Bacteria-Derived Extracellular Vesicles in Urine as a Novel Biomarker for Gastric Cancer: Integration of Liquid Biopsy and Metagenome Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Sample Collection
2.2. Bacterial and EV Isolation and DNA Extraction from Clinical Samples
2.3. PCR Amplification, Library Construction, and Sequencing of 16S rRNA Gene Variable Regions
2.4. Analysis of Bacterial Composition in the Microbiome
2.5. Diagnostic Prediction Models for Gastric Cancer
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Subjects
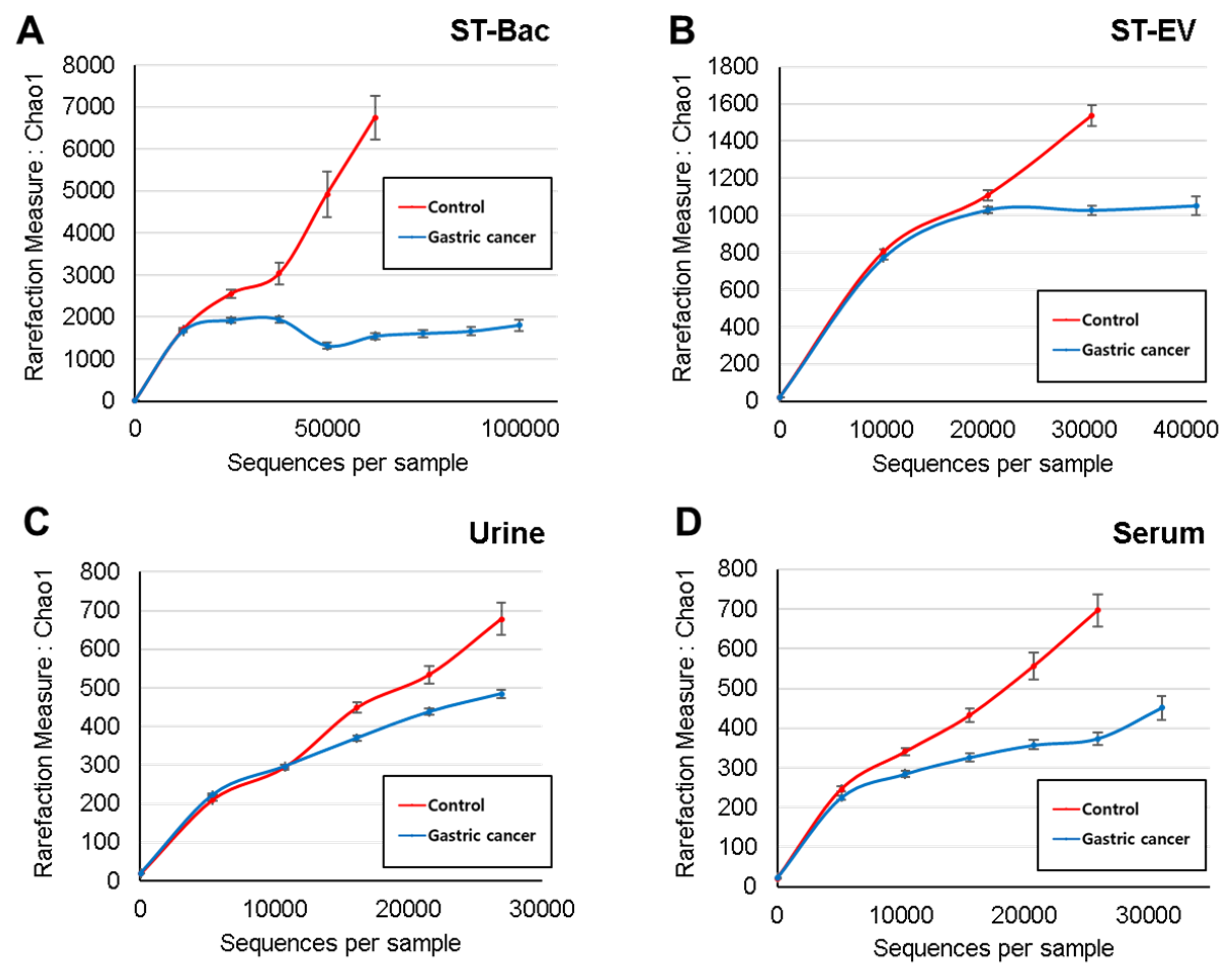
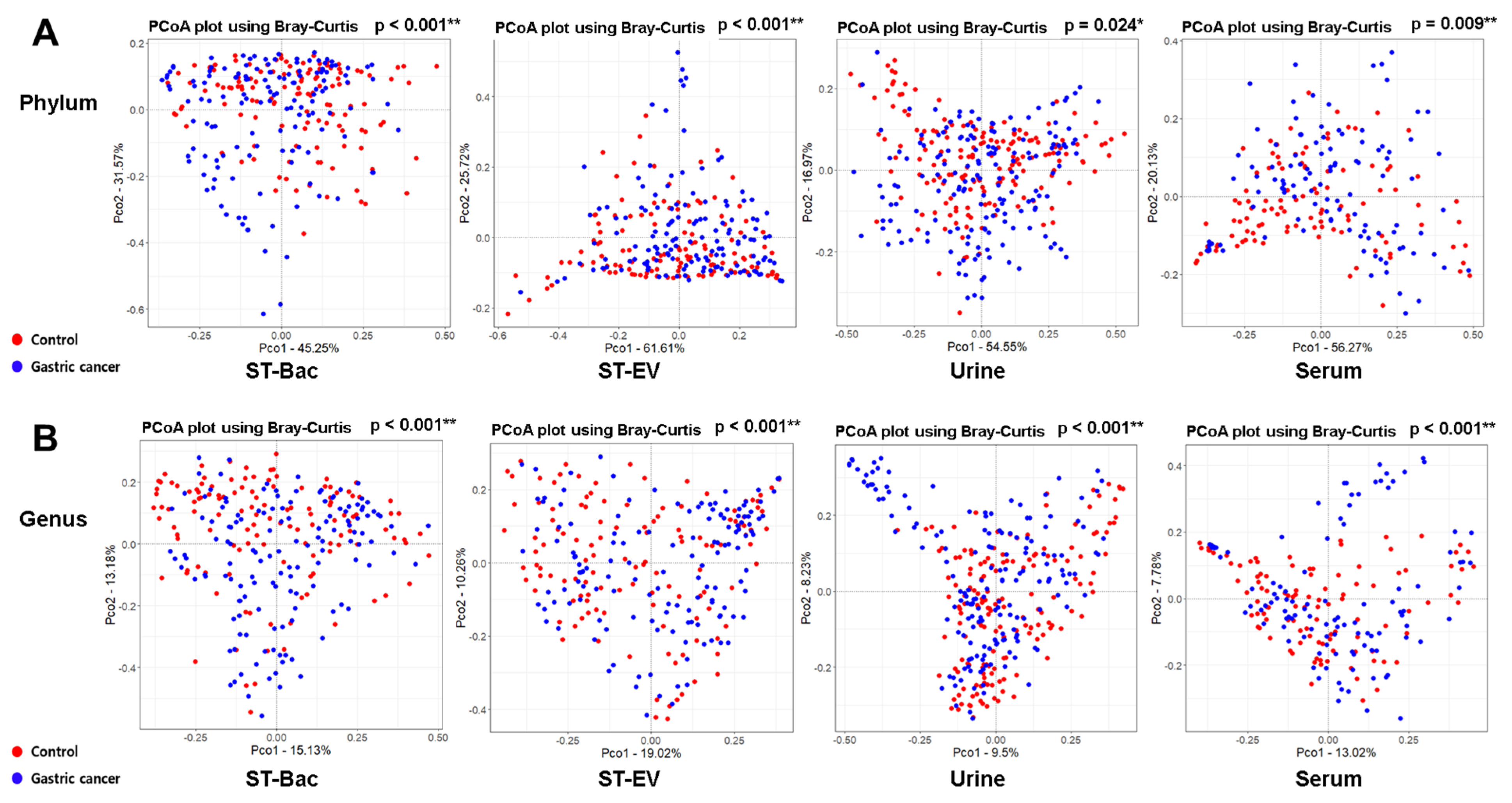
3.2. Comparison of Alpha and Beta Diversity between Healthy Controls and Gastric Cancer Patients
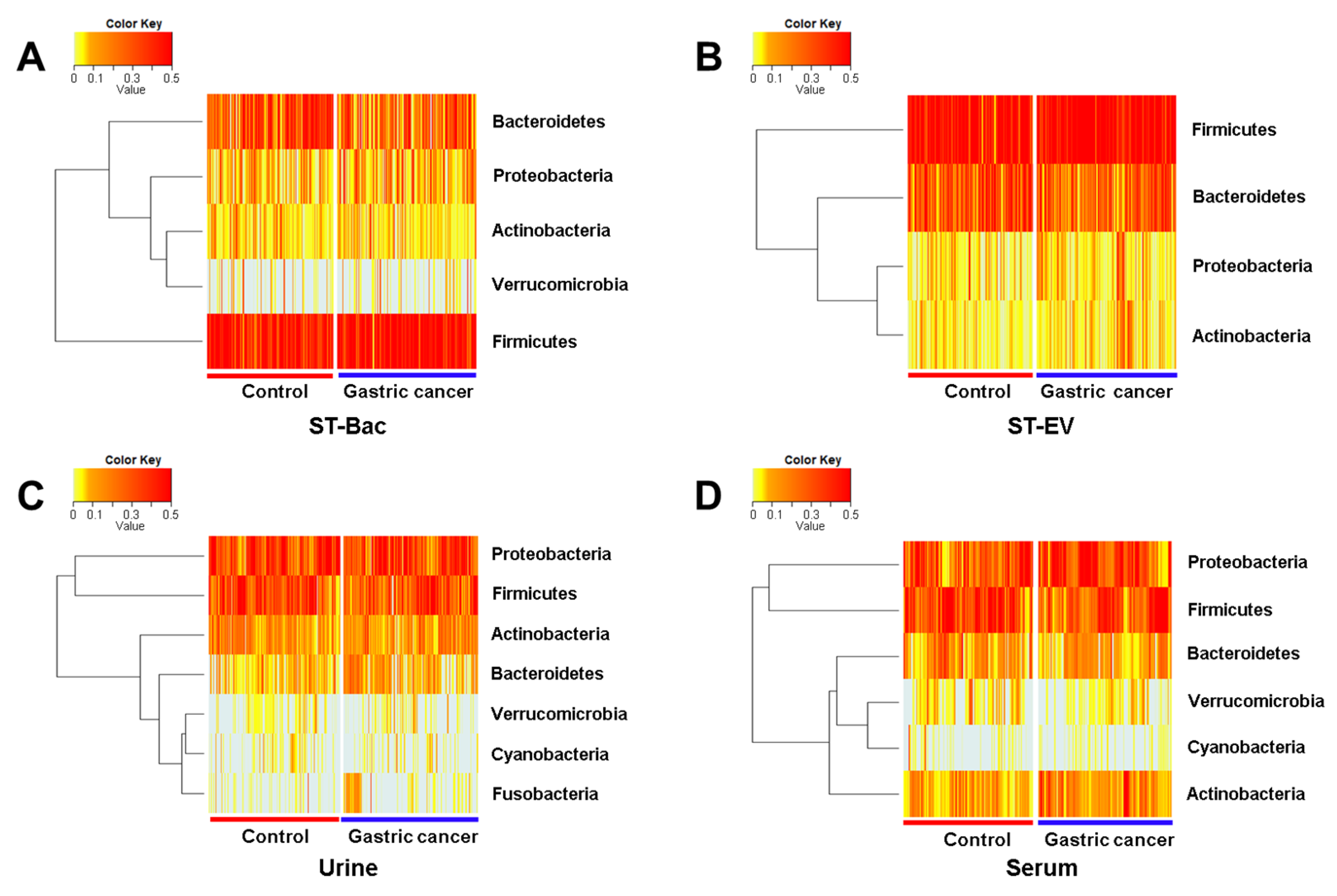
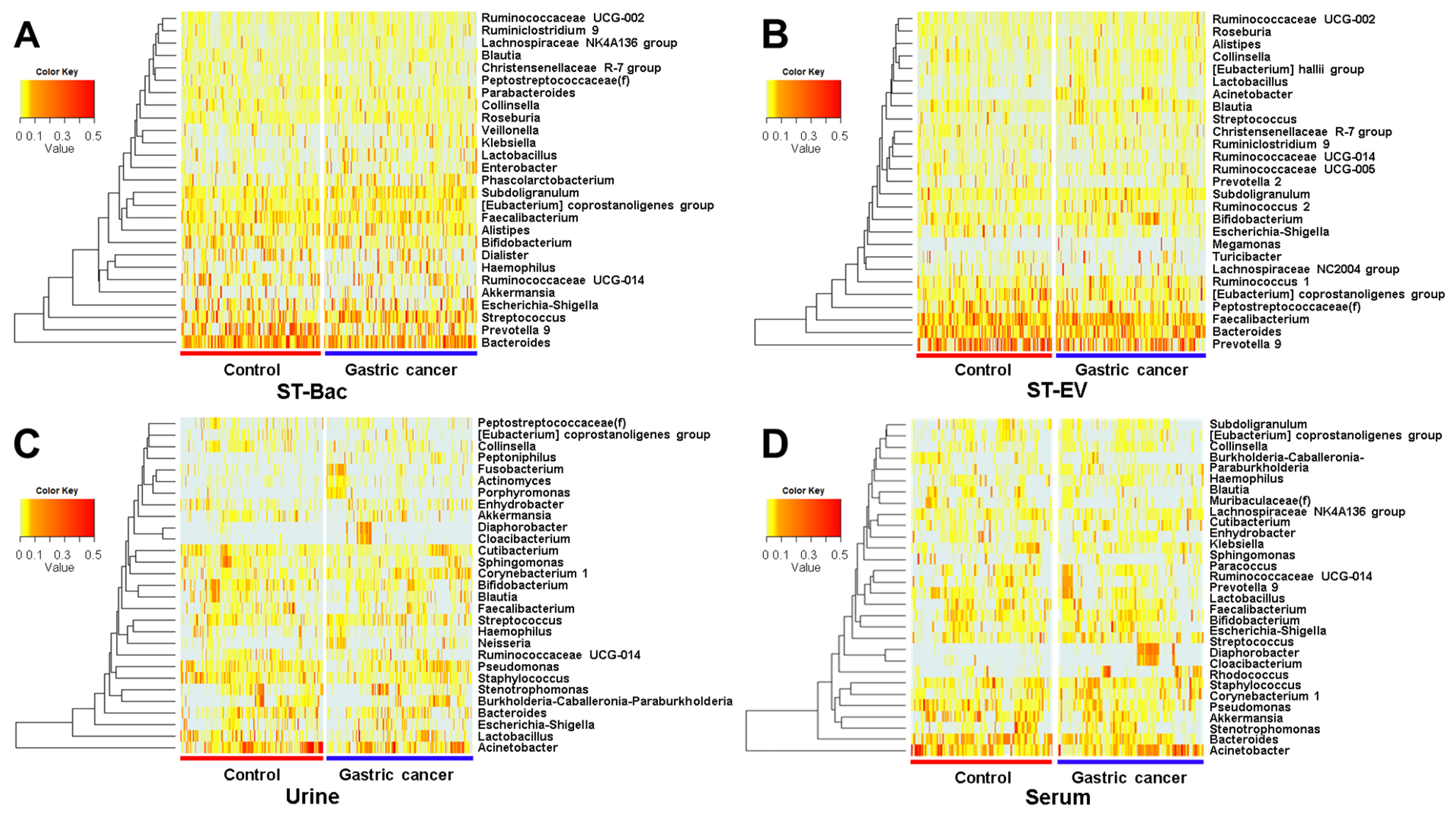
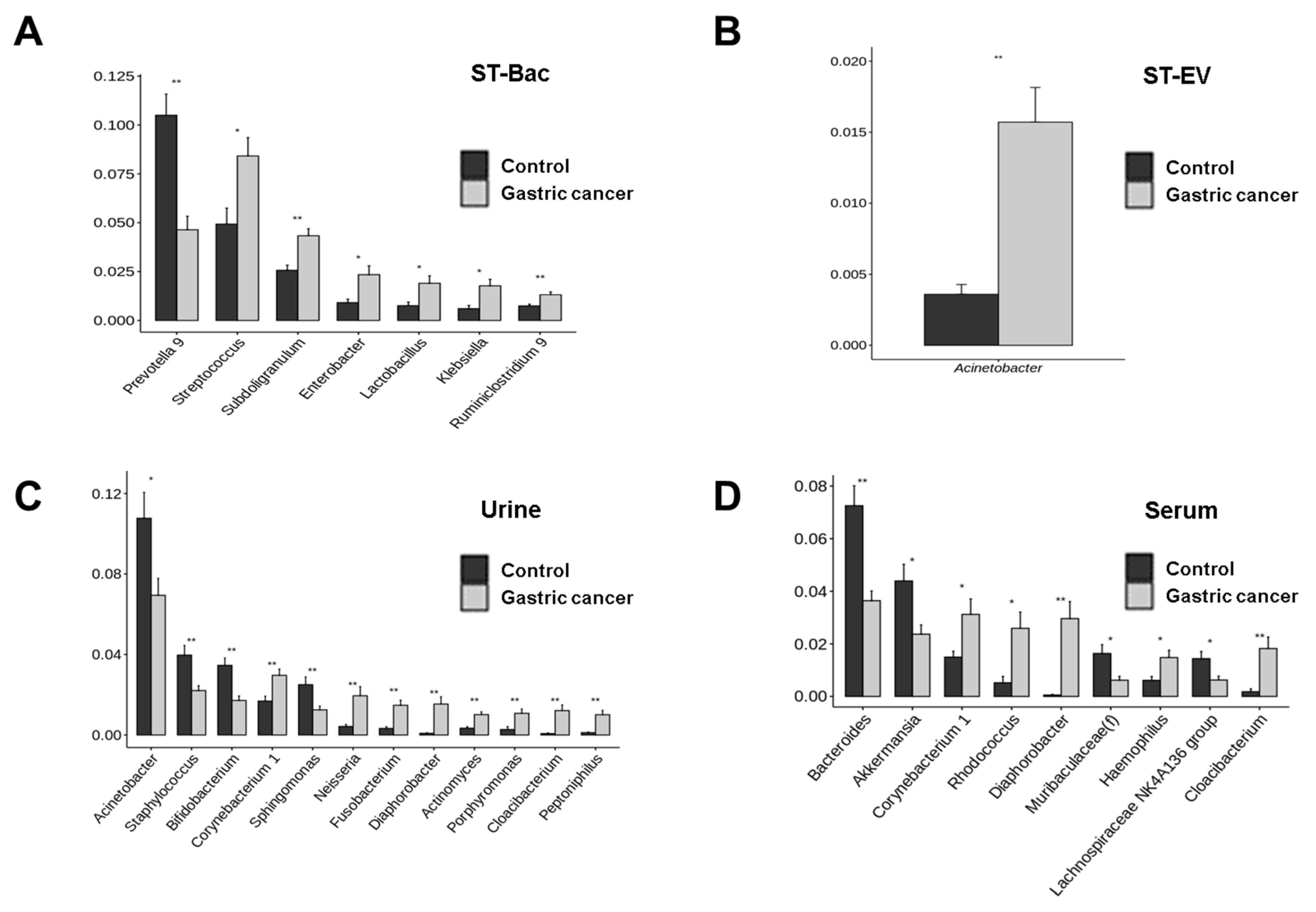
3.3. Relative Abundance Differences between Healthy Controls and Gastric Cancer Patients
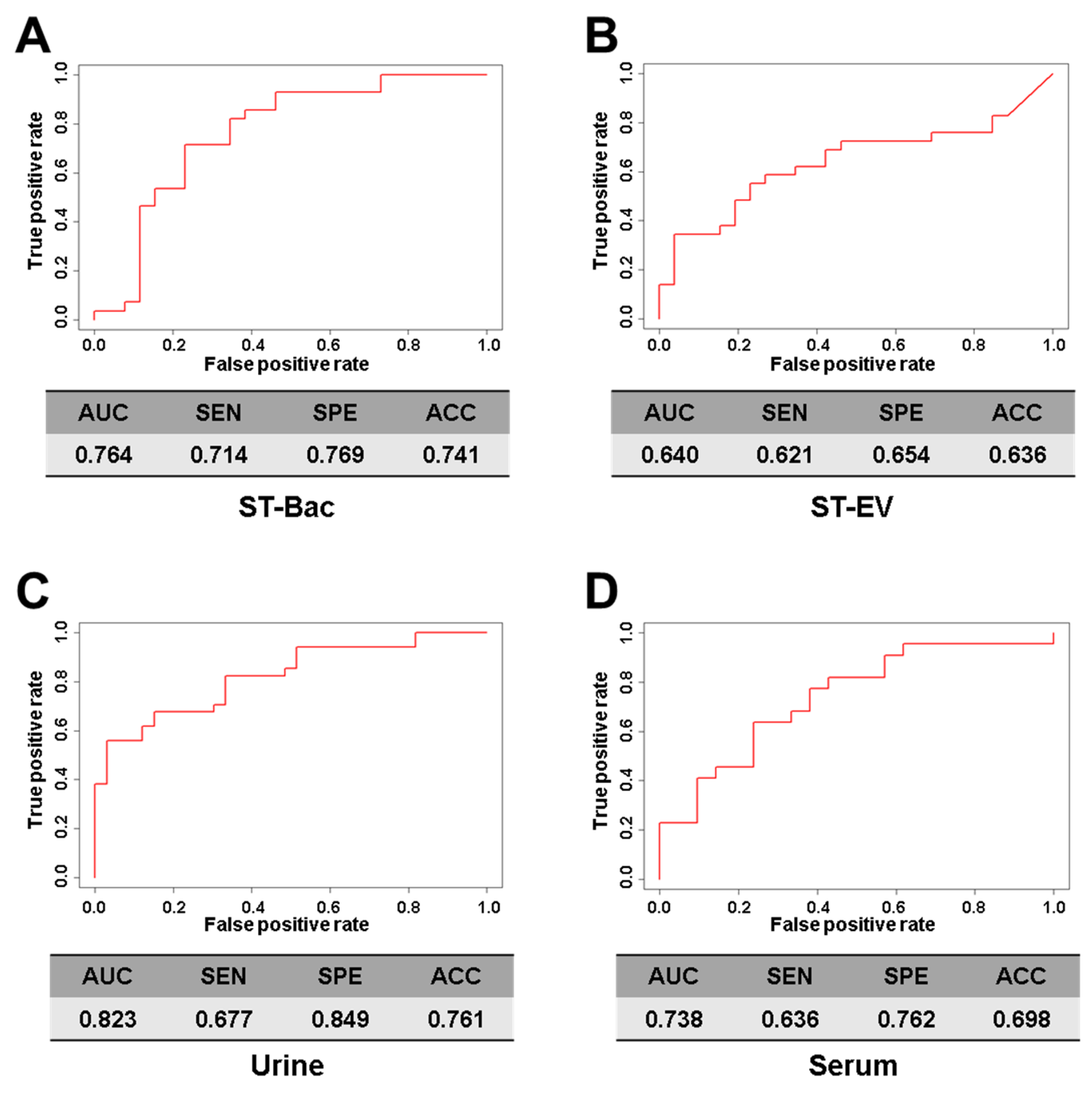
3.4. Comparison of Diagnostic Prediction Models for Gastric Cancer between Healthy Controls and Gastric Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Oh, C.M.; Shin, A.; Lee, J.S. Survival of korean adult cancer patients by stage at diagnosis, 2006–2010: National cancer registry study. Cancer Res. Treat. 2013, 45, 162–171. [Google Scholar] [CrossRef]
