Long-Term Outcomes and Causes of Death among Medullary Thyroid Carcinoma Patients with Distant Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of Distant Metastasis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants with Distant Metastasis
3.2. Comparison of Clinical Characteristics between the SDM and MDM Groups
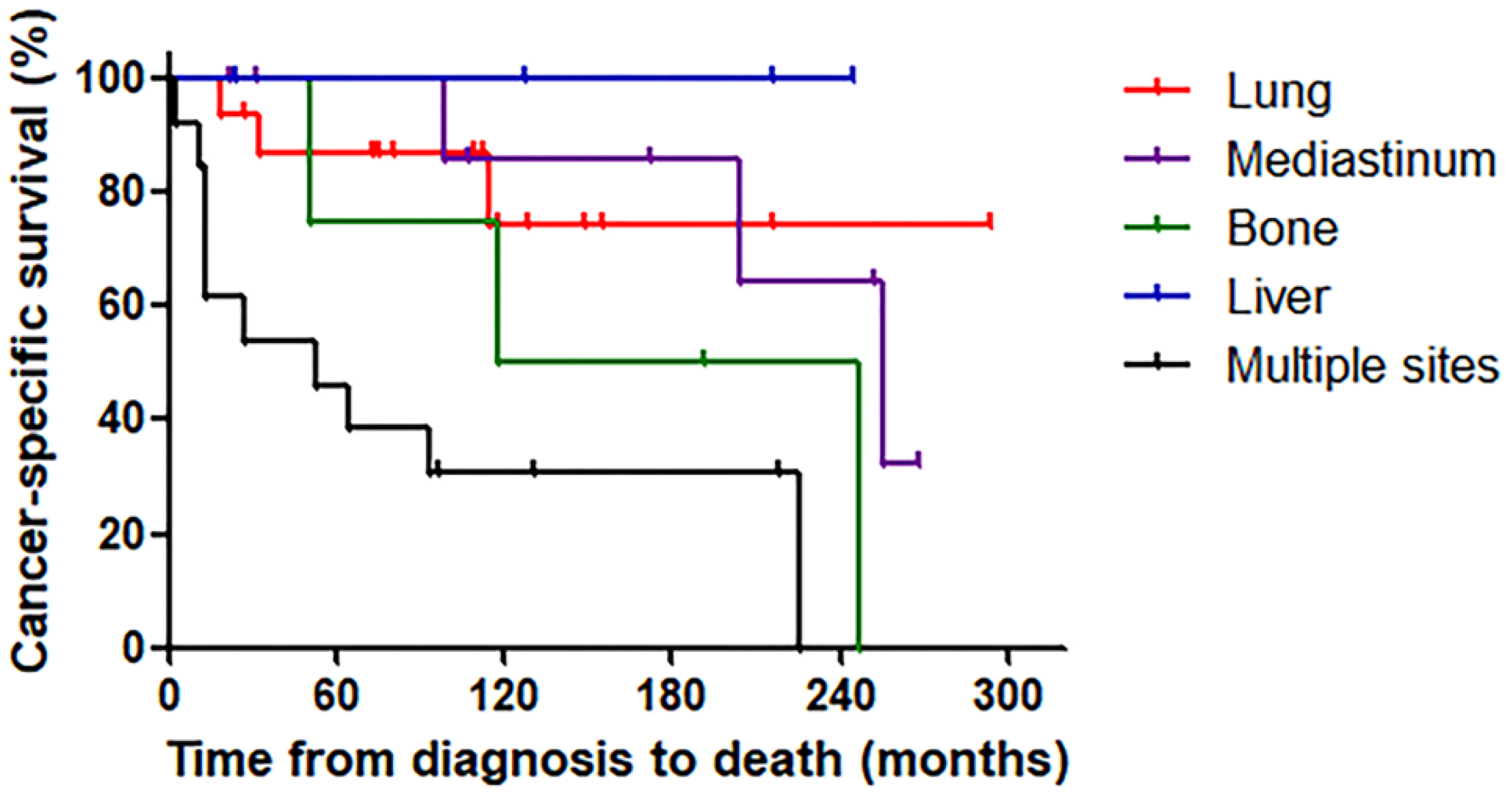
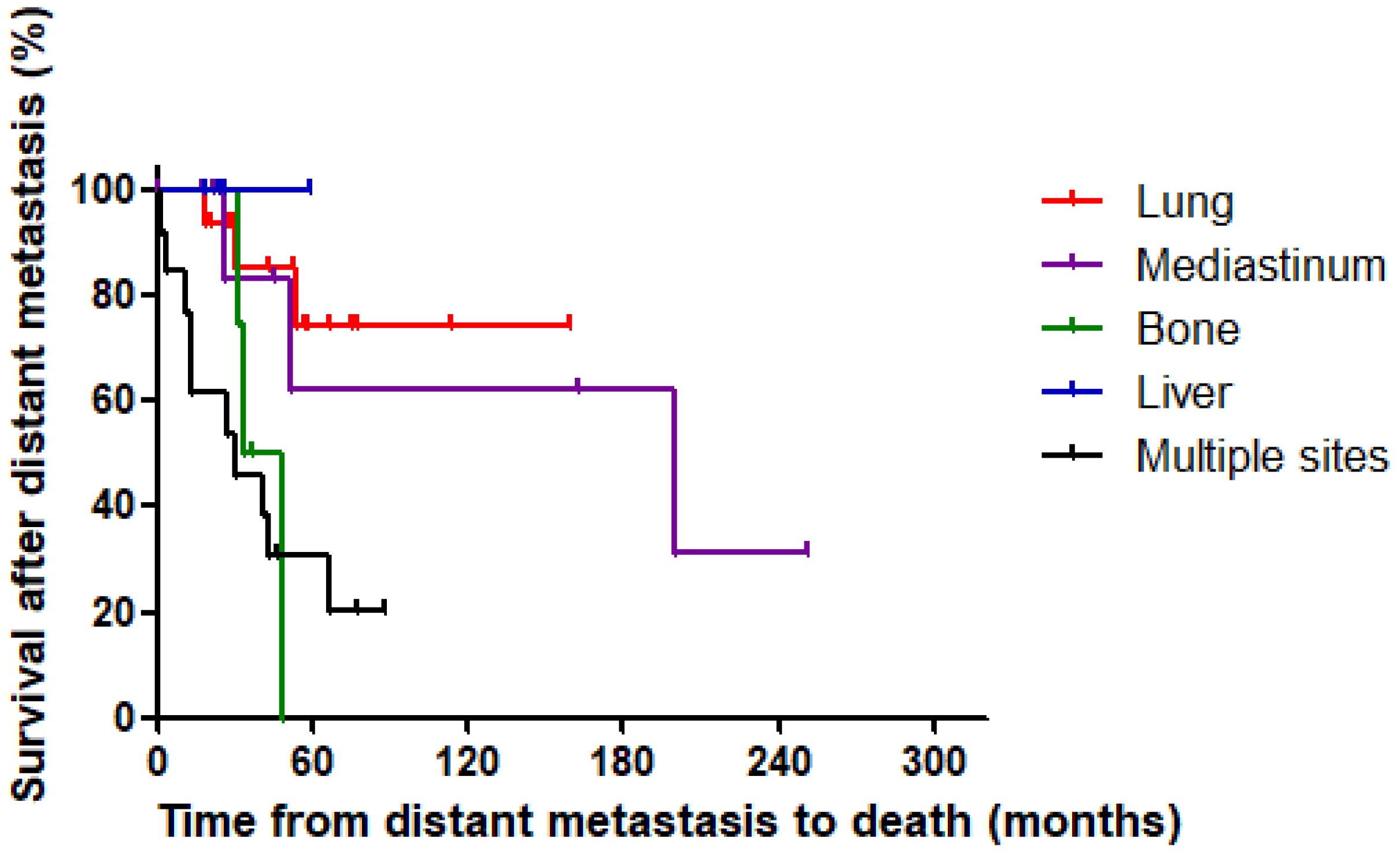
3.3. Survival According to Metastasis Site
3.4. Causes of Death among Deceased Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kebebew, E.; Ituarte, P.H.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Medullary thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000, 88, 1139–1148. [Google Scholar] [CrossRef]
- Noone, A.-M.; Cronin, K.A.; Altekruse, S.F.; Howlader, N.; Lewis, D.R.; Petkov, V.I.; Penberthy, L. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol. Biomark. Prev. 2016, 26, 632–641. [Google Scholar] [CrossRef]
- Oh, C.-M.; Jung, K.-W.; Won, Y.-J.; Shin, A.; Kong, H.-J.; Lee, J.-S. Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res. Treat. 2014, 47, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Sippel, R.S.; Kunnimalaiyaan, M.; Chen, H. Current management of medullary thyroid cancer. Oncologist 2008, 13, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Rendl, G.; Manzl, M.; Hitzl, W.; Sungler, P.; Pirich, C. Long-term prognosis of medullary thyroid carcinoma. Clin. Endocrinol. 2008, 69, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, F.D.; Hunt, W.C.; Morris, D.M.; Key, C.R. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 1997, 79, 564–573. [Google Scholar] [CrossRef]
- Wells, S.A.; Asa, S.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.Y.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Raue, F.; Kotzerke, J.; Reinwein, D.; Deckart, H.; Ritter, M.; Seif, F.; Buhr, H.; Beyer, J.; Schober, O.; Becker, W.; et al. Prognostic factors in medullary thyroid carcinoma: Evaluation of 741 patients from the German medullary thyroid carcinoma register. J. Mol. Med. 1993, 71, 7–12. [Google Scholar] [CrossRef]
- Dottorini, M.E.; Assi, A.; Sironi, M.; Sangalli, G.; Spreafico, G.; Colombo, L. Multivariate analysis of patients with medullary thyroid carcinoma: Prognostic significance and impact on treatment of clinical and pathologic variables. Cancer 1996, 77, 1556–1565. [Google Scholar] [CrossRef]
