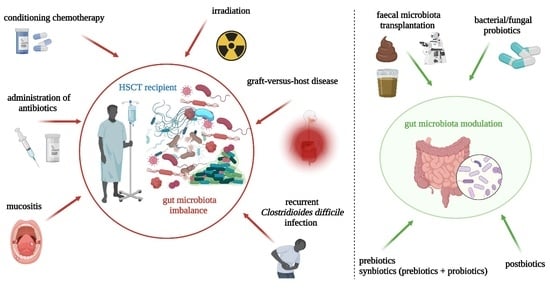
Gut Microbiome Modulation and Faecal Microbiota Transplantation Following Allogenic Hematopoietic Stem Cell Transplantation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Gut-Microbiota-Related Aspects
2.1. Gut Microbiota in Hematologic Malignancies and Allogeneic Hematopoietic Stem Cells Transplantation
2.2. Intestinal Integrity: Crosstalk between Gut Microbiota Products and Intestinal Homeostasis
3. Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation
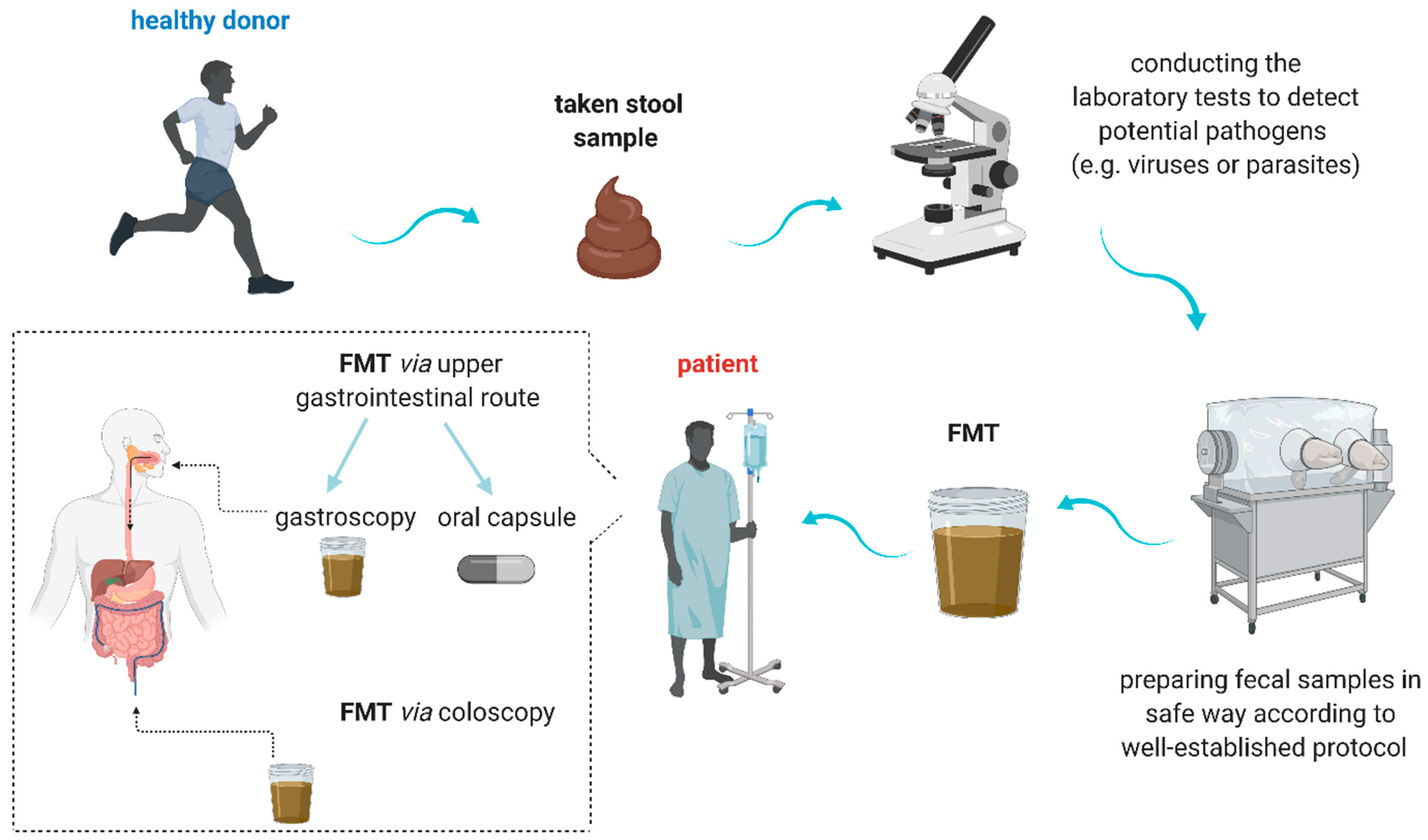
3.1. Definition, Preparation, and Implication
3.2. FMT to Treat Multidrug-Resistant Bacteria Infections
3.3. FMT and Clostridioides difficile Infection
3.4. FMT and Graft-Versus-Host Disease
4. Role of Nutritional Interventions, Probiotics, Prebiotics, Synbiotic, and Postbiotics in Hematologic Patients in the Context of Gut Microbiota Modulation
4.1. Nutritional Interventions
4.2. Probiotics
4.3. Prebiotics
4.4. Synbiotics
4.5. Postbiotics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaźmierczak-Siedlecka, K.; Daca, A.; Fic, M.; Van de Wetering, T.; Folwarski, M.; Makarewicz, W. Therapeutic methods of gut microbiota modification in colorectal cancer management–Fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 2020, 11, 1518–1530. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Dvořák, A.; Folwarski, M.; Daca, A.; Przewłócka, K.; Makarewicz, W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers 2020, 12, 1326. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Mizuiri, S.; Nishizawa, Y.; Doi, T.; Yamashita, K.; Shigemoto, K.; Usui, K.; Arita, M.; Naito, T.; Doi, S.; Masaki, T. Iron, coronary artery calcification, and mortality in patients undergoing hemodialysis. Ren. Fail. 2021, 43, 371–380. [Google Scholar] [CrossRef]
- Song, Y.; Himmel, B.; Öhrmalm, L.; Gyarmati, P. The Microbiota in Hematologic Malignancies. Curr. Treat. Options Oncol. 2020, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, G.; Zhao, Z.; Liu, Y.; Shen, Q.; Li, P.; Chen, Y.; Yin, H.; Wang, H.; Marcella, C.; et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: Clinical findings, animal studies and in vitro screening. Protein Cell 2020, 11, 251–266. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, Z.; Wen, S.; Liu, Y.; Wang, Y.; Tang, L. Transplantation of a bacterial consortium ameliorates trinitrobenzenesulfonic acid-induced colitis and intestinal dysbiosis in rats. Future Microbiol. 2016, 11, 887–902. [Google Scholar] [CrossRef]
- Zama, D.; Bossù, G.; Leardini, D.; Muratore, E.; Biagi, E.; Prete, A.; Pession, A.; Masetti, R. Insights into the role of intestinal microbiota in hematopoietic stem-cell transplantation. Ther. Adv. Hematol. 2020, 11, 2040620719896961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- McCune, V.L.; Quraishi, M.N.; Manzoor, S.; Moran, C.E.; Banavathi, K.; Steed, H.; Massey, D.C.O.; Trafford, G.R.; Iqbal, T.H.; Hawkey, P.M. Results from the first English stool bank using faecal microbiota transplant as a medicinal product for the treatment of Clostridioides difficile infection. EClinicalMedicine 2020, 20, 100301. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule– vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Hohmann, E.; Jenq, R.R.; Chen, Y.B. Fecal Microbiota Transplantation: Restoring the Injured Microbiome after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, e17–e22. [Google Scholar] [CrossRef] [Green Version]
- Giudicessi, J.R.; Ackerman, M.J. Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl. Res. 2013, 161, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Garcia Perez, L.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 2018, 9, 4832. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, J.; Liu, J.; Huang, B.; Li, J. Fecal Microbiota Taxonomic Shifts in Chinese Multiple Myeloma Patients Analyzed by Quantitative Polimerase Chain Reaction (QPCR) and 16S rRNA High-Throughput Sequencing. Med. Sci. Monit. 2019, 25, 8269–8280. [Google Scholar] [CrossRef]