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e1317. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res. Treat. 2019, 51, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Vigil, I.G.; Moreno-Martínez, A.K.; Wang, J.Y.; Roehrl, M.H.A.; Barrera-Saldaña, H.A. The dawn of the liquid biopsy in the fight against cancer. Oncotarget 2018, 9, 2912–2922. [Google Scholar] [CrossRef] [Green Version]
- Werner, S.; Chen, H.; Tao, S.; Brenner, H. Systematic review: Serum autoantibodies in the early detection of gastric cancer. Int. J. Cancer 2015, 136, 2243–2252. [Google Scholar] [CrossRef] [Green Version]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, A.R.; Lee, Y.R.; Eun, C.S.; Lee, S.K.; Han, D.S. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter 2019, 24, e12547. [Google Scholar] [CrossRef] [Green Version]
- Brestoff, J.R.; Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013, 14, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noto, J.M.; Peek, R.M., Jr. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog. 2017, 13, e1006573. [Google Scholar] [CrossRef] [Green Version]
- Macia, L.; Nanan, R.; Hosseini-Beheshti, E.; Grau, G.E. Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. Int. J. Mol. Sci 2019, 21, 107. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Moon, H.E.; Park, H.W.; McDowell, A.; Shin, T.S.; Jee, Y.K.; Kym, S.; Paek, S.H.; Kim, Y.K. Brain tumor diagnostic model and dietary effect based on extracellular vesicle microbiome data in serum. Exp. Mol. Med. 2020, 52, 1602–1613. [Google Scholar] [CrossRef]
- Jing, H.; Tang, S.; Lin, S.; Liao, M.; Chen, H.; Zhou, J. The role of extracellular vesicles in renal fibrosis. Cell Death Dis. 2019, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.P.; Jeon, S.G.; Kim, Y.K.; Cho, Y.S. Role of house dust mite-derived extracellular vesicles in a murine model of airway inflammation. Clin. Exp. Allergy 2019, 49, 227–238. [Google Scholar] [CrossRef]
- Raymond, F.; Ouameur, A.A.; Déraspe, M.; Iqbal, N.; Gingras, H.; Dridi, B.; Leprohon, P.; Plante, P.L.; Giroux, R.; Bérubé, È.; et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016, 10, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Morales-Marroquin, E.; Hanson, B.; Greathouse, L.; de la Cruz-Munoz, N.; Messiah, S.E. Comparison of methodological approaches to human gut microbiota changes in response to metabolic and bariatric surgery: A systematic review. Obes. Rev. 2020, 21, e13025. [Google Scholar] [CrossRef]