- Pazaitou-Panayiotou, K.; Chrisoulidou, A.; Mandanas, S.; Tziomalos, K.; Doumala, E.; Patakiouta, F. Predictive factors that influence the course of medullary thyroid carcinoma. Int. J. Clin. Oncol. 2013, 19, 445–451. [Google Scholar] [CrossRef]
- De Groot, J.W.B.; Plukker, J.T.M.; Wolffenbuttel, B.H.; Wiggers, T.; Sluiter, W.J.; Links, T.P. Determinants of life expectancy in medullary thyroid cancer: Age does not matter. Clin. Endocrinol. 2006, 65, 729–736. [Google Scholar] [CrossRef]
- Fromigué, J.; De Baere, T.; Baudin, E.; Dromain, C.; Leboulleux, S.; Schlumberger, M. Chemoembolization for liver metastases from medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 2496–2499. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, K.; Wolf, A.; Raffel, A.; Schott, M.; Miersch, D.; Yang, Q.; Eisenberger, C.F.; Röher, H.D.; Knoefel, W.T. Long-term clinical and biochemical follow-up in medullary thyroid carcinoma: A single institution’s experience over 20 years. Ann. Surg. 2007, 246, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, M.; Boschin, I.M.; Bernante, P.; Toniato, A.; Piotto, A.; Pagetta, C.; Nibale, O.; Rampin, L.; Muzzio, P.; Rubello, D. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur. J. Surg. Oncol. 2007, 33, 493–497. [Google Scholar] [CrossRef]
- Roman, S.; Lin, R.; Sosa, J.A. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006, 107, 2134–2142. [Google Scholar] [CrossRef]
- Sahli, Z.T.; Canner, J.K.; Zeiger, M.A.; Mathur, A. Association between age and disease specific mortality in medullary thyroid cancer. Am. J. Surg. 2020, 221, 478–484. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, S.; Son, H.; Ban, E.; Kang, S.W.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; Park, C.S. Medullary thyroid car-cinoma: A 30-year experience at one institution in Korea. Ann. Surg. Treat. Res. 2016, 91, 278–287. [Google Scholar] [CrossRef]
- Machens, A.; Dralle, H. Surgical cure rates of sporadic medullary thyroid cancer in the era of calcitonin screening. Eur. J. Endocrinol. 2016, 175, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Bastholt, L.; Dralle, H.; Jarząb, B.; Pacini, F.; Smit, J. European thyroid association task force 2012 European thyroid association guidelines for metastatic medullary Thyroid cancer. Eur. Thyroid J. 2012, 1, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Szavcsur, P.; Godény, M.; Bajzik, G.; Lengyel, E.; Repa, I.; Trón, L.; Boér, A.; Vincze, B.; Póti, Z.; Szabolcs, I.; et al. Angi-ography-proven liver metastases explain low efficacy of lymph node dissections in medullary thyroid cancer patients. Eur. J. Surg. Oncol. 2005, 31, 183–190. [Google Scholar] [CrossRef]
