The Role of Cost-Effectiveness Analysis in Patient-Centered Cancer Care in the Era of Precision Medicine
1. Introduction
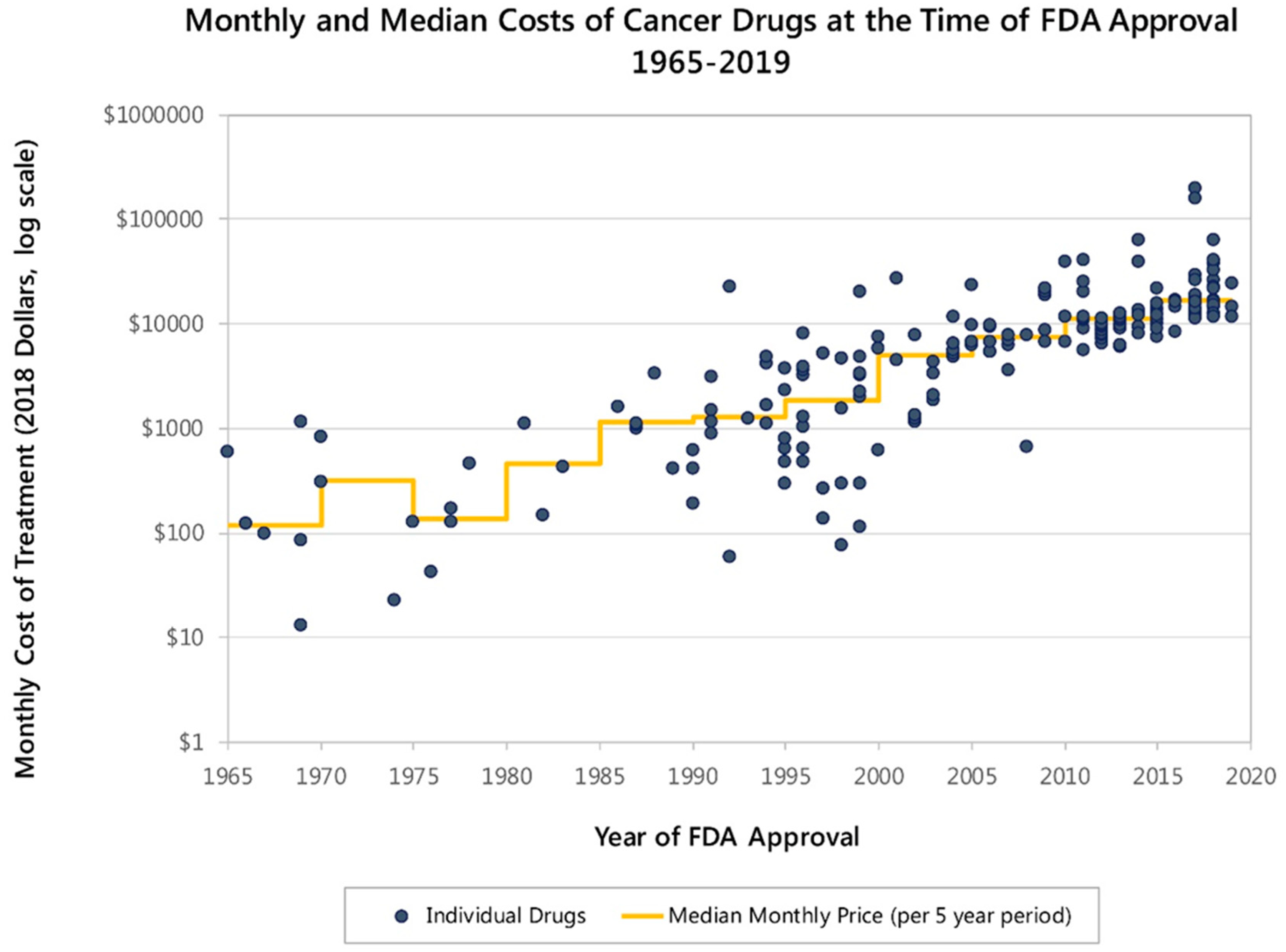
2. Patient Perspective
3. Healthcare Provider Perspective
4. Policymaker Perspective in the US and Abroad
5. Conclusions
Funding
Conflicts of Interest
References
- Markham, M.J.; Wachter, K.; Agarwal, N.; Bertagnolli, M.M.; Chang, S.M.; Dale, W.; Diefenbach, C.S.M.; Rodriguez-Galindo, C.; George, D.J.; Gilligan, T.D.; et al. Clinical Cancer Advances 2020: Annual Report on Progress Against Cancer from the American Society of Clinical Oncology. J. Clin. Oncol. 2020, 38, 1081. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Salgado, R.; Moore, H.; Martens, J.W.M.; Lively, T.; Malik, S.; McDermott, U.; Michiels, S.; Moscow, J.A.; Tejpar, S.; McKee, T.; et al. Steps forward for cancer precision medicine. Nat. Rev. Drug Discov. 2018, 17, 1–2. [Google Scholar] [CrossRef]
- Kantarjian, H.; Patel, Y. High cancer drug prices 4 years later-Progress and prospects: High Cancer Drug Prices 4 Years Later. Cancer 2017, 123, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Ganz, P.A.; Bekelman, J.E.; Chmura, S.J.; Dignam, J.J.; Efstathiou, J.A.; Jagsi, R.; Johnstone, P.A.; Steinberg, M.L.; Williams, S.B.; et al. Promoting the Appropriate Use of Advanced Radiation Technologies in Oncology: Summary of a National Cancer Policy Forum Workshop. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 450–461. [Google Scholar] [CrossRef]
- Chetty, I.J.; Martel, M.K.; Jaffray, D.A.; Benedict, S.H.; Hahn, S.M.; Berbeco, R.; Deye, J.; Jeraj, R.; Kavanagh, B.; Krishnan, S.; et al. Technology for Innovation in Radiation Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 485–492. [Google Scholar] [CrossRef]
- Yong, J.H.E.; Beca, J.; McGowan, T.; Bremner, K.E.; Warde, P.; Hoch, J.S. Cost-effectiveness of Intensity-modulated Radiotherapy in Prostate Cancer. Clin. Oncol. 2012, 24, 521–531. [Google Scholar] [CrossRef]
- Konski, A.; Speier, W.; Hanlon, A.; Beck, J.R.; Pollack, A. Is Proton Beam Therapy Cost Effective in the Treatment of Adenocarcinoma of the Prostate? J. Clin. Oncol. 2007, 25, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.R.; Tyree, G.; Guss, Z.D.; Murphy, J.D. Cost-Effectiveness of Proton Therapy in Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e403. [Google Scholar] [CrossRef]
- Yu, H.; Hevelone, N.D.; Lipsitz, S.R.; Kowalczyk, K.J.; Hu, J.C. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J. Urol. 2012, 187, 1392–1398. [Google Scholar] [CrossRef]
- Bolenz, C.; Gupta, A.; Hotze, T.; Ho, R.; Cadeddu, J.A.; Roehrborn, C.G.; Lotan, Y. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur. Urol. 2010, 57, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Childers, C.P.; Maggard-Gibbons, M. Estimation of the Acquisition and Operating Costs for Robotic Surgery. JAMA 2018, 320, 835. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, Z.; Qi, L.; Chen, M. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer: Perioperative, functional, and oncological outcomes: A Systematic review and meta-analysis. Medicine 2019, 98, e15770. [Google Scholar] [CrossRef] [PubMed]
