Liver Transplantation for HCC in HIV-Infected Patients: Long-Term Single-Center Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- -
- No previous HAART: CD4 T-cell counts ≥100 cells/µL and HIV viral load (VL) below the limit of detection.
- -
- Patient in HAART: CD4 T cell ≥100 cells/µL and HIV viral load (VL) below the limit of detection if no previous AIDS opportunist events or CD ≥200 if previous AIDS events.
- -
- No visceral Kaposi’s sarcoma or malignancies [13].
2.2. Selection Criteria
2.3. HCC Pre-LT Treatment
2.4. Surgical Procedure and Post-Operative Management
2.5. Study Design and Statistical Analysis
3. Results
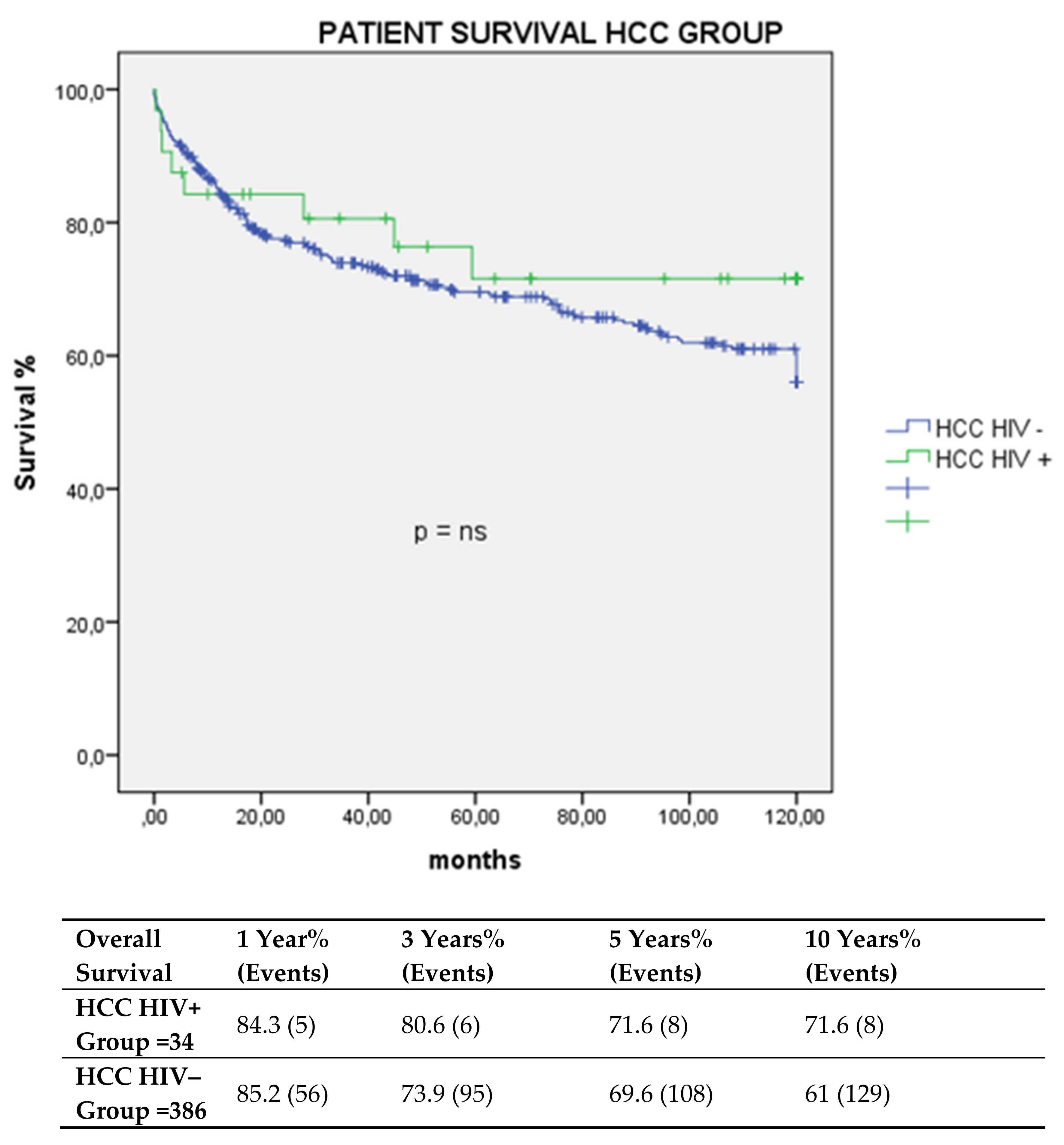
3.1. Graft and Patient Survival. Multivariate Analysis of Factors Impacting Survival Post-LT
3.2. Pathological Characteristic of Tumors of the Study Populations
3.3. Multivariate Analysis of Predictors Factors Impacting HCC Recurrence Post-LT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fauci, A.S.; Lane, H.C. Four Decades of HIV/AIDS—Much Accomplished, Much to Do. N. Engl. J. Med. 2020, 383, 1–4. [Google Scholar] [CrossRef]
- Berretta, M.; Martellotta, F.; Di Francia, R.; Spina, M.; Vaccher, E.; Balestreri, L.; Borsatti, E.; Bearz, A.; De Paoli, P.; Tirelli, U. Clinical presentation and outcome of non-AIDS defining cancers, in HIV-infected patients in the ART-era: The Italian Cooperative Group on AIDS and tumors activity. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3619–3634. [Google Scholar]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef]
- Bruden, D.J.T.; McMahon, B.J.; Townshend-Bulson, L.; Gounder, P.; Gove, J.; Plotnik, J.; Homan, C.; Hewitt, A.; Barbour, Y.; Spradling, P.R.; et al. Risk of end-stage liver disease, hepatocellular carcinoma, and liver-related death by fibrosis stage in the hepatitis C Alaska Cohort. Hepatology 2017, 66, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Althoff, K.N.; Gebo, K.A.; Moore, R.D.; Boyd, C.M.; Justice, A.C.; Wong, C.; Lucas, G.M.; Klein, M.B.; Kitahata, M.M.; Crane, H.; et al. Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: A collaboration of cohort studies. Lancet HIV 2019, 6, e93–e104. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Chun, Y.S.; Poon, R.T.; Schwartz, M.E.; Yao, F.Y.; Marsh, J.W.; Bhoori, S.; Lee, S.G. Liver transplantation for hepatocellular carcinoma. Ann. Surg. Oncol. 2008, 15, 1001–1007. [Google Scholar] [CrossRef]
- Di Benedetto, F.; De Ruvo, N.; Berretta, M.; Masetti, M.; Montalti, R.; Di Sandro, S.; Quintini, C.; Codeluppi, M.; Tirelli, U.; Gerunda, G.E. Don’t deny liver transplantation to HIV patients with hepatocellular carcinoma in the highly active antiretroviral therapy era. J. Clin. Oncol. 2006, 24, e26–e27. [Google Scholar] [CrossRef] [PubMed]