- Hyer, S.; Vini, L.; Hern, R.; Harmer, C. Medullary thyroid cancer: Multivariate analysis of prognostic factors influencing survival. Eur. J. Surg. Oncol. 2000, 26, 686–690. [Google Scholar] [CrossRef]
- Abraham, D.T.; Low, T.-H.; Messina, M.; Jackson, N.; Gill, A.; Chou, A.S.; Delbridge, L.; Learoyd, D.; Robinson, B.G.; Sidhu, S.; et al. Medullary thyroid carcinoma: Long-term outcomes of surgical treatment. Ann. Surg. Oncol. 2010, 18, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Resteghini, C.; Cavalieri, S.; Galbiati, D.; Granata, R.; Alfieri, S.; Bergamini, C.; Bossi, P.; Licitra, L.; Locati, L. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pr. Res. Clin. Endocrinol. Metab. 2017, 31, 349–361. [Google Scholar] [CrossRef]
- Chougnet, C.N.; Schlumberger, M.; Leboulleux, S.; Baudin, E. Vandetanib, in the management of patients with locally advanced or metastatic medullary thyroid carcinomas. Bull. Cancer 2014, 101, 891–895. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarząb, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Kuo, E.J.; Sho, S.; Li, N.; Zanocco, K.A.; Yeh, M.W.; Livhits, M.J. Risk factors associated with reoperation and disease-specific mortality in patients with medullary thyroid carcinoma. JAMA Surg. 2018, 153, 52–59. [Google Scholar] [CrossRef]
- Modigliani, E.; Cohen, R.; Campos, J.-M.; Conte-Devolx, B.; Maes, B.; Boneu, A.; Schlumberger, M.; Bigorgne, J.-C.; Dumontier, P.; Leclerc, L.; et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: Results in 899 patients. Clin. Endocrinol. 1998, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; Grunwell, J. Extent of disease and practice patterns for medullary thyroid cancer. J. Am. Coll. Surg. 2005, 200, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Hofmann, C.; Hauptmann, S.; Dralle, H. Locoregional recurrence and death from medullary thyroid carcinoma in a contemporaneous series: 5-year results. Eur. J. Endocrinol. 2007, 157, 85–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlumberger, M.; Carlomagno, F.; Baudin, E.; Bidart, J.M.; Santoro, M. New therapeutic approaches to treat medullary thyroid carcinoma. Nat. Clin. Pr. Endocrinol. Metab. 2008, 4, 22–32. [Google Scholar] [CrossRef]
