Simple Summary
The PD-1/PD-L1 axis is not only involved in anti-tumour immune evasion of germinal center (GC)-derived diffuse large B cell lymphomas (DLBCL), but also inherently in the fine-tuned regulation of normal GC reactions during humoral immune responses. This checkpoint axis modulates crosstalks between B and T cells that allow positive selection for survival and proliferation. Malignant DLBCL cells may deceive and take advantage of these mechanisms to establish an immunosuppressive microenvironment. This review delves into PD-1/PD-L1 role in the complex inter-cellular interactions from normal GC reactions to DLBCL progression, in order to highlight vulnerabilities that could be targeted by promising combination immunotherapies.
Abstract
Besides a recognized role of PD-1/PD-L1 checkpoint in anti-tumour immune evasion, there is accumulating evidence that PD-1/PD-L1 interactions between B and T cells also play an important role in normal germinal center (GC) reactions. Even when smaller in number, T follicular helper cells (TFH) and regulatory T (TFR) or B (Breg) cells are involved in positive selection of GC B cells and may result critical in the lymphoma microenvironment. Here, we discuss a role of PD-1/PD-L1 during tumour evolution in diffuse large B cell lymphoma (DLBCL), a paradigm of GC-derived lymphomagenesis. We depict a progression model, in two phases, where malignant B cells take advantage of positive selection signals derived from correct antigen-presentation and PD-1/PD-L1 inter-cellular crosstalks to survive and initiate tumour expansion. Later, a constant pressure for the accumulation of genetic/epigenetic alterations facilitates that DLBCL cells exhibit higher PD-L1 levels and capacity to secrete IL-10, resembling Breg-like features. As a result, a complex immunosuppressive microenvironment is established where DLBCL cells sustain proliferation and survival by impairing regulatory control of TFR cells and limiting IL-21-mediated anti-tumour functions of TFH cells and maximize the use of PD-1/PD-L1 signaling to escape from CD8+ cytotoxic activity. Integration of these molecular and cellular addictions into a framework may contribute to the better understanding of the lymphoma microenvironment and contribute to the rationale for novel PD-1/PD-L1-based combinational immunotherapies in DLBCL.
1. Introduction
Interest in the immune-checkpoint protein programmed death 1 (PD-1), in T lymphocytes, and its ligand (PD-L1), in lymphoma B cells, have increased in parallel to the remarkable clinical outcomes demonstrated with their blockade in a broad range of tumour types [1]. Beyond its role in anti-tumour immune evasion [1,2], this PD-1/PD-L1 pathway is also inherently necessary to maintain peripheral tolerance and attenuate potentially dysregulated or damaging T-cell responses [3,4]. This is especially relevant within germinal centers (GCs) at secondary lymphoid organs, where the correct orchestration of B and T cell interactions is critical for B cell activation and efficient humoral responses [5,6]. Indeed, failure of appropriate T cell signals during GC reactions results in impaired GC maintenance and immune response [7,8] and may contribute to other genetic and epigenetic determinants in GC-derived lymphomagenesis [9,10]. Particularly, diffuse large B cell lymphoma (DLBCL) is the most common lymphoid malignancy in adults worldwide and has long been regarded as a paradigm of aggressive disease originated from GC-experienced B cells [11,12]. In this review, we describe major evidence for naturally occurring PD-1/PD-L1 signaling to fine modulate GC reactions and discuss evidences for how GC-derived malignant cells may exploit this immune-checkpoint to facilitate selection and survival first, and elude anti-tumour immune responses later at advanced DLBCL stages. With the development of the immune-oncology field and the advent of promising novel immunotherapy treatments, integration of these vulnerabilities into a framework may contribute to the rationale for PD-1/PD-L1-related combinational immunotherapy in DLBCL.
2. The PD-1/PD-L1 Axis during the Germinal Center Reaction
The continuous interactions of B cells with the small fraction of various T cell populations before and during GC reactions, have been shown to be a critical limiting factor for GC maintenance and selection [6,7,8]. Only those B cells with higher affinity to the antigen are selected and clonally expanded, whereas lower affinity B cells undergo apoptosis and are dismissed [6,10,13]. Beyond the essential signaling through the B cell receptor (BCR) and the amount of antigen peptide on major histocompatibility complex-II (pMHC-II) displayed on the cell surface [14,15], T cell-derived signals work complementarily to ensure the efficiency of GC selection and preventing autoimmunity or GC lymphomagenesis. We review here that, in addition to the well-known costimulatory CD40/CD40L axis [16], CXCR4, ICOS or T-cell secreted cytokines such as IL-4 or IL-21 [17,18,19], there are accumulating evidences that immune-checkpoint signals through PD-1/PD-L1 interactions between B and T cells play an important role in GC reaction (Figure 1).
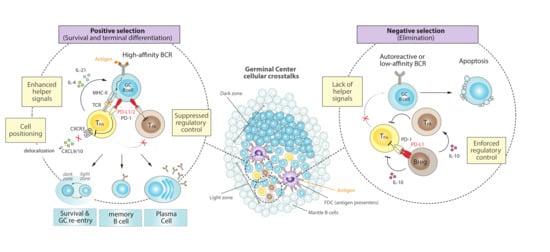
Figure 1.
Cellular crosstalks and the PD-1/PD-L1 axis during normal GC reactions. Surrounded by a mantle zone, GCs expand as a result of the fast proliferation of antigen-specific B cells that are densely packed to form a dark zone, before moving into a light zone containing FDCs and other regulatory cells that orchestrate selection. (Left) B cells that express high-affinity BCR lead to greater antigen capture and higher density of peptide-MHC-II presentation, engaging PD-1/PD-L1 signaling that promotes TFH cell positioning and signaling, suppression of regulatory control and positive selection of B cells. Survival and recirculation between the dark and light zones facilitate several iterative rounds of BCR mutation and selection, which ultimately leads to terminal differentiation into memory or antibody-secreting plasma cells. (Right) A tight regulation is necessary to avoid autoreactive or low-affinity B cells, which are unable to capture sufficient antigen and undergo apoptosis. This is also enforced by PD-L1hi Bregs and TFR cells, which dampen TFH signals and further lean towards negative selection of undesired B cells. FDC, follicular dendritic cells; Breg, regulatory B cell; TFH; T follicular helper; TFR, T follicular regulatory; BCR, B-cell receptor; TCR, T-cell receptor; MHC-II, major histocompatibility complex-II; CXCR3, C-X-C chemokine receptor type 3; CXCL9/10, C-X-C motif chemokines 9 or 10.
2.1. PD-1 in T Follicular Helper (TFH) Cells
The microanatomical distribution of PD-1 in human tonsils demonstrated that it was expressed on most T cells in the light zone (LZ) of GCs [20], which is the site where GC B cells diversified by hypermutation are selected [6]. This LZ is rich in follicular dendritic cells (FDCs) and in a smaller population of T follicular helper (TFH) cells that are critical for positive selection of B cells [13] (Figure 1, center). Indeed, a specific population of CD4+ TFH cells, which are also CXCR5+, ICOS+ and PD1+, have demonstrated to be fundamental during GC formation and a crucial source of cytokines required for a correct GC expansion and B cell isotype class switching [8,21,22]. The high expression of PD-1 by TFH cells inside the GC territory [23] might simply reflect experience of antigen stimulation [24]. However, PD-1 engagement in TFH cells can attenuate ICOS signaling [25], which is critical for TFH generation [22], and suppresses overall TFH cell development [24,26]. This is consistent with the observations in PD ligands- or PD-1-deficient mice that the lack of PD-1 signaling results in more CD4+ TFH cells [27,28]. However, these PD-1 signaling-deficient TFH cells failed to promote GC B cell survival, either because they showed a diminished capacity to synthesize the important pro-survival cytokines IL-4 and IL-21 (in the absence of PD-1) [27,29] or, on the contrary, because they displayed a hyperactive phenotype with increased IL-21 production and expression of FasL that promotes GC B cell death (in the absence of PD-L1 alone) [28]. These apparently contradictory TFH phenotypes might be capturing differences in PD-1 signaling by PD-L1 or PD-L2, but ultimately reinforce the idea that despite the negative role of PD-1 in TFH development, PD-1 is required for optimal IL-21 production by TFH cells and tight regulation of GC B cell survival [24,27,30] (Figure 1, left).
Another positive impact of PD-1 function in GC biology occurs during the orchestration of the dynamic follicular environment, where PD-1 initially ensures that activated TFH cells that are recruited into follicles are those that highly co-express ICOS and PD-1 [24,31]. Then, PD-1 helps to concentrate TFH cells in GCs by restricting CXCR3 expression and therefore preventing delocalization to interfollicular regions in response to CXCL9 and/or CXCL10 [24]. Furthermore, PD-1 can dampen T cell receptor (TCR) ligand sensitivity [32,33] and thereby help to promote affinity-based selection by TFH cells through enforcing a more stringent selection threshold for competing GC B cells [24]. Altogether, these studies support the idea that signaling through PD-1 by CD4+ TFH is essential to control both proper positioning and helper functions to GC responses (Figure 1, left).
On the other hand, CD8+ T cells have been traditionally considered as effector cytotoxic cells that protect against viruses and tumour cells releasing cytokines or differentiating into cytotoxic T lymphocytes [34,35]. However, distinct novel CXCR5+ PD-1+ subset of CD8+ T cells have recently been identified, which can directly help B cells to produce antibodies in vitro and shares similar gene expression signatures with CD4+ TFH cells, including the production of IL-21 [36]. Additional studies demonstrate the existence of CXCR5+ CD8+ that can differentiate into TFH cells [37,38], and may also express ICOS and secrete IL-21 [39,40,41], further helping B cells to form GC, proliferate and produce antibodies.
2.2. PD-1 in T Follicular Regulatory (TFR) Cells
GCs reactions are not only controlled by helper signals that promote selection and proliferation, but also by follicular regulatory T cells (TFR) that suppress the magnitude and output of the GC response [42,43] (Figure 1, right). These TFR cells are a subset of CD4+ FOXP3+ regulatory T (Treg) cells that are located within the GC and, like TFH cells, express CXCR5 (although CXCR5-independent TFR mechanisms might also exist) [44], ICOS and PD-1 [43,45,46]. Although the precise mechanisms by which TFR cells control GC B cell responses are still not fully understood, it is thought that TFR cells impair GC reactions by suppressing TFH proliferation and by inhibiting the production of TFH derived cytokines that are crucial for B cell selection and survival [47,48]. Depletion of TFR results in increased numbers of TFH and GC B cells [45], reinforcing the key role of TFR in suppressing GC reactions through the control of TFH cells.
PD-1 is also expressed in TFR, and a lack of PD-1 or its ligand PD-L1 results in a dramatic increase of TFR numbers in the draining lymph node of immunized mice, which is accompanied by a hyperactive suppressive capacity [49]. Therefore, PD-1 signaling may inhibit the number of TFR cells, even if the abundance of TFH cells is not immediately affected, skewing the TFR/TFH ratio towards a more helper phenotype that promote B cell survival and selection (Figure 1, left).
Although the interest in regulatory T cells has focused principally in CD4+ FOXP3+ cells, there is increasing attention to a subpopulation of CD8+ that may regulate GC responses and suppress TFH action [50]. Interestingly, in humans the majority of follicular CD8+ T cells are TFR that can induce TFH apoptosis and inhibit IL-21 production [51]. PD-1 has been described to be expressed in CD8+ TFR cells and to be necessary for their correct suppressive function [52], further supporting a fine-tuned involvement of PD-1/PD-L1 signaling in T cell regulatory functions.
2.3. PD-L1 Expression and PD-1 Downregulation in GC B Cells
PD-L1 is widely expressed by many different immune cells, while PD-L2 expression is much more restricted. Both of them, however, can be expressed on GC B cells [27,28,49], promoting PD-1 signaling in TFH and TFR cells, as discussed above, and thereby influencing humoral immune responses (Figure 1, left). In fact, PD-L1, but not PD-L2, can already be found on splenic resting B cells and may be up-regulated by stimulation with anti-IgM or LPS [53]. Although the underlying mechanisms for the regulation of PD-L1 expression in GC B cells remain poorly understood, the master GC regulator BCL6 has been shown to act as a key transcriptional repressor of PD-L1 and PD-L2 to dampen excessive PD-1 signaling on both TFH and TFR cells [10,54,55]. Consistently, BCL6-expressing mature GC B cells initially express low levels of PD-1 ligands [54] while late GC B cells, which turn down BCL6 to initiate terminal differentiation, upregulate PD-1 ligands and seem to be more sensitive to PD-L1/2-PD-1 signaling [27].
On the other hand, PD-1 can co-cluster with the BCR and inhibit B-cell activation and proliferation [56,57], suggesting analogous co-inhibitory receptor functions for PD-1 in both T and B cells. Consistent with this, PD-1 expression appears specifically downregulated on GC B cells and re-acquired upon GC exit, supporting a role of PD-1 in B cells as a guardian of uncontrolled activation [56].
In summary, these observations support the notion that PD-1 turnoff and PD-L1 expression in late GC B cells conditionate their selection and survival, where the interaction between PD-1-ligands on B cells and PD-1 on T cells is required for the optimal output of the GC response.
