The ‘Yin and Yang’ of Cancer Cell Growth and Mechanosensing
Simple Summary
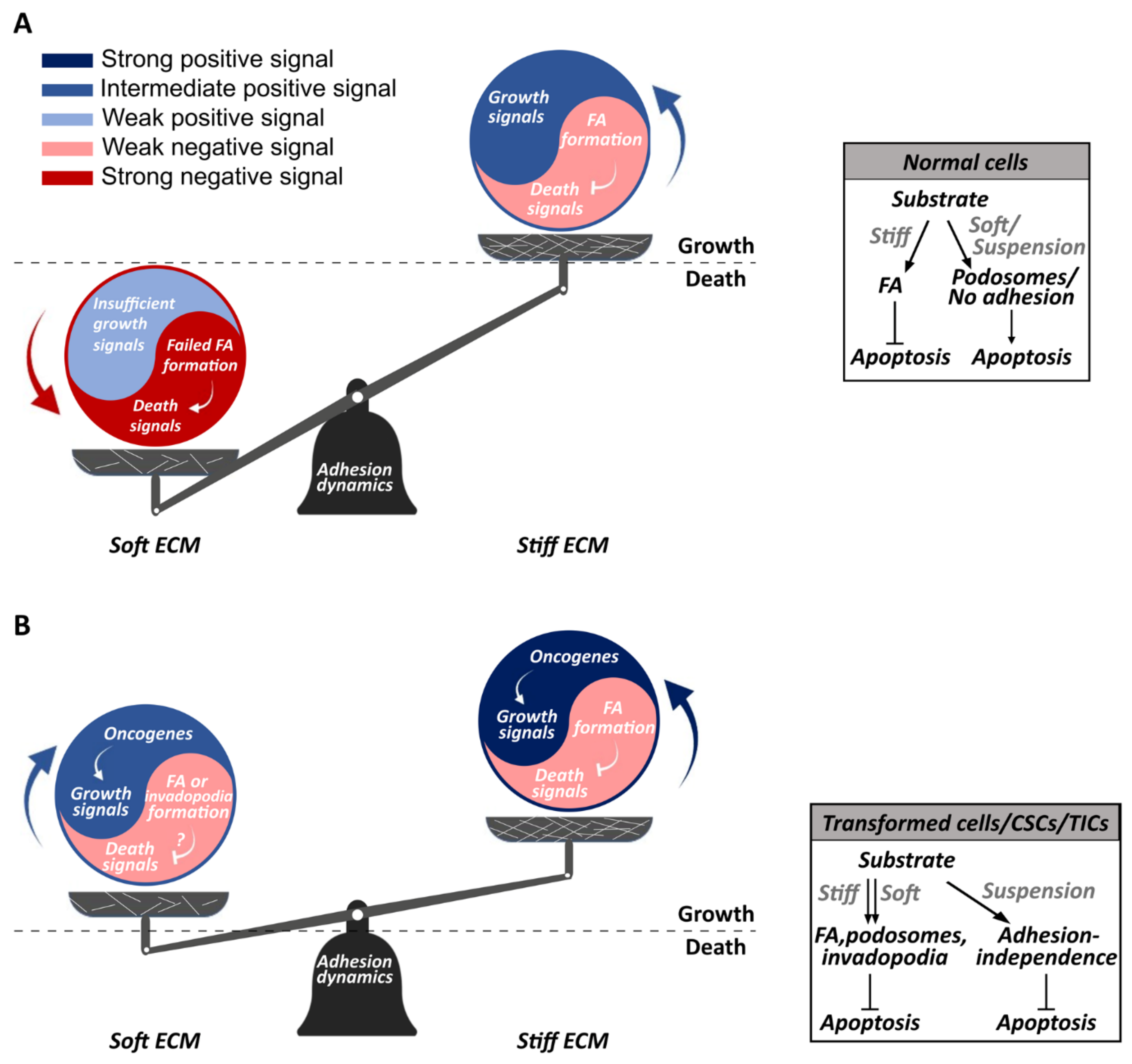
Abstract
1. Introduction
2. The ECM Provides the Biomechanical Context of Tissues and Cells
3. The Mechanical Tumor Microenvironment Affects Tumor Growth
4. ECM Mechanosensing Is a Multi-Step Process
5. Mechanotransduction at Focal Adhesions
5.1. The FAK-Src Complex
5.2. Rho Family GTPases
6. Anchorage-Independence and Mechanosensing Aberrations Characterize Metastatic Cells
7. Which Mechanobiological Processes Underlie Anchorage-Independent Cancer Cell Growth?
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Liu, H.; Seano, G.; Datta, M.; Jones, D.; Rahbari, N.; Incio, J.; Chauhan, V.P.; Jung, K.; Martin, J.D.; et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 2016, 1, 0004. [Google Scholar] [CrossRef]
- Kawano, S.; Kojima, M.; Higuchi, Y.; Sugimoto, M.; Ikeda, K.; Sakuyama, N.; Takahashi, S.; Hayashi, R.; Ochiai, A.; Saito, N. Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance. Cancer Sci. 2015, 106, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, R.; Tateishi, R.; Yoshida, H.; Sato, T.; Ohki, T.; Goto, T.; Yoshida, H.; Sato, S.; Sugioka, Y.; Ikeda, H.; et al. Assessing liver tumor stiffness by transient elastography. Hepatol. Int. 2007, 1, 394. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Leight, J.L.; Wozniak, M.A.; Chen, S.; Lynch, M.L.; Chen, C.S. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol. Biol. Cell 2012, 23, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef]
- Lim, C.T.; Bershadsky, A.; Sheetz, M.P. Mechanobiology. J. R. Soc. Interface 2010, 7, S291–S293. [Google Scholar] [CrossRef] [PubMed]
- Erler, J.T.; Weaver, V.M. Three-dimensional context regulation of metastasis. Clin. Exp. Metastasis 2009, 26, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, 23, S20. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P. Diversity of function is inherent in matricellular proteins: An appraisal of thrombospondin 1. J. Cell Biol. 1995, 130, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Oskarsson, T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013, 22, S66–S72. [Google Scholar] [CrossRef] [PubMed]
- Roycik, M.; Fang, X.; Sang, Q.-X. A Fresh Prospect of Extracellular Matrix Hydrolytic Enzymes and Their Substrates. Curr. Pharm. Des. 2009, 15, 1295–1308. [Google Scholar] [CrossRef]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef]
- Wolf, K.; Wu, Y.I.; Liu, Y.; Geiger, J.; Tam, E.; Overall, C.; Sharon Stack, M.; Friedl, P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007, 9, 893–904. [Google Scholar] [CrossRef]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Lochter, A.; Sympson, C.J.; Huey, B.; Rougier, J.-P.; Gray, J.W.; Pinkel, D.; Bissell, M.J.; Werb, Z. The Stromal Proteinase MMP3/Stromelysin-1 Promotes Mammary Carcinogenesis. Cell 1999, 98, 137–146. [Google Scholar] [CrossRef]
- Erler, J.T.; Bennewith, K.L.; Nicolau, M.; Dornhöfer, N.; Kong, C.; Le, Q.T.; Chi, J.T.A.; Jeffrey, S.S.; Giaccia, A.J. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006, 440, 1222–1226. [Google Scholar] [CrossRef]
- Kirschmann, D.A.; Seftor, E.A.; Fong, S.F.T.; Nieva, D.R.C.; Sullivan, C.M.; Edwards, E.M.; Sommer, P.; Csiszar, K.; Hendrix, M.J.C. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002, 62, 4478–4483. [Google Scholar] [PubMed]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Acharyya, S.; Zhang, X.H.F.; Vanharanta, S.; Tavazoie, S.F.; Morris, P.G.; Downey, R.J.; Manova-Todorova, K.; Brogi, E.; Massagué, J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011, 17, 867–874. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Wolfe, J.N. Breast patterns as an index of risk for developing breast cancer. Am. J. Roentgenol. 1976, 126, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.E.; Kay, E.J.; Neilson, L.J.; Henze, A.; Serneels, J.; McGhee, E.J.; Dhayade, S.; Nixon, C.; Mackey, J.B.; Santi, A.; et al. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017, 36, 2373–2389. [Google Scholar] [CrossRef]
- Wei, S.C.; Yang, J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial-Mesenchymal Transition. Trends Cell Biol. 2016, 26, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- DuChez, B.J.; Doyle, A.D.; Dimitriadis, E.K.; Yamada, K.M. Durotaxis by Human Cancer Cells. Biophys. J. 2019, 116, 670–683. [Google Scholar] [CrossRef]
- Neesse, A.; Algül, H.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef]
- Wang, H.-B.; Dembo, M.; Wang, Y.-L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 2000, 279, 1345–1350. [Google Scholar] [CrossRef]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef]
- Ulrich, T.A.; de Juan Pardo, E.M.; Kumar, S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Nocelo, M.; Raimondo, T.M.; Vining, K.H.; López-López, R.; de la Fuente, M.; Mooney, D.J. Matrix Stiffness and Tumor-Associated Macrophages Modulate Epithelial to Mesenchymal Transition of Human Adenocarcinoma Cells. Biofabrication 2018, 10, 035004. [Google Scholar] [CrossRef]
