Predict Early Recurrence of Resectable Hepatocellular Carcinoma Using Multi-Dimensional Artificial Intelligence Analysis of Liver Fibrosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
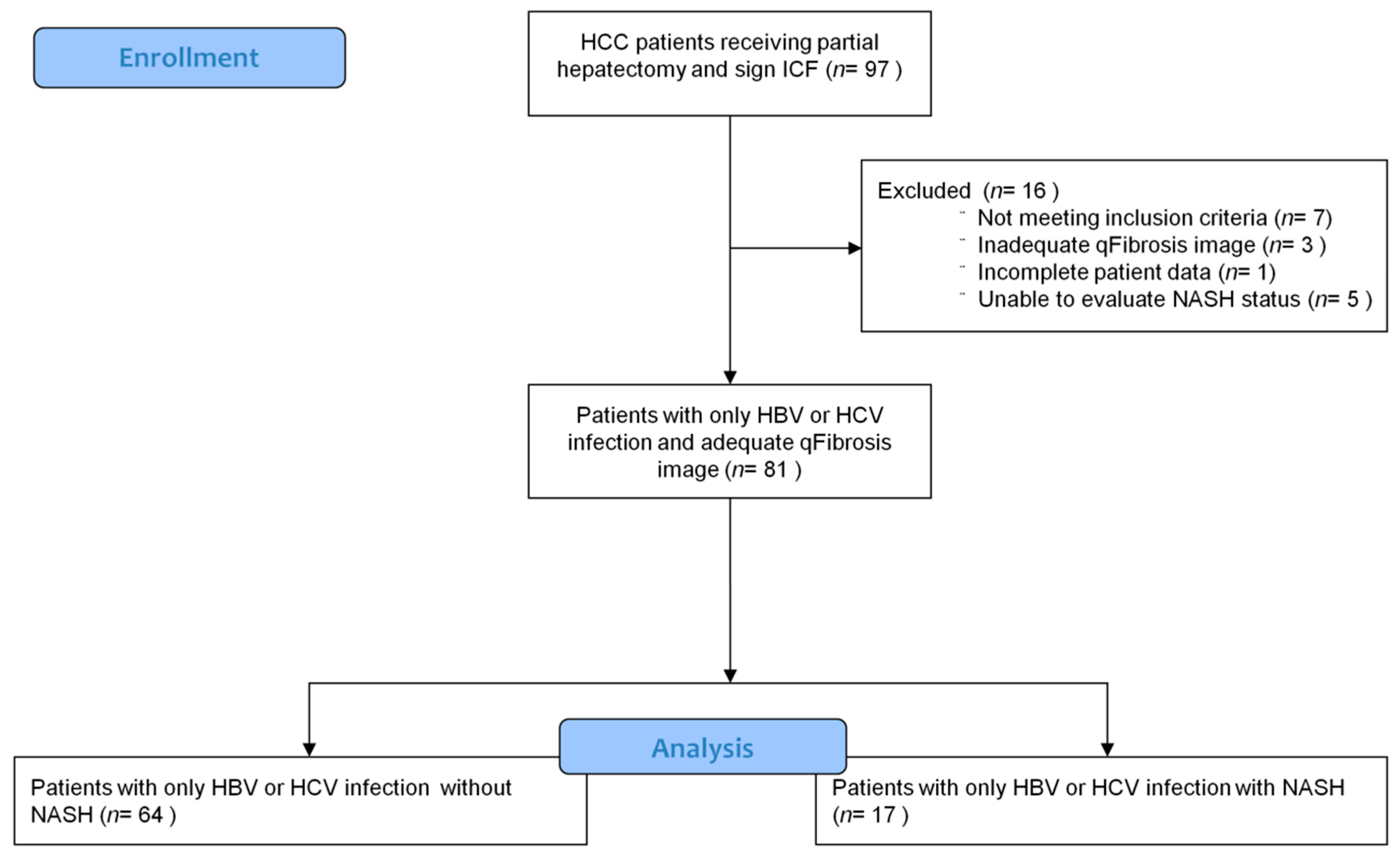
2.1. Study Population
2.2. Image Acquisition System
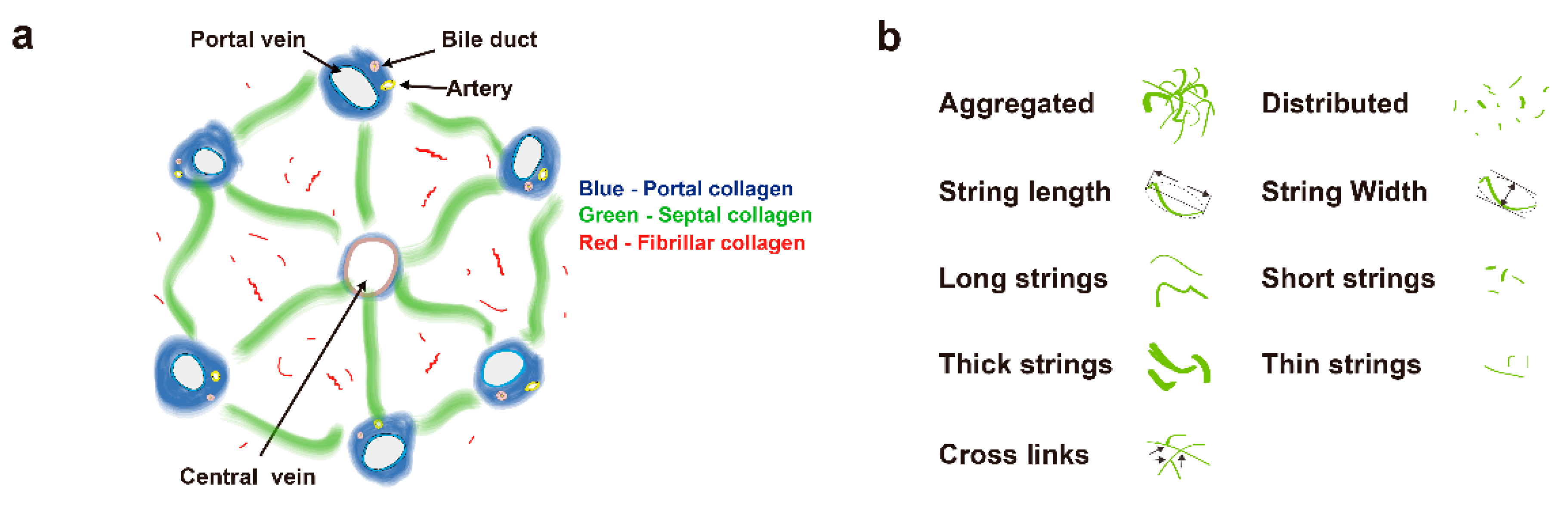
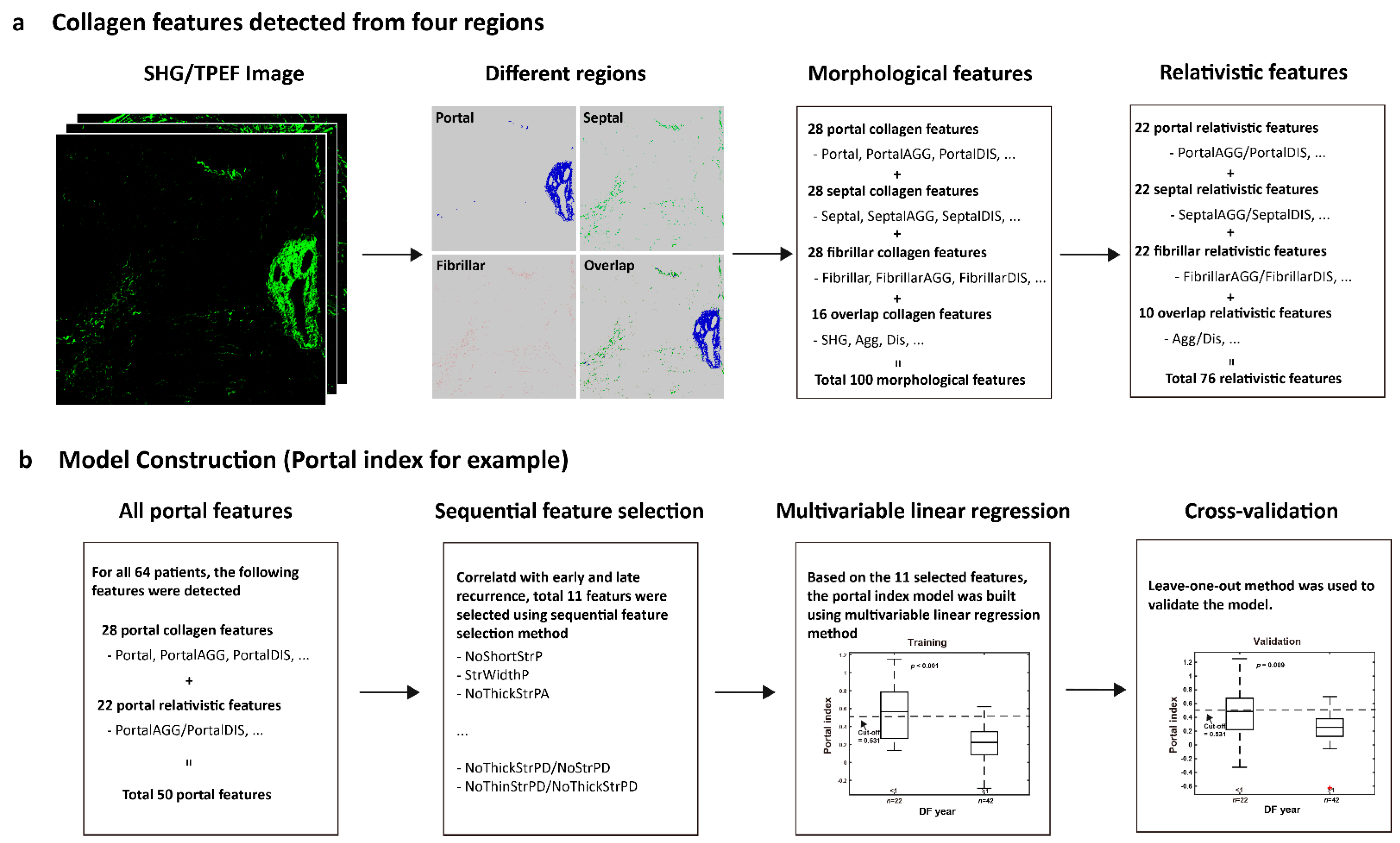
2.3. Image Quantification
2.4. Model Construction
2.5. Statistical Analysis
3. Results
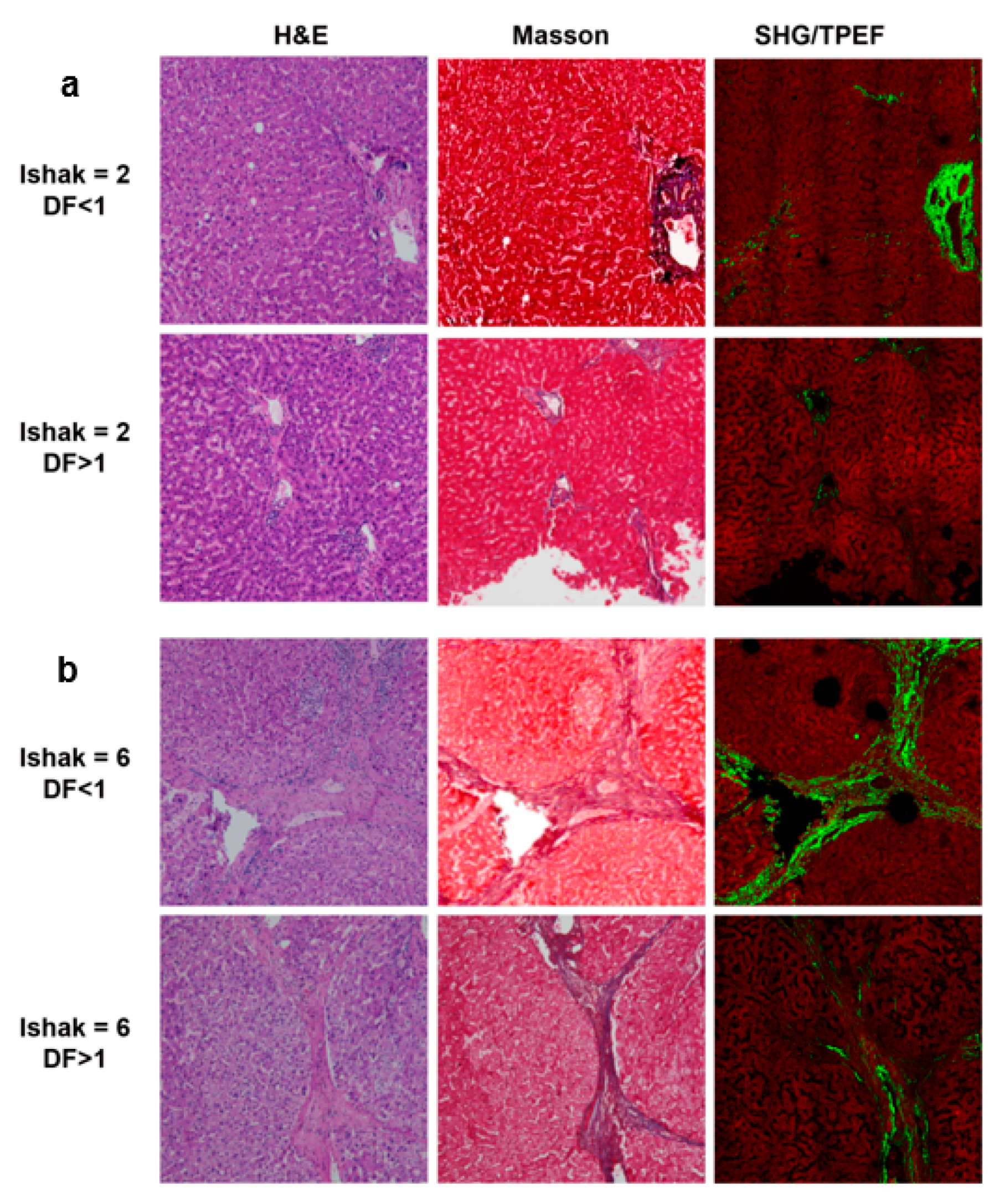
3.1. Patient Enrollment and Characteristics
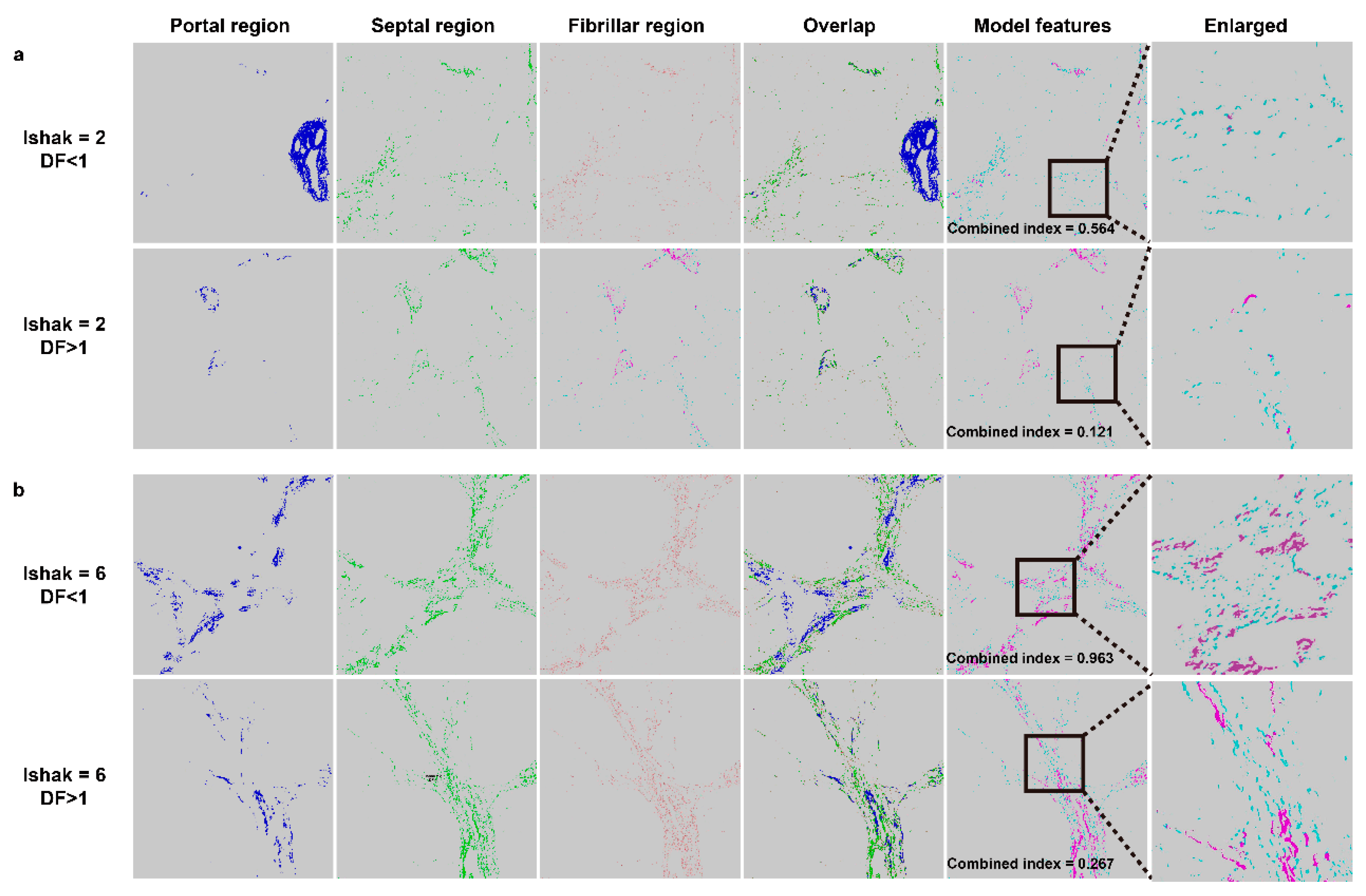
3.2. Features for Constructing the Combined Index
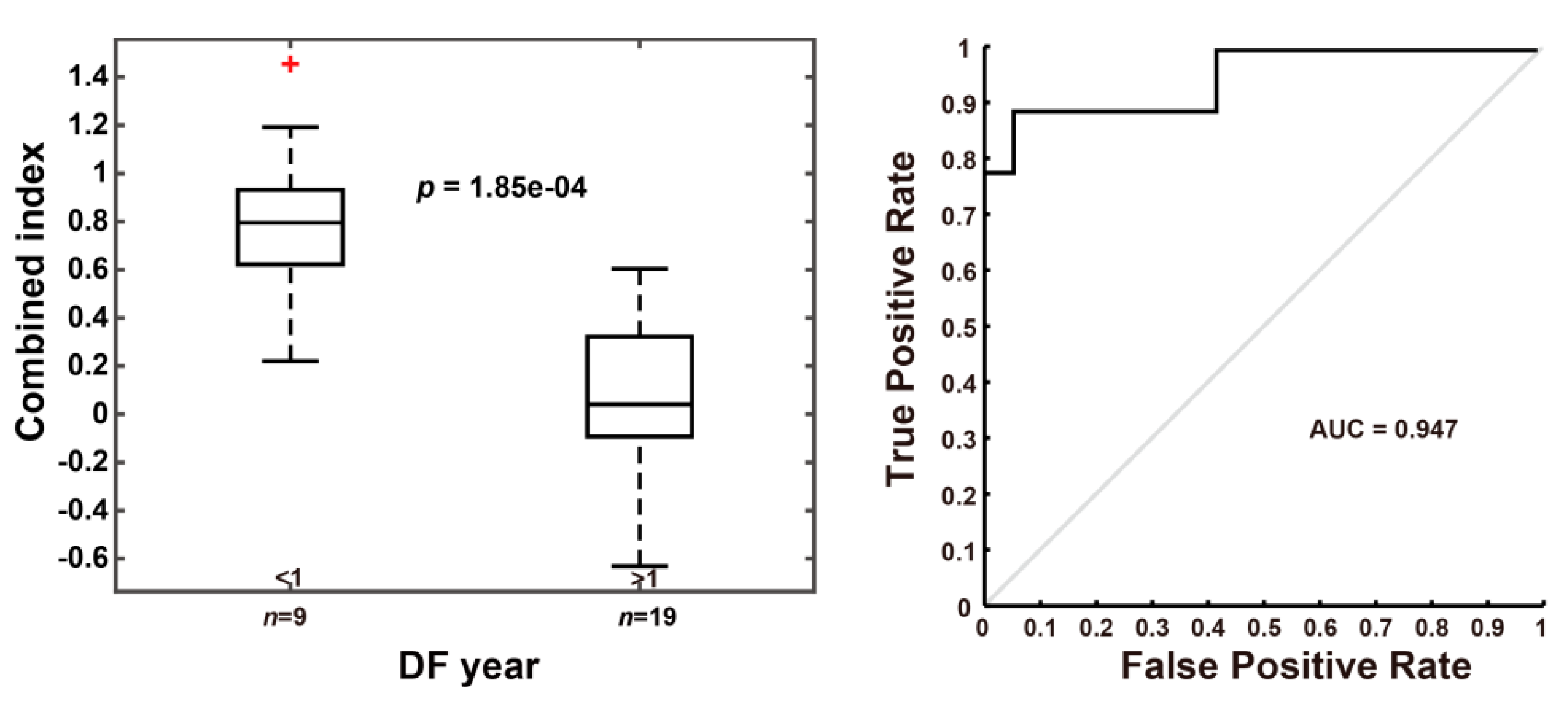
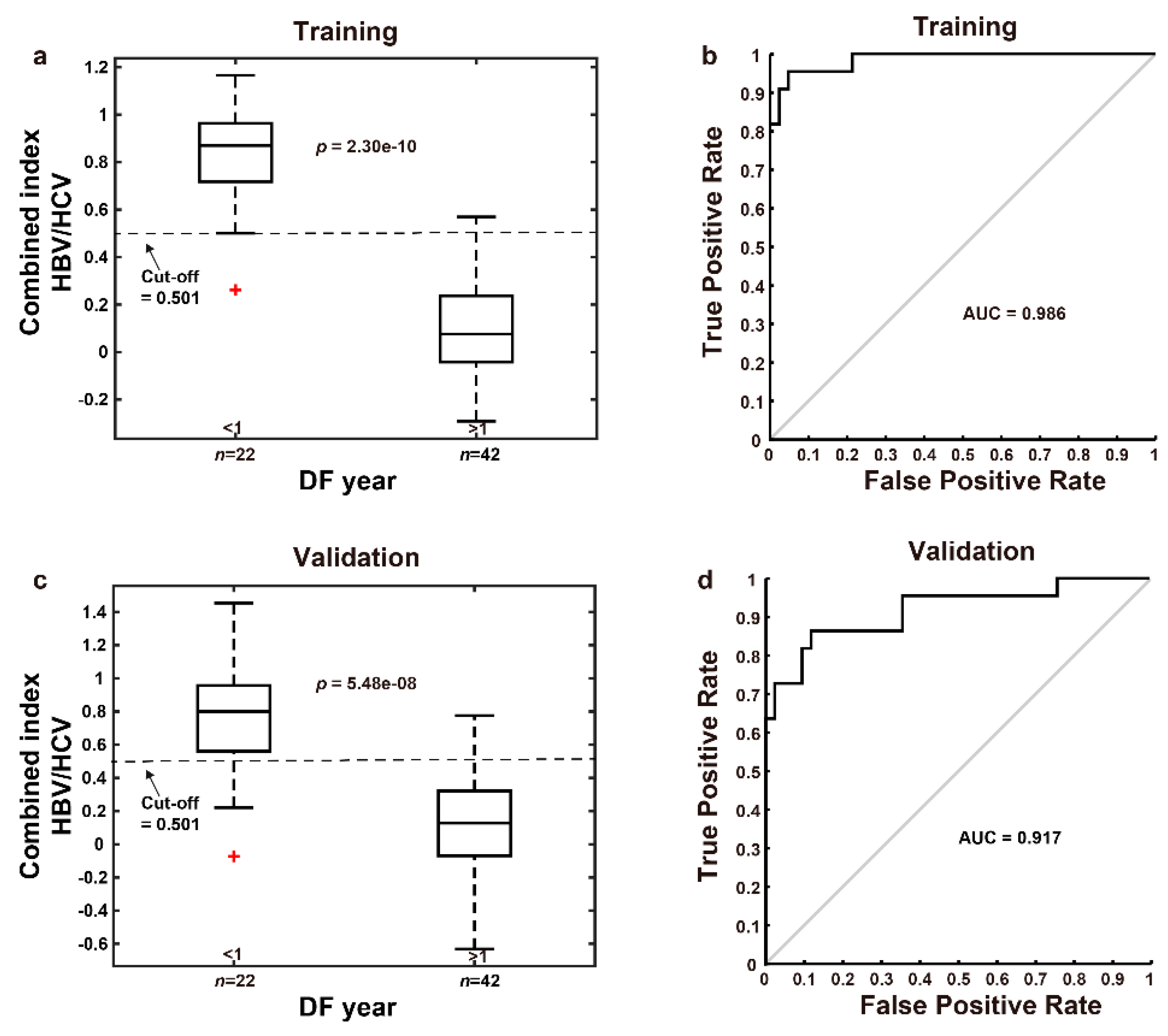
3.3. Using the Combined Index of qFibrosis to Predict Early Recurrence of HCC
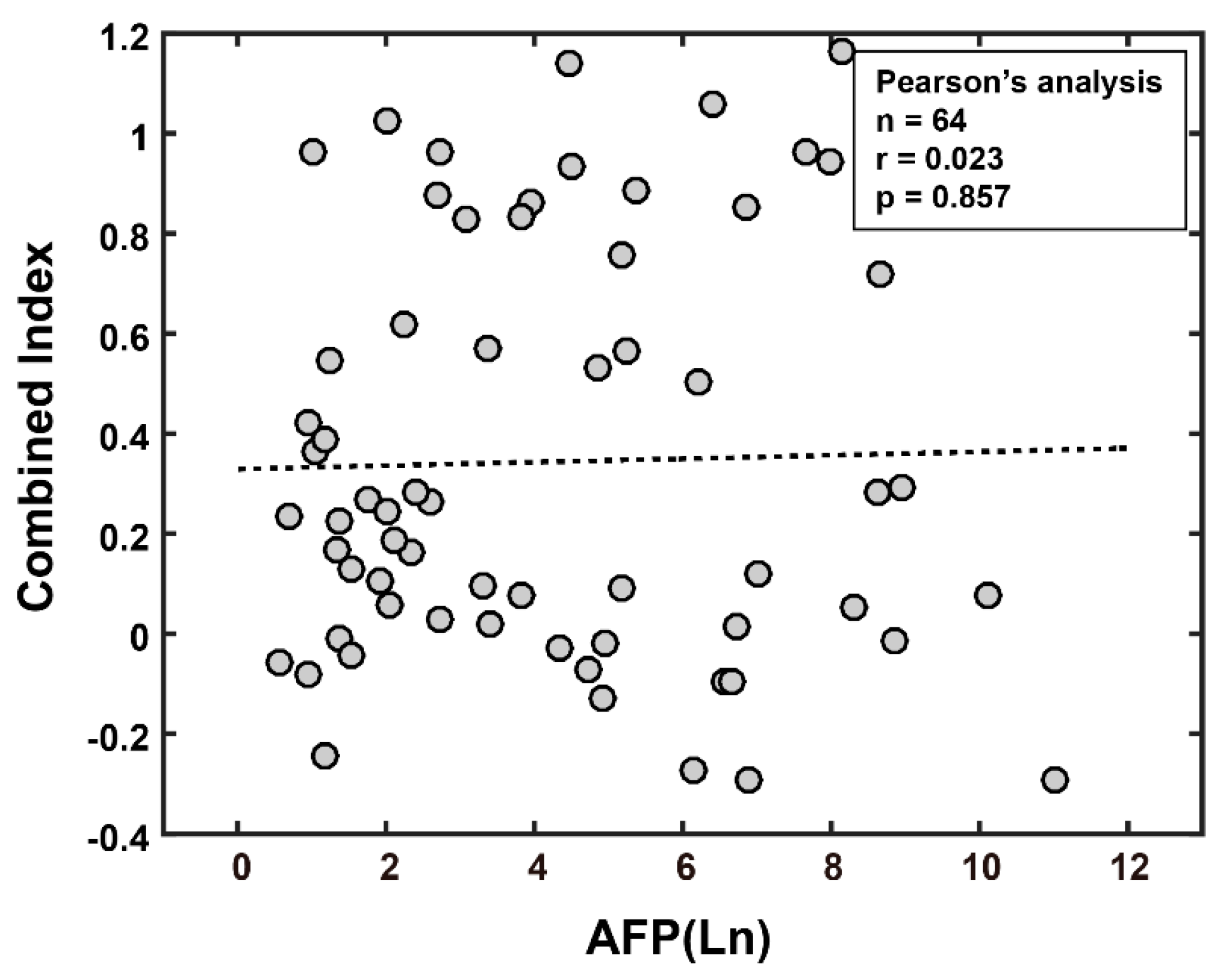
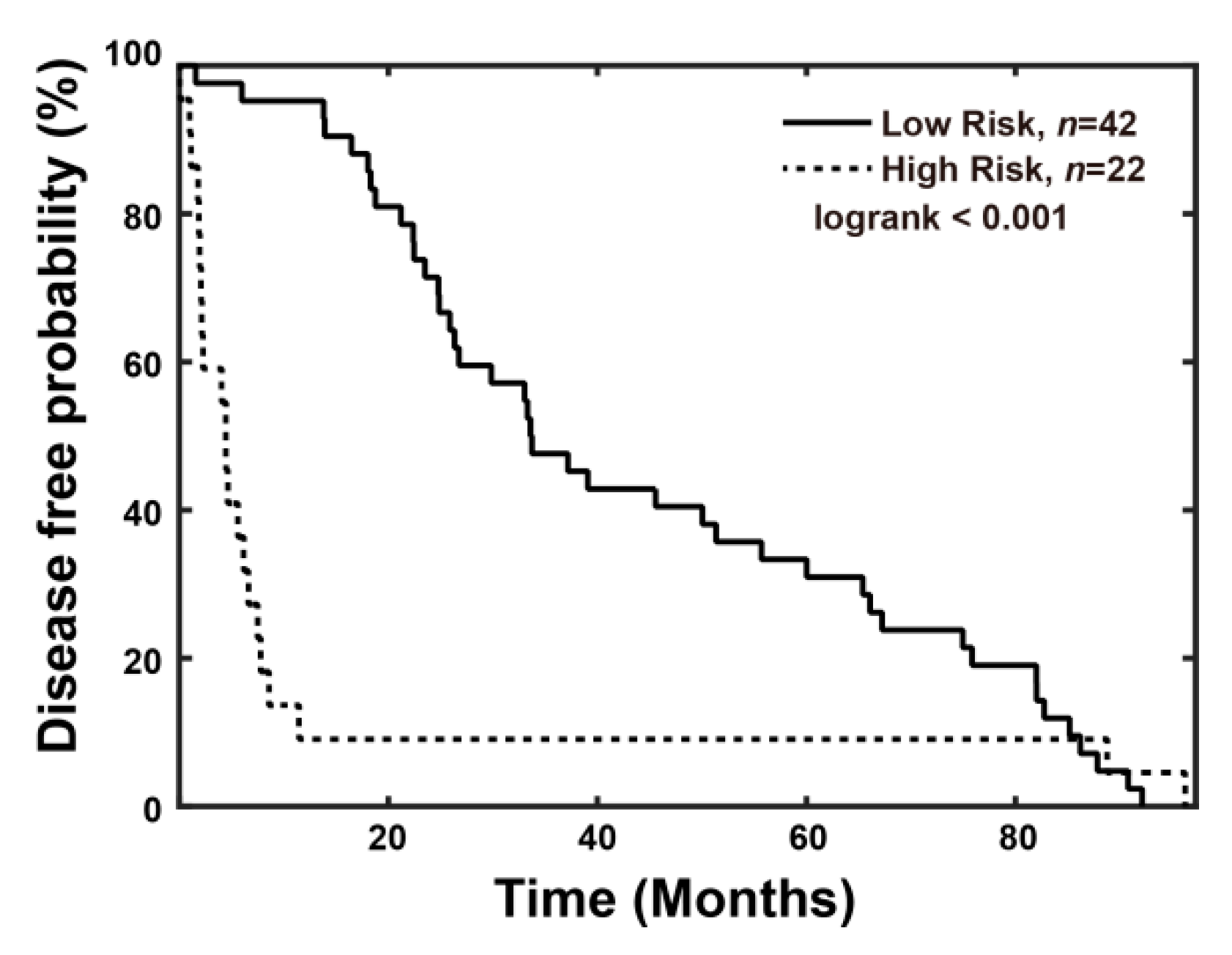
3.3.1. Combined Index Is a Better Predictor of Early Recurrence than Alpha Fetoprotein
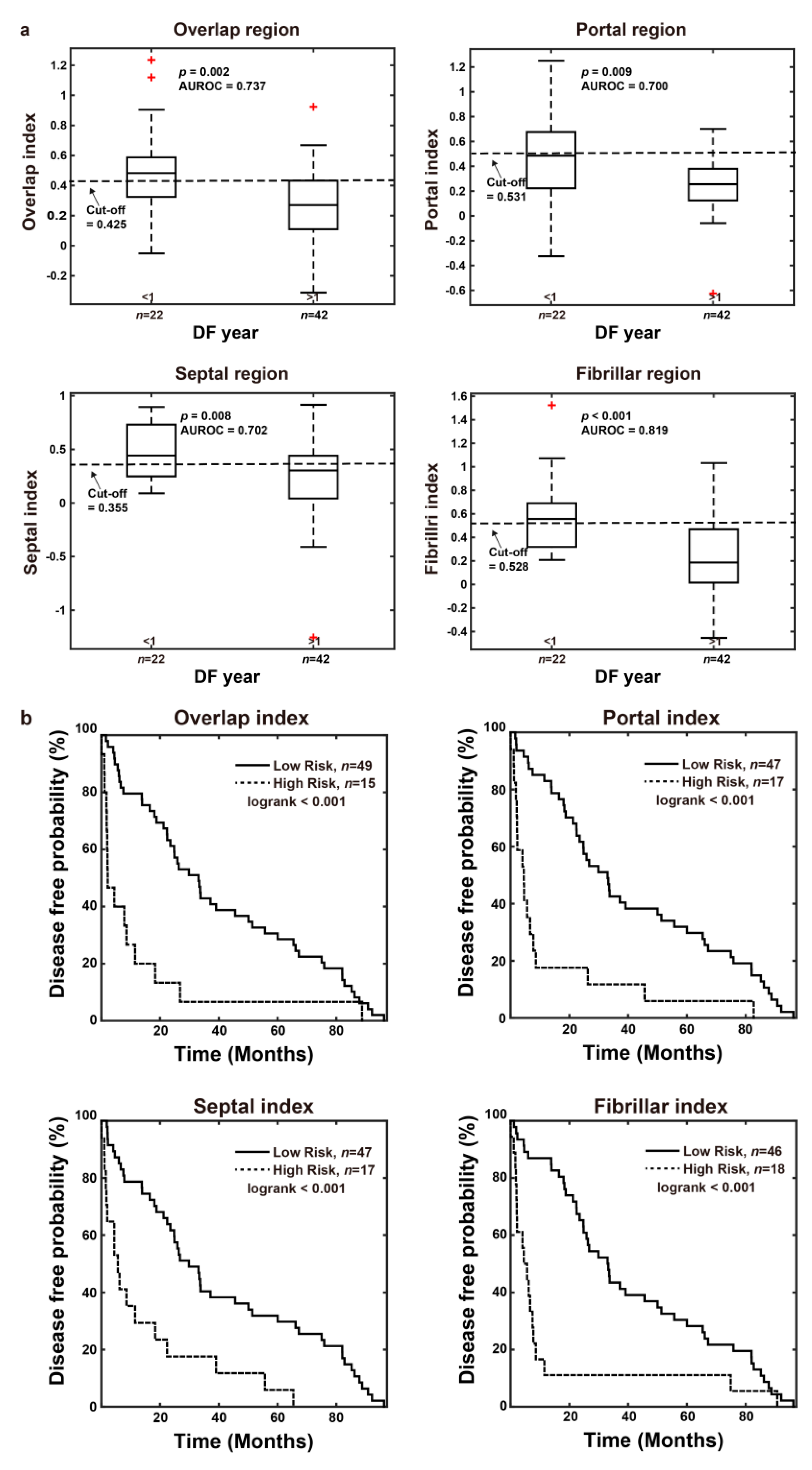
3.3.2. The Combined Index Significantly Predicts Early Recurrence as Compared to Other Regions and Features of Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | NASH Patients (n = 64) | Non-NASH Patients (n = 17) | Total (n = 81) |
|---|---|---|---|
| Age (years) | |||
| median (range) | 55 (27–81) | 60 (33–74) | 56 (27–81) |
| Gender | |||
| Male | 47 (73%) | 10 (59%) | 57 (70%) |
| Female | 17 (27%) | 7 (41%) | 24 (30%) |
| Viral hepatitis type | |||
| HBV | 51 (80%) | 13 (76%) | 64 (79%) |
| HCV | 13 (20%) | 4 (24%) | 17 (21%) |
| Liver cirrhosis | |||
| Yes | 28 (44%) | 11 (65%) | 39 (48%) |
| No | 36 (56%) | 6 (35%) | 42 (52%) |
| Histologic grade | |||
| Well | 6 (9%) | 2 (12%) | 8 (10%) |
| Moderate | 50 (78%) | 14 (82%) | 64 (79%) |
| Poor | 8 (13%) | 1 (6%) | 9 (11%) |
| Vascular invasion | |||
| Yes | 27 (42%) | 2 (12%) | 29 (36%) |
| No | 37 (58%) | 15 (88%) | 52 (64%) |
| Tumor size (cm) | |||
| ≤5 | 37 (58%) | 16 (94%) | 53 (65%) |
| >5 | 27 (42%) | 1 (6%) | 28 (35%) |
| AFP (ng/mL) | |||
| ≤20 | 27 (42%) | 10 (59%) | 37 (46%) |
| >20 | 37 (58%) | 7 (41%) | 44 (54%) |