2.4. PD-L1 and PD-1 Expression in Regulatory B (Breg) Cells
There is accumulating evidence for the existence of a subset of B cells that can exert immune regulatory functions, termed regulatory B cells (Breg) [58,59,60,61]. The majority of studies on the inhibitory function of Bregs have focused on IL-10, IL-35 and TGF-β as key players that can repress the differentiation, proliferation and effector function of various cell types, like dendritic, CD4+ and CD8+ T cells [62,63,64,65,66]. A role for Bregs during GC reaction responds to its ability to restrict TFH maturation and expand TFR cells, inhibiting subsequent antibody responses [62,67] (Figure 1, right).
Independently of IL-10 production, other suppressive mechanisms have been reported whereby Bregs can dampen T cell responses [63,68]. For example, Breg cells can induce CD4+ T cell death by expressing FasL [69], and can dramatically suppress humoral responses through the expression of high levels of PD-L1 (PD-L1hi Breg cells) [67]. This is due to PD-L1hi-mediated restriction of the expansion and function of TFH cells, partially caused by the activation of the negative regulator of TFH differentiation STAT5 [70,71]. This mechanism resulted independent from IL-10 and Treg cells, and susceptible to PD-L1 blockade, indicating a direct connection between Breg and TFH cells through the PD-1/PD-L1 axis [67], highlighting how PD-L1hi Bregs might ultimately impact GC B cell responses (Figure 1, right).
Interestingly, Breg cells are not restricted to a determined B sublineage but can be instead remodeled from B cells at different stages of development in response to different stimuli such as infections, inflammatory processes or other malignancies [72,73]. One example are regulatory plasmocytes, B cells in terminal differentiation towards antibody secretor plasma cells that in the presence of some antigens can be transcriptionally reprogramed to produce high levels of inhibitory molecules as IL-10, PD-L1 and LAG-3, repressing both myeloid and lymphoid pro-inflammatory populations [74,75]. Other examples are protumorigenic Breg populations that can affect anti-tumour immune responses through different suppressive mechanisms [61,72]. Certain subsets of these tumour-infiltrating Breg cells can upregulate PD-1 expression in response to signaling from cancer cells, promoting T-cell dysfunction via IL-10 [76] or, independently of IL-10, via PD-L1 [77]. In these cases, immune-checkpoint blockade demonstrated that PD-1-triggered production of IL-10 and PD-L1-mediated immunosuppression of T cells were instrumental for Breg protumorigenic functions. Therefore, the PD-1/PD-L1 axis may be utilized by regulatory B cells in different ways, and its blockade might offer a multifaceted benefit that recommend further investigation.
3. The PD-1/PD-L1 Axis in DLBCL
The aforementioned proper regulation of GC cells is crucial for a correct immune response, since dysregulation of proliferation, mutation and differentiation of B cells undergoing GC reactions may lead to lymphomagenesis and DLBCL. Indeed, there is an increasing appreciation of the role of the composition, function and localization of immune and stromal determinants of the tumour microenvironment in DLBCL [78,79,80,81,82,83]. Here, we review current evidence for the implication of the PD-1/PD-L1 axis in DLBCL and propose a progression model, in two phases, where increased PD-1/PD-L1 signaling and dysregulated cytokines promote anti-tumour immune escape (Figure 2). Integration of all these molecular vulnerabilities will hopefully help expanding our knowledge on the role of the microenvironment in DLBCL pathobiology and contribute to the rationale for novel PD-1/PD-L1-related combination immunotherapies for this disease.
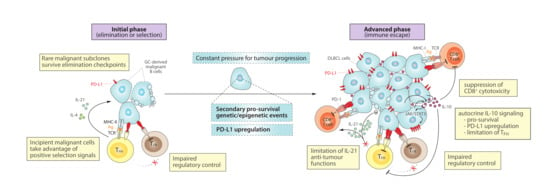
Figure 2.
Role of the PD-1/PD-L1 axis and cytokine dysregulation in a progression model for DLBCL. (Left) At an initial phase, incipient malignant B cells that arise during GC reactions might take advantage of positive selection signals derived from correct antigen-presentation and PD-1/PD-L1 checkpoint in order to survive and initiate clonal expansion. Then, constant pressures during tumour evolution promote the acquisition of additional genetic/epigenetic mutations and PD-L1 upregulation, contributing to tumour maintenance and progression. (Right) At more advanced stages, DLBCL cells tend to exhibit higher PD-L1 levels and capacity to secrete IL-10, which cooperatively promote an immunosuppressive microenvironment that helps DLBCL to escape from anti-tumour CD8+ cytotoxic activity and sustain proliferation and survival, impairing regulatory control by TFR cells and limiting IL-21-mediated anti-tumour functions of TFH cells. MHC-I, major histocompatibility complex-I; Ag, antigen.
3.1. Initial Phase: Elimination or Selection of GC-Derived Malignant Cells
During the initial state of malignant transformation from GC B cells to DLBCL cells, it is plausible that PD-1/PD-L1-related mechanisms governing normal GC reactions, as described above (Figure 1), may also be deceived into driving clonal selection and DLBCL evolution. In this early scenario, PD-L1+ transformed GC cells might continue suppressing regulatory control by TFR cells and skew the TFH/TFR ratio towards a helper phenotype, taking advantage of positive signals to enhance survival and selection of malignant subclones (Figure 2, left). Indeed, the study of DLBCL patient samples showed an enrichment of the TFH subset compared to healthy controls [84]. Then, following the general principles of cancer evolution [85], a continuous pressure for additional genetic and epigenetic alterations may provide growth advantage to selected malignant subclones, permitting DLBCL expansion.
3.1.1. Secondary Pro-Survival and Genetic/Epigenetic Events during DLBCL Progression
Numerous studies have demonstrated the large amount of genetic and epigenetic heterogeneity that characterize DLBCL [9,86,87]. How all these molecular events interact as driver or passenger alterations to impact GC B-cell fate is not yet fully understood, but they do reveal the existence of distinct DLBCL subgroups with unique biological properties and clinical behaviors that reflect cell-of-origin transcriptional hallmarks [11,88], co-occurrence of genetic alterations [89,90,91] or different microenvironment compositions [79,81]. These DLBCL hallmarks have multifaceted roles promoting cancer progression, including at times (see next section) the regulation and promotion of PD-L1 expression itself.
3.1.2. PD-L1 Upregulation in DLBCL
Upregulation of PD-L1 has been previously associated with rapid progression and poor prognosis of DLBCL [92,93,94]. Genetic abnormalities at the chromosome 9p24.1 represent crucial mechanisms affecting PD-L1 expression, but less than 27% of DLBCL patients carry copy number gains, amplifications, or translocations affecting the PD-L1/L2 locus [95,96,97,98]. In addition, only 8% of DLBCL cases exhibit structural variations disrupting the 3′-UTR of the PD-L1 gene that lead to the stabilization and elevation of aberrant PD-L1 transcripts [99]. Yet PD-L1 expression is highly diverse in DLBCL, ranging from 25–70% [100], which might reflect differences in the dynamics of tumour progression and associate more clearly with selected lymphoma subtypes. Epigenetic modulators, including promoter DNA methylation or histone modifications [101,102], might also be at the basis for the upregulation of PD-L1 without underlying genetic alterations at 9p24.1. Such heterogeneity in the prevalence of PD-L1 expression might be the reason for the current disappointing low rate of clinical responses to PD-1/PD-L1 immune-checkpoints in DLBCL patients [103,104], as well as for the controversial prognostic significance of PD-L1 expression with studies reporting its association with poorer outcome [92,93,94,96,105,106], undetectable prognostic significance [107], or trends towards better event-free survival [97]. Interestingly, PD-L1 expression is more frequently observed in the prognostically unfavorable non-GCB/activated B cell-like (ABC) subtype [92,93,94,95,105,107,108], in non-transformed de novo cases [108], or in EBV-positive DLBCL cases [107]. Indeed, PD-L1 levels escalate after infection with different viruses related with poorer prognosis of DLBCL, including Epstein–Barr virus (EBV) [109], hepatitis B virus (HBV) [110], and human immunodeficiency virus (HIV) [111].
During tumour evolution, high levels of MYC expression can result in increased expression of PD-L1 by direct binding to its promoter [112], as well as by hijacking the phospho-eIF2a dependent adaptive stress pathway to bypass the post-transcriptional control orchestrated at the 5′-UTR of the PD-L1 mRNA [113]. On the other hand, inactivating mutations of TP53, which can be detected in approximately 20% of DLBCL patients [89,114,115,116], have been shown to increase PD-L1 expression in various tumour types [101,117,118] and might result in defective p53-regulated miRNA responses that normally control PD-L1 levels [119,120]. Indeed, noncoding RNAs that may target PD-L1, such as miR-34a [120,121] or miR-195 [122] in DLBCL, as well as other immune-checkpoints, are receiving increased attention [123,124]. Hypoxia, an inevitable hallmark of tumour microenvironment resulting from deprivation of oxygen due to the abnormal vasculature and tumour mass expansion, may also enforce gene expression reprogramming in DLBCL [125] and can induce PD-L1 expression [126].
The constitutive activation of NF-kB and JAK/STAT3 signaling is also frequently associated with the most aggressive nonGCB/ABC-DLBCL subtype [127], and a coordinated activity of both pathways has been demonstrated to be important in the metabolic reprogramming of DLBCL cells [128]. NF-kB and JAK/STAT3 activation can be driven either by a complex microenvironment or intrinsic genetic lesions and are emerging as key positive regulators of PD-L1 expression [129,130,131,132]. Consistent with this idea, we recently described that NF-kB-driven lymphomagenesis in mouse models of ABC-DLBCL is strongly associated to PD-L1 upregulation and immune evasion [133], which can be efficiently blocked to improve long-term control of lymphomas and support the potential of the PD-1/PD-L1 axis as an attractive target for lymphoma immunotherapy.
3.2. Advanced Phase: Immune Escape by DLBCL Cells
As a result of the accumulation and selection of the above discussed traits, a more advanced scenario could be described where DLBCL cells have consolidated their ability for immune evasion, taking advantage of (i) preserved “select me” and “do not kill me” signals inherited from the GC B cell-of-origin, and (ii) extended mechanisms that further contribute to impaired T cell infiltration and/or cytotoxic functions (Figure 2, right). In support of this notion, deconvolution of transcriptional signatures from DLBCL microenvironment cells suggested that disease progression tends to associate with immune desertion and decreasing T cell infiltration, resulting in poorest clinical outcomes [81]. Activated T helper cells, as well as other associated regulatory T or B cells, normally release cytokines (e.g., IL-10, IL-21) into the immunological synapse toward the antigen-presenting GC B cells, which may result in dysregulation within the DLBCL microenvironment in parallel to alterations in tumour-infiltrating T cell populations. While some of these cytokines are essential for GC homeostasis (Figure 1), they may become detrimental by exerting immunosuppressive and pro-survival activities that ultimately facilitate tumour progression (Figure 2). Next, we review how DLBCL cells at more advanced stages can mimic some features of Breg cells, including high PD-L1 expression and IL-10 secretion, and promote an immunosuppressive microenvironment that reinforce anti-tumour immune evasion.
3.2.1. Autocrine IL-10 Signaling
Increasing evidences demonstrate that B cells can also produce IL-10 to mediate regulatory functions [73,134], which can even stimulate B cell proliferation under certain local conditions [135,136]. In DLBCL, increased levels of IL-10 frequently associate with the ABC-DLBCL subtype [137], and in-vitro experiments have demonstrated that DLBCL cells can sustain proliferation and survival by autocrine IL-10/JAK/STAT3 signaling [137,138]. Beyond this pro-survival effect, IL-10 production by the DLBCL cells could also promote immunosuppression by PD-L1 upregulation through intracellular mechanisms that involve JAK/STAT3 signaling [132], and mimic Breg-derived IL-10 secretion to suppress CD8 cytotoxic responses [61,73] (Figure 2, right). Yet, the exact role of IL-10 in B cell lymphomas remains controversial and urges further investigation, especially since IL-10 receptor (IL-10R) deficiency associates with lymphoma predisposition [139] and IL-10 may also possess anti-tumour functions by promoting CD8+ T cell responses in certain scenarios, including DLBCL [140,141,142]. Furthermore, IL-10 can also disturb the ratio of TFH/TFR cell populations (see below), which might exert a profound impact on lymphoma progression.
3.2.2. Impaired T-Cell Regulatory Control
The role of regulatory T cells is more intricate in hematological cancers compared to other tumour types. On the one hand, the number of tumour infiltrating TFR cells is frequently higher in DLBCL patients with less severe clinical presentation of DLBCL, but reduced in advanced DLBCL stages [143]. Similarly, a positive association between the number of intra-tumour FOXP3+ Treg cells and a better prognosis of DLBCL has been observed [144,145,146]. In fact, primary human co-cultures with Treg cells can induce the apoptosis of tumour B cells [147], and transformation of follicular lymphoma to DLBCL associates with decreased numbers of intra-tumour Tregs [148]. Altogether, these observations support a general protective role of intra-tumour Tregs in trying to restrict TFH-mediated initial pro-tumour support or as a surrogate marker for active anti-tumour immune responses [149]. PD-L1 upregulation may become an effective way to suppress any potential benefit from Treg cells in DLBCL, and help explaining the relative decrease in Treg numbers observed in murine PD-L1+ DLBCL [133].