- Trichet, L.; Le Digabel, J.; Hawkins, R.J.; Vedula, S.R.K.; Gupta, M.; Ribrault, C.; Hersen, P.; Voituriez, R.; Ladoux, B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl. Acad. Sci. USA 2012, 109, 6933–6938. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, J.; Yang, S.; Wang, H.; Wu, C.J.; Sugimoto, H.; LeBleu, V.S.; Kalluri, R. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 2021, 39, 548–565.e6. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Tilghman, R.W.; Cowan, C.R.; Mih, J.D.; Koryakina, Y.; Gioeli, D.; Slack-Davis, J.K.; Blackman, B.R.; Tschumperlin, D.J.; Parsons, J.T. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS ONE 2010, 5, e12905. [Google Scholar] [CrossRef] [PubMed]
- Dubash, A.D.; Menold, M.M.; Samson, T.; Boulter, E.; García-Mata, R.; Doughman, R.; Burridge, K. Chapter 1 Focal Adhesions: New Angles on an Old Structure. Int. Rev. Cell Mol. Biol. 2009, 277, 1–65. [Google Scholar] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Critchley, D.R.; Critchley, D.R. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem. Soc. Trans. 2004, 32, 831–836. [Google Scholar] [CrossRef]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef]
- Seong, J.; Wang, N.; Wang, Y. Mechanotransduction at focal adhesions: From physiology to cancer development. J. Cell. Mol. Med. 2013, 17, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Law, J.B.; Suryana, M.; Low, H.Y.; Sheetz, M.P. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc. Natl. Acad. Sci. USA 2011, 108, 20585–20590. [Google Scholar] [CrossRef]
- Shutova, M.; Yang, C.; Vasiliev, J.M.; Svitkina, T. Functions of nonmuscle myosin II in assembly of the cellular contractile system. PLoS ONE 2012, 7, e40814. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, H.; Meacci, G.; Liu, S.; Stachowiak, M.R.M.R.; Iskratsch, T.; Ghassemi, S.; Roca-Cusachs, P.; O’Shaughnessy, B.; Hone, J.; Sheetz, M.P.M.P. Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat. Cell Biol. 2016, 18, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Guo, W.-H.; Wang, Y.-L. Fibroblasts probe substrate rigidity with filopodia extensions before occupying an area. Proc. Natl. Acad. Sci. USA 2014, 111, 17176–17181. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, A.; Perez-Jimenez, R.; Liu, R.; Roca-Cusachs, P.; Fernandez, J.M.; Sheetz, M.P. Stretching single talin rod molecules activates vinculin binding. Science 2009, 323, 638–641. [Google Scholar] [CrossRef]
- Meacci, G.; Wolfenson, H.; Liu, S.; Stachowiak, M.R.M.R.; Iskratsch, T.; Mathur, A.; Ghassemi, S.; Gauthier, N.; Tabdanov, E.; Lohner, J.; et al. Alpha-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol. Biol. Cell 2016, 27, 3471–3479. [Google Scholar] [CrossRef]
- Roca-Cusachs, P.; del Rio, A.; Puklin-Faucher, E.; Gauthier, N.C.; Biais, N.; Sheetz, M.P. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc. Natl. Acad. Sci. USA 2013, 110, E1361–E1370. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Perez-Gonzalez, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef]
- Chan, C.E.; Odde, D.J. Traction dynamics of filopodia on compliant substrates. Science 2008, 322, 1687–1691. [Google Scholar] [CrossRef]
- Tan, S.J.; Chang, A.C.; Anderson, S.M.; Miller, C.M.; Prahl, L.S.; Odde, D.J.; Dunn, A.R. Regulation and dynamics of force transmission at individual cell-matrix adhesion bonds. Sci. Adv. 2020, 6, eaax0317. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Ahn, S.J.; Huang, B.; Kumar, A.; Schwartz, M.A. Actin flow-dependent and -independent force transmission through integrins. Proc. Natl. Acad. Sci. USA 2020, 117, 202010292. [Google Scholar] [CrossRef] [PubMed]
- Feld, L.; Kellerman, L.; Mukherjee, A.; Livne, A.; Bouchbinder, E.; Wolfenson, H. Cellular contractile forces are nonmechanosensitive. bioRxiv 2020, 6, 733303. [Google Scholar]
- Prager-Khoutorsky, M.; Lichtenstein, A.; Krishnan, R.; Rajendran, K.; Mayo, A.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011, 13, 1457–1465. [Google Scholar] [CrossRef]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate Compliance versus Ligand Density in Cell on Gel Responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef]
- Kim, D.H.; Khatau, S.B.; Feng, Y.; Walcott, S.; Sun, S.X.; Longmore, G.D.; Wirtz, D. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci. Rep. 2012, 2, 555. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.A.; Assoian, R.K. Integrins and cell proliferation. J. Cell Sci. 2001, 114, 2553–2560. [Google Scholar] [CrossRef]
- Shiu, J.Y.; Aires, L.; Lin, Z.; Vogel, V. Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nat. Cell Biol. 2018, 20, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wolfenson, H.; Chung, V.Y.; Nakazawa, N.; Liu, S.; Hu, J.; Huang, R.Y.-J.; Sheetz, M.P. Stopping transformed cancer cell growth by rigidity sensing. Nat. Mater. 2019, 19, 239–250. [Google Scholar] [CrossRef]