| Pathological Stage | |||
| I/II | 54 (84%) | 17 (100%) | 71 (88%) |
| III/IV | 10 (16%) | 0 (0%) | 10 (12%) |
| Clinical Stage | |||
| I/II | 50 (78%) | 16 (94%) | 66 (81%) |
| III/IV | 14 (22%) | 1 (6%) | 15 (19%) |
| MELD score | |||
| ≤9 | 59 (92%) | 16 (94%) | 75 (93%) |
| ≥10 | 5 (8%) | 1 (6%) | 6 (7%) |
| BCLC Stage | |||
| 0/A | 50 (78%) | 14 (82%) | 64 (79%) |
| B/C | 14 (22%) | 3 (18%) | 17 (21%) |
| Child-Pugh class | |||
| A5 | 62 (97%) | 17 (100%) | 79 (98%) |
| A6 | 2 (3%) | 0 (0%) | 2 (2%) |
| ECOG PS | |||
| 0 | 61 (95%) | 16 (94%) | 77 (95%) |
| 1 | 3 (5%) | 1 (6%) | 4 (5%) |
| Creatinine level (mg/dL) | |||
| Median (IQR) | 0.9 (0.79–1.0) | 0.8 (0.665–0.94) | 0.9 (0.705–0.995) |
| Liver function enzyme | |||
| AST (mg/dL) | |||
| Median (IQR) | 48.5 (33–73) | 37 (29–67.5) | 47 (33–71) |
| ALT (mg/dL) | |||
| Median (IQR) | 41.5 (31.5–80.25) | 33.5 (28.5–37.75) | 37.5 (30–64.5) |
| Comorbidities | |||
| Hypertension | 13 (20%) | 8 (47%) | 21 (26%) |
| Diabetes mellitus | 5 (8%) | 3 (18%) | 8 (10%) |
| Chronic kidney disease | 4 (6%) | 1 (6%) | 5 (6%) |
| Coronary artery disease | 1 (2%) | 0 (0%) | 1 (1%) |
| Cerebrovascular accident | 1 (2%) | 0 (0%) | 1 (1%) |
Appendix B
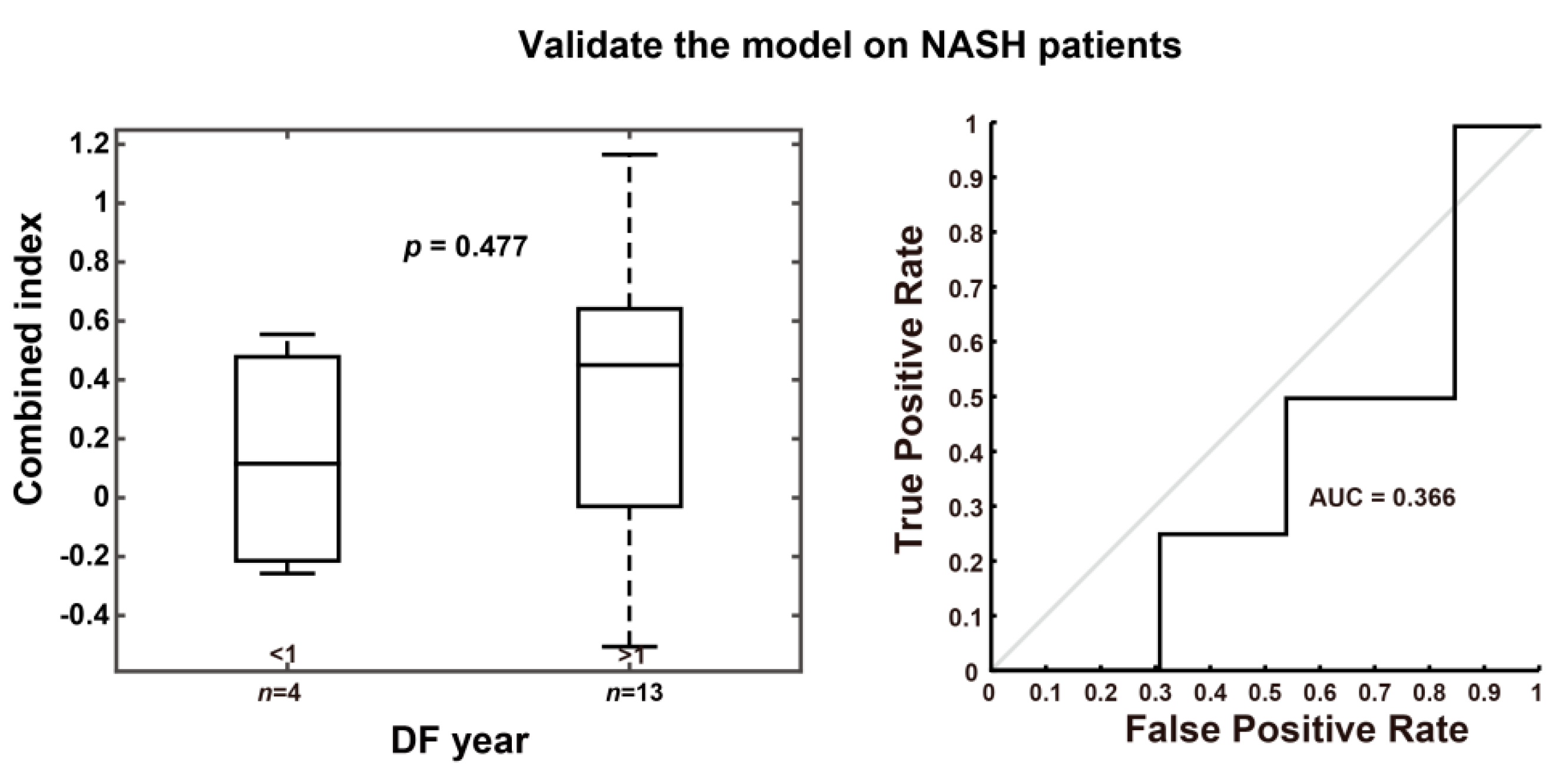
| Group | p Value between DF Year < 1 and > 1 | AUROC |
|---|---|---|
| NASH patients (n = 17) | 0.477 | 0.366 |
Appendix C
| Characteristics | Combined Index > 0.501 | AFP > 20 ng/mL |
|---|---|---|
| AUC | 0.917 | 0.595 |
| Sensitivity, % | 81.8 | 68.2 |
| Specificity, % | 90.5 | 47.6 |
| FPR, % | 9.5 | 52.4 |
| FNR, % | 18.2 | 31.8 |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.-Y.; Wang, S.-Y.; Lin, S.-M.; Chen, K.-Y.; Tseng, J.-H.; Ho, M.-C.; Lee, R.-C.; Liang, P.-C.; Liao, L.-Y.; Huang, K.-W.; et al. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med Assoc. 2021, 120, 1051–1060. [Google Scholar] [CrossRef]
- Chen, D.-S. Hepatocellular carcinoma in Taiwan. Hepatol. Res. 2007, 37, S101–S105. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Farazi, P.; DePinho, R. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.Y.; Lau, S.H.Y. The current role of radiofrequency ablation in the treatment of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 122–126. [Google Scholar] [CrossRef]