On the other hand, Tregs might support B-cell tumour growth by efficiently inhibiting anti-tumour responses [149] and by locally secreting IL-10 with pro-survival activity for the malignant B cells [136]. In this sense, increased levels of Tregs are generally found in blood [143,150] and tumour [143] of DLBCL patients compared to healthy controls, and some studies have associated increased number of these Tregs with adverse clinical outcome for DLBCL [150,151], but not always [152]. Therefore, while it remains unclear the ultimate beneficial or detrimental contribution of Tregs to the DLBCL microenvironment, future studies should also take into consideration that there are various subsets of FOXP3+ Tregs with multifaceted functions [153,154]. In this regard, TFR is a specialized subset of Tregs with particular roles in the control of TFH-driven GC responses [42,43,155], likely prone towards suppressive functions against the GC-derived lymphoma cells. Following this notion, DLBCL cells might successfully counteract TFR cell expansion and its possible protective functions, by upregulating PD-L1 at advanced stages of the disease (Figure 2, right). Even in an alternative scenario with increased TFR numbers, DLBCL cells might also be able to take advantage of IL-10 locally produced by these cells, demonstrating a complex role of TFR cells within the tumour microenvironment of DLBCL.
3.2.3. Limitation of TFH Expansion and IL-21 Anti-Tumour Functions
It has been noted that IL-21 exerts diverse regulatory effects on healthy and tumour cells depending on the type of cell, stage of differentiation and type of stimulus. After proper BCR signaling and T cell-dependent B cell response, IL-21 can induce B cell proliferation and sustain normal GC reactions [156,157] (Figure 1, left). These IL-21 pro-survival properties could also play a role during initial stages of GC-derived malignant transformation (Figure 2, left), and even constitutively persist in some hematological malignancies (i.e., follicular lymphoma, multiple myeloma and Hodgkin’s lymphoma) [158]. However, opposite outcomes are possible in more advanced stages of DLBCL, where TFH-derived IL-21 might elicit anti-lymphoma activities through at least two different mechanisms (Figure 2, right). On one hand, IL-21 has demonstrated anti-DLBCL activity through the activation of STAT3-c-Myc signaling pathway and downregulation of the anti-apoptotic Bcl-2/Bcl-XL genes [157,159]. Indeed, cytotoxic activities of IL-21 have been observed for inappropriately activated B cells and some lymphoma cells, by induction of apoptosis and growth arrest [156,159,160,161,162]. On another hand, IL-21 might also become toxic for lymphomas by expanding and enhancing tumour-infiltrating cytotoxic CD8+ cells and NK cells [163,164,165,166]. Such immunotherapeutic potential of IL-21 to stimulate anti-tumour immune responses is being clinically investigated in different cancers [160,167,168].
A priori paradoxical, the number of circulating CD4+ TFH cells, which likely reflects the type of TFH cells infiltrating tumours, appears to be significantly increased in DLBCL cases, especially in more advanced stages of the disease [84]. However, these TFH cells secrete significantly less IL-21 than other circulating CD4+ T cells [169] and associate with decreased serum IL-21 levels [170], suggesting a severe limitation of the expected anti-tumour functions of TFH cells in DLBCL (Figure 2, right). This would be consistent with an immunosuppressive tumour microenvironment where DLBCL cells may directly restrict TFH functions through increased surface PD-L1 expression and autocrine IL-10 secretion, acquiring immune evasion characteristics that resemble Breg-like functions [58,59,61,63,67,72,73]. These IL-21-impaired TFH cells in DLBCL may also promote a cytokine switch towards IL-10 production by TFR cells [169,171], further contributing to lymphoma maintenance and progression.
3.2.4. Suppression of CD8 Cytotoxicity
It is plausible to expect that tumour-infiltrating CD8+ effector T cells may result immunosuppressed in DLBCL, as a consequence of aforementioned mechanisms of cytokine dysregulation (decreased TFH-derived cytotoxic IL-21 and increased autocrine IL-10) and PD-L1 upregulation, compromising efficient anti-lymphoma immune responses and facilitating immune escape (Figure 2, right). Consistent with this, the amount of intra-tumour PD-1+ T cells correlates positively with PD-L1+ lymphoma B cells in human [100,107,172] and murine DLBCL [133]. Interestingly, a high number of PD-1+ tumour-infiltrating lymphocytes is a favorable prognostic factor in DLBCL [107,173], suggesting ongoing anti-lymphoma T cell responses, still with a chance to respond to combinational PD-1/PD-L1 blockade [133] and yet not fully progressed into the immune deserted scenario with poorer clinical outcomes [81]. Altogether, these observations suggest that, while still controversial due to technical differences, challenges and cut-off values in PD-1 detection [100,174], there is a biological connection between CD8+ PD-1+ effector T cells and DLBCL progression with potential prognostic and clinical relevance that justifies further research for this fatal disease.
4. Immunotherapy Perspectives in DLBCL
Despite the significant curative outcomes with the current standard of care R-CHOP treatment, including an anti-CD20 antibody (R, rituximab) in combination with multiagent chemotherapy (CHOP), approximately 40% of DLBCL patients experience relapse or refractory (R/R) disease [12,175]. Given the critical crosstalk between DLBCL cells and surrounding immune cells during lymphoma evolution and maintenance (Figure 2), it is reasonable to hold expectations on novel immunotherapy approaches aiming at restoring or “normalizing” [176] effective anti-tumour immunity for DLBCL. Here, we outline most promising highlights in using immunotherapy against DLBCL, with a special focus on those that directly target the PD-1/PD-L1 axis or might indirectly cooperate with this therapeutic blockade through combination (Figure 3).
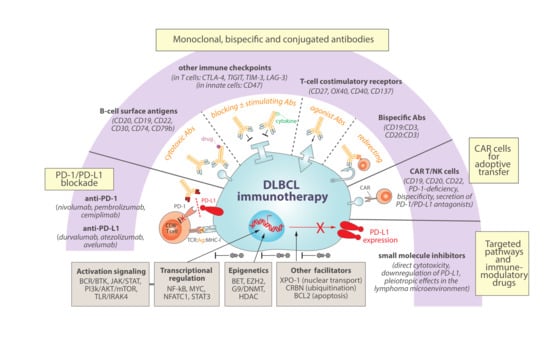
Figure 3.
Immunotherapy strategies for DLBCL with promising rationale to involve or cooperate with PD-1/PD-L1 blockade. Combinational immunotherapy offers a potential to improve the current limited therapeutic efficacy of PD-1/PD-L1 blockade in DLBCL. For example, exploring novel monoclonal, bispecific or conjugated antibodies might cooperate to target a continuously extending portfolio of B-cell specific and tumour antigens, to block other immune-checkpoint inhibitors, to enhance immune stimulation by cytokine delivery or T-cell costimulatory receptor targeting, or to strategically redirect T cells trying to enhance anti-tumour engagements. Adoptive transfer therapies of T or NK cells with rapidly evolving new generations of engineered chimeric antigen receptors (CAR) are also emerging as promising alternatives to DLBCL patients. Finally, and profoundly connected with precision medicine for DLBCL patients, a plethora of small molecule inhibitors to key DLBCL deregulated pathways is continuously being investigated with the goal of inducing direct cytotoxicity of lymphoma cells. Interestingly, most of these targeted pathways are intertwined with PD-L1 regulation and might offer concomitant opportunities as immune-modulatory drugs, further offering promising clinical synergistic opportunities.
4.1. PD-1/PD-L1 Immune-Checkpoint Blockade
Among numerous anti-PD1-/PD-L1 monoclonal antibodies (mAbs) in the global market, six currently FDA/EMA-approved mAbs to either target PD-1 (i.e., nivolumab, pembrolizumab, cemiplimab) or PD-L1 (i.e., durvalumab, atezolizumab, avelumab) are under intense investigation across multiple cancer types [177]. Despite efficacy enhancing T cell proliferation and cytotoxic functions with these antibodies in many clinical trials, PD-1 blockade with nivolumab failed to show a benefit for R/R DLBCL patients [103,178]. However, as earlier discussed, significant PD-L1 expression by the lymphoma cells, which might evidence tumour progression and more clearly associates with specific subtypes of DLBCL, may become a critical limiting factor for anti-PD-1/PD-L1 efficacy and requires further investigation as a predictive biomarker of immunotherapy responses in DLBCL. On the other hand, and contrary to de novo DLBCL, rare PD-L1 expression but high PD-1 expression are observed in the large neoplastic B cells in Richter syndrome (RS), which represents transformation of chronic lymphocytic leukemia (CLL) to DLBCL with dismal prognosis [179,180,181]. Interestingly, this distinct PD-1hi phenotype in the RS lymphoma cells resembles that of protumorigenic PD-1hi Breg subsets recently identified in certain cancer types [76,77], for what it has been proposed that RS patients might specially benefit from therapy targeting PD-1/PD-L1 [179,182,183,184,185,186]. Yet some discrepancies exist [187], and ongoing research is expected to shed light about the potential benefit of PD-1/PD-L1 blockade for the treatment of the DLBCL histologic variant of RS.
Finally, there is still a potential to improve the therapeutic activity of PD-1/PD-L1 blockade in B-cell malignancies and DLBCL by exploring the utility of novel combinational immunotherapy strategies, as well as by considering these therapies in a selected subset of patients with higher PD-L1 levels. Indeed, ongoing clinical trials are evaluating the efficacy of PD-1/PD-L1 blockade in combination with other agents, including antibodies targeting B-cell antigens, other suppressor or costimulatory checkpoints, bispecific antibodies to engage T cells, chimeric antigen receptor (CAR) cells, and other targeted small molecules or immunomodulatory drugs [104,174,188,189]. In this regard, preclinical data from our group support the notion that combinational immunotherapy with anti-CD20 and anti-PD-1 can reinvigorate T cells and anti-tumour specificity, improving long-term overall survival in animal models of PD-L1-positive DLBCL [133].
4.2. Monoclonal, Bispecific and Conjugated Antibodies
Since the approval of first-generation rituximab (anti-CD20) for the treatment of DLBCL, additional mAbs are being developed to target surface antigens in the lymphoma cells, either as unconjugated mAbs or conjugated to radioactive or cytotoxic drugs [190]. It is plausible to predict that some of these agents that directly target B-cell expressing markers (e.g., CD19, CD22, CD30, CD74, CD79b, and next generation of anti-CD20 mAbs) might also offer promising therapeutic opportunities in combination with the inhibition of PD-1/PD-L1 and other immune-checkpoints (e.g., CTLA-4, TIGIT, TIM-3, LAG-3) [189,190]. Failure to benefit from PD-1/PD-L1 blockade might also be due to effector T-cell exhaustion, which further inspires the development of mAbs targeting costimulatory receptors (e.g., CD27, OX40, CD40, 4-1BB/CD137) [189,191,192,193] to work as agonists that might overcome the immunosuppressive DLBCL microenvironment, stimulating effector T-cells and regulating T-cell memory responses. Innate immune-checkpoint blockade with anti-CD47 antibodies have also shown activity in combination with rituximab by enhancing phagocytosis of malignant tumour cells in heavily pretreated DLBCL patients [194].
On the other hand, instead of unique targeting of immune cells, bispecific antibodies targeting two antigens simultaneously are innovative synthetic constructions rapidly developing for the management of hematologic malignancies [195]. Frequently, these bispecific antibodies target one tumour antigen (e.g., CD19 or CD20 in B-cell lymphomas) and one immune-related molecule (e.g., CD3), aiming at redirecting T cells against tumour cells and working as cytotoxic T-cell engagers [196]. In DLBCL, several bispecific antibodies are under clinical evaluation with promising results, including a CD19:CD3 antibody [197], a CD20:CD3 antibody [198] and a next-generation 2:1 CD20:CD3 antibody with two CD20 binders and higher potency for hematologic malignancies [199,200].
Antibody-cytokine conjugated proteins represent another class of biopharmaceuticals in cancer immunotherapy [201]. Combination therapy of IL-21 with PD-1 or CTLA-4 previously showed efficacy in mouse tumour models [202], and an engineered IL-21 fused to a PD-1 antibody showed promising results by providing superior anti-tumour immunity than anti-PD-1 alone in preclinical studies [167].
4.3. CAR T-Cells and NK-Cells
Targeting DLBCL cells with anti-CD19 CAR T cells are rapidly emerging as a promising cellular immunotherapy in R/R DLBCL [203,204]. Tisagenlecleucel (tisa-cel) [205], axicabtagene ciloleucel (axi-cel) [206] and lisocabtagene maraleucel (liso-cel) [207] have shown potent therapeutic efficacy against CD19+ DLBCL cells. However, loss of CD19 expression or upregulation of PD-L1 have been associated with relapses after CD19 CAR T cell therapy [206,208]. In this regard, CAR T cells engineered to secrete PD-1/PD-L1 antagonists [209,210,211], to be deficient in PD-1 expression [212,213], to bispecifically target other B-cell markers (such as CD20 or CD22) in addition to CD19 [214,215], or to be administered in combination with immune-checkpoint inhibitors [216,217] are promising alternatives in the treatment of DLBCL. Furthermore, NK cells are demonstrating inherent properties that make them strong candidates for genetic modification in a CAR NK-cell format [218,219]. Indeed, clinical perspectives are continuously expanding with novel cell-based engineering strategies against CD19+ lymphoid tumours, many of which try to simultaneously coordinate checkpoint inhibition to enhance the function of adoptively transferred cells [220].