- Kim, D.H.; Chambliss, A.B.; Wirtz, D. The multi-faceted role of the actin cap in cellular mechanosensation and mechanotransduction. Soft Matter 2013, 9, 5516–5523. [Google Scholar] [CrossRef] [PubMed]
- Herzog, F.A.; Braun, L.; Schoen, I.; Vogel, V. Structural Insights How PIP2 Imposes Preferred Binding Orientations of FAK at Lipid Membranes. J. Phys. Chem. B 2017, 121, 3523–3535. [Google Scholar] [CrossRef] [PubMed]
- Goñi, G.M.; Epifano, C.; Boskovic, J.; Camacho-Artacho, M.; Zhou, J.; Bronowska, A.; Martín, M.T.; Eck, M.J.; Kremer, L.; Gräter, F.; et al. Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes. Proc. Natl. Acad. Sci. USA 2014, 111, E3177–E3186. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Mui, K.L.; Hsu, B.Y.; Liu, S.L.; Cretu, A.; Razinia, Z.; Xu, T.; Puré, E.; Assoian, R.K.; Pure, E.; et al. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci. Signal 2014, 7, ra57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Westhoff, M.A.; Serrels, B.; Fincham, V.J.; Frame, M.C.; Carragher, N.O. Src-Mediated Phosphorylation of Focal Adhesion Kinase Couples Actin and Adhesion Dynamics to Survival Signaling. Mol. Cell. Biol. 2004, 24, 8113–8133. [Google Scholar] [CrossRef]
- Bolós1, V.; Gasent2, J.M.; López-Tarruella3, S.; Grande, E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer R. Ther. Ther. 2010, 3, 83–97. [Google Scholar] [CrossRef]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef]
- Verbeek, B.S.; Vroom, T.M.; Adriaansen-Slot, S.S.; Ottenhoff-Kalff, A.E.; Geertzema, J.G.; Hennipman, A.; Rijksen, G. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J. Pathol. 1996, 180, 383–388. [Google Scholar] [CrossRef]
- Fizazi, K. The role of Src in prostate cancer. Ann. Oncol. 2007, 18, 1765–1773. [Google Scholar] [CrossRef]
- Lutz, M.P.; Eßer, I.B.S.; Flossmann-Kast, B.B.M.; Vogelmann, R.; Lührs, H.; Friess, H.; Büchler, M.W.; Adler, G. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem. Biophys. Res. Commun. 1998, 243, 503–508. [Google Scholar] [CrossRef]
- Chen, J.; Elfiky, A.; Han, M.; Chen, C.; Saif, M.W. The role of Src in colon cancer and its therapeutic implications. Clin. Colorectal Cancer 2014, 13, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kanteti, R.; Batra, S.K.; Lennon, F.E.; Salgia, R. FAK and paxillin, two potential targets in pancreatic cancer. Oncotarget 2016, 7, 31586–31601. [Google Scholar] [CrossRef]
- Hyder, C.L.; Lazaro, G.; Pylvänäinen, J.W.; Roberts, M.W.G.; Qvarnström, S.M.; Eriksson, J.E. Nestin regulates prostate cancer cell invasion by influencing the localisation and functions of FAK and integrins. J. Cell Sci. 2014, 127, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Nana, F.A.; Hoton, D.; Ambroise, J.; Lecocq, M.; Vanderputten, M.; Sibille, Y.; Vanaudenaerde, B.; Pilette, C.; Bouzin, C.; Ocak, S. Increased Expression and Activation of FAK in Small-Cell Lung Cancer Compared to Non-Small-Cell Lung Cancer. Cancers 2019, 11, 1526. [Google Scholar]
- Weiner, T.M.; Liu, E.T.; Craven, R.; Cance, W.G. Expression of focal adhesion kinase gene and invasive cancer. Lancet 1993, 342, 1024–1025. [Google Scholar] [CrossRef]
- Owens, L.V.; Xu, L.; Craven, R.J.; Dent, G.A.; Weiner, T.M.; Kornberg, L.; Liu, E.T.; Cance, W.G. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995, 55, 2752–2755. [Google Scholar]
- Masaki, T.; Igarashi, K.; Tokuda, M.; Yukimasa, S.; Han, F.; Jin, Y.J.; Li, J.Q.; Yoneyama, H.; Uchida, N.; Fujita, J.; et al. pp60c-src activation in lung adenocarcinoma. Eur. J. Cancer 2003, 39, 1447–1455. [Google Scholar] [CrossRef]
- Hauck, C.R.; Hsia, D.A.; Ilic, D.; Schlaepfer, D.D. v-Src SH3-enhanced interaction with focal adhesion kinase at β1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 2002, 277, 12487–12490. [Google Scholar] [CrossRef] [PubMed]
- Gatesman Ammer, A.; Kelley, L.C.; Hayes, K.E.; Evans, J.V.; Lopez-Skinner, L.A.; Martin, K.H.; Frederick, B.; Rothschild, B.L.; Raben, D.; Elvin, P.; et al. Saracatinib Impairs Head and Neck Squamous Cell Carcinoma Invasion by Disrupting Invadopodia Function. J. Cancer Sci. Ther. 2009, 01, 052–061. [Google Scholar] [CrossRef]
- Weaver, A.M. Invadopodia: Specialized cell structures for cancer invasion. Clin. Exp. Metastasis 2006, 23, 97–105. [Google Scholar] [CrossRef]
- Kim, L.C.; Song, L.; Haura, E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009, 6, 587–595. [Google Scholar] [CrossRef]
- Fu, Q.F.; Liu, Y.; Fan, Y.; Hua, S.N.; Qu, H.Y.; Dong, S.W.; Li, R.L.; Zhao, M.Y.; Zhen, Y.; Yu, X.L.; et al. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J. Hematol. Oncol. 2015, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Y.; Gao, H.; Xu, Y.; Jing, P.; Wu, J.; Zhang, X.; Xiong, J.; Dong, C.; Yao, L.; et al. Activation of the aryl hydrocarbon receptor leads to resistance to EGFR TKIs in non–small cell lung cancer by activating src-mediated bypass signaling. Clin. Cancer Res. 2018, 24, 1227–1239. [Google Scholar] [CrossRef]