- Ko, S.; Kanehiro, H.; Hisanaga, M.; Nagao, M.; Ikeda, N.; Nakajima, Y. Liver fibrosis increases the risk of intrahepatic recur-rence after hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2002, 89, 57–62. [Google Scholar] [CrossRef]
- 8Tai, D.C.; Tan, N.; Xu, S.; Kang, C.H.; Chia, S.M.; Cheng, C.L.; Wee, A.; Wei, C.L.; Raja, A.M.; Xiao, G.; et al. Fibro-C-Index: Comprehensive, morphology-based quantification of liver fibrosis using second harmonic generation and two-photon microscopy. J. Biomed. Opt. 2009, 14, 044013. [Google Scholar]
- He, Y.; Kang, C.H.; Xu, S.; Tuo, X.; Trasti, S.; Tai, D.C.S.; Raja, A.M.; Peng, Q.; So, P.T.C.; Rajapakse, J.C.; et al. Toward surface quantification of liver fibrosis progression. J. Biomed. Opt. 2010, 15, 056007. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tai, D.; Wee, A.; Welsch, R.; So, P.; Yu, H.; Rajapakse, J. Automated scoring of liver fibrosis through combined features from different collagen groups. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–1 September 2011; pp. 4503–4506. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Tai, D.C.; Wang, S.; Cheng, C.L.; Peng, Q.; Yan, J.; Chen, Y.; Sun, J.; Liang, X.; et al. qFibrosis: A fully-quantitative innovative method incorporating histological features to facilitate accurate fibrosis scoring in animal model and chronic hepatitis B patients. J. Hepatol. 2014, 61, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhou, J.; Wu, X.; Chen, Y.; Piao, H.; Lu, L.; Ding, H.; Nan, Y.; Jiang, W.; Wang, T.; et al. Quantitative assessment of liver fibrosis (qFibrosis) reveals precise outcomes in Ishak “stable” patients on anti-HBV therapy. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wong, G.L.-H.; He, F.-P.; Sun, J.; Chan, A.W.-H.; Yang, J.; Shu, S.S.-T.; Liang, X.; Tse, Y.K.; Fan, X.