- Botha, J.; Fabian, J.; Etheredge, H.; Conradie, F.; Tiemessen, C.T. HIV and Solid Organ Transplantation: Where Are we Now. Curr. HIV/AIDS Rep. 2019, 16, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Tan-Tam, C.C.; Frassetto, L.A.; Stock, P.G. Liver and kidney transplantation in HIV-infected patients. AIDS Rev. 2009, 11, 190–204. [Google Scholar] [PubMed]
- Jayakrishnan, T.; Bakalov, V.; Finley, G.; Monga, D.; Wegner, R.E. Influence of Social Determinants of Health on Timeliness to Treatment for Metastatic HCC and the Impact of Affordable Care Act. World Cancer Res. J. 2021, 8, e2073. [Google Scholar]
- Berretta, M.; Garlassi, E.; Cacopardo, B.; Cappellani, A.; Guaraldi, G.; Cocchi, S.; De Paoli, P.; Lleshi, A.; Izzi, I.; Torresin, A.; et al. Hepatocellular carcinoma in HIV-infected patients: Check early, treat hard. Oncologist 2011, 16, 1258–1269. [Google Scholar] [CrossRef]
- Nissen, N.N.; Barin, B.; Stock, P.G. Malignancy in the HIV-infected patients undergoing liver and kidney transplantation. Curr. Opin. Oncol. 2012, 24, 517–521. [Google Scholar] [CrossRef]
- Morabito, V.; Grossi, P.; Lombardini, L.; Ricci, A.; Trapani, S.; Peritore, D.; Gaeta, A.; Ballanti, D.; Del Sordo, E.; Rizzato, L.; et al. Solid Organ Transplantation in HIV+ Recipients: Italian Experience. Transplant. Proc. 2016, 48, 424–430. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Yao, F.Y.; Xiao, L.; Bass, N.M.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Validation of the UCSF-expanded criteria based on preoperative imaging. Am. J. Transplant. 2007, 7, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Burra, P.; Mazzaferro, V.; Belli, L.; Pinna, A.D.; Spada, M.; Nanni Costa, A.; Toniutto, P. A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a “Blended Principle Model”. Am. J. Transplant. 2015, 15, 2552–2561. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Assirati, G.; Magistri, P. Full robotic ALPPS for HCC with intrahepatic portal vein thrombosis. Int. J. Med. Robot. 2020, 16, e2087. [Google Scholar] [CrossRef]
- Magistri, P.; Catellani, B.; Frassoni, S.; Guidetti, C.; Olivieri, T.; Assirati, G.; Caporali, C.; Pecchi, A.; Serra, V.; Ballarin, R.; et al. Robotic Liver Resection Versus Percutaneous Ablation for Early HCC: Short- and Long-Term Results. Cancers 2020, 12, 3578. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transplant. 2015, 21, 1142–1152. [Google Scholar] [CrossRef]