- Ochi, N.; Takigawa, N.; Harada, D.; Yasugi, M.; Ichihara, E.; Hotta, K.; Tabata, M.; Tanimoto, M.; Kiura, K. Src mediates ERK reactivation in gefitinib resistance in non-small cell lung cancer. Exp. Cell Res. 2014, 322, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, S.; Zhang, W.Q.; Yang, Y.L.; Hang, P.; Wang, H.; Cheng, L.; Hsu, P.C.; Wang, Y.C.; Xu, Z.; et al. YAP1 regulates ABCG2 and cancer cell side population in human lung cancer cells. Oncotarget 2017, 8, 4096–4109. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Luo, Q.Q.; Xu, Y.H.; Tang, N.W.; Niu, X.M.; Li, Z.M.; Shen, S.P.; Lu, S.; Chen, Z.W. 17-AAG suppresses growth and invasion of lung adenocarcinoma cells via regulation of the LATS1/YAP pathway. J. Cell. Mol. Med. 2015, 19, 651–663. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Q.; Zhang, Q.; Li, Z.; Wang, E.; Qiu, X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010, 101, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Yang, C.-T.; Jablons, D.M.; You, L. The Crosstalk between Src and Hippo/YAP Signaling Pathways in Non-Small Cell Lung Cancer (NSCLC). Cancers 2020, 12, 1361. [Google Scholar] [CrossRef]
- Thamilselvan, V.; Craig, D.H.; Basson, M.D. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007, 21, 1730–1741. [Google Scholar] [CrossRef]
- Jin, W. Regulation of Src Family Kinases during Colorectal Cancer Development and Its Clinical Implications. Cancers 2020, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.S.; Wu, M.S.; Bien, M.Y.; Chen, C.H.; Lin, C.H.; Chen, B.C.; GS, L.; MS, W.; MY, B.; CH, C.; et al. Epidermal growth factor stimulates nuclear factor-κB activation and heme oxygenase-1 expression via c-Src, NADPH oxidase, PI3K, and Akt in human colon cancer cells. PLoS ONE 2014, 9, e104891. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.; Soreghan, B.; Szabo, I.L.; Pavelka, M.; Baatar, D.; Tarnawski, A.S. Prostaglandin E2 transactivates EGF receptor: A novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 2002, 8, 289–293. [Google Scholar] [CrossRef]
- Darmoul, D.; Gratio, V.; Devaud, H.; Laburthe, M. Protease-activated receptor 2 in colon cancer: Trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J. Biol. Chem. 2004, 279, 20927–20934. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Bowman, T.L.; Niu, G.; Yu, H.; Minton, S.; Muro-Cacho, C.A.; Cox, C.E.; Falcone, R.; Fairclough, R.; Parsons, S.; et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 2001, 20, 2499–2513. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, G.; Smith, C.; Goddard, L.; Jordan, N.; McClelland, R.; Barrett-Lee, P.; Nicholson, R.I.; Hiscox, S. Targeting focal adhesion kinase in ER+/HER2+ breast cancer improves trastuzumab response. Endocr.-Relat. Cancer 2013, 20, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Zhao, C.; Zhai, L. Binding of MMP-9-degraded fibronectin to β6 integrin promotes invasion via the FAK-Src-related Erk1/2 and PI3K/Akt/Smad-1/5/8 pathways in breast cancer. Oncol. Rep. 2015, 34, 1345–1352. [Google Scholar] [CrossRef]
- Lamar, J.M.; Xiao, Y.; Norton, E.; Jiang, Z.G.; Gerhard, G.M.; Kooner, S.; Warren, J.S.A.; Hynes, R.O. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J. Biol. Chem. 2019, 294, 2302–2317. [Google Scholar] [CrossRef]
- Si, Y.; Ji, X.; Cao, X.; Dai, X.; Xu, L.; Zhao, H.; Guo, X.; Yan, H.; Zhang, H.; Zhu, C.; et al. Src Inhibits the Hippo Tumor Suppressor Pathway through Tyrosine Phosphorylation of Lats1. Cancer Res. 2017, 77, 4868–4880. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Y.; Zhang, X.-X.; Yang, M.-W.; Hu, L.-P.; Jiang, S.-H.; Tian, G.-A.; Zhu, L.-L.; Li, Q.; Sun, Y.-W.; Zhang, Z.-G. Aberrant upregulation of KLK10 promotes metastasis via enhancement of EMT and FAK/SRC/ERK axis in PDAC. Biochem. Biophys. Res. Commun. 2018, 499, 584–593. [Google Scholar] [CrossRef]
- Freeman, J.W.; DeArmond, D.; Lake, M.; Huang, W.; Venkatasubbarao, K.; Zhao, S. Alterations of cell signaling pathways in pancreatic cancer. Front. Biosci. 2004, 9, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- An, E.-J.; Kim, Y.; Lee, S.-H.; Ko, H.M.; Chung, W.-S.; Jang, H.-J. Anti-Cancer Potential of Oxialis obtriangulata in Pancreatic Cancer Cell through Regulation of the ERK/Src/STAT3-Mediated Pathway. Molecules 2020, 25, 2301. [Google Scholar] [CrossRef] [PubMed]
- Bartscht, T.; Rosien, B.; Rades, D.; Kaufmann, R.; Biersack, H.; Lehnerta, H.; Ungefroren, H. Inhibition of TGF-β Signaling in Tumor Cells by Small Molecule Src Family Kinase Inhibitors. Anti-Cancer Agents Med. Former. Curr. Med. Chem. Anti-Cancer Agents 2017, 17, 1351–1356. [Google Scholar] [CrossRef]
