, A.H.; Vroomen, L.G.P.H.; Puijk, R.S.; Geboers, B.; Nieuwenhuizen, S.; Bemd, B.A.T.V.D.; Nielsen, K.; de Vries, J.J.J.; van Lienden, K.P.; et al. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology 2021, 299, 470–480. [Google Scholar] [CrossRef]
- Comprehensive Cancer Organisation the Netherlands (I.K.N.L.). National Evidence-Based Guideline. Colorectaalcarcinoom. 2014. Available online: http://oncoline.nl/ (accessed on 25 November 2020).
- Crocetti, L.; de Baere, T.; Lencioni, R. Quality Improvement Guidelines for Radiofrequency Ablation of Liver Tumours. Cardiovasc. Interv. Radiol. 2009, 33, 11–17. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 25 November 2020).
- IBM Corp. IBM® SPSS® Statistics for Windows, Version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R for Windows Version 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Adair, R.A.; Young, A.L.; Cockbain, A.J.; Malde, D.; Prasad, K.R.; Lodge, J.P.A.; Toogood, G.J. Repeat hepatic resection for colorectal liver metastases. BJS 2012, 99, 1278–1283. [Google Scholar] [CrossRef]
- Brachet, D.; Lermite, E.; Rouquette, A.; Lorimier, G.; Hamy, A.; Arnaud, J.-P. Prognostic Factors of Survival in Repeat Liver Resection for Recurrent Colorectal Metastases. Dis. Colon Rectum 2009, 52, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kobayashi, T.; Ishiyama, K.; Ide, K.; Ohira, M.; Tahara, H.; Kuroda, S.; Hamaoka, M.; Iwako, H.; Okimoto, M.; et al. Efficacy of repeat hepatectomy for recurrence following curative hepatectomy for colorectal liver metastases: A Retrospective Cohort Study of 128 patients. Int. J. Surg. 2016, 36, 96–103. [Google Scholar] [CrossRef]
- Matsuoka, H.; Morise, Z.; Tanaka, C.; Hayashi, T.; Ikeda, Y.; Maeda, K.; Masumori, K.; Koide, Y.; Katsuno, H.; Tanahashi, Y.; et al. Repeat hepatectomy with systemic chemotherapy might improve survival of recurrent liver metastasis from colorectal cancer—A retrospective observational study. World J. Surg. Oncol. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Imai, K.; Yamashita, Y.-I.; Miyamoto, Y.; Nakagawa, S.; Okabe, H.; Hashimoto, D.; Chikamoto, A.; Baba, H. The predictors and oncological outcomes of repeat surgery for recurrence after hepatectomy for colorectal liver metastases. Int. J. Clin. Oncol. 2018, 23, 908–916. [Google Scholar] [CrossRef]
- Homayounfar, K.; Bleckmann, A.; Conradi, L.C.; Sprenger, T.; Lorf, T.; Niessner, M.; Sahlmann, C.O.; Meller, J.; Liersch, T.; Ghadimi, B.M. Metastatic recurrence after complete resection of colorectal liver metastases: Impact of surgery and chemotherapy on survival. Int. J. Color. Dis. 2013, 28, 1009–1017. [Google Scholar] [CrossRef]
- Ishiguro, S.; Akasu, T.; Fujimoto, Y.; Yamamoto, J.; Sakamoto, Y.; Sano, T.; Shimada, K.; Kosuge, T.; Yamamoto, S.; Fujita, S.; et al. Second Hepatectomy for Recurrent Colorectal Liver Metastasis: Analysis of Preoperative Prognostic Factors. Ann. Surg. Oncol. 2006, 13, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Castro-Benitez, C.; Allard, M.-A.; Vibert, E.; Cunha, A.S.; Cherqui, D.; Castaing, D.; Bismuth, H.; Baba, H.; Adam, R. Impact of Surgical Treatment for Recurrence After 2-Stage Hepatectomy for Colorectal Liver Metastases, on Patient Outcome. Ann. Surg. 2019, 269, 322–330. [Google Scholar] [CrossRef]