4.4. Small Molecules for Targeted Pathways and Immunomodulatory Drugs
Other agents targeting deregulated pathways in DLBCL have shown limited activity as monotherapy but are being investigated in various combinations guided by patient underlying lymphoma characteristics that might foster precision medicine for DLBCL [91,189,190]. As alluded to earlier in this review, most of these deregulated pathways have revealed capacity to ultimately impact PD-L1 levels within the DLBCL cell, highlighting a secondary immune-modulatory potential that might be further exploited by current immune-checkpoint modalities. For example, chronically-active BCR signaling in DLBCL might be inhibited with MALT1 paracaspase inhibitors [221,222] or with various kinase inhibitors such as ibrutinib or acalabrutinib (BTK inhibitors), which have shown promising clinical activities [223,224] and a potential to be efficiently combined with a variety of other agents to kill DLBCL cells [225]. Interestingly, BTK inhibition can indirectly downregulate PD-L1 signaling in DLBCL cells [132], and enhance the anti-tumour therapeutic effects of PD-1 blockade in mouse models of lymphoma [226], which might be proven synergistic with anti-PD-1 immunotherapy (NCT02362035) [227]. Inhibition of JAK/STAT3 or PI3k/AKT/mTOR pathways, which have shown potential therapeutic implications in DLBCL [228,229,230], may also ultimately impact PD-L1 expression in the tumour cells [132,231,232], providing additional rationale for future clinical evaluation with PD-1/PD-L1 blockade combinations. In selected DLBCL subtypes with aberrant toll-like receptor (TLR) signaling, IRAK4 inhibitors may have clinical utility, either alone or in combination with BTK or BCL2 inhibitors [233], with capacity to inhibit the expression of PD-L1 and provide novel opportunities for hematologic malignancies [234]. Indeed, apoptosis might be targeted with selective BCL-2 inhibitors such as venetoclax (ABT199) when added to R-CHOP [235], and is being further explored in combination with anti-PD-1 and anti-CD20 in R/R DLBCL (NCT03276468) [236]. Lenalidomide is an immunomodulatory drug with direct anti-tumour activity via binding to cereblon (CRBN) to inhibit downstream NF-kB signaling, and also indirect effects in the tumour microenvironment through multifaceted functions on different immune cells [237], including downregulation of PD-L1 on the surface of lymphoma and myeloma cells [238,239]. While discrepant results came from the addition of lenalidomide to R-CHOP in DLBCL [240,241], promising activity has been observed for triplet rituximab (anti-CD20), ibrutinib (BTKi) and lenalidomide [242,243] or in combination with rafasitamab (anti-CD19) for R/R DLBCL [244], reopening the door for this immunomodulatory drug to future clinical trials in combination [245], some of which currently involve PD-1/PD-L1 inhibitors [188].
Inhibition of exportin 1 (XPO-1) with selinexor can prevent nuclear export of key cargo proteins, including pro-tumour eIF4E-mRNAs complexes to be translated in the cytoplasm (e.g., mRNAs for c-Myc, Bcl2, Bcl-XL, Bcl6, survivin, and cyclin D1) or factors implicated in PD-1/PD-L1 upregulation (e.g., NFATC1, and STAT1/3), and this has shown substantial improved survival in R/R DLBCL patients [246] and promising efficacy in mouse syngeneic tumour models in combination with immune-checkpoint blockade [247,248].
From an epigenetic perspective, selected genetic subtypes of DLBCL might respond to targeting MYC activity with BET-bromodomain inhibitors, alone or in combination with BCL2 inhibition [249], and BET inhibition can repress PD-L1 expression and synergize with PD-1/PD-L1 blockade in mice bearing Myc-driven lymphomas [250]. Analogously, growing evidences support the promising opportunity of combining epigenetic modulators that have previously shown activity in DLBCL with immune-checkpoint blockade [251], including inhibitors for EZH2 histone methyltransferase [252,253,254], G9a/DNMT methyltransferase [255,256], or histone deacetylases [102,257,258].
5. Conclusions
The complex interplay between DLBCL cells and surrounding tumour microenvironment demonstrates lymphoma evolution towards the acquisition of immunosuppression and immunoescaping traits that may ensure tumour maintenance and progression. The PD-1/PD-L1 axis is a key player in these processes, likely inherited from the GC B cell-of-origin of DLBCL and engaged in disturbed inter-cellular signaling with tumour-infiltrating T cells. Evidence highlights the importance of a fine equilibrium in the proportion of intra-tumour TFH and TFR populations, as DLBCL cells deceive normal function of these cells to take advantage of helper pro-survival signals, while impairing regulatory control and suppressing cytotoxic functions. Selected cytokines may result pivotal in the orchestration of such pathogenic DLBCL microenvironment, where deprivation of IL-21 or addiction to IL-10 can support tumour survival and modulate PD-L1 levels in the lymphoma cells. Yet it urges us to better understand all these intricated cellular and molecular interactions in DLBCL, as this would improve the rationale for combinational immunotherapy with the constantly increasing portfolio of novel monoclonal, bispecific and conjugated antibodies, CAR T and NK cells, and small molecule inhibitors with immunomodulatory activities that hold the potential to bring more favorable outcomes for DLBCL patients.
Author Contributions
Conceptualization, M.G.-L., S.C.G. and S.R.; writing—original draft preparation, M.G.-L., S.C.G., J.M., A.A.-L. and S.R.; writing—review and editing, M.G.-L. and S.R.; visualization, S.R.; funding acquisition, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Agencia Estatal de Investigación (PID2020-112994RB-I00/AEI/10.13039/501100011033) and Ministerio de Economía, Industria y Competitividad (SAF2017-82309-R/MINECO/AEI/FEDER, EU) to S.R. JM was supported by Plan de Formación y de I+D 2019 from Gobierno de Navarra (Gobierno de Navarra 34E/2020). SR was supported by Ministerio de Economía y Competitividad through Programa Ramón y Cajal (RYC-2014-16399/MEC).
Acknowledgments
We acknowledge Francisco Javier Novo (Universidad de Navarra), Jose Angel Martinez-Climent (Cima Universidad de Navarra) and Carlos Panizo (Clínica Universidad de Navarra) for stimulating discussions.
Conflicts of Interest
S.R. receives research support from Roche/Genentech (imCORE) and Gilead. The rest of the authors declare no conflict of interest.
References
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- McDermott, D.F.; Atkins, M.B. PD-1 as a potential target in cancer therapy. Cancer Med. 2013, 2, 662–673. [Google Scholar] [CrossRef]
- De Silva, N.S.; Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15, 137–148. [Google Scholar] [CrossRef]
- Victora, G.D.; Dominguez-Sola, D.; Holmes, A.B.; Deroubaix, S.; Dalla-Favera, R.; Nussenzweig, M.C. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood 2012, 120, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.T.; Sharpe, A.H. T follicular regulatory cells. Immunol. Rev. 2016, 271, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef]
- Mlynarczyk, C.; Fontan, L.; Melnick, A. Germinal center-derived lymphomas: The darkest side of humoral immunity. Immunol. Rev. 2019, 288, 214–239. [Google Scholar] [CrossRef]
- Basso, K.; Dalla-Favera, R. Germinal centres and B cell lymphomagenesis. Nat. Rev. Immunol. 2015, 15, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Susanibar-Adaniya, S.; Barta, S.K. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am. J. Hematol. 2021, 96, 617–629. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef]
- Adler, L.N.; Jiang, W.; Bhamidipati, K.; Millican, M.; Macaubas, C.; Hung, S.C.; Mellins, E.D. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Barnett, L.G.; Simkins, H.M.; Barnett, B.E.; Korn, L.L.; Johnson, A.L.; Wherry, E.J.; Wu, G.F.; Laufer, T.M. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J. Immunol. 2014, 192, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Naka, T.; Yoshida, K.; Tanaka, T.; Fujiwara, H.; Suematsu, S.; Yoshida, N.; Kishimoto, T.; Kikutani, H. The immune responses in CD40-deficient mice: Impaired immunoglobulin class switching and germinal center formation. Immunity 1994, 1, 167–178. [Google Scholar] [CrossRef]
- Reinhardt, R.L.; Liang, H.E.; Locksley, R.M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 2009, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Spolski, R.; Feng, C.G.; Qi, C.F.; Cheng, J.; Sher, A.; Morse, H.C., 3rd; Liu, C.; Schwartzberg, P.L.; Leonard, W.J. A critical role for IL-21 in regulating immunoglobulin production. Science 2002, 298, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Zotos, D.; Coquet, J.M.; Zhang, Y.; Light, A.; D’Costa, K.; Kallies, A.; Corcoran, L.M.; Godfrey, D.I.; Toellner, K.M.; Smyth, M.J.; et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 2010, 207, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Okazaki, T.; Nishimura, H.; Kawasaki, A.; Yagita, H.; Honjo, T. Microanatomical localization of PD-1 in human tonsils. Immunol. Lett. 2002, 83, 215–220. [Google Scholar] [CrossRef]
- Arnold, C.N.; Campbell, D.J.; Lipp, M.; Butcher, E.C. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur. J. Immunol. 2007, 37, 100–109. [Google Scholar] [CrossRef]
- Akiba, H.; Takeda, K.; Kojima, Y.; Usui, Y.; Harada, N.; Yamazaki, T.; Ma, J.; Tezuka, K.; Yagita, H.; Okumura, K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 2005, 175, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Haynes, N.M.; Allen, C.D.; Lesley, R.; Ansel, K.M.; Killeen, N.; Cyster, J.G. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 2007, 179, 5099–5108. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018, 49, 264–274.e4. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.; Luxenberg, D.; Ling, V.; Wang, I.M.; Marquette, K.; Lowe, D.; Khan, N.; Veldman, G.; Jacobs, K.A.; Valge-Archer, V.E.; et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: Attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 2003, 170, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Hams, E.; McCarron, M.J.; Amu, S.; Yagita, H.; Azuma, M.; Chen, L.; Fallon, P.G. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J. Immunol. 2011, 186, 5648–5655. [Google Scholar] [CrossRef]
- Good-Jacobson, K.L.; Szumilas, C.G.; Chen, L.; Sharpe, A.H.; Tomayko, M.M.; Shlomchik, M.J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 2010, 11, 535–542. [Google Scholar] [CrossRef]
- Hamel, K.M.; Cao, Y.; Wang, Y.; Rodeghero, R.; Kobezda, T.; Chen, L.; Finnegan, A. B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis. Eur. J. Immunol. 2010, 40, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Ma, C.S. Regulation of the germinal center and humoral immunity by interleukin-21. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Kawamoto, S.; Tran, T.H.; Maruya, M.; Suzuki, K.; Doi, Y.; Tsutsui, Y.; Kato, L.M.; Fagarasan, S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 2012, 336, 485–489. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Liu, D.; Li, J.; Zhang, X.; Chen, X.; Hou, S.; Peng, L.; Xu, C.; Liu, W.; et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 2013, 496, 523–527. [Google Scholar] [CrossRef]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Zinselmeyer, B.H.; Heydari, S.; Sacristan, C.; Nayak, D.; Cammer, M.; Herz, J.; Cheng, X.; Davis, S.J.; Dustin, M.L.; McGavern, D.B. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 2013, 210, 757–774. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. CD8(+) T cells: Foot soldiers of the immune system. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, M.; Zheng, Y.; Fu, G.; Xin, G.; Zhu, W.; Luo, L.; Burns, R.; Li, Q.Z.; Dent, A.L.; et al. CXCR5(+)PD-1(+) follicular helper CD8 T cells control B cell tolerance. Nat. Commun. 2019, 10, 4415. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Zhang, X.; Wagner, U.G.; Yang, H.; Beckenbaugh, R.D.; Kurtin, P.J.; Goronzy, J.J.; Weyand, C.M. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J. Exp. Med. 2002, 195, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Zeng, Z.; Xing, S.; Li, F.; Hartwig, S.M.; Gullicksrud, J.A.; Kurup, S.P.; Van Braeckel-Budimir, N.; Su, Y.; Martin, M.D.; et al. The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage. Nat. Immunol. 2017, 18, 931–939. [Google Scholar] [CrossRef]