- Dosch, A.R.; Dai, X.; Reyzer, M.L.; Mehra, S.; Srinivasan, S.; Willobee, B.A.; Kwon, D.; Kashikar, N.; Caprioli, R.; Merchant, N.B.; et al. Combined Src/EGFR Inhibition Targets STAT3 Signaling and Induces Stromal Remodeling to Improve Survival in Pancreatic Cancer. Mol. Cancer Res. 2020, 18, 623–631. [Google Scholar] [CrossRef]
- Cooper, C.R.; Chay, C.H.; Pienta, K.J. The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia 2002, 4, 191–194. [Google Scholar] [CrossRef]
- Vlaeminck-Guillem, V.; Gillet, G.; Rimokh, R. SRC: Marker or actor in prostate cancer aggressiveness. Front. Oncol. 2014, 4, 222. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Daaka, Y.; Lefkowitz, R.J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 1999, 11, 177–183. [Google Scholar] [CrossRef]
- Roediger, J.; Hessenkemper, W.; Bartsch, S.; Manvelyan, M.; Huettner, S.S.; Liehr, T.; Esmaeili, M.; Foller, S.; Petersen, I.; Grimm, M.O.; et al. Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol. Cancer 2014, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Babic, I.; Wei, X.; Huang, J.; Witte, O.N. Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res. 2011, 71, 862–872. [Google Scholar] [CrossRef]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Burridge, K.; Wennerberg, K. Rho and Rac Take Center Stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Svensmark, J.H.; Brakebusch, C. Rho GTPases in cancer: Friend or foe? Oncogene 2019, 38, 7447–7456. [Google Scholar] [CrossRef]
- Pillé, J.Y.; Denoyelle, C.; Varet, J.; Bertrand, J.R.; Soria, J.; Opolon, P.; Lu, H.; Pritchard, L.L.; Vannier, J.P.; Malvy, C.; et al. Anti-RhoA and Anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol. Ther. 2005, 11, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, Z.F.; Rosenthal, D.T.; Rhee, E.M.; Merajver, S.D. Characterization of the roles of RHOC and RHOA GTPases in invasion, motility, and matrix adhesion in inflammatory and aggressive breast cancers. Cancer 2010, 116, 2768–2782. [Google Scholar] [CrossRef]
- Kalpana, G.; Figy, C.; Yeung, M.; Yeung, K.C. Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci. Rep. 2019, 9, 16351. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.; Wang, Z.; Li, Y.; Jhan, J.R.; Jiang, Y.; Yang, C. ARHGAP18 downregulation by miR-200b suppresses metastasis of triple-negative breast cancer by enhancing activation of RhoA. Cancer Res. 2017, 77, 4051–4064. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.; Wang, Z.; Yang, C. Rho GTPases: Big Players in Breast Cancer Initiation, Metastasis and Therapeutic Responses. Cells 2020, 9, 2167. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, K.; Rasheed, Z.A.; Karisch, R.; Wang, Q.; Kowalski, J.; Susky, E.; Pereira, K.; Karamboulas, C.; Moghal, N.; Rajeshkumar, N.V.; et al. Tumor-Initiating Cells Are Rare in Many Human Tumors. Cell Stem Cell 2010, 7, 279–282. [Google Scholar] [CrossRef]
- Odoux, C.; Fohrer, H.; Hoppo, T.; Guzik, L.; Stolz, D.B.; Lewis, D.W.; Gollin, S.M.; Gamblin, T.C.; Geller, D.A.; Lagasse, E. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008, 68, 6932–6941. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Huang, Y.; Pei, Q.; Liu, H.; Pei, H.; Zhu, H. Matrix stiffness mediates stemness characteristics via activating the Yes-associated protein in colorectal cancer cells. J. Cell. Biochem. 2018, 120, 2213–2225. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Luo, Q.; Ju, Y.; Song, G. A Soft Matrix Enhances the Cancer Stem Cell Phenotype of HCC Cells. Int. J. Mol. Sci. 2019, 20, 2831. [Google Scholar] [CrossRef]
- Tan, Y.; Tajik, A.; Chen, J.; Jia, Q.; Chowdhury, F.; Wang, L.; Chen, J.; Zhang, S.; Hong, Y.; Yi, H.; et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat. Commun. 2014, 5, 4619. [Google Scholar] [CrossRef]
- Burridge, K.; Guilluy, C. Focal adhesions, stress fibers and mechanical tension. Exp. Cell Res. 2016, 343, 14–20. [Google Scholar] [CrossRef]
- Panciera, T.; Citron, A.; Di Biagio, D.; Battilana, G.; Gandin, A.; Giulitti, S.; Forcato, M.; Bicciato, S.; Panzetta, V.; Fusco, S.; et al. Reprogramming normal cells into tumour precursors requires ECM stiffness and oncogene-mediated changes of cell mechanical properties. Nat. Mater. 2020, 19, 797–806. [Google Scholar] [CrossRef]
- Sanford, K.K.; Likely, G.D.; Earle, W.R. The development of variations in transplantability and morphology within a clone of mouse fibroblasts transformed to sarcoma-producing cells in vitro. J. Natl. Cancer Inst. 1954, 15, 215–237. [Google Scholar]
- Temin, H.M.; Rubin, H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology 1958, 6, 669–688. [Google Scholar] [CrossRef]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Poste, G.; Fidler, I.J. The pathogenesis of cancer metastasis. Nature 1980, 283, 139–146. [Google Scholar] [CrossRef]