- Yao, F.Y.; Mehta, N.; Flemming, J.; Dodge, J.; Hameed, B.; Fix, O.; Hirose, R.; Fidelman, N.; Kerlan, R.K., Jr.; Roberts, J.P. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015, 61, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Arzu, G.D.; De Ruvo, N.; Montalti, R.; Masetti, M.; Begliomini, B.; Di Benedetto, F.; Rompianesi, G.; Di Sandro, S.; Smerieri, N.; D’Amico, G.; et al. Temporary porto-caval shunt utility during orthotopic liver transplantation. Transplant. Proc. 2008, 40, 1937–1940. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Di Sandro, S.; De Ruvo, N.; Montalti, R.; Ballarin, R.; Guerrini, G.P.; Spaggiari, M.; Guaraldi, G.; Gerunda, G. First report on a series of HIV patients undergoing rapamycin monotherapy after liver transplantation. Transplantation 2010, 89, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Weimer, L.E.; Fragola, V.; Giacometti, A.; Ancarani, F.; Maracci, M.; Gabrielli, E.; Marchionni, E.; Pirillo, M.F.; Vella, S. A Simplified HAART Regimen with Raltegravir and Lamivudine, and Pharmacokinetic Interactions with a Combined Immunosuppressive Therapy with Tacrolimus and Everolimus in an HIV/HCV/HBV/HDV Patient after Liver Transplantation. West Indian Med. J. 2014, 63, 779–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi, D.; Agarwal, K. Role of liver transplantation in human immunodeficiency virus positive patients. World J. Gastroenterol. 2015, 21, 12311–12321. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.; Eghtesad, B.; Patel-Tom, K.; DeVera, M.; Chapman, H.; Ragni, M. Liver transplantation in patients with HIV infection. Liver Transplant. 2004, 10, S39–S53. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, F.; Di Sandro, S.; De Ruvo, N.; Berretta, M.; Montalti, R.; Guerrini, G.P.; Ballarin, R.; De Blasiis, M.G.; Spaggiari, M.; Smerieri, N.; et al. Human immunodeficiency virus and liver transplantation: Our point of view. Transplant. Proc. 2008, 40, 1965–1971. [Google Scholar] [CrossRef]
- Fox, A.N.; Vagefi, P.A.; Stock, P.G. Liver transplantation in HIV patients. Semin Liver Dis. 2012, 32, 177–185. [Google Scholar] [CrossRef]
- Joshi, D.; O’Grady, J.; Taylor, C.; Heaton, N.; Agarwal, K. Liver transplantation in human immunodeficiency virus-positive patients. Liver Transplant. 2011, 17, 881–890. [Google Scholar] [CrossRef]
- Locke, J.E.; Durand, C.; Reed, R.D.; MacLennan, P.A.; Mehta, S.; Massie, A.; Nellore, A.; DuBay, D.; Segev, D.L. Long-term Outcomes After Liver Transplantation Among Human Immunodeficiency Virus-Infected Recipients. Transplantation 2016, 100, 141–146. [Google Scholar] [CrossRef]