- Shen, J.; Luo, X.; Wu, Q.; Huang, J.; Xiao, G.; Wang, L.; Yang, B.; Li, H.; Wu, C. A Subset of CXCR5(+)CD8(+) T Cells in the Germinal Centers From Human Tonsils and Lymph Nodes Help B Cells Produce Immunoglobulins. Front. Immunol. 2018, 9, 2287. [Google Scholar] [CrossRef] [PubMed]
- Le, K.S.; Ame-Thomas, P.; Tarte, K.; Gondois-Rey, F.; Granjeaud, S.; Orlanducci, F.; Foucher, E.D.; Broussais, F.; Bouabdallah, R.; Fest, T.; et al. CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas. Blood Adv. 2018, 2, 1889–1900. [Google Scholar] [CrossRef]
- Valentine, K.M.; Davini, D.; Lawrence, T.J.; Mullins, G.N.; Manansala, M.; Al-Kuhlani, M.; Pinney, J.M.; Davis, J.K.; Beaudin, A.E.; Sindi, S.S.; et al. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. J. Immunol. 2018, 201, 31–40. [Google Scholar] [CrossRef]
- Sage, P.T.; Sharpe, A.H. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015, 36, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, I.; Agua-Doce, A.; Hernandez, A.; Almeida, C.; Oliveira, V.G.; Faro, J.; Graca, L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 2011, 187, 4553–4560. [Google Scholar] [CrossRef] [PubMed]
- Vanderleyden, I.; Fra-Bido, S.C.; Innocentin, S.; Stebegg, M.; Okkenhaug, H.; Evans-Bailey, N.; Pierson, W.; Denton, A.E.; Linterman, M.A. Follicular Regulatory T Cells Can Access the Germinal Center Independently of CXCR5. Cell Rep. 2020, 30, 611–619.e4. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Tanaka, S.; Chu, F.; Nurieva, R.I.; Martinez, G.J.; Rawal, S.; Wang, Y.H.; Lim, H.; Reynolds, J.M.; Zhou, X.H.; et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011, 17, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A.; Pierson, W.; Lee, S.K.; Kallies, A.; Kawamoto, S.; Rayner, T.F.; Srivastava, M.; Divekar, D.P.; Beaton, L.; Hogan, J.J.; et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011, 17, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, A.; Liu, G.; Wu, F.; Li, Z. T follicular regulatory cells suppress Tfh-mediated B cell help and synergistically increase IL-10-producing B cells in breast carcinoma. Immunol. Res. 2019, 67, 416–423. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Liu, H.; Xu, L.L.; Teuscher, P.; Wang, S.; Lu, S.; Dent, A.L. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur. J. Immunol. 2016, 46, 1152–1161. [Google Scholar] [CrossRef]
- Sage, P.T.; Francisco, L.M.; Carman, C.V.; Sharpe, A.H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 2013, 14, 152–161. [Google Scholar] [CrossRef]
- Kim, H.J.; Verbinnen, B.; Tang, X.; Lu, L.; Cantor, H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 2010, 467, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.; Miller, S.M.; Folkvord, J.M.; Levy, D.N.; Rakasz, E.G.; Skinner, P.J.; Connick, E. Follicular Regulatory CD8 T Cells Impair the Germinal Center Response in SIV and Ex Vivo HIV Infection. PLoS Pathog. 2016, 12, e1005924. [Google Scholar] [CrossRef]
- Dai, H.; Wan, N.; Zhang, S.; Moore, Y.; Wan, F.; Dai, Z. Cutting edge: Programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J. Immunol. 2010, 185, 803–807. [Google Scholar] [CrossRef]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef]
- Peng, C.; Hu, Q.; Yang, F.; Zhang, H.; Li, F.; Huang, C. BCL6-Mediated Silencing of PD-1 Ligands in Germinal Center B Cells Maintains Follicular T Cell Population. J. Immunol. 2019, 202, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Basso, K.; Saito, M.; Sumazin, P.; Margolin, A.A.; Wang, K.; Lim, W.K.; Kitagawa, Y.; Schneider, C.; Alvarez, M.J.; Califano, A.; et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood 2010, 115, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Thibult, M.L.; Mamessier, E.; Gertner-Dardenne, J.; Pastor, S.; Just-Landi, S.; Xerri, L.; Chetaille, B.; Olive, D. PD-1 is a novel regulator of human B-cell activation. Int. Immunol. 2013, 25, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. USA 2001, 98, 13866–13871. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Fillatreau, S. Antibody-independent functions of B cells: A focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Baba, Y.; Matsumoto, M.; Kurosaki, T. Signals controlling the development and activity of regulatory B-lineage cells. Int. Immunol. 2015, 27, 487–493. [Google Scholar] [CrossRef]
- Catalan, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillon, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Achour, A.; Simon, Q.; Mohr, A.; Seite, J.F.; Youinou, P.; Bendaoud, B.; Ghedira, I.; Pers, J.O.; Jamin, C. Human regulatory B cells control the TFH cell response. J. Allergy Clin. Immunol. 2017, 140, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.A.; Vasconcellos, R.; Rosser, E.C.; Tulone, C.; Munoz-Suano, A.; Kamanaka, M.; Ehrenstein, M.R.; Flavell, R.A.; Mauri, C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J. Immunol. 2011, 186, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Khoder, A.; Sarvaria, A.; Alsuliman, A.; Chew, C.; Sekine, T.; Cooper, N.; Mielke, S.; de Lavallade, H.; Muftuoglu, M.; Fernandez Curbelo, I.; et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood 2014, 124, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Hams, E.; Floudas, A.; Sparwasser, T.; Weaver, C.T.; Fallon, P.G. PD-L1hi B cells are critical regulators of humoral immunity. Nat. Commun. 2015, 6, 5997. [Google Scholar] [CrossRef]
- Dasgupta, S.; Dasgupta, S.; Bandyopadhyay, M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell Immunol. 2020, 352, 104076. [Google Scholar] [CrossRef]
- Tian, J.; Zekzer, D.; Hanssen, L.; Lu, Y.; Olcott, A.; Kaufman, D.L. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol. 2001, 167, 1081–1089. [Google Scholar] [CrossRef]
- Johnston, R.J.; Choi, Y.S.; Diamond, J.A.; Yang, J.A.; Crotty, S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 2012, 209, 243–250. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Podd, A.; Chen, Y.; Alekseev, A.M.; Yu, M.; Qi, X.; Huang, H.; Wen, R.; Wang, J.; Li, H.S.; et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J. Biol. Chem. 2012, 287, 11234–11239. [Google Scholar] [CrossRef]
- Michaud, D.; Steward, C.R.; Mirlekar, B.; Pylayeva-Gupta, Y. Regulatory B cells in cancer. Immunol. Rev. 2021, 299, 74–92. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S. Natural regulatory plasma cells. Curr. Opin. Immunol. 2018, 55, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S. Regulatory functions of B cells and regulatory plasma cells. Biomed. J. 2019, 42, 233–242. [Google Scholar] [CrossRef]
- Xiao, X.; Lao, X.M.; Chen, M.M.; Liu, R.X.; Wei, Y.; Ouyang, F.Z.; Chen, D.P.; Zhao, X.Y.; Zhao, Q.; Li, X.F.; et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016, 6, 546–559. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Wang, Z.; Liu, B.; Han, N.; Li, J.; Lu, C.; Liu, X.; Zhang, Q.; Yang, Q.; et al. PD-1-expressing B cells suppress CD4(+) and CD8(+) T cells via PD-1/PD-L1-dependent pathway. Mol. Immunol. 2019, 109, 20–26. [Google Scholar] [CrossRef]
- Monti, S.; Savage, K.J.; Kutok, J.L.; Feuerhake, F.; Kurtin, P.; Mihm, M.; Wu, B.; Pasqualucci, L.; Neuberg, D.; Aguiar, R.C.; et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 2005, 105, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef]
- Scott, D.W.; Gascoyne, R.D. The tumour microenvironment in B cell lymphomas. Nat. Rev. Cancer 2014, 14, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Kotlov, N.; Bagaev, A.; Revuelta, M.V.; Phillip, J.M.; Cacciapuoti, M.T.; Antysheva, Z.; Svekolkin, V.; Tikhonova, E.; Miheecheva, N.; Kuzkina, N.; et al. Clinical and Biological Subtypes of B-cell Lymphoma Revealed by Microenvironmental Signatures. Cancer Discov. 2021, 11, 1468–1489. [Google Scholar] [CrossRef]
- Opinto, G.; Vegliante, M.C.; Negri, A.; Skrypets, T.; Loseto, G.; Pileri, S.A.; Guarini, A.; Ciavarella, S. The Tumor Microenvironment of DLBCL in the Computational Era. Front. Oncol. 2020, 10, 351. [Google Scholar] [CrossRef]
- Ciavarella, S.; Vegliante, M.C.; Fabbri, M.; De Summa, S.; Melle, F.; Motta, G.; De Iuliis, V.; Opinto, G.; Enjuanes, A.; Rega, S.; et al. Dissection of DLBCL microenvironment provides a gene expression-based predictor of survival applicable to formalin-fixed paraffin-embedded tissue. Ann. Oncol. 2018, 29, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Cha, Z.; Guo, H.; Tu, X.; Zang, Y.; Gu, H.; Song, H.; Qian, B. Alterations of circulating follicular helper T cells and interleukin 21 in diffuse large B-cell lymphoma. Tumor Biol. 2014, 35, 7541–7546. [Google Scholar] [CrossRef]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef]
- Bakhshi, T.J.; Georgel, P.T. Genetic and epigenetic determinants of diffuse large B-cell lymphoma. Blood Cancer J. 2020, 10, 123. [Google Scholar] [CrossRef]
- Pasqualucci, L. Molecular pathogenesis of germinal center-derived B cell lymphomas. Immunol. Rev. 2019, 288, 240–261. [Google Scholar] [CrossRef]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e14. [Google Scholar] [CrossRef] [PubMed]
- Kiyasu, J.; Miyoshi, H.; Hirata, A.; Arakawa, F.; Ichikawa, A.; Niino, D.; Sugita, Y.; Yufu, Y.; Choi, I.; Abe, Y.; et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 2015, 126, 2193–2201. [Google Scholar] [CrossRef]
- Andorsky, D.J.; Yamada, R.E.; Said, J.; Pinkus, G.S.; Betting, D.J.; Timmerman, J.M. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 4232–4244. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Chapuy, B.; Ouyang, J.; Sun, H.H.; Roemer, M.G.; Xu, M.L.; Yu, H.; Fletcher, C.D.; Freeman, G.J.; Shipp, M.A.; et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.; Chen, L.; Berglund, M.; Ren, W.; de Miranda, N.F.; Lisboa, S.; Fangazio, M.; Zhu, S.; Hou, Y.; Wu, K.; et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood 2016, 127, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, J.; Tumuluru, S.; Bao, R.; Leukam, M.; Venkataraman, G.; Phillip, J.; Fitzpatrick, C.; McElherne, J.; MacNabb, B.W.; Orlowski, R.; et al. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood 2019, 133, 2279–2290. [Google Scholar] [CrossRef]
- Wang, Y.; Wenzl, K.; Manske, M.K.; Asmann, Y.W.; Sarangi, V.; Greipp, P.T.; Krull, J.E.; Hartert, K.; He, R.; Feldman, A.L.; et al. Amplification of 9p24.1 in diffuse large B-cell lymphoma identifies a unique subset of cases that resemble primary mediastinal large B-cell lymphoma. Blood Cancer J. 2019, 9, 73. [Google Scholar] [CrossRef]
- Xie, W.; Medeiros, L.J.; Li, S.; Yin, C.C.; Khoury, J.D.; Xu, J. PD-1/PD-L1 Pathway and Its Blockade in Patients with Classic Hodgkin Lymphoma and Non-Hodgkin Large-Cell Lymphomas. Curr. Hematol. Malig. Rep. 2020, 15, 372–381. [Google Scholar] [CrossRef]
- Kataoka, K.; Shiraishi, Y.; Takeda, Y.; Sakata, S.; Matsumoto, M.; Nagano, S.; Maeda, T.; Nagata, Y.; Kitanaka, A.; Mizuno, S.; et al. Aberrant PD-L1 expression through 3’-UTR disruption in multiple cancers. Nature 2016, 534, 402–406. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Zhou, J.; Young, K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018, 131, 68–83. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, L.; Li, J.; Li, Y.; Wang, Y.; Xu, Z.X. Recent Findings in the Regulation of Programmed Death Ligand 1 Expression. Front. Immunol. 2019, 10, 1337. [Google Scholar] [CrossRef]