- Fidler, I.J. Critical Factors in the Biology of Human Cancer Metastasis: Twenty-eighth G. H. A. Clowes Memorial Award Lecture. Cancer Res. 1990, 50, 6130–6138. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer Review evolve progressively from normalcy via a series of pre. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Taddei, M.L.; Giannoni, E.; Fiaschi, T.; Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2012, 226, 380–393. [Google Scholar] [CrossRef]
- Mori, S.; Chang, J.T.; Andrechek, E.R.; Matsumura, N.; Baba, T.; Yao, G.; Kim, J.W.; Gatza, M.; Murphy, S.; Nevins, J.R. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene 2009, 28, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Guadamillas, M.C.; Cerezo, A.; del Pozo, M.A. Overcoming anoikis—Pathways to anchorageindependent growth in cancer. J. Cell Sci. 2011, 124, 3189–3197. [Google Scholar] [CrossRef]
- Huang, S.; Ingber, D.E. Cell tension, matrix mechanics, and cancer development. Cancer Cell 2005, 8, 175–176. [Google Scholar] [CrossRef]
- Liang, S.; Slattery, M.J.; Wagner, D.; Simon, S.I.; Dong, C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann. Biomed. Eng. 2008, 36, 661–671. [Google Scholar] [CrossRef]
- Clark, E.A.; Golub, T.R.; Lander, E.S.; Hynes, R.O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000, 406, 532–535. [Google Scholar] [CrossRef]
- Gunning, P.W.; Hardeman, E.C.; Lappalainen, P.; Mulvihill, D.P. Tropomyosin—Master regulator of actin filament function in the cytoskeleton. J. Cell Sci. 2015, 128, 2965–2974. [Google Scholar] [CrossRef]
- Bryce, N.S.; Schevzov, G.; Ferguson, V.; Percival, J.M.; Lin, J.J.C.; Matsumura, F.; Bamburg, J.R.; Jeffrey, P.L.; Hardeman, E.C.; Gunning, P.; et al. Specification of actin filament function and molecular composition by tropomyosin isoforms. Mol. Biol. Cell 2003, 14, 1002–1016. [Google Scholar] [CrossRef]
- Bugyi, B.; Papp, G.; Hild, G.; Lõrinczy, D.; Nevalainen, E.M.; Lappalainen, P.; Somogyi, B.; Nyitrai, M. Formins regulate actin filament flexibility through long range allosteric interactions. J. Biol. Chem. 2006, 281, 10727–10736. [Google Scholar] [CrossRef]
- McMichael, B.K.; Lee, B.S. Tropomyosin 4 regulates adhesion structures and resorptive capacity in osteoclasts. Exp. Cell Res. 2008, 314, 564–573. [Google Scholar] [CrossRef]
- Novy, R.E.; Lin, J.L.; Lin, C.-S.; Lin, J.J. Human fibroblast tropomyosin isoforms: Characterization of cDNA clones and analysis of tropomyosin isoform expression in human tissues and in normal and transformed cells. Cell Motil. Cytoskelet. 1993, 25, 267–281. [Google Scholar] [CrossRef]
- Boyd, J.; Risinger, J.I.; Wiseman, R.W.; Merrick, B.A.; Selkirk, J.K.; Barrett, J.C. Regulation of microfilament organization and anchorage-independent growth by tropomyosin 1. Proc. Natl. Acad. Sci. USA 1995, 92, 11534–11538. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Thanawala, R.; Bon, G.; Falcioni, R.; Prasad, G.L. Resensitization of breast cancer cells to anoikis by tropomyosin-1: Role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene 2005, 24, 8291–8303. [Google Scholar] [CrossRef]
- Desouza-Armstrong, M.; Gunning, P.W.; Stehn, J.R. Tumor suppressor tropomyosin Tpm2.1 regulates sensitivity to apoptosis beyond anoikis characterized by changes in the levels of intrinsic apoptosis proteins. Cytoskeleton 2017, 74, 233–248. [Google Scholar] [CrossRef]
- Miyado, K.; Kimura, M.; Taniguchi, S. Decreased expression of a single tropomyosin isoform, TM5/TM30nm, results in reduction in motility of highly metastatic B16-F10 mouse melanoma cells. Biochem. Biophys. Res. Commun. 1996, 225, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Schevzov, G.; Kee, A.J.; Wang, B.; Sequeira, V.B.; Hook, J.; Coombes, J.D.; Lucas, C.A.; Stehn, J.R.; Musgrove, E.A.; Cretu, A.; et al. Regulation of cell proliferation by ERK and signal-dependent nuclear translocation of ERK is dependent on Tm5NM1-containing actin filaments. Mol. Biol. Cell 2015, 26, 2475–2490. [Google Scholar] [CrossRef]
- Stehn, J.R.; Schevzov, G.; O’Neill, G.M.; Gunning, P.W. Specialisation of the tropomyosin composition of actin filaments provides new potential targets for chemotherapy. Curr. Cancer Drug Targets 2006, 6, 245–256. [Google Scholar] [CrossRef]
- Alanko, J.; Mai, A.; Jacquemet, G.; Schauer, K.; Kaukonen, R.; Saari, M.; Goud, B.; Ivaska, J. Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 2015, 17, 1412–1421. [Google Scholar] [CrossRef]
- Kuo, J.-C.; Wang, W.-J.; Yao, C.-C.; Wu, P.-R.; Chen, R.-H. The tumor suppressor DAPK inhibits cell motility by blocking the integrin-mediated polarity pathway. J. Cell Biol. 2006, 172, 619–631. [Google Scholar] [CrossRef]
- Ivanovska, J.; Mahadevan, V.; Schneider-Stock, R. DAPK and cytoskeleton-associated functions. Apoptosis 2014, 19, 329–338. [Google Scholar] [CrossRef]
- Qin, R.; Wolfenson, H.; Saxena, M.; Sheetz, M. Tumor suppressor DAPK1 catalyzes adhesion assembly on rigid but anoikis on soft matrices. bioRxiv 2018, 320739. [Google Scholar]