-T.; et al. Quantifying and monitoring fibrosis in non-alcoholic fatty liver disease using dual-photon microscopy. Gut 2019, 69, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Soon, G.; Wee, A. Updates in the quantitative assessment of liver fibrosis for nonalcoholic fatty liver disease: Histological perspective. Clin. Mol. Hepatol. 2021, 27, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Rückstieß, T.; Osendorfer, C.; van der Smagt, P. Sequential feature selection for classification. Adv. Artif. Intell. 2011, 7106, 132–141. [Google Scholar]
- Rushing, C.; Bulusu, A.; Hurwitz, H.I.; Nixon, A.B.; Pang, H. A leave-one-out cross-validation SAS macro for the identification of markers associated with survival. Comput. Biol. Med. 2015, 57, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.-T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recognit. 2015, 48, 2839–2846. [Google Scholar] [CrossRef]
- Ma, W.-J.; Wang, H.-Y.; Teng, L.-S. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J. Surg. Oncol. 2013, 11, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Q.; Melandro, F.; Pinheiro, R.S.; Donfrancesco, A.; Fadel, B.A.; Sandri, G.B.L.; Rossi, M.; Berloco, P.B.; Frattaroli, F.M. Alpha-Fetoprotein and novel tumor biomarkers as predictors of hepatocellular carcinoma recurrence after surgery: A brilliant star raises again. Int. J. Hepatol. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Chung, H.A.; Kim, J.-H.; Hwang, Y.; Choi, H.S.; Ko, S.Y.; Choe, W.H.; Kwon, S.Y. Noninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablation. Saudi J. Gastroenterol. 2016, 22, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Leow, W.-Q.; Bedossa, P.; Liu, F.; Wei, L.; Lim, K.-H.; Wan, W.-K.; Ren, Y.; Chang, J.P.-E.; Tan, C.-K.; Wee, A.; et al. An improved qfibrosis algorithm for precise screening and enrollment into non-alcoholic steatohepatitis (NASH) clinical trials. Diagnostics 2020, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Goh, B.B.G.; Tiniakos, D.; Wee, A.; Leow, W.; Zhao, J.; Rao, H.; Wang, X.; Wang, Q.; Wan, W.; et al. qFIBS: An automated technique for quantitative evaluation of fibrosis, inflammation, ballooning, and steatosis in patients with nonalcoholic steatohepatitis. Hepatology 2019, 71, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Castera, L.; Halfon, P.; Pol, S.; Mangia, A.; Di Marco, V.; Pirisi, M.; Voiculescu, M.; Bourliere, M.; Alberti, A. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: An international study of 2411 cases. Aliment. Pharmacol. Ther. 2011, 34, 1202–1216. [Google Scholar] [CrossRef]
- Naveau, S.; Raynard, B.; Ratziu, V.; Abella, A.; Imbert–Bismut, F.; Messous, D.; Beuzen, F.; Capron, F.; Thabut, D.; Munteanu, M.; et al. Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. Clin. Gastroenterol. Hepatol. 2005, 3, 167–174. [Google Scholar] [CrossRef]
- Naveau, S.; Gaudé, G.; Asnacios, A.; Agostini, H.; Abella, A.; Barri-Ova, N.; Dauvois, B.; Prévot, S.; Ngo, Y.; Munteanu, M.; et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology 2008, 49, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.; George, J.; Bugianesi, E.; Rossi, E.; De Boer, W.B.; Van Der Poorten, D.; Ching, H.L.; Bulsara, M.; Jeffrey, G.P. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2011, 26, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Guha, I.N.; Parkes, J.; Roderick, P.R.; Harris, S.; Rosenberg, W. Non-invasive markers associated with liver fibrosis in non-alcoholic fatty liver disease. Gut 2006, 55, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Almpanis, Z.; Demonakou, M.; Tiniakos, D. Evaluation of liver fibrosis: “Something old, something new…”. Ann. Gastroenterol. 2016, 29, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Toyoda, H.; Kobayashi, M.; Koiwa, Y.; Fujii, H.; Fujita, K.; Maeda, A.; Kaneoka, Y.; Hazama, S.; Nagano, H.; et al. Prediction of early recurrence of hepatocellular carcinoma after resection using digital pathology images assessed by machine learning. Mod. Pathol. 2021, 34, 417–425. [Google Scholar] [CrossRef]
- Schoenberg, M.B.; Bucher, J.N.; Koch, D.; Börner, N.; Hesse, S.; De Toni, E.N.; Seidensticker, M.; Angele, M.K.; Klein, C.; Bazhin, A.V.; et al. A novel machine learning algorithm to predict disease free survival after resection of hepatocellular carcinoma. Ann. Transl. Med. 2020, 8, 434. [Google Scholar] [CrossRef]