- Alkharabsheh, O.; Hasib Sidiqi, M.; Aljama, M.A.; Gertz, M.A.; Frankel, A.E. The Human Microbiota in Multiple Myeloma and Proteasome Inhibitors. Acta Haematol. 2020, 143, 118–123. [Google Scholar] [CrossRef]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 2020, 8, 74. [Google Scholar] [CrossRef]
- Shono, Y.; van den Brink, M.R.M. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat. Rev. Cancer 2018, 18, 283–295. [Google Scholar] [CrossRef]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.-C.; Jeha, S.; et al. Gut Microbiome Composition Predicts Infection Risk during Chemotherapy in Children with Acute Lymphoblastic Leukemia. Clin. Infect. Dis. 2018, 67, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, M.J.; Tissing, W.J.E.; Dun, C.A.J.; Meessen, N.E.L.; Kamps, W.A.; de Bont, E.S.J.M.; Harmsen, H.J.M. Chemotherapy Treatment in Pediatric Patients with Acute Myeloid Leukemia Receiving Antimicrobial Prophylaxis Leads to a Relative Increase of Colonization with Potentially Pathogenic Bacteria in the Gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, L.L.; Rajasuriar, R.; Ai Lian Lim, Y.; Ling Woo, Y.; Loke, P.; Ariffin, H. Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy. BMC Cancer 2020, 20, 151. [Google Scholar] [CrossRef] [Green Version]
- Gałązka, P.; Styczyński, J.; Czyżewski, K.; Salamonowicz-Bodzioch, M.; Frączkiewicz, J.; Zając-Spychała, O.; Zaucha-Prażmo, A.; Goździk, J.; Biliński, J.; Basak, G.W.; et al. Impact of decontamination therapy on gastrointestinal acute graft-versus-host disease after allogeneic hematopoietic cell transplantation in children: Decontamination therapy in allo-HCT. Curr. Res. Transl. Med. 2021, 69, 103298. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28711. [Google Scholar] [CrossRef]
- Biagi, E.; Zama, D.; Nastasi, C.; Consolandi, C.; Fiori, J.; Rampelli, S.; Turroni, S.; Centanni, M.; Severgnini, M.; Peano, C.; et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015, 50, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2017, 23, 820–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagi, E.; Zama, D.; Rampelli, S.; Turroni, S.; Brigidi, P.; Consolandi, C.; Severgnini, M.; Picotti, E.; Gasperini, P.; Merli, P.; et al. Early gut microbiota signature of aGvHD in children given allogeneic hematopoietic cell transplantation for hematological disorders. BMC Med. Genom. 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Romick-Rosendale, L.E.; Haslam, D.B.; Lane, A.; Denson, L.; Lake, K.; Wilkey, A.; Watanabe, M.; Bauer, S.; Litts, B.; Luebbering, N.; et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2018, 24, 2418–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, A.; Innao, V.; Allegra, A.G.; Ettari, R.; Pugliese, M.; Pulvirenti, N.; Musolino, C. Role of the microbiota in hematologic malignancies. Neth. J. Med. 2019, 77, 67–80. [Google Scholar]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal Domination and the Risk of Bacteremia in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Staffas, A.; da Silva, M.B.; van den Brink, M.R.M. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 2017, 129, 927–933. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. Intestinal permeability defects: Is it time to treat? Clin. Gastroenterol. Hepatol. 2013, 11, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Palaniyandi, S.; Hildebrandt, G.C. Microbiome: An Emerging New Frontier in Graft-Versus-Host Disease. Dig. Dis. Sci. 2019, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.C.; Bscheider, M.; Eisenkolb, G.; Lin, C.C.; Wintges, A.; Otten, V.; Lindemans, C.A.; Heidegger, S.; Rudelius, M.; Monette, S.; et al. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl. Med. 2017, 9, eaag2513. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Kang, M.; Choi, E.Y. TLR/MyD88-mediated Innate Immunity in Intestinal Graft-versus-Host Disease. Immune Netw. 2017, 17, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyszka, M.; Biliński, J.; Basak, G.W. Advances in Intestinal Barrier Preservation and Restoration in the Allogeneic Hematopoietic Cell Transplantation Setting. J. Clin. Med. 2021, 10, 2508. [Google Scholar] [CrossRef]
- Zhou, Z.; Shang, T.; Li, X.; Zhu, H.; Qi, Y.B.; Zhao, X.; Chen, X.; Shi, Z.-X.; Pan, G.; Wang, Y.-F.; et al. Protecting Intestinal Microenvironment Alleviates Acute Graft-Versus-Host Disease. Front. Physiol. 2021, 11, 608279. [Google Scholar] [CrossRef]
- Routy, B.; Letendre, C.; Enot, D.; Chénard-Poirier, M.; Mehraj, V.; Séguin, N.C.; Guenda, K.; Gagnon, K.; Woerther, P.-L.; Ghez, D.; et al. The influence of gut-decontamination prophylactic antibiotics on acute graft-versus-host disease and survival following allogeneic hematopoietic stem cell transplantation. Oncoimmunology 2016, 6, e1258506. [Google Scholar] [CrossRef] [Green Version]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Horton-Sparks, K.; Hull, V.; Li, R.W.; Martínez-Cerdeño, V. The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol. Autism 2018, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Chang, L.; Shen, Y.; Gao, W.H.; Wu, Y.N.; Dou, H.B.; Huang, M.-M.; Wang, Y.; Fang, W.-Y.; Shan, J.-H.; et al. Valproic Acid Ameliorates Graft-versus-Host Disease by Downregulating Th1 and Th17 Cells. J. Immunol. 2015, 195, 1849–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, H.; Docampo, M.D.; Riwes, M.; Peltier, D.; Toubai, T.; Henig, I.; Wu, S.J.; Kim, S.; Taylor, A.; Brabbs, S.; et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 2018, 9, 3674. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.R.; Sun, Y.; Rossi, C.; et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zama, D.; Biagi, E.; Masetti, R.; Gasperini, P.; Prete, A.; Candela, M.; Brigidi, P.; Pession, A. Gut microbiota and hematopoietic stem cell transplantation: Where do we stand? Bone Marrow Transplant. 2017, 52, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaźmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef]
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef]
- Lübbert, C.; Salzberger, B.; Mössner, J. Fecal microbiota transplantation. Internist 2017, 58, 456–468. [Google Scholar] [CrossRef]
- Bhutiani, N.; Schucht, J.E.; Miller, K.R.; McClave, S.A. Technical Aspects of Fecal Microbial Transplantation (FMT). Curr. Gastroenterol. Rep. 2018, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; FMT-standardization Study Group. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell. 2018, 9, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Gulati, M.; Singh, S.K.; Corrie, L.; Kaur, I.P.; Chandwani, L. Delivery routes for faecal microbiota transplants: Available, anticipated and aspired. Pharmacol. Res. 2020, 159, 104954. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Yin, W.; Liu, W. Is frozen fecal microbiota transplantation as effective as fresh fecal microbiota transplantation in patients with recurrent or refractory Clostridium difficile infection: A meta-analysis? Diagn. Microbiol. Infect. Dis. 2017, 88, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lucas, A.L.; Grinspan, A.M. Fecal Transplants by Colonoscopy and Capsules Are Cost-Effective Strategies for Treating Recurrent Clostridioides difficile Infection. Dig. Dis. Sci. 2020, 65, 1125–1133. [Google