- Randle, R.W.; Balentine, C.J.; Leverson, G.E.; Havlena, J.A.; Sippel, R.S.; Schneider, D.F.; Pitt, S.C. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2016, 161, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Hong, N.; Park, S.H.; Shin, D.Y.; Lee, C.R.; Kang, S.-W.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; et al. The relationship of comorbidities to mortality and cause of death in patients with differentiated thyroid carcinoma. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beasley, N.J.P.; Walfish, P.G.; Witterick, I.; Freeman, J.L. Cause of death in patients with well-differentiated thyroid carcinoma. Laryngoscope 2001, 111, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.; Park, S.Y.; Kim, T.H.; Kim, S.W.; Chung, J.H. Clinical course from diagnosis to death in patients with well-differentiated thyroid cancer. Cancers 2020, 12, 2323. [Google Scholar] [CrossRef]
- Kitamura, Y.; Shimizu, K.; Nagahama, M.; Sugino, K.; Ozaki, O.; Mimura, T.; Ito, K.; Ito, K.; Tanaka, S. Immediate causes of death in thyroid carcinoma: Clinicopathological analysis of 161 fatal cases. J. Clin. Endocrinol. Metab. 1999, 84, 4043–4049. [Google Scholar] [CrossRef]
| Variables | Data |
|---|---|
| Age, years (mean ± SD) | 45.9 (15.0) |
| Sex, women (n, %) | 23 (50.0) |
| Tumor type (n, %) | |
| Sporadic | 43 (93.5) |
| Hereditary (MEN 2A) | 3 (6.5) |
| Primary tumor size, cm (median, IQR) † | 3.0 (2.0–4.4) |
| Cervical lymph node metastasis † (n, %) | |
| N0/Nx | 8 (17.3) |
| N1a | 3 (6.5) |
| N1b | 35 (76.1) |
| Extent of initial surgery (n, %) | |
| Total thyroidectomy | 41 (89.1) |
| Lobectomy | 1 (2.2) |
| Inoperable | 4 (8.7) |
| Initial central lymph node dissection (n, %) | |
| Yes | 41 (89.1) |
| No | 1 (2.2) |
| Inoperable | 4 (8.7) |
| Initial lateral lymph node dissection (n, %) | |
| Yes | 34 (73.9) |
| No | 8 (17.4) |
| Inoperable | 4 (8.7) |
| 8th AJCC/TNM stage † (n, %) | |
| Stage I | 1 (2.2) |
| Stage II | 3 (6.5) |
| Stage III | 3 (6.5) |
| Stage IVA | 20 (43.5) |
| Stage IVB | 2 (4.3) |
| Stage IVC | 15 (32.6) |
| No available data | 2 (4.3) |
| SDM Group (n = 15) | MDM Group (n = 31) | p-Value | |
|---|---|---|---|
| Age, years (mean ± SD) | 47.3 (18.5) | 45.3 (13.3) | 0.675 |
| Sex, women (n, %) | 7 (46.7) | 16 (51.6) | 0.753 |
| Tumor type (n, %) | |||
| Sporadic | 15 (100.0) | 28 (90.3) | 0.213 |
| Hereditary (MEN 2A) | 0 (0.0) | 3 (9.7) | |
| Primary tumor size, cm (median, IQR) † | 4.0 (2.6–5.0) | 2.7 (1.8–4.0) | 0.097 |