- Mai, R.-Y.; Zeng, J.; Meng, W.-D.; Lu, H.-Z.; Liang, R.; Lin, Y.; Wu, G.-B.; Li, L.-Q.; Ma, L.; Ye, J.-Z.; et al. Artificial neural network model to predict post-hepatectomy early recurrence of hepatocellular carcinoma without macroscopic vascular invasion. BMC Cancer 2021, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Zeng, Y.; Liu, Z.; Ma, H.; Liu, J. Development and validation of a machine learning prognostic model for hepatocellular carcinoma recurrence after surgical resection. Front. Oncol. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Yamaguchi, T.; Nagamatsu, H.; Shiina, S. AI-based radiological imaging for HCC: Current status and future of ultrasound. Diagnostics 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centonze, L.; Sandro, S.D.; Lauterio, A.; Carlis, R.D.; Sgrazzutti, C.; Ciulli, C.; Vella, I.; Vicentin, I.; Incarbone, N.; Bagnardi, V.; et al. A retrospective single-centre analysis of the oncological impact of LI-RADS classification applied to Metroticket 2.0 cal-culator in liver transplantation: Every nodule matters. Transpl. Int. 2021, 34, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
| No. | Features | Estimated Coefficients | Region |
|---|---|---|---|
| 0 | Intercept | 3.838 | - |
| 1 | SHG | 4.300 | Overlap |
| 2 | StrOrientation | 1.280 | Overlap |
| 3 | StrAreaPA | −2.413 | Portal |
| 4 | StrAreaPD | −1.269 | Portal |
| 5 | NoThickStrS | 3.182 | Septal |
| 6 | NoThinStrSA | −2.486 | Septal |
| 7 | Fibrillar | −2.591 | Fibrillar |
| 8 | NoThickStrF | −1.889 | Fibrillar |
| 9 | NoThickStrFA | 1.735 | Fibrillar |
| 10 | NoShortStr/NoLongStr | −3.733 | Overlap/Overlap |
| 11 | StrLengthP/StrWidthP | −0.859 | Portal/Portal |
| 12 | NoThickStrPD/NoStrPD | −0.771 | Portal/Portal |
| 13 | NoThinStrPD/NoThickStrPD | −0.599 | Portal/Portal |
| 14 | SeptalAGG/Septal | −1.782 | Septal/Septal |
| 15 | StrLengthSD/StrWidthSD | −0.761 | Fibrillar/Fibrillar |
| 16 | NoThinStrFA/NoThickStrFA | 0.957 | Fibrillar/Fibrillar |
| 17 | NoThickStrFD/NoStrFD | −0.443 | Fibrillar/Fibrillar |