Scholar] [CrossRef]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A. Oral Fecal Microbiota Transplant Capsules Are Safe and Effective for Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2021, 55, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Battipaglia, G.; Malard, F.; Rubio, M.T.; Ruggeri, A.; Mamez, A.C.; Brissot, E.; Giannotti, F.; Dulery, R.; Joly, A.C.; Baylatry, M.T.; et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 2019, 104, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Tariq, R.; Tosh, P.K.; Pardi, D.S.; Khanna, S. Faecal microbiota transplantation for eradicating carriage of multidrug-resistant organisms: A systematic review. Clin. Microbiol. Infect. 2019, 25, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Aira, A.; Fehér, C.; Rubio, E.; Soriano, A. The Intestinal Microbiota as a Reservoir and a Therapeutic Target to Fight Multi-Drug-Resistant Bacteria: A Narrative Review of the Literature. Infect. Dis. Ther. 2019, 8, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Laffin, M.; Millan, B.; Madsen, K.L. Fecal microbial transplantation as a therapeutic option in patients colonized with antibiotic resistant organisms. Gut Microbes 2017, 8, 221–224. [Google Scholar] [CrossRef]
- Bilinski, J.; Grzesiowski, P.; Sorensen, N.; Madry, K.; Muszynski, J.; Robak, K.; Wroblewska, M.; Dzieciatkowski, T.; Dulny, G.; Dwilewicz-Trojaczek, J.; et al. Fecal Microbiota Transplantation in Patients with Blood Disorders Inhibits Gut Colonization with Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin. Infect. Dis. 2017, 65, 364–370. [Google Scholar] [CrossRef]
- Kim, S.B.; Min, Y.H.; Cheong, J.W.; Kim, J.S.; Kim, S.J.; Ku, N.S.; Jeong, S.J.; Han, S.H.; Choi, J.Y.; Song, Y.G.; et al. Incidence and risk factors for carbapenem- and multidrug-resistant Acinetobacter baumannii bacteremia in hematopoietic stem cell transplantation recipients. Scand. J. Infect. Dis. 2014, 46, 81–88. [Google Scholar] [CrossRef]
- Bilinski, J.; Robak, K.; Peric, Z.; Marchel, H.; Karakulska-Prystupiuk, E.; Halaburda, K.; Rusicka, P.; Swoboda-Kopec, E.; Wroblewska, M.; Wiktor-Jedrzejczak, W.; et al. Impact of Gut Colonization by Antibiotic-Resistant Bacteria on the Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective, Single-Center Study. Biol. Blood Marrow Transplant. 2016, 22, 1087–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caselli, D.; Cesaro, S.; Ziino, O.; Zanazzo, G.; Manicone, R.; Livadiotti, S.; Cellini, M.; Frenos, S.; Milano, G.M.; Cappelli, B.; et al. Multidrug resistant Pseudomonas aeruginosa infection in children undergoing chemotherapy and hematopoietic stem cell transplantation. Haematologica 2010, 95, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Peled, J.U.; Li, S.; Mahabamunuge, J.; Dagher, Z.; Slingerland, A.E.; Del Rio, C.; Valles, B.; Kempner, M.E.; Smith, M.; et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018, 2, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Misch, E.A.; Safdar, N. Clostridioides difficile Infection in the Stem Cell Transplant and Hematologic Malignancy Population. Infect. Dis. Clin. N. Am. 2019, 33, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Gouliouris, T.; Petrosillo, N. Fecal microbiota transplantation (FMT) for Clostridium difficile infection: Focus on immunocompromised patients. J. Infect. Chemother. 2015, 21, 230–237. [Google Scholar] [CrossRef]
- Chopra, T.; Chandrasekar, P.; Salimnia, H.; Heilbrun, L.K.; Smith, D.; Alangaden, G.J. Recent epidemiology of Clostridium difficile infection during hematopoietic stem cell transplantation. Clin. Transplant. 2011, 25, E82–E87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, B.J.; Brunner, A.; Ford, C.D.; Gazdik, M.A.; Petersen, F.B.; Hoda, D. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2016, 18, 628–633. [Google Scholar] [CrossRef]