- Campos-Varela, I.; Dodge, J.L.; Berenguer, M.; Adam, R.; Samuel, D.; Di Benedetto, F.; Karam, V.; Belli, L.S.; Duvoux, C.; Terrault, N.A. Temporal Trends and Outcomes in Liver Transplantation for Recipients With HIV Infection in Europe and United States. Transplantation 2020, 104, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Caraglia, M.; Martellotta, F.; Zappavigna, S.; Lombardi, A.; Fierro, C.; Atripaldi, L.; Muto, T.; Valente, D.; De Paoli, P.; et al. Drug-Drug Interactions Based on Pharmacogenetic Profile between Highly Active Antiretroviral Therapy and Antiblastic Chemotherapy in Cancer Patients with HIV Infection. Front. Pharmacol. 2016, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.E.; Barin, B.; Carlson, L.; Frassetto, L.A.; Terrault, N.A.; Hirose, R.; Freise, C.E.; Benet, L.Z.; Ascher, N.L.; Roberts, J.P.; et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am. J. Transplant. 2008, 8, 355–365. [Google Scholar] [CrossRef]
- Kardashian, A.A.; Price, J.C. Hepatitis C virus-HIV-coinfected patients and liver transplantation. Curr. Opin. Organ. Transplant. 2015, 20, 276–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Merwat, S.N.; Vierling, J.M. HIV infection and the liver: The importance of HCV-HIV coinfection and drug-induced liver injury. Clin. Liver Dis. 2011, 15, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Manzardo, C.; Londoño, M.C.; Castells, L.; Testillano, M.; Luis Montero, J.; Peñafiel, J.; Subirana, M.; Moreno, A.; Aguilera, V.; Luisa González-Diéguez, M.; et al. Direct-acting antivirals are effective and safe in HCV/HIV-coinfected liver transplant recipients who experience recurrence of hepatitis C: A prospective nationwide cohort study. Am. J. Transplant. 2018, 18, 2513–2522. [Google Scholar] [CrossRef]
- Sikavi, C.; Chen, P.H.; Lee, A.D.; Saab, E.G.; Choi, G.; Saab, S. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: No longer a difficult-to-treat population. Hepatology 2018, 67, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Naggie, S.; Cooper, C.; Saag, M.; Workowski, K.; Ruane, P.; Towner, W.J.; Marks, K.; Luetkemeyer, A.; Baden, R.P.; Sax, P.E.; et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N. Engl. J. Med. 2015, 373, 705–713. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Childs, K.; Thornton, A.; Bhagani, S.; Demma, S.; Srivastava, A.; Leen, C.; Agarwal, K.; Rodger, A.J.; Sabin, C.A. Cirrhosis and liver transplantation in patients co-infected with HIV and hepatitis B or C: An observational cohort study. Infection 2017, 45, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Cavaliere, C.; Alessandrini, L.; Stanzione, B.; Facchini, G.; Balestreri, L.; Perin, T.; Canzonieri, V. Serum and tissue markers in hepatocellular carcinoma and cholangiocarcinoma: Clinical and prognostic implications. Oncotarget 2017, 8, 14192–14220. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Berretta, M.; Tarantino, G.; Magistri, P.; Pecchi, A.; Ballarin, R.; Di Benedetto, F. Multimodal oncological approach in patients affected by recurrent hepatocellular carcinoma after liver transplantation. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3421–3435. [Google Scholar] [PubMed]