- Crew, B. Worth the cost? A closer look at the da Vinci robot’s impact on prostate cancer surgery. Nature 2020, 580, S5–S7. [Google Scholar] [CrossRef]
- OECD. Health at a Glance—OECD Indicators; OECD Publishing: Paris, France, 2005. [Google Scholar]
- National Center for Health Statistics. Health, UnitedStates, 2015: With Special Feature on Racial and Ethnic Health Disparities; National Center for Health Statistics: Hyattsville, MD, USA, 2016. [Google Scholar]
- Ledford, H. Immunotherapy’s cancer remit widens. Nature 2013, 497, 544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banegas, M.P.; Guy, G.P.; de Moor, J.S.; Ekwueme, D.U.; Virgo, K.S.; Kent, E.E.; Nutt, S.; Zheng, Z.; Rechis, R.; Yabroff, K.R. For Working-Age Cancer Survivors, Medical Debt and Bankruptcy Create Financial Hardships. Health Aff. 2016, 35, 54–61. [Google Scholar] [CrossRef]
- Park, J.; Look, K.A. Relationship Between Objective Financial Burden and the Health-Related Quality of Life and Mental Health of Patients with Cancer. J. Oncol. Pract. 2018, 14, e113–e121. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.Y. Financial Toxicity of Cancer Care: It’s Time to Intervene. J. Natl. Cancer Inst. 2015, 108, djv370. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Chino, F.; Ubel, P.A.; Rushing, C.; Samsa, G.; Altomare, I.; Nicolla, J.; Schrag, D.; Tulsky, J.A.; Abernethy, A.P.; et al. The utility of cost discussions between patients with cancer and oncologists. Am. J. Manag. Care 2015, 21, 607–615. [Google Scholar]
- Adler, N.E.; Page, A.E.K. (Eds.) Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs; National Academies Press: Washington, DC, USA, 2008; ISBN 978-0-309-11107-2. [Google Scholar]
- Tirodkar, M.A.; Roth, L.; Fuld Nasso, S.; Friedberg, M.W.; Scholle, S.H. Facilitators and Barriers to Implementing a Patient-Centered Oncology Care Model. JCO Oncol. Pract. 2020, 16, e1441–e1450. [Google Scholar] [CrossRef]
- Liang, H.; Tao, L.; Ford, E.W.; Beydoun, M.A.; Eid, S.M. The patient-centered oncology care on health care utilization and cost: A systematic review and meta-analysis. Health Care Manag. Rev. 2020, 45, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.M.; Tirodkar, M.; Patel, T.; Friedberg, M.; Smith-McLallen, A.; Scholle, S.H. Patient-centered oncology care: Impact on utilization, patient experiences, and quality. Am. J. Manag. Care 2020, 26, 372–380. [Google Scholar] [PubMed]