- Kishi, Y.; Nara, S.; Esaki, M.; Shimada, K. Feasibility of “Watch-and-Wait” Management before Repeat Hepatectomy for Colorectal Liver Metastases. Dig. Surg. 2018, 36, 233–240. [Google Scholar] [CrossRef]
- Andreou, A.; Brouquet, A.; Abdalla, E.K.; Aloia, T.A.; Curley, S.A.; Vauthey, J.-N. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB 2011, 13, 774–782. [Google Scholar] [CrossRef]
- Ayez, N.; van der Stok, E.; Grünhagen, D.; Rothbarth, J.; van Meerten, E.; Eggermont, A.; Verhoef, C. The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: Clinical risk score as possible discriminator. Eur. J. Surg. Oncol. 2015, 41, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Galvanin, J.; Poretti, D.; Del Fabbro, D.; Gentile, D.; Pedicini, V.; Solbiati, L.; Torzilli, G. Percutaneous ablation of post-surgical solitary early recurrence of colorectal liver metastases is an effective “test-of-time” approach. Updat. Surg. 2021, 73, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Kooby, D.A. Impact of steatosis on perioperative outcome following hepatic resection. J. Gastrointest. Surg. 2003, 7, 1034–1044. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Pawlik, T.M.; Ribero, D.; Wu, T.-T.; Zorzi, D.; Hoff, P.M.; Xiong, H.Q.; Eng, C.; Lauwers, G.Y.; Mino-Kenudson, M.; et al. Chemotherapy Regimen Predicts Steatohepatitis and an Increase in 90-Day Mortality After Surgery for Hepatic Colorectal Metastases. J. Clin. Oncol. 2006, 24, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Wiering, B.; Oyen, W.J.G.; Adang, E.M.M.; van der Sijp, J.R.M.; Roumen, R.M.; de Jong, K.P.; Ruers, T.J.M.; Krabbe, P.F.M. Long-term global quality of life in patients treated for colorectal liver metastases. BJS 2010, 98, 565–571. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab Plus Irinotecan, Fluorouracil, and Leucovorin As First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Zimmitti, G.; Kopetz, S.; Shindoh, J.; Chen, S.S.; Andreou, A.; Curley, S.A.; Aloia, T.A.; Maru, D.M. RAS Mutation Status Predicts Survival and Patterns of Recurrence in Patients Undergoing Hepatectomy for Colorectal Liver Metastases. Ann. Surg. 2013, 258, 619–627. [Google Scholar] [CrossRef]
- Taieb, J.; Zaanan, A.; Le Malicot, K.; Julié, C.; Blons, H.; Mineur, L.; Bennouna, J.; Tabernero, J.; Mini, E.; Folprecht, G.; et al. Prognostic Effect ofBRAFandKRASMutations in Patients With Stage III Colon Cancer Treated With Leucovorin, Fluorouracil, and Oxaliplatin With or Without Cetuximab. JAMA Oncol. 2016, 2, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Kopetz, S.; Li, L.; Conrad, C.; Aloia, T.A.; Vauthey, J. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. BJS 2015, 102, 1175–1183. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Ye, M.; Weng, W.; Tan, C.; Ni, S.; Huang, D.; Sheng, W.; Wang, L. KRAS Mutation Predicted More Mirometastases and Closer Resection Margins in Patients with Colorectal Cancer Liver Metastases. Ann. Surg. Oncol. 2019, 27, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Mise, Y.; Chung, M.H.; Chun, Y.S.; Kopetz, S.E.; Passot, G.; Conrad, C.; Maru, D.M.; Aloia, T.A.; Vauthey, J.-N.; et al. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2016, 23, 2635–2643. [Google Scholar] [CrossRef]