- Deng, S.; Hu, Q.; Zhang, H.; Yang, F.; Peng, C.; Huang, C. HDAC3 Inhibition Upregulates PD-L1 Expression in B-Cell Lymphomas and Augments the Efficacy of Anti-PD-L1 Therapy. Mol. Cancer Ther. 2019, 18, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Merryman, R.W.; Armand, P.; Wright, K.T.; Rodig, S.J. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017, 1, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Dresser, K.; Zhang, R.; Evens, A.M.; Yu, H.; Woda, B.A.; Chen, B.J. PD-L1 expression in EBV-negative diffuse large B-cell lymphoma: Clinicopathologic features and prognostic implications. Oncotarget 2016, 7, 59976–59986. [Google Scholar] [CrossRef]
- Rossille, D.; Gressier, M.; Damotte, D.; Maucort-Boulch, D.; Pangault, C.; Semana, G.; Le Gouill, S.; Haioun, C.; Tarte, K.; Lamy, T.; et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: Results from a French multicenter clinical trial. Leukemia 2014, 28, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Kim, S.; Kim, P.J.; Go, H.; Nam, S.J.; Paik, J.H.; Kim, Y.A.; Kim, T.M.; Heo, D.S.; Kim, C.W.; et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology 2016, 68, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinska, A.; Tsesmetzis, N.; Ghaderi, M.; Kis, L.; Saft, L.; Rassidakis, G.Z. CD274 (PD-L1)/PDCD1 (PD-1) expression in de novo and transformed diffuse large B-cell lymphoma. Br. J. Haematol. 2018, 180, 744–748. [Google Scholar] [CrossRef]
- Lu, T.X.; Liang, J.H.; Miao, Y.; Fan, L.; Wang, L.; Qu, X.Y.; Cao, L.; Gong, Q.X.; Wang, Z.; Zhang, Z.H.; et al. Epstein-Barr virus positive diffuse large B-cell lymphoma predict poor outcome, regardless of the age. Sci. Rep. 2015, 5, 12168. [Google Scholar] [CrossRef]
- Rong, X.; Wang, H.; Ma, J.; Pan, S.; Wang, H.; Jing, S.; Su, Y.; Wang, L.; Zhao, C. Chronic hepatitis B virus infection is associated with a poorer prognosis in diffuse large B-cell lymphoma: A meta-analysis and systemic review. J. Cancer 2019, 10, 3450–3458. [Google Scholar] [CrossRef]
- Re, A.; Cattaneo, C.; Rossi, G. Hiv and Lymphoma: From Epidemiology to Clinical Management. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019004. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gutgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef]
- Xu, Y.; Poggio, M.; Jin, H.Y.; Shi, Z.; Forester, C.M.; Wang, Y.; Stumpf, C.R.; Xue, L.; Devericks, E.; So, L.; et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med. 2019, 25, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Xu-Monette, Z.Y.; Wu, L.; Visco, C.; Tai, Y.C.; Tzankov, A.; Liu, W.M.; Montes-Moreno, S.; Dybkaer, K.; Chiu, A.; Orazi, A.; et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: Report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2012, 120, 3986–3996. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, L.; Trifonov, V.; Fabbri, G.; Ma, J.; Rossi, D.; Chiarenza, A.; Wells, V.A.; Grunn, A.; Messina, M.; Elliot, O.; et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat. Genet. 2011, 43, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Chapuy, B.; Takeyama, K.; Rodig, S.J.; Hao, Y.; Yeda, K.T.; Inguilizian, H.; Mermel, C.; Currie, T.; Dogan, A.; et al. Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell 2012, 22, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Buchakjian, M.R.; Merritt, N.M.; Moose, D.L.; Dupuy, A.J.; Tanas, M.R.; Henry, M.D. A Trp53fl/flPtenfl/fl mouse model of undifferentiated pleomorphic sarcoma mediated by adeno-Cre injection and in vivo bioluminescence imaging. PLoS ONE 2017, 12, e0183469. [Google Scholar] [CrossRef] [PubMed]
- Thiem, A.; Hesbacher, S.; Kneitz, H.; di Primio, T.; Heppt, M.V.; Hermanns, H.M.; Goebeler, M.; Meierjohann, S.; Houben, R.; Schrama, D. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. J. Exp. Clin. Cancer Res. 2019, 38, 397. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Indovina, P.; Mattioli, E.; Forte, I.M.; Iannuzzi, C.A.; Luzzi, L.; Bellan, C.; De Summa, S.; Bucci, E.; Di Marzo, D.; et al. P53-regulated miR-320a targets PDL1 and is downregulated in malignant mesothelioma. Cell Death Dis. 2020, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K.; et al. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Dong, K.; Lin, F.; Long, M.; Ouyang, Y.; Wei, J.; Chen, X.; Weng, Y.; He, T.; et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell. Signal. 2015, 27, 443–452. [Google Scholar] [CrossRef]
- He, B.; Yan, F.; Wu, C. Overexpressed miR-195 attenuated immune escape of diffuse large B-cell lymphoma by targeting PD-L1. Biomed. Pharmacother. 2018, 98, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Calin, H.N.; Pichler, M.; Calin, G.A. Noncoding RNAs and immune checkpoints-clinical implications as cancer therapeutics. FEBS J. 2017, 284, 1952–1966. [Google Scholar] [CrossRef] [PubMed]
- Huemer, F.; Leisch, M.; Geisberger, R.; Zaborsky, N.; Greil, R. miRNA-Based Therapeutics in the Era of Immune-Checkpoint Inhibitors. Pharmaceuticals 2021, 14, 89. [Google Scholar] [CrossRef]
- Bhalla, K.; Jaber, S.; Nahid, M.N.; Underwood, K.; Beheshti, A.; Landon, A.; Bhandary, B.; Bastian, P.; Evens, A.M.; Haley, J.; et al. Author Correction: Role of hypoxia in Diffuse Large B-cell Lymphoma: Metabolic repression and selective translation of HK2 facilitates development of DLBCL. Sci. Rep. 2018, 8, 7221. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef]
- Ding, B.B.; Yu, J.J.; Yu, R.Y.; Mendez, L.M.; Shaknovich, R.; Zhang, Y.; Cattoretti, G.; Ye, B.H. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 2008, 111, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Feist, M.; Schwarzfischer, P.; Heinrich, P.; Sun, X.; Kemper, J.; von Bonin, F.; Perez-Rubio, P.; Taruttis, F.; Rehberg, T.; Dettmer, K.; et al. Cooperative STAT/NF-kappaB signaling regulates lymphoma metabolic reprogramming and aberrant GOT2 expression. Nat. Commun. 2018, 9, 1514. [Google Scholar] [CrossRef]
- Antonangeli, F.; Natalini, A.; Garassino, M.C.; Sica, A.; Santoni, A.; Di Rosa, F. Regulation of PD-L1 Expression by NF-kappaB in Cancer. Front. Immunol. 2020, 11, 584626. [Google Scholar] [CrossRef]
- Marzec, M.; Zhang, Q.; Goradia, A.; Raghunath, P.N.; Liu, X.; Paessler, M.; Wang, H.Y.; Wysocka, M.; Cheng, M.; Ruggeri, B.A.; et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 2008, 105, 20852–20857. [Google Scholar] [CrossRef] [PubMed]
- Ritprajak, P.; Azuma, M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral. Oncol. 2015, 51, 221–228. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Chen, J.; Xu-Monette, Z.Y.; Miao, Y.; Xiao, M.; Young, K.H.; Wang, S.; Medeiros, L.J.; Wang, M.; et al. B-cell receptor-mediated NFATc1 activation induces IL-10/STAT3/PD-L1 signaling in diffuse large B-cell lymphoma. Blood 2018, 132, 1805–1817. [Google Scholar] [CrossRef]
- Pascual, M.; Mena-Varas, M.; Robles, E.F.; Garcia-Barchino, M.J.; Panizo, C.; Hervas-Stubbs, S.; Alignani, D.; Sagardoy, A.; Martinez-Ferrandis, J.I.; Bunting, K.L.; et al. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphomas. Blood 2019, 133, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef]
- Rousset, F.; Garcia, E.; Defrance, T.; Peronne, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Lu, Y.; Amezquita, R.A.; Weinstein, J.S.; Vander Heiden, J.A.; Gupta, N.T.; Kleinstein, S.H.; Kaech, S.M.; Craft, J. Interleukin-10 from CD4(+) follicular regulatory T cells promotes the germinal center response. Sci. Immunol. 2017, 2, eaan4767. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.T.; Wright, G.; Davis, R.E.; Lenz, G.; Farinha, P.; Dang, L.; Chan, J.W.; Rosenwald, A.; Gascoyne, R.D.; Staudt, L.M. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood 2008, 111, 3701–3713. [Google Scholar] [CrossRef] [PubMed]
- Beguelin, W.; Sawh, S.; Chambwe, N.; Chan, F.C.; Jiang, Y.; Choo, J.W.; Scott, D.W.; Chalmers, A.; Geng, H.; Tsikitas, L.; et al. IL10 receptor is a novel therapeutic target in DLBCLs. Leukemia 2015, 29, 1684–1694. [Google Scholar] [CrossRef]
- Neven, B.; Mamessier, E.; Bruneau, J.; Kaltenbach, S.; Kotlarz, D.; Suarez, F.; Masliah-Planchon, J.; Billot, K.; Canioni, D.; Frange, P.; et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood 2013, 122, 3713–3722. [Google Scholar] [CrossRef]
- Emmerich, J.; Mumm, J.B.; Chan, I.H.; LaFace, D.; Truong, H.; McClanahan, T.; Gorman, D.M.; Oft, M. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012, 72, 3570–3581. [Google Scholar] [CrossRef]
- Mumm, J.B.; Emmerich, J.; Zhang, X.; Chan, I.; Wu, L.; Mauze, S.; Blaisdell, S.; Basham, B.; Dai, J.; Grein, J.; et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 2011, 20, 781–796. [Google Scholar] [CrossRef]
- Qiu, H.; Hu, X.; Gao, L.; Chen, L.; Chen, J.; Yuan, J.; Huang, C.; Xu, X.; Yang, J. Interleukin 10 enhanced CD8+ T cell activity and reduced CD8(+) T cell apoptosis in patients with diffuse large B cell lymphoma. Exp. Cell Res. 2017, 360, 146–152. [Google Scholar] [CrossRef]
- Cha, Z.; Gu, H.; Zang, Y.; Wang, Z.; Li, J.; Huang, W.; Qin, A.; Zhu, L.; Tu, X.; Cheng, N.; et al. The prevalence and function of CD4(+)CXCR5(+)Foxp3(+) follicular regulatory T cells in diffuse large B cell lymphoma. Int. Immunopharmacol. 2018, 61, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Tzankov, A.; Meier, C.; Hirschmann, P.; Went, P.; Pileri, S.A.; Dirnhofer, S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 2008, 93, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Song, E.K.; Jang, K.Y.; Choi, H.N.; Moon, W.S.; Kwon, K.; Lee, J.H.; Yim, C.Y.; Kwak, J.Y. Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk. Lymphoma 2008, 49, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Yokote, T.; Akioka, T.; Hiraoka, N.; Nishiwaki, U.; Miyoshi, T.; Iwaki, K.; Takayama, A.; Masuda, Y.; Hatooka, J.; et al. Infiltration of effector regulatory T cells predicts poor prognosis of diffuse large B-cell lymphoma, not otherwise specified. Blood Adv. 2017, 1, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, C.A.; Christiansson, L.H.; Thorn, I.; Mangsbo, S.; Paul-Wetterberg, G.; Sundstrom, C.; Totterman, T.H.; Simonsson, B.; Enblad, G.; Frisk, P.; et al. Both CD4+ FoxP3+ and CD4+ FoxP3- T cells from patients with B-cell malignancy express cytolytic markers and kill autologous leukaemic B cells in vitro. Immunology 2011, 133, 296–306. [Google Scholar] [CrossRef]
- Carreras, J.; Lopez-Guillermo, A.; Fox, B.C.; Colomo, L.; Martinez, A.; Roncador, G.; Montserrat, E.; Campo, E.; Banham, A.H. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 2006, 108, 2957–2964. [Google Scholar] [CrossRef]
- Nicholas, N.S.; Apollonio, B.; Ramsay, A.G. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochim. Biophys. Acta 2016, 1863, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, S.Y.; Kang, Y.W.; Lin, K.P.; Chen, T.Y.; Medeiros, L.J.; Chang, K.C. High levels of regulatory T cells in blood are a poor prognostic factor in patients with diffuse large B-cell lymphoma. Am. J. Clin. Pathol. 2015, 144, 935–944. [Google Scholar] [CrossRef]
- Saez, A.I.; Garcia-Cosio, M.; Saez, A.J.; Hernandez, J.M.; Sanchez-Verde, L.; Alvarez, D.; de la Cueva, P.; Arranz, R.; Conde, E.; Grande, C.; et al. Identification of biological markers of sensitivity to high-clinical-risk-adapted therapy for patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 2009, 50, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Hasselblom, S.; Sigurdadottir, M.; Hansson, U.; Nilsson-Ehle, H.; Ridell, B.; Andersson, P.O. The number of tumour-infiltrating TIA-1+ cytotoxic T cells but not FOXP3+ regulatory T cells predicts outcome in diffuse large B-cell lymphoma. Br. J. Haematol. 2007, 137, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ke, X.Y. The four types of Tregs in malignant lymphomas. J. Hematol. Oncol. 2011, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.T.; Sharpe, A.H. The multifaceted functions of follicular regulatory T cells. Curr. Opin. Immunol. 2020, 67, 68–74. [Google Scholar] [CrossRef]