- Hefazi, M.; Patnaik, M.M.; Hogan, W.J.; Litzow, M.R.; Pardi, D.S.; Khanna, S. Safety and Efficacy of Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection in Patients with Cancer Treated with Cytotoxic Chemotherapy: A Single-Institution Retrospective Case Series. Mayo Clin. Proc. 2017, 92, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Naymagon, S.; Naymagon, L.; Wong, S.Y.; Ko, H.M.; Renteria, A.; Levine, J.; Colombel, J.-F.; Ferrara, J. Acute graft-versus-host disease of the gut: Considerations for the gastroenterologist. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 711–726. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Bilinski, J.; Lis, K.; Tomaszewska, A.; Pechcinska, A.; Grzesiowski, P.; Dzieciatkowski, T.; Walesiak, A.; Gierej, B.; Ziarkiewicz-Wróblewska, B.; Tyszka, M.; et al. Eosinophilic gastroenteritis and graft-versus-host disease induced by transmission of Norovirus with fecal microbiota transplant. Transpl. Infect. Dis. 2021, 23, e13386. [Google Scholar]
- Spindelboeck, W.; Schulz, E.; Uhl, B.; Kashofer, K.; Aigelsreiter, A.; Zinke-Cerwenka, W.; Mulabecirovic, A.; Kump, P.K.; Halwachs, B.; Gorkiewicz, G.; et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica 2017, 102, e210–e213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef]
- Kakihana, K. Fecal microbiota transplantation for acute graft-versus-host disease of the gut. Rinsho Ketsueki 2017, 58, 499–505. [Google Scholar] [PubMed]
- Qi, X.; Li, X.; Zhao, Y.; Wu, X.; Chen, F.; Ma, X.; Zhang, F.; Wu, D. Treating Steroid Refractory Intestinal Acute Graft-vs.-Host Disease with Fecal Microbiota Transplantation: A Pilot Study. Front. Immunol. 2018, 9, 2195. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Lis, K.; Tomaszewska, A.; Grzesiowski, P.; Dzieciatkowski, T.; Tyszka, M.; Karakulska-Prystupiuk, E.; Boguradzki, P.; Tormanowska, M.; Halaburda, K.; et al. Fecal microbiota transplantation in patients with acute and chronic graft-versus-host disease-spectrum of responses and safety profile. Results from a prospective, multicenter study. Am. J. Hematol. 2021, 96, E88–E91. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, Y.; Gao, J.; Jiao, Y.; Zhu, B.; Wu, D.; Qi, X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results from FMT2017002 Trial. Front. Immunol. 2021, 12, 678476. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Soto, M.T.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant, E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, S.R.; Witt, J.; Targownik, L.E.; Kao, D.; Lee, C.; Smieliauskas, F.; Rubin, D.T.; Singh, H.; Bernstein, C.N. Cost-effectiveness analysis of a fecal microbiota transplant center for treating recurrent C. difficile infection. J. Infect. 2020, 81, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Varier, R.U.; Biltaji, E.; Smith, K.J.; Roberts, M.S.; Jensen, M.K.; LaFleur, J.; Nelson, R.E. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect. Control. Hosp. Epidemiol. 2015, 36, 438–444. [Google Scholar] [CrossRef]
- Arbel, L.T.; Hsu, E.; McNally, K. Cost-Effectiveness of Fecal Microbiota Transplantation in the Treatment of Recurrent Clostridium Difficile Infection: A Literature Review. Cureus 2017, 9, e1599. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Sun, H.; Cao, W.; Han, L.; Song, Y.; Wan, D.; Jiang, Z. Applications of gut microbiota in patients with hematopoietic stem-cell transplantation. Exp. Hematol. Oncol. 2020, 9, 35. [Google Scholar]
- D’Amico, F.; Biagi, E.; Rampelli, S.; Fiori, J.; Zama, D.; Soverini, M.; Barone, M.; Leardini, D.; Muratore, E.; Prete, A.; et al. Enteral nutrition in pediatric patients undergoing hematopoietic SCT promotes the recovery of gut microbiome homeostasis. Nutrients 2019, 11, 2958. [Google Scholar] [CrossRef] [Green Version]