- Odisio, B.C.; Yamashita, S.; Huang, S.Y.; Harmoush, S.; Kopetz, S.; Ahrar, K.; Chun, Y.S.; Conrad, C.; Aloia, T.A.; Gupta, S.; et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. BJS 2017, 104, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Vakiani, E.; Ziv, E.; Gonen, M.; Brown, K.T.; Kemeny, N.E.; Solomon, S.B.; Solit, D.B.; Sofocleous, C.T. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 2017, 8, 66117–66127. [Google Scholar] [CrossRef] [PubMed]
- Calandri, M.; Yamashita, S.; Gazzera, C.; Fonio, P.; Veltri, A.; Bustreo, S.; Sheth, R.A.; Yevich, S.M.; Vauthey, J.-N.; Odisio, B.C. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur. Radiol. 2018, 28, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Brudvik, K.W.; Kopetz, S.; Maru, D.; Clarke, C.N.; Passot, G.; Conrad, C.; Chun, Y.S.; Aloia, T.A.; Vauthey, J.-N. Embryonic Origin of Primary Colon Cancer Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colon Cancer Liver Metastases. Ann. Surg. 2018, 267, 514–520. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total | Neoadjuvant Chemotherapy Group | Upfront Repeat Local Treatment Group | p-Value | |
|---|---|---|---|---|---|
| Number of patients | 152 | 32 (21.1) | 120 (78.9) | ||
| Patient characteristics | |||||
| Gender | Male | 115 (75.7) | 26 (81.3) | 89 (74.2) | |
| Female | 37 (24.3) | 6 (18.8) | 31 (25.8) | 0.492 a | |
| Age (years) * | 65.4 (11.0) | 63.9 (10.8) | 65.7 (11.0) | 0.399 c | |
| ASA physical status | 1 | 9 (5.9) | 1 (3.1) | 8 (6.7) | |
| 2 | 105 (69.1) | 23 (71.9) | 82 (68.3) | ||
| 3 | 38 (25.0) | 8 (25.0) | 30 (25.0) | 0.748 b | |
| Comorbidities | None | 75 (49.3) | 17 (53.1) | 58 (48.3) | |
| Minimal | 56 (36.8) | 11 (34.4) | 45 (37.5) | ||
| Major | 21 (13.8) | 4 (12.5) | 17 (14.2) | 0.889 b | |
| BMI (kg/cm2) * | 26.1 (4.1) | 25.4 (3.8) | 26.2 (4.2) | 0.306 c | |
| Primary tumor location | Rectum | 40 (26.3) | 9 (28.1) | 31 (25.8) | |
| Colon left-sided | 74 (48.7) | 15 (46.9) | 59 (49.2) | ||
| Colon right-sided | 38 (25.0) | 8 (25.0) | 30 (25.0) | 0.962 b | |
| Characteristics initial local treatment of CRLM | |||||
| Initial CRLM diagnosis | Synchronous | 80 (53.7) | 22 (68.8) | 58 (49.6) | |
| Metachronous | 69 (46.3) | 10 (31.3) | 59 (50.4) | 0.071 a | |
| Missing | 3 | 0 | 3 | ||
| Number of tumors | 1 | 44 (28.9) | 8 (25.0) | 36 (30.0) | |
| 2–5 | 69 (45.4) | 13 (40.6) | 56 (46.7) | ||
| >5 | 39 (25.7) | 11 (34.4) | 28 (23.3) | 0.444 b | |
| Size of largest metastasis (mm) | Small (1–30) | 84 (62.7) | 15 (60.0) | 69 (63.3) | |
| Intermediate (31–50) | 36 (29.1) | 6 (24.0) | 33 (30.3) | ||
| Large (>50) | 11 (8.2) | 4 (16.0) | 7 (6.4) | 0.275 b | |
| Missing | 18 | 7 | 11 | ||
| Extrahepatic disease at time of initial diagnosis CRLM | No | 125 (91.9) | 6 (83.9) | 99 (94.3) | |