- Wing, J.B.; Lim, E.L.; Sakaguchi, S. Control of foreign Ag-specific Ab responses by Treg and Tfr. Immunol. Rev. 2020, 296, 104–119. [Google Scholar] [CrossRef]
- Jin, H.; Carrio, R.; Yu, A.; Malek, T.R. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 2004, 173, 657–665. [Google Scholar] [CrossRef]
- Bhatt, S.; Sarosiek, K.A.; Lossos, I.S. Interleukin 21—its potential role in the therapy of B-cell lymphomas. Leuk. Lymphoma 2017, 58, 17–29. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, K.A.; Malumbres, R.; Nechushtan, H.; Gentles, A.J.; Avisar, E.; Lossos, I.S. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B-cell lymphomas. Blood 2010, 115, 570–580. [Google Scholar] [CrossRef]
- Croce, M.; Rigo, V.; Ferrini, S. IL-21: A pleiotropic cytokine with potential applications in oncology. J. Immunol. Res. 2015, 2015, 696578. [Google Scholar] [CrossRef]
- De Totero, D.; Meazza, R.; Zupo, S.; Cutrona, G.; Matis, S.; Colombo, M.; Balleari, E.; Pierri, I.; Fabbi, M.; Capaia, M.; et al. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood 2006, 107, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Gelebart, P.; Zak, Z.; Anand, M.; Dien-Bard, J.; Amin, H.M.; Lai, R. Interleukin-21 effectively induces apoptosis in mantle cell lymphoma through a STAT1-dependent mechanism. Leukemia 2009, 23, 1836–1846. [Google Scholar] [CrossRef][Green Version]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Parrish-Novak, J.; Foster, D.C.; Holly, R.D.; Clegg, C.H. Interleukin-21 and the IL-21 receptor: Novel effectors of NK and T cell responses. J. Leukoc. Biol. 2002, 72, 856–863. [Google Scholar] [CrossRef]
- Zeng, R.; Spolski, R.; Finkelstein, S.E.; Oh, S.; Kovanen, P.E.; Hinrichs, C.S.; Pise-Masison, C.A.; Radonovich, M.F.; Brady, J.N.; Restifo, N.P.; et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005, 201, 139–148. [Google Scholar] [CrossRef]
- Moroz, A.; Eppolito, C.; Li, Q.; Tao, J.; Clegg, C.H.; Shrikant, P.A. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: Comparative evaluation of IL-2, IL-15, and IL-21. J. Immunol. 2004, 173, 900–909. [Google Scholar] [CrossRef]
- Shen, S.; Sckisel, G.; Sahoo, A.; Lalani, A.; Otter, D.D.; Pearson, J.; DeVoss, J.; Cheng, J.; Casey, S.C.; Case, R.; et al. Engineered IL-21 Cytokine Muteins Fused to Anti-PD-1 Antibodies Can Improve CD8+ T Cell Function and Anti-tumor Immunity. Front. Immunol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Davis, M.R.; Zhu, Z.; Hansen, D.M.; Bai, Q.; Fang, Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015, 358, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cha, Z.; Qian, G.; Zang, Y.; Gu, H.; Huang, Y.; Zhu, L.; Li, J.; Liu, Y.; Tu, X.; Song, H.; et al. Circulating CXCR5+CD4+ T cells assist in the survival and growth of primary diffuse large B cell lymphoma cells through interleukin 10 pathway. Exp. Cell Res. 2017, 350, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Cha, Z.; Gu, H.; Guo, H.; Tu, X.; Zang, Y.; Zhao, C.; Hua, M.; Rechlic, J.R.; Olasnova, L.M.; Song, H.; et al. Effect of interleukin 21 and its receptor on CD8(+) T cells in the pathogenesis of diffuse large B-cell lymphoma. Oncol. Lett. 2014, 8, 421–425. [Google Scholar] [CrossRef]
- Ma, X.; Zha, J.; He, J.; Chen, L.; Huang, J.; Wu, W.; Tian, P.; Qian, B.H.; Yu, L.; Jiang, Y.; et al. T follicular helper cell-mediated IL-21 production suppresses FOXP3 expression of T follicular regulatory-like cells in diffuse large B cell lymphoma patients. Hum. Immunol. 2020, 81, 452–459. [Google Scholar] [CrossRef]
- Menter, T.; Bodmer-Haecki, A.; Dirnhofer, S.; Tzankov, A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum. Pathol. 2016, 54, 17–24. [Google Scholar] [CrossRef]
- Fang, X.; Xiu, B.; Yang, Z.; Qiu, W.; Zhang, L.; Zhang, S.; Wu, Y.; Zhu, X.; Chen, X.; Xie, S.; et al. The expression and clinical relevance of PD-1, PD-L1, and TP63 in patients with diffuse large B-cell lymphoma. Medicine 2017, 96, e6398. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Park, B.B.; Uhm, J. Understanding Immune Evasion and Therapeutic Targeting Associated with PD-1/PD-L1 Pathway in Diffuse Large B-cell Lymphoma. Int. J. Mol. Sci. 2019, 20, 1326. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.; Neftelino, S.T.; Hodge, J.P.; Oliva, C.; Campbell, J.R.; Yu, J.X. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat. Rev. Drug Discov. 2021, 20, 168–169. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients with Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Behdad, A.; Griffin, B.; Chen, Y.H.; Ma, S.; Kelemen, K.; Lu, X.; Chen, Q.C. PD-1 is highly expressed by neoplastic B-cells in Richter transformation. Br. J. Haematol. 2019, 185, 370–373. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ding, W.; Viswanatha, D.S.; Chen, D.; Shi, M.; Van Dyke, D.; Tian, S.; Dao, L.N.; Parikh, S.A.; Shanafelt, T.D.; et al. PD-1 Expression in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) and Large B-cell Richter Transformation (DLBCL-RT): A Characteristic Feature of DLBCL-RT and Potential Surrogate Marker for Clonal Relatedness. Am. J. Surg. Pathol. 2018, 42, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Auge, H.; Notarantonio, A.B.; Morizot, R.; Quinquenel, A.; Fornecker, L.M.; Hergalant, S.; Feugier, P.; Broseus, J. Microenvironment Remodeling and Subsequent Clinical Implications in Diffuse Large B-Cell Histologic Variant of Richter Syndrome. Front. Immunol. 2020, 11, 594841. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; LaPlant, B.R.; Call, T.G.; Parikh, S.A.; Leis, J.F.; He, R.; Shanafelt, T.D.; Sinha, S.; Le-Rademacher, J.; Feldman, A.L.; et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017, 129, 3419–3427. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Basu, S.; Thompson, P.A.; Ohanian, M.; Ferrajoli, A.; Pemmaraju, N.; Cortes, J.E.; Estrov, Z.; Burger, J.A.; Neelapu, S.S.; et al. Nivolumab Combined with Ibrutinib for CLL and Richter Transformation: A Phase II Trial. Blood 2016, 128, 59. [Google Scholar] [CrossRef]
- Younes, A.; Brody, J.; Carpio, C.; Lopez-Guillermo, A.; Ben-Yehuda, D.; Ferhanoglu, B.; Nagler, A.; Ozcan, M.; Avivi, I.; Bosch, F.; et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: A phase 1/2a study. Lancet Haematol. 2019, 6, e67–e78. [Google Scholar] [CrossRef]
- Armand, P.; Murawski, N.; Molin, D.; Zain, J.; Eichhorst, B.; Gulbas, Z.; Hawkes, E.A.; Pagel, J.M.; Phillips, T.; Ribrag, V.; et al. Pembrolizumab in relapsed or refractory Richter syndrome. Br. J. Haematol. 2020, 190, e117–e120. [Google Scholar] [CrossRef] [PubMed]
- Pula, B.; Salomon-Perzynski, A.; Prochorec-Sobieszek, M.; Jamroziak, K. Immunochemotherapy for Richter syndrome: Current insights. Immunotargets Ther. 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Huang, Y.; Dotson, E.; Lundberg, J.; Andritsos, L.A.; Awan, F.T.; Woyach, J.A.; Byrd, J.C. Use of PD-1 (PDCD1) inhibitors for the treatment of Richter syndrome: Experience at a single academic centre. Br. J. Haematol. 2019, 185, 363–366. [Google Scholar] [CrossRef]
- Zhang, J.; Medeiros, L.J.; Young, K.H. Cancer Immunotherapy in Diffuse Large B-Cell Lymphoma. Front. Oncol. 2018, 8, 351. [Google Scholar] [CrossRef]
- Armengol, M.; Santos, J.C.; Fernandez-Serrano, M.; Profitos-Peleja, N.; Ribeiro, M.L.; Roue, G. Immune-Checkpoint Inhibitors in B-Cell Lymphoma. Cancers 2021, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.R.; Young, K.H. New agents and regimens for diffuse large B cell lymphoma. J. Hematol. Oncol. 2020, 13, 175. [Google Scholar] [CrossRef]
- Lundqvist, A.; van Hoef, V.; Zhang, X.; Wennerberg, E.; Lorent, J.; Witt, K.; Sanz, L.M.; Liang, S.; Murray, S.; Larsson, O.; et al. 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer (SITC 2016): Part one. J. Immunother. Cancer 2016, 4, 82. [Google Scholar] [CrossRef]
- De Vos, S.; Forero-Torres, A.; Ansell, S.M.; Kahl, B.; Cheson, B.D.; Bartlett, N.L.; Furman, R.R.; Winter, J.N.; Kaplan, H.; Timmerman, J.; et al. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J. Hematol. Oncol. 2014, 7, 44. [Google Scholar] [CrossRef]
- Ansell, S.M.; Flinn, I.; Taylor, M.H.; Sikic, B.I.; Brody, J.; Nemunaitis, J.; Feldman, A.; Hawthorne, T.R.; Rawls, T.; Keler, T.; et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv. 2020, 4, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Tun, A.M.; Ansell, S.M. Immunotherapy in Hodgkin and non-Hodgkin lymphoma: Innate, adaptive and targeted immunological strategies. Cancer Treat Rev. 2020, 88, 102042. [Google Scholar] [CrossRef]
- Viardot, A.; Goebeler, M.E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Arnason, J.; Advani, R.; Brown, J.R.; Allan, J.; Ansell, S.; O’Brien, S.; Chavez, J.; Duell, J.; Rosenwald, A.; et al. CLINICAL ACTIVITY OF REGN1979, AN ANTI-CD20 X ANTI-CD3 BISPECIFIC ANTIBODY (AB) IN PATIENTS (PTS) WITH (W/) RELAPSED/REFRACTORY (R/R) B-CELL NON-HODGKIN LYMPHOMA (B-NHL). Hematol. Oncol. 2019, 37, 90–92. [Google Scholar] [CrossRef]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martinez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef]
- Bacac, M.; Colombetti, S.; Herter, S.; Sam, J.; Perro, M.; Chen, S.; Bianchi, R.; Richard, M.; Schoenle, A.; Nicolini, V.; et al. CD20-TCB with Obinutuzumab Pretreatment as Next-Generation Treatment of Hematologic Malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4785–4797. [Google Scholar] [CrossRef]
- Murer, P.; Neri, D. Antibody-cytokine fusion proteins: A novel class of biopharmaceuticals for the therapy of cancer and of chronic inflammation. New Biotechnol. 2019, 52, 42–53. [Google Scholar] [CrossRef]
- Lewis, K.E.; Selby, M.J.; Masters, G.; Valle, J.; Dito, G.; Curtis, W.R.; Garcia, R.; Mink, K.A.; Waggie, K.S.; Holdren, M.S.; et al. Interleukin-21 combined with PD-1 or CTLA-4 blockade enhances antitumor immunity in mouse tumor models. Oncoimmunology 2017, 7, e1377873. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Upadhaya, S.; Tatake, R.; Barkalow, F.; Hubbard-Lucey, V.M. Cancer cell therapies: The clinical trial landscape. Nat. Rev. Drug Discov. 2020, 19, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, O.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Bukhari, A.; El Chaer, F.; Koka, R.; Singh, Z.; Hutnick, E.; Ruehle, K.; Lee, S.T.; Kocoglu, M.H.; Shanholtz, C.; Badros, A.; et al. Rapid relapse of large B-cell lymphoma after CD19 directed CAR-T-cell therapy due to CD-19 antigen loss. Am. J. Hematol. 2019, 94, E273–E275. [Google Scholar] [CrossRef]
- Suarez, E.R.; Chang de, K.; Sun, J.; Sui, J.; Freeman, G.J.; Signoretti, S.; Zhu, Q.; Marasco, W.A. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget 2016, 7, 34341–34355. [Google Scholar] [CrossRef]
- Rafiq, S.; Yeku, O.O.; Jackson, H.J.; Purdon, T.J.; van Leeuwen, D.G.; Drakes, D.J.; Song, M.; Miele, M.M.; Li, Z.; Wang, P.; et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018, 36, 847–856. [Google Scholar] [CrossRef]
- Li, S.; Siriwon, N.; Zhang, X.; Yang, S.; Jin, T.; He, F.; Kim, Y.J.; Mac, J.; Lu, Z.; Wang, S.; et al. Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor-Modified T Cells Engineered to Secrete Checkpoint Inhibitors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6982–6992. [Google Scholar] [CrossRef]
- Cherkassky, L.; Morello, A.; Villena-Vargas, J.; Feng, Y.; Dimitrov, D.S.; Jones, D.R.; Sadelain, M.; Adusumilli, P.S. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Investig. 2016, 126, 3130–3144. [Google Scholar] [CrossRef]