- Neumann, P.J.; Palmer, J.A.; Nadler, E.; Fang, C.; Ubel, P. Cancer Therapy Costs Influence Treatment: A National Survey of Oncologists. Health Aff. 2010, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A.; Levit, L.A. Charting a new course for the delivery of high-quality cancer care. J. Clin. Oncol. 2013, 31, 4485–4487. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Robin Yabroff, K.; Shao, Y.; Feuer, E.J.; Brown, M.L. Projections of the Cost of Cancer Care in the United States: 2010-2020. JNCI J. Natl. Cancer Inst. 2011, 103, 117–128. [Google Scholar] [CrossRef]
- Johnson, J.R.; Ning, Y.-M.; Farrell, A.; Justice, R.; Keegan, P.; Pazdur, R. Accelerated Approval of Oncology Products: The Food and Drug Administration Experience. JNCI J. Natl. Cancer Inst. 2011, 103, 636–644. [Google Scholar] [CrossRef]
- Kurzrock, R.; Kantarjian, H.M.; Kesselheim, A.S.; Sigal, E.V. New drug approvals in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 140–146. [Google Scholar] [CrossRef]
- Ladanie, A.; Schmitt, A.M.; Speich, B.; Naudet, F.; Agarwal, A.; Pereira, T.V.; Sclafani, F.; Herbrand, A.K.; Briel, M.; Martin-Liberal, J.; et al. Clinical Trial Evidence Supporting US Food and Drug Administration Approval of Novel Cancer Therapies Between 2000 and 2016. JAMA Netw. Open 2020, 3, e2024406. [Google Scholar] [CrossRef]
- Chen, E.Y.; Raghunathan, V.; Prasad, V. An Overview of Cancer Drugs Approved by the US Food and Drug Administration Based on the Surrogate End Point of Response Rate. JAMA Intern. Med. 2019, 179, 915. [Google Scholar] [CrossRef]
- Gill, J.; Prasad, V. A reality check of the accelerated approval of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Dayal, A.; Kircher, S.M.; Chen, R.C.; Royce, T.J. Analysis of Price Transparency via National Cancer Institute–Designated Cancer Centers’ Chargemasters for Prostate Cancer Radiation Therapy. JAMA Oncol. 2020, 6, 409. [Google Scholar] [CrossRef]
- Paravati, A.J.; Boero, I.J.; Triplett, D.P.; Hwang, L.; Matsuno, R.K.; Xu, B.; Mell, L.K.; Murphy, J.D. Variation in the Cost of Radiation Therapy Among Medicare Patients with Cancer. J. Oncol. Pract. 2015, 11, 403–409. [Google Scholar] [CrossRef]
- Marta, G.N.; Ramiah, D.; Kaidar-Person, O.; Kirby, A.; Coles, C.; Jagsi, R.; Hijal, T.; Sancho, G.; Zissiadis, Y.; Pignol, J.-P.; et al. The Financial Impact on Reimbursement of Moderately Hypofractionated Postoperative Radiation Therapy for Breast Cancer: An International Consortium Report. Clin. Oncol. 2021, 33, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Mazzitello, C.; Esposito, S.; De Francesco, A.; Capuano, A.; Russo, E.; De Sarro, G. Pharmacovigilance in Italy: An overview. J. Pharm. Pharm. 2013, 4, 20. [Google Scholar]
- Davis, C.; Naci, H.; Gurpinar, E.; Poplavska, E.; Pinto, A.; Aggarwal, A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: Retrospective cohort study of drug approvals 2009-13. BMJ 2017, 359, j4530. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA) Website. Available online: https://www.ema.europa.eu/en/partners-networks/health-technology-assessment-bodies (accessed on 5 May 2021).