| Yes | 11 (8.1) | 5 (16.1) | 6 (5.7) | 0.125 a | |
| Missing | 16 | 1 | 15 | ||
| Type of initial procedure | Resection | 50 (32.9) | 13 (40.6) | 37 (30.8) | |
| Thermal ablation | 47 (30.9) | 5 (15.6) | 42 (35.0) | ||
| Resection and thermal ablation | 51 (33.6) | 14 (43.8) | 37 (30.8) | ||
| IRE | 2 (1.3) | 0 (0.0) | 2 (1.7) | ||
| SBRT | 2 (1.3) | 0 (0.0) | 2 (1.7) | 0.190 b | |
| Characteristics repeat local treatment of CRLM | |||||
| Time between initial treatment and diagnosis recurrence (months) * | 6.8 (4.0–13.0) | 7.6 (3.9–14.7) | 6.8 (4.0–12.6) | 0.733 d | |
| Number of tumors | 1 | 90 (59.2) | 13 (40.6) | 77 (64.2) | |
| 2–5 | 59 (38.8) | 16 (50.0) | 43 (35.8) | ||
| >5 | 3 (2.0) | 3 (9.4) | 0 (0.0) | 0.001 b | |
| Size of largest metastasis (mm) | Small (1–30) | 111 (84.7) | 25 (89.3) | 86 (83.5) | |
| Intermediate (31–50) | 16 (15.5) | 2 (7.1) | 16 (15.5) | ||
| Large (>50) | 2 (1.5) | 1 (1.0) | 1 (3.6) | 0.334 b | |
| Missing | 21 | 4 | 17 | ||
| Repeat local treatment | Resection | 37 (24.3) | 7 (21.9) | 30 (25.0) | |
| Thermal ablation | 102 (67.1) | 22 (68.8) | 80 (66.7) | ||
| Combination | 13 (8.6) | 3 (9.4) | 10 (8.3) | 0.928 b | |
| Characteristics | Neoadjuvant Chemotherapy Group N = 32 | Upfront Repeat Local Treatment Group N = 120 | p-Value | |
|---|---|---|---|---|
| Type of repeat local treatment | Thermal ablation | 22 (68.8) | 80 (66.7) | 0.928 a |
| Partial hepatectomy | 7 (21.9) | 30 (25.0) | ||
| Combination | 3 (9.4) | 10 (8.3) | ||
| Approach | Open | 15 (46.9) | 44 (37.9) | 0.372 a |
| Laparoscopic | 0 (0.0) | 5 (4.3) | ||
| Percutaneous | 17 (53.1) | 67 (57.8) | ||
| Missing | 0 | 4 | ||
| Characteristics | Neoadjuvant Chemotherapy Group N = 74 | Upfront Repeat Local Treatment Group N = 193 | p-Value | |
|---|---|---|---|---|
| Type of repeat thermal ablation | RFA | <0.001 a | ||
| Le VeenTM | 7 (9.5) | 58 (30.4) | ||
| Cool-tipTM | 7 (9.5) | 5 (2.6) | ||
| Others | 1 (1.4) | 1 (0.5) | ||
| MWA | ||||
| EmprintTM | 33 (44.6) | 61 (31.9) | ||
| Covidien EvidentTM | 3 (4.1) | 2 (1.0) | ||
| Others | 0 (0.0) | 14 (7.3) | ||
| Type of repeat resection | Minor (<3 segments) | 23 (31.1) | 48 (25.1) | |
| Major (≥3 segments) | 0 (0.0) | 2 (1.0) | ||
| Missing | 0 | 2 | ||
| Characteristics | Neoadjuvant Chemotherapy Group N = 32 | ||
|---|---|---|---|
| Chemotherapeutic regimen | CAPOX | 22 (73.3) | |
| Capecitabine | 2 (6.7) | ||
| Irinotecan | 3 (10.0) | ||
| FOLFOX | 1 (3.3) | ||
| CAPIRI | 1 (3.3) | ||
| FOLFIRI | 1 (3.3) | ||
| Additional monoclonal antibodies | Bevacizumab | 21 (70.0) | |
| Panitumumab | 1 (3.3) | ||
| Missing | 2 | ||
| Number of cycles | 1–6 | 19 (63.3) | |
| >6 | 11 (36.7) | ||
| Missing | 2 | ||
| Grade | Total | Neoadjuvant Chemotherapy Group N = 32 | Upfront Repeat Local Treatment Group N = 120 | p-Value |
|---|---|---|---|---|
| None | 110 (79.7) | 24 (80.0) | 86 (79.6) | 0.843 a |
| Grade 1 | 8 (5.8) | 1 (3.3) | 7 (6.5) | |
| Grade 2 | 8 (58) | 2 (6.7) | 6 (5.6) | |
| Grade 3 | 10 (7.2) | 3 (10.0) | 7 (6.5) | |
| Grade 4 | 2 (1.4) | 0 (0.0) | 2 (1.9) | |
| Grade 5 | NR | NR | NR | |