- Song, Y.; Joung, H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Rho, M.; You, Y.A.; Kwon, E.J.; Kim, M.H.; Kym, S.; Jee, Y.K.; Kim, Y.K.; Kim, Y.J. 16S rRNA gene-based metagenomic analysis reveals differences in bacteria-derived extracellular vesicles in the urine of pregnant and non-pregnant women. Exp. Mol. Med. 2016, 48, e208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; McDowell, A.; Seo, H.; Kim, S.; Min, T.K.; Jee, Y.K.; Choi, Y.; Park, H.S.; Pyun, B.Y.; Kim, Y.K. Diagnostic Models for Atopic Dermatitis Based on Serum Microbial Extracellular Vesicle Metagenomic Analysis: A Pilot Study. Allergy Asthma Immunol. Res. 2020, 12, 792–805. [Google Scholar] [CrossRef]
- Yang, J.; McDowell, A.; Kim, E.K.; Seo, H.; Yum, K.; Lee, W.H.; Jee, Y.K.; Kim, Y.K. Consumption of a Leuconostoc holzapfelii-enriched synbiotic beverage alters the composition of the microbiota and microbial extracellular vesicles. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.J.; Yang, J.; Seo, H.; Lee, W.H.; Ho Lee, D.; Kym, S.; Park, Y.S.; Kim, J.G.; Jang, I.J.; Kim, Y.K.; et al. Colorectal cancer diagnostic model utilizing metagenomic and metabolomic data of stool microbial extracellular vesicles. Sci Rep. 2020, 10, 2860. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [Green Version]