- Tafuri, G.; Pagnini, M.; Moseley, J.; Massari, M.; Petavy, F.; Behring, A.; Catalan, A.; Gajraj, E.; Hedberg, N.; Obach, M.; et al. How aligned are the perspectives of EU regulators and HTA bodies? A comparative analysis of regulatory-HTA parallel scientific advice. Br. J. Clin. Pharmacol. 2016, 82, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Dominguez, P.; Naci, H.; Osipenko, L.; Mossialos, E. NICE’s evaluations of medicines authorized by EMA with conditional marketing authorization or under exceptional circumstances. Int. J. Technol. Assess. Health Care 2020, 36, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Lipska, I.; Hoekman, J.; McAuslane, N.; Leufkens, H.; Hövels, A. Does conditional approval for new oncology drugs in Europe lead to differences in health technology assessment decisions? Clin. Pharmacol. Ther. 2015, 98, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Herold, R.; Camarero, J.; Melchiorri, D.; Sebris, Z.; Enzmann, H.; Pignatti, F. Revocation of the conditional marketing authorisation of a cancer medicine: The olaratumab experience. Eur. J. Cancer 2019, 123, 25–27. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Grau, C. Health Economics in Radiation Oncology: Introducing the ESTRO HERO project. Radiother. Oncol. 2012, 103, 109–112. [Google Scholar] [CrossRef]
- Lievens, Y.; Defourny, N.; Corral, J.; Gasparotto, C.; Grau, C.; Borras, J.M.; Kozma, E.; Sedlmayer, F.; Slobina, E.; Daisne, J.-F.; et al. How public health services pay for radiotherapy in Europe: An ESTRO–HERO analysis of reimbursement. Lancet Oncol. 2020, 21, e42–e54. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, F.; Vera, A.; Kim, E.; Golinelli, D.; Vila-Reyes, H.; Bteich, F.; Schernberg, A.; Seban, R.-D.; Yeh, R.; Dercle, L. The Role of Cost-Effectiveness Analysis in Patient-Centered Cancer Care in the Era of Precision Medicine. Cancers 2021, 13, 4272. https://doi.org/10.3390/cancers13174272
Toscano F, Vera A, Kim E, Golinelli D, Vila-Reyes H, Bteich F, Schernberg A, Seban R-D, Yeh R, Dercle L. The Role of Cost-Effectiveness Analysis in Patient-Centered Cancer Care in the Era of Precision Medicine. Cancers. 2021; 13(17):4272. https://doi.org/10.3390/cancers13174272
Chicago/Turabian StyleToscano, Fabrizio, Alberto Vera, Eleanor Kim, Davide Golinelli, Helena Vila-Reyes, Fernand Bteich, Antoine Schernberg, Romain-David Seban, Randy Yeh, and Laurent Dercle. 2021. "The Role of Cost-Effectiveness Analysis in Patient-Centered Cancer Care in the Era of Precision Medicine" Cancers 13, no. 17: 4272. https://doi.org/10.3390/cancers13174272
APA StyleToscano, F., Vera, A., Kim, E., Golinelli, D., Vila-Reyes, H., Bteich, F., Schernberg, A., Seban, R.-D., Yeh, R., & Dercle, L. (2021). The Role of Cost-Effectiveness Analysis in Patient-Centered Cancer Care in the Era of Precision Medicine. Cancers, 13(17), 4272. https://doi.org/10.3390/cancers13174272