| Cervical lymph node metastasis (n, %) † | |||
| N0/Nx | 2 (13.3) | 6 (19.4) | 0.635 |
| N1a | 0 (0.0) | 3 (9.7) | |
| N1b | 13 (86.7) | 22 (71.0) | |
| Initial extent of surgery (n, %) | |||
| Total thyroidectomy | 11 (73.3) | 30 (96.8) | 0.008 |
| Lobectomy | 0 (0) | 1 (3.2) | |
| Inoperable | 4 (26.7) | 0 (0.0) | |
| Initial central lymph node dissection (n, %) | |||
| Yes | 11 (73.3) | 30 (96.8) | 0.008 |
| No | 0 (0.0) | 1 (3.2) | |
| Inoperable | 4 (26.7) | 0 (0.0) | |
| Initial lateral lymph node dissection (n, %) | |||
| Yes | 10 (66.7) | 24 (77.4) | 0.008 |
| No | 1 (6.7) | 7 (22.6) | |
| Inoperable | 4 (26.7) | 0 (0.0) | |
| Number of dead patients | 9 (60.0) | 10 (32.3) | 0.073 |
| Metastatic Sites | Synchronous Distant Metastasis (n, %) | Metachronous Distant Metastasis (n, %) | Total (n, %) |
|---|---|---|---|
| Lung | 6 (40.0) | 10 (32.3) | 16 (34.8) |
| Mediastinum | 2 (13.3) | 7 (22.6) | 9 (19.6) |
| Bone | 0 (0.0) | 4 (12.9) | 4 (8.7) |
| Liver | 1 (6.7) | 3 (9.7) | 4 (8.7) |
| Multiple sites | |||
| Lung + bone | 2 (13.3) | 2 (6.5) | 4 (8.7) |
| Liver + bone | 1 (6.7) | 2 (6.5) | 3 (6.5) |
| Lung + liver | 1 (6.7) | 0 (0.0) | 1 (2.2) |
| Adrenal + lung | 1 (6.7) | 0 (0.0) | 1 (2.2) |
| Adrenal + liver | 0 (0.0) | 1 (3.2) | 1 (2.2) |
| Brain + lung | 0 (0.0) | 1 (3.2) | 1 (2.2) |
| Brain + pancreas + lung + liver + bone | 1 (6.7) | 0 (0.0) | 1 (2.2) |
| Kidney + liver + bone | 0 (0.0) | 1 (3.2) | 1 (2.2) |
| Site of Metastasis | Number of Patients | Number of Cancer-Specific Deaths | 5-Year Survival from the Detection of Distant Metastasis (%) | HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Overall | 46 | 19 | 52.9 | ||
| Lung | 16 | 3 | 74.6 | Reference | |
| Mediastinum | 9 | 3 | 62.5 | 1.43 (0.24–8.54) | 0.679 |
| Bone | 4 | 3 | 0.0 | 5.42 (1.05–27.99) | 0.044 |
| Liver | 4 | 0 | 100.0 | - | - |
| Multiple sites | 13 | 10 | 30.8 | 6.11 (1.67–22.34) | 0.006 |
| No. | Age | Sex | RET Mutation | 8th TNM Stage | Site of Distant Metastasis | Cause of Death | Duration (Months) |
|---|---|---|---|---|---|---|---|
| 1 | 56 | M | NA | Not available | Lung, bone | Airway obstruction | 13 |
| 2 | 60 | F | NA | IVC | Lung, adrenal | Airway obstruction | 3 |
| 3 | 25 | F | NA | IVC | Lung | Airway obstruction | 19 |
| 4 | 48 | M | Wild-type | III | Mediastinum | Airway obstruction | 99 |
| 5 | 35 | M | Wild-type | IVA | Lung, bone, liver | Respiratory failure | 115 |