| 18 | StrLengthFD/StrWidthFD | 1.067 | Fibrillar/Fibrillar |
| Variable | Number of Patients | Death | p-Value | |
|---|---|---|---|---|
| Number | Percent | |||
| Gender | 0.126 | |||
| Male | 47 | 21 | 44.7 | |
| Female | 17 | 4 | 23.5 | |
| Groups | 0.703 | |||
| HBV + cirrhosis | 20 | 7 | 35.0 | |
| HBV | 31 | 13 | 41.9 | |
| HCV+cirrhosis | 8 | 4 | 50.0 | |
| HCV | 5 | 1 | 20.0 | |
| Liver cirrhosis | 0.974 | |||
| No | 36 | 14 | 38.9 | |
| Yes | 28 | 11 | 39.3 | |
| Histologic grade | 0.431 | |||
| Well | 6 | 1 | 16.7 | |
| Moderate | 50 | 20 | 40.0 | |
| Poor | 8 | 4 | 50.0 | |
| Vascular invasion | 0.021 * | |||
| No | 37 | 10 | 27.0 | |
| Yes | 27 | 15 | 55.6 | |
| Tumor size (cm) | 0.005 * | |||
| ≤5 | 37 | 9 | 24.3 | |
| > 5 | 27 | 16 | 59.3 | |
| AFP (ng/mL) | 0.066 | |||
| ≤20 | 27 | 7 | 25.9 | |
| >20 | 37 | 18 | 48.6 | |
| Pathological Stage | 0.029 * | |||
| Stage I, II | 54 | 18 | 33.3 | |
| Stage III, IV | 10 | 7 | 70.0 | |
| Clinical Stage | 0.005 * | |||
| Stage I, II | 50 | 15 | 30.0 | |
| Stage III, IV | 14 | 10 | 71.4 | |
| Combined Index | <0.001 * | |||
| ≤0.501 | 42 | 9 | 21.4 | |
| >0.501 | 22 | 16 | 72.7 | |
| Factors | Multivariate | |||
|---|---|---|---|---|
| HR | 95%CI | p-Value | ||
| Disease-free survival | ||||
| Gender (Female vs. Male) | 0.804 | 0.352 | 1.838 | 0.605 |
| Liver cirrhosis (Yes vs. No) | 1.344 | 0.660 | 2.735 | 0.415 |
| Histologic grade (Moderate vs. Well) | 0.815 | 0.260 | 2.554 | 0.725 |
| Histologic grade (Poor vs. Well) | 1.361 | 0.324 | 5.709 | 0.674 |
| Vascular invasion (Yes vs. No) | 0.635 | 0.265 | 1.518 | 0.307 |
| Tumor size (>5 vs. ≤5) | 1.546 | 0.688 | 3.476 | 0.291 |
| AFP (>20 vs. ≤20) | 1.510 | 0.651 | 3.501 | 0.337 |
| Pathological Stage (III/IV vs. I/II) | 1.960 | 0.692 | 5.547 | 0.205 |
| Clinical Stage (III/IV vs. I/II) | 2.029 | 0.816 | 5.047 | 0.128 |
| MELD score (≥10 vs. ≤9) | 4.167 | 1.173 | 14.803 | 0.027 * |
| BCLC Stage (B/C vs. 0/A) | 1.444 | 0.601 | 3.466 | 0.411 |
| Combined Index (High risk vs. Low risk) | 3.821 | 1.596 | 9.153 | 0.003 * |
| Overall survival | ||||