- Pierre, J.F. Gastrointestinal immune and microbiome changes during parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, 246–256. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [Green Version]
- Andermann, T.M.; Rezvani, A.; Bhatt, A.S. Microbiota Manipulation with Prebiotics and Probiotics in Patients Undergoing Stem Cell Transplantation. Curr. Hematol. Malig. Rep. 2016, 11, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gerbitz, A.; Schultz, M.; Wilke, A.; Linde, H.J.; Schölmerich, J.; Andreesen, R.; Holler, E. Probiotic effects on experimental graft-versus-host disease: Let them eat yogurt. Blood 2004, 103, 4365–4367. [Google Scholar] [CrossRef] [Green Version]
- Ciernikova, S.; Kasperova, B.; Drgona, L.; Smolkova, B.; Stevurkova, V.; Mego, M. Targeting the gut microbiome: An emerging trend in hematopoietic stem cell transplantation. Blood Rev. 2021, 48, 100790. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Mego, M.; Semanova, M.; Wachsmannova, L.; Adamcikova, Z.; Stevurkova, V.; Drgona, L.; Zajac, V. Probiotic Survey in Cancer Patients Treated in the Outpatient Depart-ment in a Comprehensive Cancer Center. Integr. Cancer Ther. 2017, 16, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Gorshein, E.; Ambrosy, S.; Budney, S.; Vivas, J.; Manago, J.; McGrath, M.K.; Tyno, A.; Strair, R. Probiotic Enteric Regimen for Easing the Complications of Transplant. Blood 2014, 124, 5877. [Google Scholar] [CrossRef]
- Reyna-Figueroa, J.; Barrón-Calvillo, E.; García-Parra, C.; Galindo-Delgado, P.; Contreras-Ochoa, C.; Lagunas-Martínez, A.; Campos-Romero, F.H.; Silva-Estrada, J.A.; Limón-Rojas, A.E. Probiotic Supplementation Decreases Chemotherapy-induced Gastrointestinal Side Effects in Patients with Acute Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar]
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef]
- Koyama, S.; Fujita, H.; Shimosato, T.; Kamijo, A.; Ishiyama, Y.; Yamamoto, E.; Ishii, Y.; Hattori, Y.; Hagihara, M.; Yamazaki, E.; et al. Septicemia from Lactobacillus rhamnosus GG, from a Probiotic Enriched Yogurt, in a Patient with Autologous Stem Cell Transplantation. Probiotics Antimicrob. Proteins 2019, 11, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ladas, E.J.; Bhatia, M.; Chen, L.; Sandler, E.; Petrovic, A.; Berman, D.M.; Hamblin, F.; Gates, M.; Hawks, R.; Sung, L.; et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016, 51, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Sadanand, A.; Newland, J.G.; Bednarski, J.J. Safety of Probiotics among High-Risk Pediatric Hematopoietic Stem Cell Transplant Recipients. Infect. Dis Ther. 2019, 8, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaźmierczak-Siedlecka, K.; Fic, M.; Ruszkowski, J.; Folwarski, M.; Makarewicz, W. Saccharomyces boulardii (CNCM I-745): A non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. 2020, 77, 1987–1996. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Piekarska, A.; Lubieniecka-Archutowska, E.; Bicz, M.; Folwarski, M.; Makarewicz, W.; Zaucha, J.M. Nutritional status in patients after hematopoietic cell transplantation. Acta Haematol. Pol. 2019, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cesaro, S.; Chinello, P.; Rossi, L.; Zanesco, L. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharomyces boulardii. Support. Care Cancer 2000, 8, 504–505. [Google Scholar] [CrossRef]
- Lolis, N.; Veldekis, D.; Moraitou, H.; Kanavaki, S.; Velegraki, A.; Triandafyllidis, C.; Tasioudis, C.; Pefanis, A.; Pneumatikos, I. Saccharomyces boulardii fungaemia in an intensive care unit patient treated with caspofungin. Crit. Care 2008, 12, 414. [Google Scholar]