- Huse, S.M.; Dethlefsen, L.; Huber, J.A.; Mark Welch, D.; Relman, D.A.; Sogin, M.L. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet. 2008, 4, e1000255. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Iraci, N.; Gaude, E.; Leonardi, T.; Costa, A.S.H.; Cossetti, C.; Peruzzotti-Jametti, L.; Bernstock, J.D.; Saini, H.K.; Gelati, M.; Vescovi, A.L.; et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat. Chem. Biol. 2017, 13, 951–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Zeng, B.; Wang, P.; Wang, L.; Wen, B.; Li, Y.; Liu, H.; Bai, S.; Jia, G. Microbiome of Total Versus Live Bacteria in the Gut of Rex Rabbits. Front. Microbiol. 2018, 9, 733. [Google Scholar] [CrossRef]
- Emerson, J.B.; Adams, R.I.; Román, C.M.B.; Brooks, B.; Coil, D.A.; Dahlhausen, K.; Ganz, H.H.; Hartmann, E.M.; Hsu, T.; Justice, N.B.; et al. Schrödinger’s microbes: Tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 2017, 5, 86. [Google Scholar] [CrossRef]
- Palviainen, M.; Saraswat, M.; Varga, Z.; Kitka, D.; Neuvonen, M.; Puhka, M.; Joenväärä, S.; Renkonen, R.; Nieuwland, R.; Takatalo, M.; et al. Extracellular vesicles from human plasma and serum are carriers of extravesicular cargo-Implications for biomarker discovery. PLoS ONE 2020, 15, e0236439. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Chiam, K.; Mayne, G.C.; Wang, T.; Watson, D.I.; Irvine, T.S.; Bright, T.; Smith, L.T.; Ball, I.A.; Bowen, J.M.; Keefe, D.M.; et al. Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus. World J. Gastroenterol. 2020, 26, 2570–2583. [Google Scholar] [CrossRef]
- Yeaman, M.R. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 1997, 25, 951–968. [Google Scholar] [CrossRef] [Green Version]
- Drago, L.; Bortolin, M.; Vassena, C.; Romanò, C.L.; Taschieri, S.; Del Fabbro, M. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: An in vitro study. PLoS ONE 2014, 9, e107813. [Google Scholar] [CrossRef] [Green Version]
- Šimundić, A.M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar] [PubMed]
- Samra, M.S.; Lim, D.H.; Han, M.Y.; Jee, H.M.; Kim, Y.K.; Kim, J.H. Bacterial Microbiota-derived Extracellular Vesicles in Children with Allergic Airway Diseases: Compositional and Functional Features. Allergy Asthma Immunol. Res. 2021, 13, 56–74. [Google Scholar] [CrossRef]
- Samra, M.; Nam, S.K.; Lim, D.H.; Kim, D.H.; Yang, J.; Kim, Y.K.; Kim, J.H. Urine Bacteria-Derived Extracellular Vesicles and Allergic Airway Diseases in Children. Int. Arch. Allergy Immunol. 2019, 178, 150–158. [Google Scholar] [CrossRef] [PubMed]