| 6 | 60 | M | NA | IVC | Lung, bone | Respiratory failure | 33 |
| 7 | 41 | M | NA | IVA | Mediastinal soft tissue, lung, pleural | Respiratory failure | 255 |
| 8 | 34 | M | Wild-type | IVC | Lung, bone | Complications due to bone metastasis | 247 |
| 9 | 46 | F | Wild-type | III | Bone | Complications due to bone metastasis | 11 |
| 10 | 57 | M | Wild-type | II | Bone, liver | Complications due to bone metastasis | 93 |
| 11 | 65 | F | Wild-type | IVC | Lung, bone, liver, pancreas, brain | Complications due to brain metastasis | 65 |
| 12 | 77 | F | NA | IVC | Bone, liver | Complications related to conventional chemotherapy (capecitabine) | 13 |
| 13 | 63 | M | NA | IVC | Mediastinal soft tissue, bone | Complications related to targeted therapy (vandetanib) | 204 |
| 14 | 64 | M | NA | IVA | Lung, bone, liver | Complications related to targeted therapy (vandetanib) | 13 |
| 15 | 55 | M | Wild-type | IVC | Lung, liver, bone, pancreas, brain | Complications related to targeted therapy (lenvatinib) | 118 |
| 16 | 46 | M | Wild-type | Not available | Bone, lung, pleural, pancreas | Complications related to targeted therapy (vandetanib) | 226 |
| 17 | 54 | M | Wild-type | IVA | Bone | Unspecified | 51 |
| 18 | 24 | F | Wild-type | IVC | Bone, lung | Unspecified | 27 |
| 19 | 71 | M | Wild-type | IVA | Liver, kidney, bone | Unspecified | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Yang, H.; Heo, J.; Kim, T.H.; Kim, S.W.; Chung, J.H. Long-Term Outcomes and Causes of Death among Medullary Thyroid Carcinoma Patients with Distant Metastases. Cancers 2021, 13, 4670. https://doi.org/10.3390/cancers13184670
Park H, Yang H, Heo J, Kim TH, Kim SW, Chung JH. Long-Term Outcomes and Causes of Death among Medullary Thyroid Carcinoma Patients with Distant Metastases. Cancers. 2021; 13(18):4670. https://doi.org/10.3390/cancers13184670
Chicago/Turabian StylePark, Hyunju, Heera Yang, Jung Heo, Tae Hyuk Kim, Sun Wook Kim, and Jae Hoon Chung. 2021. "Long-Term Outcomes and Causes of Death among Medullary Thyroid Carcinoma Patients with Distant Metastases" Cancers 13, no. 18: 4670. https://doi.org/10.3390/cancers13184670
APA StylePark, H., Yang, H., Heo, J., Kim, T. H., Kim, S. W., & Chung, J. H. (2021). Long-Term Outcomes and Causes of Death among Medullary Thyroid Carcinoma Patients with Distant Metastases. Cancers, 13(18), 4670. https://doi.org/10.3390/cancers13184670