- Michie, A.M.; McCaig, A.M.; Nakagawa, R.; Vukovic, M. Death-associated protein kinase (DAPK) and signal transduction: Regulation in cancer. FEBS J. 2010, 277, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Rafiq, N.B.; Krishnasamy, A.; Hartman, K.L.; Jones, G.E.; Bershadsky, A.D.; Sheetz, M.P. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep. 2013, 5, 1456–1468. [Google Scholar] [CrossRef]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol. 2018, 81, 585–605. [Google Scholar] [CrossRef]
- Rafiq, N.B.M.; Nishimura, Y.; Plotnikov, S.V.; Thiagarajan, V.; Zhang, Z.; Shi, S.; Natarajan, M.; Viasnoff, V.; Kanchanawong, P.; Jones, G.E.; et al. A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat. Mater. 2019, 18, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Samaržija, I.; Humphries, J.D.; Humphries, M.J.; Ambriović-Ristov, A. KANK family proteins in cancer. Int. J. Biochem. Cell Biol. 2021, 131, 105903. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Olson, M.F.; Marshall, C.J. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001, 20, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Marrocco, I.; Yarden, Y. Roles for receptor tyrosine kinases in tumor progression and implications for cancer treatment. Adv. Cancer Res. 2020, 147, 1–57. [Google Scholar] [PubMed]
- Maa, M.C.; Leu, T.H.; Mccarley, D.J.; Schatzman, R.C.; Parsons, S.J. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: Implications for the etiology of multiple human cancers. Proc. Natl. Acad. Sci. USA 1995, 92, 6981–6985. [Google Scholar] [CrossRef]
- Benlimame, N.; He, Q.; Jie, S.; Xiao, D.; Ying, J.X.; Loignon, M.; Schlaepfer, D.D.; Alaoui-Jamali, M.A. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J. Cell Biol. 2005, 171, 505–516. [Google Scholar] [CrossRef]
- Demers, M.J.; Thibodeau, S.; Noël, D.; Fujita, N.; Tsuruo, T.; Gauthier, R.; Arguin, M.; Vachon, P.H. Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J. Cell. Biochem. 2009, 107, 639–654. [Google Scholar] [CrossRef]
- Havel, L.S.; Kline, E.R.; Salgueiro, A.M.; Marcus, A.I. Vimentin regulates lung cancer cell adhesion through a VAV2-Rac1 pathway to control focal adhesion kinase activity. Oncogene 2015, 34, 1979–1990. [Google Scholar] [CrossRef]
- Shukla, V.C.; Higuita-Castro, N.; Nana-Sinkam, P.; Ghadiali, S.N. Substrate stiffness modulates lung cancer cell migration but not epithelial to mesenchymal transition. J. Biomed. Mater. Res. A 2016, 104, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, S.; Sun, H.; Sun, T.; Ji, F.; Wang, Y.; Xu, L.; Zhou, D. Keap1 Inhibits Metastatic Properties of NSCLC Cells by Stabilizing Architectures of F-Actin and Focal Adhesions. Mol. Cancer Res. 2018, 16, 508–516. [Google Scholar] [CrossRef]
- Anguiano, M.; Morales, X.; Castilla, C.; Pena, A.R.; Ederra, C.; Martínez, M.; Ariz, M.; Esparza, M.; Amaveda, H.; Mora, M.; et al. The use of mixed collagen-Matrigel matrices of increasing complexity recapitulates the biphasic role of cell adhesion in cancer cell migration: ECM sensing, remodeling and forces at the leading edge of cancer invasion. PLoS ONE 2020, 15, e0220019e0220019. [Google Scholar] [CrossRef]
- Al Haddad, M.; El-Rif, R.; Hanna, S.; Jaafar, L.; Dennaoui, R.; Abdellatef, S.; Miskolci, V.; Cox, D.; Hodgson, L.; El-Sibai, M. Differential regulation of rho GTPases during lung adenocarcinoma migration and invasion reveals a novel role of the tumor suppressor StarD13 in invadopodia regulation. Cell Commun. Signal. 2020, 18, 144. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Gong, H.; Yuan, Y.; Li, Y.; Wang, C.; Li, W.; Zhang, Z.; Liu, M.; Liu, H.; et al. miR-182 suppresses invadopodia formation and metastasis in non-small cell lung cancer by targeting cortactin gene. J. Exp. Clin. Cancer Res. 2018, 37, 141. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.R.; Branch, K.M.; Parekh, A.; Clark, E.S.; Iwueke, I.C.; Guelcher, S.A.; Weaver, A.M. Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol. 2008, 18, 1295–1299. [Google Scholar] [CrossRef]
- Parekh, A.; Ruppender, N.S.; Branch, K.M.; Sewell-Loftin, M.K.; Lin, J.; Boyer, P.D.; Candiello, J.E.; Merryman, W.D.; Guelcher, S.A.; Weaver, A.M. Sensing and modulation of invadopodia across a wide range of rigidities. Biophys. J. 2011, 100, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Pang, E.M.; Adebowale, K.; Wisdom, K.M.; Chaudhuri, O. Increased Stiffness Inhibits Invadopodia Formation and Cell Migration in 3D. Biophys. J. 2020, 119, 726–736. [Google Scholar] [CrossRef]
- Burdyga, A.; Conant, A.; Haynes, L.; Zhang, J.; Jalink, K.; Sutton, R.; Neoptolemos, J.; Costello, E.; Tepikin, A. cAMP inhibits migration, ruffling and paxillin accumulation in focal adhesions of pancreatic ductal adenocarcinoma cells: Effects of PKA and EPAC. Biochim. Biophys. Acta 2013, 1833, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McNiven, M.A. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J. Cell Biol. 2012, 196, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Baik, M.; Byers, J.T.; Chen, K.T.; French, S.W.; Díaz, B. TKS5-positive invadopodia-like structures in human tumor surgical specimens. Exp. Mol. Pathol. 2019, 106, 17–26. [Google Scholar] [CrossRef]