| Sample Type | Age & Sex | Control | Gastric Cancer | p-Value |
|---|---|---|---|---|
| ST-Bac | Age (mean ± SD) Sex (M:F) | 63.6 ± 8.3 127 (93:34) | 63.6 ± 9.5 140 (95:45) | 0.9815 0.4088 |
| ST-EV | Age (mean ± SD) Sex (M:F) | 63.6 ± 8.3 127 (93:34) | 63.6 ± 9.5 141 (96:45) | 0.9853 0.4307 |
| Urine | Age (mean ± SD) Sex (M:F) | 63.5 ± 9.8 164 (114:50) | 63.8 ± 9.8 168 (114:54) | 0.8207 0.8362 |
| Serum | Age (mean ± SD) Sex (M:F) | 62.3 ± 9.4 105 (74:31) | 63.7 ± 10.3 108 (73:35) | 0.2891 0.7590 |
| Sample Type | Group | Read Count | OTU | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | SE | Mean | Median | SE | ||
| ST-Bac | Control Gastric cancer | 18,352.6 19,987.9 | 16,243.0 16,138.5 | ±1062.7 ±1420.5 | 939.4 890.4 | 810.0 763.5 | ±64.5 ±46.6 |
| ST-EV | Control Gastric cancer | 19,262.0 22,555.8 | 18,378.0 19,753.0 | ±961.6 ±1227.5 | 550.7 556.9 | 453.0 510.0 | ±32.7 ±22.7 |
| Urine | Control Gastric cancer | 12,766.7 12,968.7 | 12,412.5 9282.0 | ±598.8 ±787.5 | 176.6 167.4 | 140.5 141.5 | ±11.6 ±8.6 |
| Serum | Control Gastric cancer | 11,340.4 14,259.4 | 10,642.0 10,763.0 | ±841.3 ±977.6 | 189.6 226.8 | 144.0 181.0 | ±17.3 ±16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Kang, C.-S.; Seo, H.-C.; Shin, J.-C.; Kym, S.