- McGowan, E.; Lin, Q.; Ma, G.; Yin, H.; Chen, S.; Lin, Y. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed. Pharmacother. 2020, 121, 109625. [Google Scholar] [CrossRef]
- Hossain, N.; Sahaf, B.; Abramian, M.; Spiegel, J.Y.; Kong, K.; Kim, S.; Mavroukakis, S.; Oak, J.; Natkunam, Y.; Meyer, E.H.; et al. Phase I Experience with a Bi-Specific CAR Targeting CD19 and CD22 in Adults with B-Cell Malignancies. Blood 2018, 132, 490. [Google Scholar] [CrossRef]
- Sang, W.; Shi, M.; Yang, J.; Cao, J.; Xu, L.; Yan, D.; Song, X.; Sun, C.; Li, D.; Zhu, F.; et al. Combination of Anti-CD19 and Anti-CD20 Chimeric Antigen Receptor T Cells for Relapsed and Refractory Diffuse Larger B Cell Lymphoma: An Open-Label, Single-Arm, Phase Ⅰ/Ⅱ Trial. Blood 2019, 134, 1590. [Google Scholar] [CrossRef]
- Ardeshna, K.M.; Marzolini, M.A.V.; Norman, J.; Al-Hajj, M.; Thomas, S.; Faulkner, J.; Kotsopoulou, E.; Pule, M.; Peddareddigari, V.G.R.; Khokhar, N.Z.; et al. Phase 1/2 Study of AUTO3 the First Bicistronic Chimeric Antigen Receptor (CAR) Targeting CD19 and CD22 Followed by an Anti-PD1 in Patients with Relapsed/Refractory (r/r) Diffuse Large B Cell Lymphoma (DLBCL): Results of Cohort 1 and 2 of the Alexander Study. Blood 2019, 134, 246. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Locke, F.L.; Miklos, D.B.; Herrera, A.F.; Westin, J.R.; Lee, J.; Rossi, J.M.; Zheng, L.; Avanzi, M.P.; Roberts, Z.J.; et al. End of Phase 1 Results from Zuma-6: Axicabtagene Ciloleucel (Axi-Cel) in Combination with Atezolizumab for the Treatment of Patients with Refractory Diffuse Large B Cell Lymphoma. Blood 2018, 132, 4192. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Patel, S.; Burga, R.A.; Powell, A.B.; Chorvinsky, E.A.; Hoq, N.; McCormack, S.E.; Van Pelt, S.N.; Hanley, P.J.; Cruz, C.R.Y. Beyond CAR T Cells: Other Cell-Based Immunotherapeutic Strategies Against Cancer. Front. Oncol. 2019, 9, 196. [Google Scholar] [CrossRef]
- Fontan, L.; Qiao, Q.; Hatcher, J.M.; Casalena, G.; Us, I.; Teater, M.; Durant, M.; Du, G.; Xia, M.; Bilchuk, N.; et al. Specific covalent inhibition of MALT1 paracaspase suppresses B cell lymphoma growth. J. Clin. Investig. 2018, 128, 4397–4412. [Google Scholar] [CrossRef]
- Fontan, L.; Goldstein, R.; Casalena, G.; Durant, M.; Teater, M.R.; Wilson, J.; Phillip, J.; Xia, M.; Shah, S.; Us, I.; et al. Identification of MALT1 feedback mechanisms enables rational design of potent antilymphoma regimens for ABC-DLBCL. Blood 2021, 137, 788–800. [Google Scholar] [CrossRef]
- Dyer, M.J.; Vos, S.D.; Ruan, J.; Flowers, C.; Maddocks, K.J.; Rule, S.; Hamdy, A.M.; Izumi, R.; Slatter, J.G.; Cheung, J.; et al. Acalabrutinib monotherapy in patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2018, 36, 7547. [Google Scholar] [CrossRef]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef]
- Mathews Griner, L.A.; Guha, R.; Shinn, P.; Young, R.M.; Keller, J.M.; Liu, D.; Goldlust, I.S.; Yasgar, A.; McKnight, C.; Boxer, M.B.; et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc. Natl. Acad. Sci. USA 2014, 111, 2349–2354. [Google Scholar] [CrossRef] [PubMed]
- Sagiv-Barfi, I.; Kohrt, H.E.; Czerwinski, D.K.; Ng, P.P.; Chang, B.Y.; Levy, R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. USA 2015, 112, E966–E972. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Maddocks, K.J.; Vos, S.D.; Lyons, R.M.; Edenfield, W.J.; Sharman, J.P.; Vose, J.; Yimer, H.A.; Wei, H.; Chan, E.M.; et al. Phase 1/2 trial of acalabrutinib plus pembrolizumab (Pem) in relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2019, 37, 7519. [Google Scholar] [CrossRef]
- Phillips, T.J.; Forero-Torres, A.; Sher, T.; Diefenbach, C.S.; Johnston, P.; Talpaz, M.; Pulini, J.; Zhou, L.; Scherle, P.; Chen, X.; et al. Phase 1 study of the PI3Kdelta inhibitor INCB040093 +/- JAK1 inhibitor itacitinib in relapsed/refractory B-cell lymphoma. Blood 2018, 132, 293–306. [Google Scholar] [CrossRef]
- Forero-Torres, A.; Ramchandren, R.; Yacoub, A.; Wertheim, M.S.; Edenfield, W.J.; Caimi, P.; Gutierrez, M.; Akard, L.; Escobar, C.; Call, J.; et al. Parsaclisib, a potent and highly selective PI3Kdelta inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood 2019, 133, 1742–1752. [Google Scholar] [CrossRef]
- Kallam, A.; Witzig, T.E.; Roschewski, M.J.; Lyden, E.; Lunning, M.A.; Bierman, P.J.; Bociek, G.; Armitage, J.O.; Vose, J. Phase II multi-center study of ruxolitinib phosphate for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma (PTCL). J. Clin. Oncol. 2019, 37, e19063. [Google Scholar] [CrossRef]
- Lastwika, K.J.; Wilson, W., III; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S.; et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016, 76, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.D.; Proud, C. Dissecting the signaling pathways that mediate cancer in PTEN and LKB1 double-knockout mice. Sci. Signal. 2015, 8, pe1. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Romero, D.L.; Yang, Y.; Shaffer, A.L., III; Chaudhary, D.; Robinson, S.; Miao, W.; Rui, L.; Westlin, W.F.; Kapeller, R.; et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J. Exp. Med. 2015, 212, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Markovtsov, V.V.; Lamagna, C.; Chan, M.; Yi, S.; Young, C.; Frances, R.; Siu, S.; Braselmann, S.; Li, H.; Singh, R.; et al. Abstract 346: Potential role for R191, potent and selective IRAK4 kinase inhibitor, in treatment of hematologic malignancies. Cancer Res. 2016, 76, 346. [Google Scholar] [CrossRef]
- Morschhauser, F.; Feugier, P.; Flinn, I.W.; Gasiorowski, R.; Greil, R.; Illes, A.; Johnson, N.A.; Larouche, J.F.; Lugtenburg, P.J.; Patti, C.; et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 2021, 137, 600–609. [Google Scholar] [CrossRef]
- Herbaux, C.; Casasnovas, O.; Feugier, P.; Damaj, G.; Bouabdallah, R.; Guidez, S.; Ysebaert, L.; Tilly, H.; Gouill, S.L.; Fornecker, L.; et al. Atezolizumab + obinutuzumab + venetoclax in patients with relapsed or refractory diffuse large B-cell Lymphomas (R/R DLBCL): Primary analysis of a phase II trial from LYSA. J. Clin. Oncol. 2020, 38, 8053. [Google Scholar] [CrossRef]
- Gribben, J.G.; Fowler, N.; Morschhauser, F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J. Clin. Oncol. 2015, 33, 2803–2811. [Google Scholar] [CrossRef]
- Ramsay, A.G.; Clear, A.J.; Fatah, R.; Gribben, J.G. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: Establishing a reversible immune evasion mechanism in human cancer. Blood 2012, 120, 1412–1421. [Google Scholar] [CrossRef]
- Benson, D.M., Jr.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef]
- Nowakowski, G.S.; Hong, F.; Scott, D.W.; Macon, R.; King, R.L.; Habermann, T.M.; Wagner-Johnston, N.; Casulo, C.; Wade, J.L.; Nagargoje, G.G.; et al. Addition of lenalidomide to R-CHOP (R2CHOP) improves outcomes in newly diagnosed diffuse large B-cell lymphoma (DLBCL): First report of ECOG-ACRIN1412 a randomized phase 2 US intergroup study of R2CHOP vs R-CHOP. Hematol. Oncol. 2019, 37, 37–38. [Google Scholar] [CrossRef]
- Vitolo, U.; Witzig, T.E.; Gascoyne, R.D.; Scott, D.W.; Zhang, Q.; Jurczak, W.; Özcan, M.; Hong, X.; Zhu, J.; Jin, J.; et al. ROBUST: First report of phase III randomized study of lenalidomide/R-CHOP (R2-CHOP) vs placebo/R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Hematol. Oncol. 2019, 37, 36–37. [Google Scholar] [CrossRef]
- Goy, A.; Ramchandren, R.; Ghosh, N.; Munoz, J.; Morgan, D.S.; Dang, N.H.; Knapp, M.; Delioukina, M.; Kingsley, E.; Ping, J.; et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood 2019, 134, 1024–1036. [Google Scholar] [CrossRef]
- Westin, J.R.; Nastoupil, L.J.; Fayad, L.; Hagemeister, F.B.; Oki, Y.; Turturro, F.; Ahmed, S.; Rodriguez, M.A.; Lee, H.J.; Steiner, R.E.; et al. Smart Start: Rituximab, Lenalidomide, and Ibrutinib Alone and in Combination with Standard Chemotherapy for Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma: Final Phase II Results. Blood 2019, 134, 1581. [Google Scholar] [CrossRef]
- Salles, G.; Duell, J.; Gonzalez Barca, E.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; Andre, M.; et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): A multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef]
- Goldfinger, M.; Cooper, D.L. Lenalidomide in DLBCL: Are we past the cell of origin? Clin. Adv. Hematol. Oncol. 2021, 19, 320–325. [Google Scholar]
- Kalakonda, N.; Maerevoet, M.; Cavallo, F.; Follows, G.; Goy, A.; Vermaat, J.S.P.; Casasnovas, O.; Hamad, N.; Zijlstra, J.M.; Bakhshi, S.; et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): A single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020, 7, e511–e522. [Google Scholar] [CrossRef]
- Farren, M.R.; Hennessey, R.C.; Shakya, R.; Elnaggar, O.; Young, G.; Kendra, K.; Landesman, Y.; Elloul, S.; Crochiere, M.; Klebanov, B.; et al. The Exportin-1 Inhibitor Selinexor Exerts Superior Antitumor Activity when Combined with T-Cell Checkpoint Inhibitors. Mol. Cancer Ther. 2017, 16, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Trott, J.; Anderson, K.; Kim, J.; Graef, A.; Shacham, S.; Landesman, Y.; Sarver, A.; Modiano, J.; Weiss, R.H. Abstract LB-086: Combination therapy of immune checkpoint and nuclear exporter inhibitors in a renal cell carcinoma mouse model. Cancer Res. 2016, 76, Abstract nr LB-086. [Google Scholar] [CrossRef]
- Li, W.; Gupta, S.K.; Han, W.; Kundson, R.A.; Nelson, S.; Knutson, D.; Greipp, P.T.; Elsawa, S.F.; Sotomayor, E.M.; Gupta, M. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J. Hematol. Oncol. 2019, 12, 73. [Google Scholar] [CrossRef]
- Hogg, S.J.; Vervoort, S.J.; Deswal, S.; Ott, C.J.; Li, J.; Cluse, L.A.; Beavis, P.A.; Darcy, P.K.; Martin, B.P.; Spencer, A.; et al. BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1. Cell Rep. 2017, 18, 2162–2174. [Google Scholar] [CrossRef]
- Sermer, D.; Pasqualucci, L.; Wendel, H.G.; Melnick, A.; Younes, A. Emerging epigenetic-modulating therapies in lymphoma. Nat. Rev. Clin. Oncol. 2019, 16, 494–507. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.T.; Ott, H.M.; Ganji, G.; Korenchuk, S.; Thompson, C.; Van Aller, G.S.; Liu, Y.; Graves, A.P.; Della Pietra, A., III; Diaz, E.; et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 2012, 492, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Jin, L.L.; Liu, C.Q.; Wang, Y.C.; Meng, Y.M.; Zhou, Z.G.; Chen, J.; Yu, X.J.; Zhang, Y.J.; Xu, J.; et al. EZH2 negatively regulates PD-L1 expression in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 300. [Google Scholar] [CrossRef]
- Morin, R.D.; Arthur, S.E.; Assouline, S. Treating lymphoma is now a bit EZ-er. Blood Adv. 2021, 5, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- San Jose-Eneriz, E.; Agirre, X.; Rabal, O.; Vilas-Zornoza, A.; Sanchez-Arias, J.A.; Miranda, E.; Ugarte, A.; Roa, S.; Paiva, B.; de Mendoza, A.E.-H.; et al. Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies. Nat. Commun. 2017, 8, 15424. [Google Scholar] [CrossRef] [PubMed]
- Segovia, C.; San Jose-Eneriz, E.; Munera-Maravilla, E.; Martinez-Fernandez, M.; Garate, L.; Miranda, E.; Vilas-Zornoza, A.; Lodewijk, I.; Rubio, C.; Segrelles, C.; et al. Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat. Med. 2019, 25, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Waschke, B.C.; Woolaver, R.A.; Chen, Z.; Zhang, G.; Piscopio, A.D.; Liu, X.; Wang, J.H. Histone Deacetylase Inhibition Sensitizes PD1 Blockade-Resistant B-cell Lymphomas. Cancer Immunol. Res. 2019, 7, 1318–1331. [Google Scholar] [CrossRef]
- Herrera, A.F.; Chen, L.; Popplewell, L.L.; Budde, L.E.; Mei, M.; Armenian, S.H.; Darrah, J.; Nikolaenko, L.; Chen, R.W.; Peters, L.; et al. Preliminary Results from a Phase I Trial of Pembrolizumab Plus Vorinostat in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, and Hodgkin Lymphoma. Blood 2019, 134, 759. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).