| Gender (Female vs. Male) | 0.330 | 0.092 | 1.176 | 0.087 |
| Liver cirrhosis (Yes vs. No) | 1.517 | 0.580 | 3.970 | 0.395 |
| Histologic grade (Moderate vs. Well) | 1.998 | 0.168 | 23.790 | 0.584 |
| Histologic grade (Poor vs. Well ) | 3.534 | 0.218 | 57.208 | 0.374 |
| Vascular invasion (Yes vs. No) | 2.137 | 0.728 | 6.277 | 0.167 |
| Tumor size (>5 vs. ≤5) | 1.467 | 0.470 | 4.584 | 0.509 |
| AFP (>20 vs. ≤20) | 4.639 | 1.358 | 15.840 | 0.014 * |
| Pathological Stage (III/IV vs. I/II) | 0.396 | 0.110 | 1.425 | 0.156 |
| Clinical Stage (III/IV vs. I/II) | 4.285 | 1.160 | 15.825 | 0.029 * |
| MELD score (≥10 vs. ≤9) | 7.628 | 1.393 | 41.757 | 0.019 * |
| BCLC Stage (B/C vs. 0/A) | 1.061 | 0.367 | 3.069 | 0.913 |
| Combined Index (High risk vs. Low risk) | 4.059 | 1.366 | 12.058 | 0.012 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, I.-T.; Yen, C.-S.; Wang, W.-L.; Tsai, H.-W.; Chu, C.-Y.; Chang, M.-Y.; Hou, Y.-F.; Yen, C.-J. Predict Early Recurrence of Resectable Hepatocellular Carcinoma Using Multi-Dimensional Artificial Intelligence Analysis of Liver Fibrosis. Cancers 2021, 13, 5323. https://doi.org/10.3390/cancers13215323
Liu I-T, Yen C-S, Wang W-L, Tsai H-W, Chu C-Y, Chang M-Y, Hou Y-F, Yen C-J. Predict Early Recurrence of Resectable Hepatocellular Carcinoma Using Multi-Dimensional Artificial Intelligence Analysis of Liver Fibrosis. Cancers. 2021; 13(21):5323. https://doi.org/10.3390/cancers13215323
Chicago/Turabian StyleLiu, I-Ting, Chia-Sheng Yen, Wen-Lung Wang, Hung-Wen Tsai, Chang-Yao Chu, Ming-Yu Chang, Ya-Fu Hou, and Chia-Jui Yen. 2021. "Predict Early Recurrence of Resectable Hepatocellular Carcinoma Using Multi-Dimensional Artificial Intelligence Analysis of Liver Fibrosis" Cancers 13, no. 21: 5323. https://doi.org/10.3390/cancers13215323
APA StyleLiu, I.-T., Yen, C.-S., Wang, W.-L., Tsai, H.-W., Chu, C.-Y., Chang, M.-Y., Hou, Y.-F., & Yen, C.-J. (2021). Predict Early Recurrence of Resectable Hepatocellular Carcinoma Using Multi-Dimensional Artificial Intelligence Analysis of Liver Fibrosis. Cancers, 13(21), 5323. https://doi.org/10.3390/cancers13215323