- Biondi, A.; Malaguarnera, G.; Vacante, M.; Berretta, M.; D’Agata, V.; Malaguarnera, M.; Basile, F.; Drago, F.; Bertino, G. Elevated serum levels of Chromogranin A in hepatocellular carcinoma. BMC Surg. 2012, 12 (Suppl. 1), S7. [Google Scholar] [CrossRef][Green Version]
- D’Andrea, F.; Rullo, E.V.; Marino, A.; Moscatt, V.; Celesia, B.M.; Cacopardo, B.; Condorelli, F.; La Rocca, G.; Di Rosa, M.; Pellicano, G.F.; et al. Hepatitis b virus infection and hepatocellular carcinoma in plwh: Epidemiology, pathogenesis and treatment. World Cancer Res. J. 2020, 7, e1537. [Google Scholar]
- Torgersen, J.; Kallan, M.J.; Carbonari, D.M.; Park, L.S.; Mehta, R.L.; D’Addeo, K.; Tate, J.P.; Lim, J.K.; Goetz, M.B.; Rodriguez-Barradas, M.C.; et al. HIV RNA, CD4+ Percentage, and Risk of Hepatocellular Carcinoma by Cirrhosis Status. J. Natl. Cancer Inst. 2020, 112, 747–755. [Google Scholar] [CrossRef]
- Nunnari, G.; Berretta, M.; Pinzone, M.R.; Di Rosa, M.; Berretta, S.; Cunsolo, G.; Malaguarnera, M.; Cosentino, S.; De Paoli, P.; Schnell, J.M.; et al. Hepatocellular carcinoma in HIV positive patients. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1257–1270. [Google Scholar]
- Puoti, M.; Bruno, R.; Soriano, V.; Donato, F.; Gaeta, G.B.; Quinzan, G.P.; Precone, D.; Gelatti, U.; Asensi, V.; Vaccher, E. Hepatocellular carcinoma in HIV-infected patients: Epidemiological features, clinical presentation and outcome. AIDS 2004, 18, 2285–2293. [Google Scholar] [CrossRef]
- Bräu, N.; Fox, R.K.; Xiao, P.; Marks, K.; Naqvi, Z.; Taylor, L.E.; Trikha, A.; Sherman, M.; Sulkowski, M.S.; Dieterich, D.T.; et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: A U.S.-Canadian multicenter study. J. Hepatol. 2007, 47, 527–537. [Google Scholar] [CrossRef]
- Di Benedetto, F.; De Ruvo, N.; Berretta, M.; Masetti, M.; Montalti, R.; Di Sandro, S.; Ballarin, R.; Codeluppi, M.; Guaraldi, G.; Gerunda, G.E. Hepatocellular carcinoma in HIV patients treated by liver transplantation. Eur. J. Surg. Oncol. 2008, 34, 422–427. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Tarantino, G.; Ercolani, G.; Baccarani, U.; Montalti, R.; De Ruvo, N.; Berretta, M.; Adani, G.L.; Zanello, M.; Tavio, M.; et al. Multicenter italian experience in liver transplantation for hepatocellular carcinoma in HIV-infected patients. Oncologist 2013, 18, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Vibert, E.; Duclos-Vallée, J.C.; Ghigna, M.R.; Hoti, E.; Salloum, C.; Guettier, C.; Castaing, D.; Samuel, D.; Adam, R. Liver transplantation for hepatocellular carcinoma: The impact of human immunodeficiency virus infection. Hepatology 2011, 53, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Bhangui, P.; Yao, F.Y.; Mazzaferro, V.; Toso, C.; Akamatsu, N.; Durand, F.; Ijzermans, J.; Polak, W.; Zheng, S.; et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Goldaracena, N.; Laurence, J.M.; Dib, M.; Barbas, A.; Ghanekar, A.; Cleary, S.P.; Lilly, L.; Cattral, M.S.; Marquez, M.; et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology 2016, 64, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Pinelli, D.; Di Benedetto, F.; Marini, E.; Corno, V.; Guizzetti, M.; Aluffi, A.; Zambelli, M.; Fagiuoli, S.; Lucà, M.G.; et al. Predictive value of nodule size and differentiation in HCC recurrence after liver transplantation. Surg. Oncol. 2016, 25, 419–428. [Google Scholar] [CrossRef] [PubMed]

| Study Population = 420 | ||||||
|---|---|---|---|---|---|---|
| Variable | HIV Group n = 34 | Control Group n = 386 | p Value | |||
| Number | % | Number | % | |||
| Sex | M | 30 | 88.2 | 320 | 82.9 | ns |
| F | 4 | 11.8 | 66 | 17.1 | ||
| Age | mean and sd | 50.3 | ±5.6 | 57.6 | 8.1 | 0.001 |
| Child | A/B | 22 | 64.8 | 258 | 66.9 | ns |
| C | 12 | 35.2 | 128 | 33.1 | ||
| Meld at LT | mean and sd | 17.5 | ±9.6 | 15.3 | ±7.2 | ns |
| CD4 | mean and sd | 328 | ±214 | |||
| BMI | mean and sd | 23.9 | ±3.7 | 26 | 6 | ns |
| Type of LT | Isolated | 33 | 97 | 382 | 98 | ns |
| Combined Kidney-Liver | 1 | 3 | 4 | 2 | ||
| Etiology | HCV | 24 | 70.5 | 209 | 54.1 | 0.019 |
| HBV | 8 | 23.5 | 97 | 25.1 | ns | |
| Alcohol | 2 | 6 | 44 | 11.4 | ns | |
| Biliary | 0 | 3 | 3 | 0.8 | ns | |
| Metabolic | 0 | 22 | 5.7 | ns | ||
| Other | 0 | 11 | 2.9 | ns | ||
| Waiting list time days | mean and sd | 150.3 | ±33 | 146.1 | ±9.6 | ns |
| Re-OLT | yes | 2 | 5.8 | 18 | 4.7 | ns |
| Post-Transplant Complications Study Population = 420 | ||||||
|---|---|---|---|---|---|---|
| Variable | HIV Group n = 34 | Control Group n = 386 | p Value | |||
| Number | % | Number | % | |||
| Acute Cellular Rejection | yes | 6 | 17.6 | 45 | 11.6% | ns |
| Biliary Stenosis | yes | 7 | 20.5 | 42 | 10.8 | ns |
| Early Hepatic Artery Thrombosis | yes | 1 | 2.9 | 10 | 2.5 | ns |
| Late Hepatic Artery Thrombosis | yes | 1 | 2.9 | 12 | 3.1 | ns |
| Portal Vein Thrombosis | yes | 0 | 0 | 9 | 2.3 | ns |
| De Novo Tumor | yes | 1 | 2.9 | 26 | 6.7 | ns |
| Multivariate Analysis | |||||
|---|---|---|---|---|---|
| Variable | B | S.E. | HR | 95% C.I. for EXP | p Value |
| Cox regression of overall survival | |||||
| HCV etiology | 0.608 | 0.268 | 1.837 | 1.085–3.109 | 0.024 |
| HIV Infection | 0.72 | 0.51 | 2.06 | 0.75–5.65 | ns (0.159) |
| Logistic regression for HCC recurrence in the study population | |||||
| Numbers of HCC nodules | 0.233 | 0.079 | 1.262 | 1.081–1.474 | 0.003 |