- Burkhardt, O.; Köhnlein, T.; Pletz, M.; Welte, T. Saccharomyces boulardii induced sepsis: Successful therapy with voriconazole after treatment failure with fluconazole. Scand. J. Infect. Dis. 2005, 37, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sulik-Tyszka, B.; Snarski, E.; Niedźwiedzka, M.; Augustyniak, M.; Myhre, T.N.; Kacprzyk, A.; Swoboda-Kopeć, E.; Roszkowska, M.; Dwilewicz-Trojaczek, J.; Wiktor Jędrzejczak, W.; et al. Experience with Saccharomyces boulardii Probiotic in Oncohaematological Patients. Probiotics Antimicrob. Proteins 2018, 10, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Ambalam, P.; Kondepudi, K.K.; Pithva, S.; Kothari, C.; Patel, A.T.; Purama, R.K.; Dave, J.M.; Vyas, B.R.M. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes 2013, 4, 181–192. [Google Scholar] [PubMed] [Green Version]
- Iyama, S.; Sato, T.; Tatsumi, H.; Hashimoto, A.; Tatekoshi, A.; Kamihara, Y.; Horiguchi, H.; Ibata, S.; Ono, K.; Murase, K.; et al. Efficacy of Enteral Supplementation Enriched with Glutamine, Fiber, and Oligosaccharide on Mucosal Injury following Hematopoietic Stem Cell Transplantation. Case Rep. Oncol. 2014, 7, 692–699. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Claus, S.P.; Le Roy, C.I.; Grangette, C.; Pot, B.; Martinez, I.; Walter, J.; Cani, P.D.; Delzenne, N.M. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016, 10, 1456–1470. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.E.G.; Luke, J.J. The Impact of the Fecal Microbiome on Cancer Immunotherapy. BioDrugs 2019, 33, 1–7. [Google Scholar] [CrossRef]
- Segain, J.P.; de la Blétière, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rask, C.; Adlerberth, I.; Berggren, A.; Ahrén, I.L.; Wold, A.E. Differential effect on cell-mediated immunity in human volunteers after intake of different lactobacilli. Clin. Exp. Immunol. 2013, 172, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut microbiota promotes hematopoiesis tocontrol bacterial infection. Cell Host. Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riwes, M.; Reddy, P. Short chain fatty acids: Postbiotics/metabolites and graft versus host disease colitis. Semin. Hematol. 2020, 57, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.A.; Schluter, J.; Gomes, A.L.C.; Littmann, E.R.; Pickard, A.J.; Taylor, B.P.; Giardina, P.A.; Weber, D.; Dai, A.; Docampo, M.D.; et al. The microbe-derived short-chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood 2020, 136, 130–136. [Google Scholar]
- Teshima, T.; Reddy, P.; Zeiser, R. Acute Graft-versus-Host Disease: Novel Biological Insights. Biol. Blood Marrow Transplant. 2016, 22, 11–16. [Google Scholar]
- Haring, E.; Uhl, F.M.; Andrieux, G.; Proietti, M.; Bulashevska, A.; Sauer, B.; Braun, L.M.; de Vega Gomez, E.; Esser, P.R.; Martin, S.F.; et al. Bile acids regulate intestinal antigen presentation and reduce graft-versus-host disease without impairing the graft-versus-leukemia effect. Haematologica 2021, 106, 2131–2146. [Google Scholar]
- Michonneau, D.; Latis, E.; Curis, E.; Dubouchet, L.; Ramamoorthy, S.; Ingram, B.; Peffault de Latour, R.; Robin, M.; Sicre de Fontbrune, F.; Chevret, S.; et al. Metabolomics analysis of human acute graft-versus-host disease reveals changes in host and microbiota-derived metabolites. Nat. Commun. 2019, 10, 5695. [Google Scholar] [PubMed] [Green Version]
- Cai, S.Y.; Ouyang, X.; Chen, Y.; Soroka, C.J.; Wang, J.; Mennone, A.; Wang, Y.; Mehal, W.Z.; Jain, D.; Boyer, J.L. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017, 2, e90780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Xia, D.; Ke, Y.; Lu, L.; Wang, D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 802–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| The Most Common Preliminary Tests for FMT | |
|---|---|
| Bacterial serology | Treponema palladium |