- Meirson, T.; Gil-Henn, H. Targeting invadopodia for blocking breast cancer metastasis. Drug Resist. Updat. 2018, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Species | Elevated Kinase Activity | Growth-Associated Signaling Pathways | References (PMID) | |
|---|---|---|---|---|---|
| FAK | Src | ||||
| Lung | Human | + | + | PI3K/Akt | [93,94] |
| Human | + | MEK/MAPK | [94,95] | ||
| Human | + | YAP/Hippo | [96,97,98,99] | ||
| Colorectal | Human | + | + | PI3K/Akt | [100] |
| Human | + | EGFR | [101,102] | ||
| Human, rat | + | EGFR/ERK | [103] | ||
| Human | + | ERK | [104] | ||
| Breast | Human | + | STAT3 | [105] | |
| Human | + | + | HER receptors/PI3k/Akt /MAPK | [106,107] | |
| Human | + | YAP/Hippo | [108,109] | ||
| Pancreatic | Human | + | + | Ras/Raf, PI3K/Akt | [81,83,110] |
| Human, mouse | + | + | ERK | [108] | |
| Human | + | EGFR/Erb2, ERK, STATs, TGF-β | [111,112,113] | ||
| Human, mouse | + | EGFR/STAT3 | [114] | ||
| Prostate | Human, mouse | + | SFK (Src family kinases) Lyn | [80] | |
| Human | + | PI3K/Akt | [115] | ||
| Human | + | EGFR/Akt/ERK | [116,117,118] | ||
| Mouse | + | MAPK | [119] | ||
| Cancer Type | Adhesion Types | Adhesion Rigidity Dependency | Signaling Pathways | Cancer-Related Effects | In Vitro/In Vivo/Ex Vivo | Publicat-ion |
|---|---|---|---|---|---|---|
| Lung | FA | N/A | VAV2/FAK/Rac1 | Promotion of metastasis | In vitro: human H1299 and H460 cells In vivo: transplantation in nude mice | [178] |
| FA | Promotion of FA formation on soft ECM | FAK | Increase in migration velocity and distance | In vitro: human A549 cells | [179] | |
| FA | N/A | Keap1 upregulation of RhoA activity | Inhibition of cell motility caused by FA turnover inhibition | In vitro: human A549 cells | [180] | |
| FA | Increase in FA formation and size on stiff ECM | N/A | Decrease in cell motility | In vitro: human H1299 cells | [181] | |
| FA & invadopodia | N/A | StarD13/RhoA/ Rac1/FA, SrarD13/Cdc42/ invadopodia | Inhibition of cell motility (immature FA); promotion of cell invasion (invadopodia) | In vitro: human A549 cells | [182] | |
| Invadopodia | N/A | Cortactin/ Cdc42/N-WASP | Promotion of cell invasion | In vitro: human H1299 and A549 cells | [183] | |
| Breast | FA | Increase in FA formation on stiff ECM | Integrins/PI3K/Akt | Promotion of cell invasion and malignancy | In vitro: human MCF10 and Ha-ras MCF10 AT MEC cells In vivo: MMTV-Neu mice model, transplantation in mice | [29] |
| FA | Increase in FA assembly and size on stiff ECM | ERK/Rho/ Src/FAK | Increase in cell growth and perturbation of tissue architecture | In vitro: Human HMT-3522 S1 cells In vivo: Transplantation in transgenic mice | [184] | |
| FA | No difference in FA areas across rigidities | N/A | Increase in cell proliferation | In vitro: human MDA-MB-231 cells | [70] | |
| Invadopodia | Increase in invadopodia quantity and activity on stiff ECM | Rho/p130Cas/ FAK | Promotion of cellular invasion | In vitro: human MCF10CA1d cells | [185] | |
| Invadopodia | Increase in invadopodia formation at ~30 kPa and 1.8 Gpa | N/A | Increase in ECM degradation | In vitro: human MCF10CA1d cells | [186] | |
| Invadopodia | Decrease in invadopodia formation in stiff 3D networks | Rac1/ROCK | Increase in cell migration | In vitro: human MDA-MB-231 cells | [187] | |
| Pancreatic | FA | No difference in FA areas across rigidities | N/A | Increase in cell proliferation | In vitro: human PANC-1 cells | [70] |
| FA | N/A | Inhibition of FA turnover by cAMP | Inhibition of cell migration | In vitro: human PANC-1, BxPC3, Capan-2, MiaPaca-2 and SUIT-2 cells | [188] | |
| FA & invadopodia | N/A | Src/FAK/ p130Cas (FA); Src (invadopodia) | Promotion of cellular invasion and ECM degradation by FA | In vitro: human PANC-1 and BxPC3 cells | [189] | |
| Invadopodia | N/A | N/A | Presence of invadopodia within human tumors | In vitro: human PANC-1, BxPC3, Capan-2, MiaPaca-2, SU86.86, MRC-5 and L3.6pl cells Ex vivo: human tumor surgical specimen | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, M.; Shi, L.; Wolfenson, H. The ‘Yin and Yang’ of Cancer Cell Growth and Mechanosensing. Cancers 2021, 13, 4754. https://doi.org/10.3390/cancers13194754
Amer M, Shi L, Wolfenson H. The ‘Yin and Yang’ of Cancer Cell Growth and Mechanosensing. Cancers. 2021; 13(19):4754. https://doi.org/10.3390/cancers13194754
Chicago/Turabian StyleAmer, Malak, Lidan Shi, and Haguy Wolfenson. 2021. "The ‘Yin and Yang’ of Cancer Cell Growth and Mechanosensing" Cancers 13, no. 19: 4754. https://doi.org/10.3390/cancers13194754
APA StyleAmer, M., Shi, L., & Wolfenson, H. (2021). The ‘Yin and Yang’ of Cancer Cell Growth and Mechanosensing. Cancers, 13(19), 4754. https://doi.org/10.3390/cancers13194754