| Missing | 14 | 2 | 12 |
| Grade | Neoadjuvant Chemotherapy Group N = 32 |
|---|---|
| None | 16 (53.3) |
| Grade 1 | NR |
| Grade 2 | 9 (30.0) |
| Grade 3 | 5 (16.7) |
| Grade 4 | NR |
| Grade 5 | NR |
| Missing | 2 |
| Characteristics | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| HR (CI) | p-Value | HR (CI) | p-Value | ||
| Repeat local treatment | Upfront repeat local treatment | Reference | 0.335 | Reference | 0.407 |
| Neoadjuvant chemotherapy | 0.621 (0.236–1.635) | 0.662 (0.249–1.756) | |||
| Patient-related factors | |||||
| Gender | Male | Reference | 0.272 | ||
| Female | 1.554 (0.708–3.414) | ||||
| Age (years) | 0.998 (0.966–1.031) | 0.892 | |||
| ASA physical status | 1 | Reference | 0.591 | ||
| 2 | 0.935 (0.220–3.978) | ||||
| 3 | 0.569 (0.110–2.933) | ||||
| Comorbidities | None | Reference | 0.446 | ||
| Minimal | 1.500 (0.705–3.191) | ||||
| Major | 0.731 (0.165–3.239) | ||||
| BMI (kg/cm2) | 1.074 (0.992–1.163) | 0.079 | 1.032 (0.952–1.118) | 0.448 | |
| Primary tumor location | Rectum | Reference | 0.960 | ||
| Colon left-sided | 0.886 (0.383–2.052) | ||||
| Colon right-sided | 0.948 (0.361–2.494) | ||||
| Factors regarding initial local treatment of CRLM | |||||
| Initial CRLM diagnosis | Synchronous | Reference | 0.004 | Reference | 0.022 |
| Metachronous | 3.086 (1.424–6.688) | 2.559 (1.148–5.705) | |||
| Number of tumors | 1 | Reference | 0.567 | ||
| 2–5 | 1.645 (0.592–4.572) | ||||
| >5 | 1.736 (0.593–5.081) | ||||
| Size of largest metastasis (mm) | Small (1–-30) | Reference | 0.289 | ||
| Intermediate (31–50) | 0.370 (0.108–1.275) | ||||
| Large (>50) | * | ||||
| Extrahepatic disease 1 | No | Reference | 0.369 | ||
| Yes | 0.400 (0.054–2.955) | ||||
| Type of initial procedure | Resection | Reference | 0.997 | ||
| Thermal ablation | 0.949 (0.375–2.407) | ||||
| Resection and thermal ablation | 1.124 (0.477–2.646) | ||||
| IRE | * | ||||
| SBRT | * | ||||
| Factors regarding repeat local treatment of CRLM | |||||
| Time between initial treatment and diagnosis recurrence (months) | 1.029 (1.009–1.048) | 0.004 | 1.023 (1.004–1.043) | 0.018 | |
| Number of tumors | 1 | Reference | 0.027 | Reference | 0.278 |
| 2–5 | 0.359 (0.168–0.766) | 0.544 (0.237–1.251) | |||
| >5 | 0.428 (0.056–3.273) | 1.370 (0.128–14.679) | |||
| Size of metastasis (mm) | Small (1–30) | Reference | 0.242 | ||
| Intermediate (31–50) | 2.580 (0.856–7.774) | ||||
| Large (>50) | * | ||||
| Repeat local treatment | Resection | Reference | 0.982 | ||
| Thermal ablation | 1.021 (0.426–2.449) | ||||
| Combination | 0.918 (0.268–3.144) | ||||
| Margin size | <5 mm | Reference | 0.513 | ||
| >5 mm | 3.491 (0.082–148.0) | ||||
| Characteristics | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| HR (CI) | p-Value | HR (CI) | p-Value | ||
| Repeat local treatment | Upfront repeat local treatment | Reference | 0.378 | Reference | 0.503 |
| Neoadjuvant chemotherapy | 0.798 (0.483–1.318) | 0.822 (0.464–1.457) | |||
| Patient-related factors | |||||
| Gender | Male | Reference | 0.904 | ||
| Female | 1.027 (0.662–1.594) | ||||