| Viral serology | Hepatitis A virus IgM, hepatitis B surface antigen, hepatitis C antibody, cytomegalovirus, and Epstein–Barr virus |
| Parasite serology | Strongyloides stercoralis and Entamoeba histolytica |
| Blood tests | Complete blood count, complete metabolic panel, liver tests (i.e., aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, and C-reactive protein) |
| Stool tests | Stool Clostridium difficile studies (toxin polymerase chain reaction (PCR), enzyme-linked immunoassay (ELISA), and toxigenic culture) |
| Bacterial stool tests | Salmonella, Shigella, Campylobacter cultures, E. coli O157 culture, H. pylori immunoassay, and vancomycin-resistant Enterococcus culture |
| Viral stool tests | Adenovirus ELISA, norovirus ELISA or quantitative PCR, and rotavirus ELISA |
| Parasite stool tests | Ova and parasite microscopy, Microsporidia microscopy, Giardia fecal antigen ELISA, Cryptosporidium ELISA, and Isospora and Cyclospora microscopy |
| Identifier | Title of the Study | Study Type | Disease/ Condition | Sample Size (n) | Interventions/ Treatment | Primary Outcomes | Current Status |
|---|---|---|---|---|---|---|---|
| NCT03922035 | “CBM588 in improving clinical outcomes in patients who have undergone donor hematopoietic stem cell transplant” | Pilot study | Hematopoietic and lymphoid cell neoplasm | 36 | Clostridium butyricum CBM 588 probiotic strain | Adverse events | Recruiting |
| NCT04269850 | “Fecal microbiota transplantation with ruxolitinib and steroids as an upfront treatment of severe acute intestinal GVHD” | Pilot study | Intestinal GVHD | 20 | Allogenic FMT | Overall survival | Recruiting |
| NCT03678493 | “A study of FMT in patients with AML allo HSCT in recipients” | Randomized placebo- controlled trial | AML, ASCT | 120 | FMT | Incidence of infections | Recruiting |
| NCT03819803 | “Fecal microbiota transplantation in aGvHD after ASCT” | Interventional | GvHD in GI Tract | 15 | FMT | GI-aGvHD remission | Recruiting |
| NCT04593368 | “Faecal microbiome transplantation (FMT) in pediatric patients colonized with antibiotic-resistant pathogens before hematopoietic stem cell transplantation (HSCT)” | Prospective non-randomized phase II trial | Pediatric patients colonized with antibiotic-resistant pathogens before HSCT | 15 | oral dosing of fecal microbiome from allogeneic donor | Frequency of decolonization | Not yet recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Biliński, J.; Roviello, G.; Iannone, L.F.; Atzeni, A.; Sobocki, B.K.; Połom, K. Gut Microbiome Modulation and Faecal Microbiota Transplantation Following Allogenic Hematopoietic Stem Cell Transplantation. Cancers 2021, 13, 4665. https://doi.org/10.3390/cancers13184665
Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Biliński J, Roviello G, Iannone LF, Atzeni A, Sobocki BK, Połom K. Gut Microbiome Modulation and Faecal Microbiota Transplantation Following Allogenic Hematopoietic Stem Cell Transplantation. Cancers. 2021; 13(18):4665. https://doi.org/10.3390/cancers13184665
Chicago/Turabian StyleKaźmierczak-Siedlecka, Karolina, Karolina Skonieczna-Żydecka, Jarosław Biliński, Giandomenico Roviello, Luigi Francesco Iannone, Alessandro Atzeni, Bartosz Kamil Sobocki, and Karol Połom. 2021. "Gut Microbiome Modulation and Faecal Microbiota Transplantation Following Allogenic Hematopoietic Stem Cell Transplantation" Cancers 13, no. 18: 4665. https://doi.org/10.3390/cancers13184665
APA StyleKaźmierczak-Siedlecka, K., Skonieczna-Żydecka, K., Biliński, J., Roviello, G., Iannone, L. F., Atzeni, A., Sobocki, B. K., & Połom, K. (2021). Gut Microbiome Modulation and Faecal Microbiota Transplantation Following Allogenic Hematopoietic Stem Cell Transplantation. Cancers, 13(18), 4665. https://doi.org/10.3390/cancers13184665