-M.; Park, Y.-S.; Shin, T.-S.; Kim, J.-G.; Kim, Y.-K. Bacteria-Derived Extracellular Vesicles in Urine as a Novel Biomarker for Gastric Cancer: Integration of Liquid Biopsy and Metagenome Analysis. Cancers 2021, 13, 4687. https://doi.org/10.3390/cancers13184687
Park J-Y, Kang C-S, Seo H-C, Shin J-C, Kym S-M, Park Y-S, Shin T-S, Kim J-G, Kim Y-K. Bacteria-Derived Extracellular Vesicles in Urine as a Novel Biomarker for Gastric Cancer: Integration of Liquid Biopsy and Metagenome Analysis. Cancers. 2021; 13(18):4687. https://doi.org/10.3390/cancers13184687
Chicago/Turabian StylePark, Jae-Yong, Chil-Sung Kang, Ho-Chan Seo, Jin-Chul Shin, Sung-Min Kym, Young-Soo Park, Tae-Seop Shin, Jae-Gyu Kim, and Yoon-Keun Kim. 2021. "Bacteria-Derived Extracellular Vesicles in Urine as a Novel Biomarker for Gastric Cancer: Integration of Liquid Biopsy and Metagenome Analysis" Cancers 13, no. 18: 4687. https://doi.org/10.3390/cancers13184687
APA StylePark, J.-Y., Kang, C.-S., Seo, H.-C., Shin, J.-C., Kym, S.-M., Park, Y.-S., Shin, T.-S., Kim, J.-G., & Kim, Y.-K. (2021). Bacteria-Derived Extracellular Vesicles in Urine as a Novel Biomarker for Gastric Cancer: Integration of Liquid Biopsy and Metagenome Analysis. Cancers, 13(18), 4687. https://doi.org/10.3390/cancers13184687