| Age (years) | 0.979 (0.961–0.998) | 0.030 | 0.989 (0.968–1.010) | 0.290 | |
| ASA physical status | 1 | Reference | 0.691 | ||
| 2 | 1.293 (0.561–2.983) | ||||
| 3 | 1.457 (0.598–3.550) | ||||
| Comorbidities | None | Reference | 0.538 | ||
| Minimal | 0.931 (0.613–1.413) | ||||
| Major | 1.329 (0.721–2.450) | ||||
| BMI (kg/cm2) | 0.969 (0.921–1.020) | 0.225 | |||
| Primary tumor location | Rectum | Reference | 0.777 | ||
| Colon left-sided | 0.911 (0.573–1.447) | ||||
| Colon right-sided | 1.078 (0.637–1.822) | ||||
| Factors regarding initial local treatment of CRLM | |||||
| Initial CRLM diagnosis | Synchronous | Reference | 0.013 | Reference | 0.092 |
| Metachronous | 0.600 (0.401–0.898) | 0.663 (0.411–1.069) | |||
| Number of tumors | 1 | Reference | 0.083 | Reference | 0.891 |
| 2–5 | 1.114 (0.690–1.800) | 1.144 (0.660–1.984) | |||
| >5 | 1.726 (1.019–2.923) | 1.086 (0.567–2.081) | |||
| Size of largest metastasis (mm) | Small (1–30) | Reference | 0.330 | ||
| Intermediate (31–50) | 0.723 (0.449–1.162) | ||||
| Large (>50) | 0.689 (0.296–1.605) | ||||
| Extrahepatic disease 1 | No | Reference | 0.521 | ||
| Yes | 0.776 (0.357–1.684) | ||||
| Type of initial procedure | Resection | Reference | 0.613 | ||
| Thermal ablation | 1.362 (0.838–2.214) | ||||
| Resection and thermal ablation | 0.936 (0.575–1.524) | ||||
| IRE | 1.149 (0.275–4.805) | ||||
| SBRT | 1.065 (0.255–4.450) | ||||
| Factors regarding repeat local treatment of CRLM | |||||
| Time between initial treatment and diagnosis recurrence (months) | 0.981 (0.963–0.998) | 0.031 | 0.972 (0.952–0.993) | 0.011 | |
| Number of tumors | 1 | Reference | 0.027 | Reference | 0.101 |
| 2–5 | 1.538 (1.037–2.282) | 1.320 (0.830–2.100) | |||
| >5 | 3.231 (0.998–10.455) | 3.980 (1.047–15.122) | |||
| Size of largest metastasis (mm) | Small (1–30) | Reference | 0.006 | Reference | 0.001 |
| Intermediate (31–50) | 1.689 (0.963–2.964) | 2.114 (1.182–3.781) | |||
| Large (>50) | 7.707 (1.823–32.580) | 10.734 (2.385–48.308) | |||
| Repeat local treatment | Resection | Reference | 0.201 | ||
| Thermal ablation | 1.140 (0.715–1.817) | ||||
| Combination | 1.901 (0.929–3.891) | ||||
| Characteristics | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| HR (CI) | p-Value | HR (CI) | p-Value | ||
| Repeat local treatment | Upfront repeat local treatment | Reference | 0.834 | Reference | 0.624 |
| Neoadjuvant chemotherapy | 0.928 (0.463–1.861) | 0.839 (0.416–1.691) | |||
| Patient-related factors | |||||
| Gender | Male | Reference | 0.519 | ||
| Female | 0.801 (0.409–1.570) | ||||
| Age (years) | 1.027 (0.996–1.060) | 0.092 | 1.021 (0.987–1.057) | 0.222 | |
| ASA physical status | 1 | Reference | 0.290 | ||
| 2 | 3.155 (0.752–13.235) | ||||
| 3 | 3.046 (0.673–13.787) | ||||
| Comorbidities | None | Reference | 0.019 | Reference | 0.010 |
| Minimal | 1.585 (0.850–2.955) | 1.776 (0.945–3.339) | |||
| Major | 3.165 (1.410–7.104) | 3.489 (1.536–7.923) | |||
| BMI (kg/cm2) | 0.977 (0.909–1.049) | 0.518 | |||
| Primary tumor location | Rectum | Reference | 0.054 | Reference | 0.023 |
| Colon left-sided | 0.757 (0.383–1.494) | 0.735 (0.369–1.464) | |||