| Diameter of the largest nodule | 0.152 | 0.069 | 1.164 | 1.017–1.333 | 0.028 |
| Microvascular invasion | −1.645 | 0.455 | 5.18 | 2.12–12.64 | 0.001 |
| HIV infection | −0.107 | 0.77 | 0.89 | 0.195–9.130 | ns (0.88) |
| HCC LT POPULATION =420 | ||||||
|---|---|---|---|---|---|---|
| Variable | HIV Group Number = 34 | Control Group Number = 386 | p Value | |||
| HCC Diameter | mean and sd | 2.4 | ±1.7 | 3 | ±2.1 | ns |
| HCC N. Nodules | mean and sd | 2.7 | ±2.4 | 2.3 | ±1.8 | 0.024 |
| α-FETO-Protein ng/mL | mean and sd | 65 | ±14.1 | 68 | ±186 | ns |
| HCC Focality | uni | 16 | 47% | 158 | 40.9% | ns |
| multi | 18 | 53% | 228 | 59.1% | ||
| HCC Treatment Pre-LT Downstaging | no | 8 | 23.6% | 76 | 19.6% | ns |
| yes | 26 | 76.4% | 310 | 80.4% | ||
| Microvascular Invasion | no | 29 | 85.3% | 321 | 83.2% | ns |
| yes | 5 | 14.7% | 65 | 16.8% | ||
| Capsule Invasion | no | 28 | 82.3% | 340 | 88 % | ns |
| yes | 6 | 17.7% | 46 | 12% | ||
| Satellitosis | no | 28 | 82.3% | 353 | 91.4% | ns |
| yes | 6 | 17.7% | 33 | 8.6% | ||
| Grading | G1–2 | 25 | 73.5% | 317 | 82.1% | ns |
| G3–4 | 9 | 26.5% | 69 | 19.9% | ||
| Milan Criteria | In | 21 | 61.7% | 245 | 63.4% | ns |
| out | 13 | 38.2% | 141 | 36.6% | ||
| USCF | In | 23 | 67.6% | 276 | 71.5% | ns |
| out | 11 | 32.4% | 110 | 28.5% | ||
| Up-to Seven Criteria | In | 27 | 79.4% | 297 | 76.9% | ns |
| out | 7 | 20.6% | 89 | 23.1% | ||
| Recurrence | yes | 5 | 14.7% | 49 | 12.6% | ns |
| no | 29 | 85.3% | 337 | 87.4% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, G.P.; Berretta, M.; Guaraldi, G.; Magistri, P.; Esposito, G.; Ballarin, R.; Serra, V.; Di Sandro, S.; Di Benedetto, F. Liver Transplantation for HCC in HIV-Infected Patients: Long-Term Single-Center Experience. Cancers 2021, 13, 4727. https://doi.org/10.3390/cancers13184727
Guerrini GP, Berretta M, Guaraldi G, Magistri P, Esposito G, Ballarin R, Serra V, Di Sandro S, Di Benedetto F. Liver Transplantation for HCC in HIV-Infected Patients: Long-Term Single-Center Experience. Cancers. 2021; 13(18):4727. https://doi.org/10.3390/cancers13184727
Chicago/Turabian StyleGuerrini, Gian Piero, Massimiliano Berretta, Giovanni Guaraldi, Paolo Magistri, Giuseppe Esposito, Roberto Ballarin, Valentina Serra, Stefano Di Sandro, and Fabrizio Di Benedetto. 2021. "Liver Transplantation for HCC in HIV-Infected Patients: Long-Term Single-Center Experience" Cancers 13, no. 18: 4727. https://doi.org/10.3390/cancers13184727
APA StyleGuerrini, G. P., Berretta, M., Guaraldi, G., Magistri, P., Esposito, G., Ballarin, R., Serra, V., Di Sandro, S., & Di Benedetto, F. (2021). Liver Transplantation for HCC in HIV-Infected Patients: Long-Term Single-Center Experience. Cancers, 13(18), 4727. https://doi.org/10.3390/cancers13184727