| Colon right-sided | 1.762 (0.835–3.718) | 1.958 (0.921–4.160) | |||
| Factors regarding initial local treatment of CRLM | |||||
| Initial CRLM diagnosis | Synchronous | Reference | 0.634 | ||
| Metachronous | 0.868 (0.486–1.553) | ||||
| Number of tumors | 1 | Reference | 0.754 | ||
| 2–5 | 1.022 (0.532–1.963) | ||||
| >5 | 0.784 (0.360–1.710) | ||||
| Size of largest metastasis (mm) | Small (1–30) | Reference | 0.503 | ||
| Intermediate (31–50) | 0.915 (0.477–1.755) | ||||
| Large (>50) | 0.485 (0.144–1.629) | ||||
| Extrahepatic disease 1 | No | Reference | 0.219 | ||
| Yes | 0.287 (0.039–2.099) | ||||
| Type of initial procedure | Resection | Reference | 0.456 | ||
| Thermal ablation | 1.722 (0.861–3.443) | ||||
| Resection and thermal ablation | 0.955 (0.459–1.987) | ||||
| IRE | 1.348 (0.176–10.316) | ||||
| SBRT | * | ||||
| Factors regarding repeat local treatment of CRLM | |||||
| Time between initial treatment and diagnosis recurrence (months) | 1.003 (0.982–1.025) | 0.785 | |||
| Number of tumors | 1 | Reference | 0.564 | ||
| 2–5 | 1.367 (0.771–2.424) | ||||
| >5 | * | ||||
| Size of largest metastasis (mm) | Small (1–30) | Reference | 0.130 | ||
| Intermediate (31–50) | 2.092 (0.984–4.449) | ||||
| Large (>50) | 1.874 (0.420–8.371) | ||||
| Repeat localtreatment | Resection | Reference | 0.685 | ||
| Thermal ablation | 1.041 (0.554–1.956) | ||||
| Combination | 0.614 (0.176–2.145) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.S.; Timmer, F.E.F.; Geboers, B.; Schouten, E.A.C.; Opperman, J.; Scheffer, H.J.; de Vries, J.J.J.; Versteeg, K.S.; et al. Repeat Local Treatment of Recurrent Colorectal Liver Metastases, the Role of Neoadjuvant Chemotherapy: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers 2021, 13, 4997. https://doi.org/10.3390/cancers13194997
Dijkstra M, Nieuwenhuizen S, Puijk RS, Timmer FEF, Geboers B, Schouten EAC, Opperman J, Scheffer HJ, de Vries JJJ, Versteeg KS, et al. Repeat Local Treatment of Recurrent Colorectal Liver Metastases, the Role of Neoadjuvant Chemotherapy: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers. 2021; 13(19):4997. https://doi.org/10.3390/cancers13194997
Chicago/Turabian StyleDijkstra, Madelon, Sanne Nieuwenhuizen, Robbert S. Puijk, Florentine E. F. Timmer, Bart Geboers, Evelien A. C. Schouten, Jip Opperman, Hester J. Scheffer, Jan J. J. de Vries, Kathelijn S. Versteeg, and et al. 2021. "Repeat Local Treatment of Recurrent Colorectal Liver Metastases, the Role of Neoadjuvant Chemotherapy: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study" Cancers 13, no. 19: 4997. https://doi.org/10.3390/cancers13194997
APA StyleDijkstra, M., Nieuwenhuizen, S., Puijk, R. S., Timmer, F. E. F., Geboers, B., Schouten, E. A. C., Opperman, J., Scheffer, H. J., de Vries, J. J. J., Versteeg, K. S., Lissenberg-Witte, B. I., Meijerink, M. R., & van den Tol, M. P. (2021). Repeat Local Treatment of Recurrent Colorectal Liver Metastases, the Role of Neoadjuvant Chemotherapy: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers, 13(19), 4997. https://doi.org/10.3390/cancers13194997








