Development and Validation of an HPLC Method for Analysis of Topotecan in Human Cerebrospinal Fluid and Its Application in Elimination Evaluation of Topotecan after Intraventricular Injection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Chromatographic Separation
2.4. Stock and Working Solutions
2.5. Calibration and Quality Control Samples
2.6. Sample Preparation
2.7. Validation of the Method
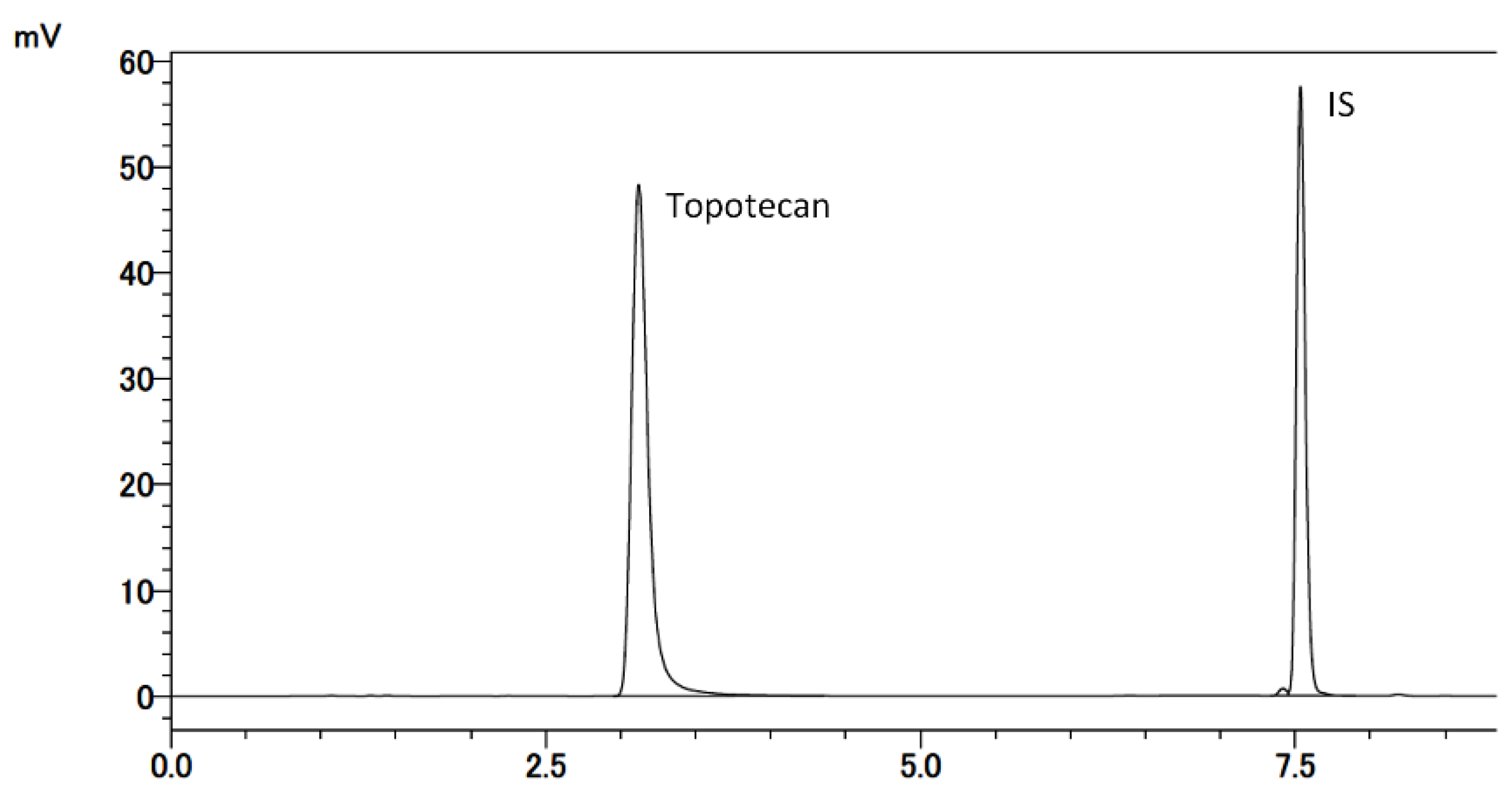
2.7.1. Selectivity and LLOQ
2.7.2. Carry-Over and Linearity
2.7.3. Accuracy and Precision
2.7.4. Recovery Rate
2.7.5. Stability
2.8. Clinical Monitoring of Topotecan
2.9. Ethics Approval
2.10. Statistical Analysis
3. Results
3.1. Method Validation for HPLC
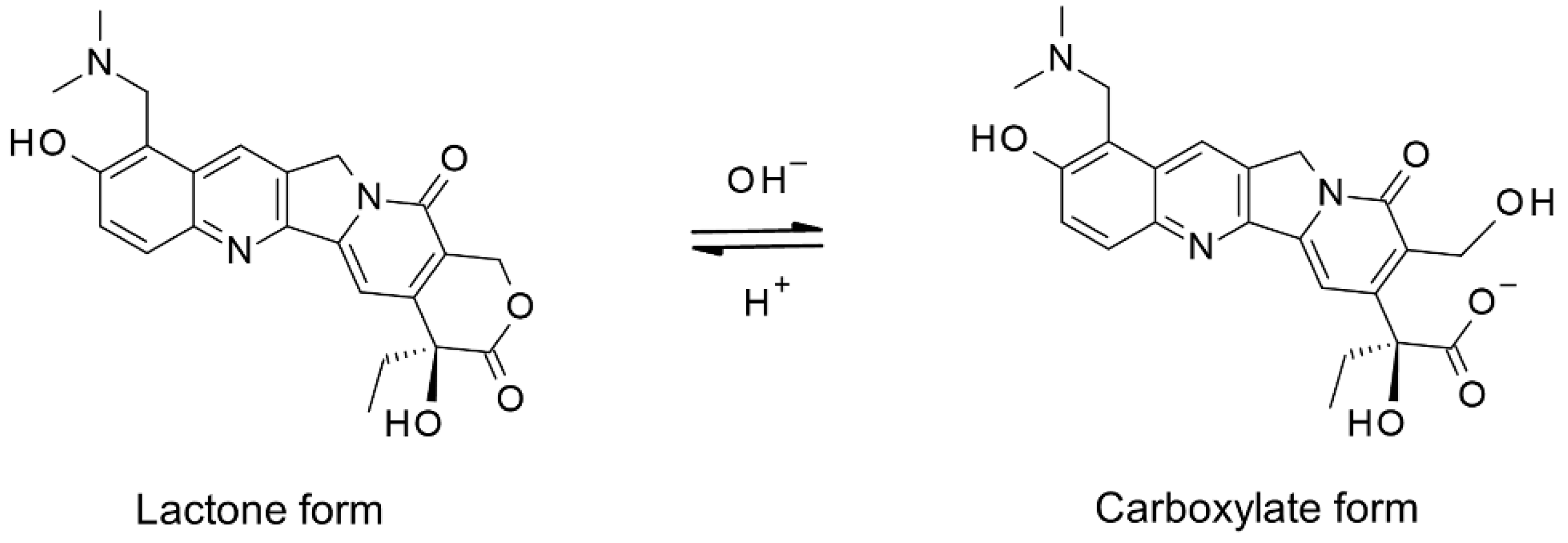
3.1.1. Ring-Form Conversion of Topotecan
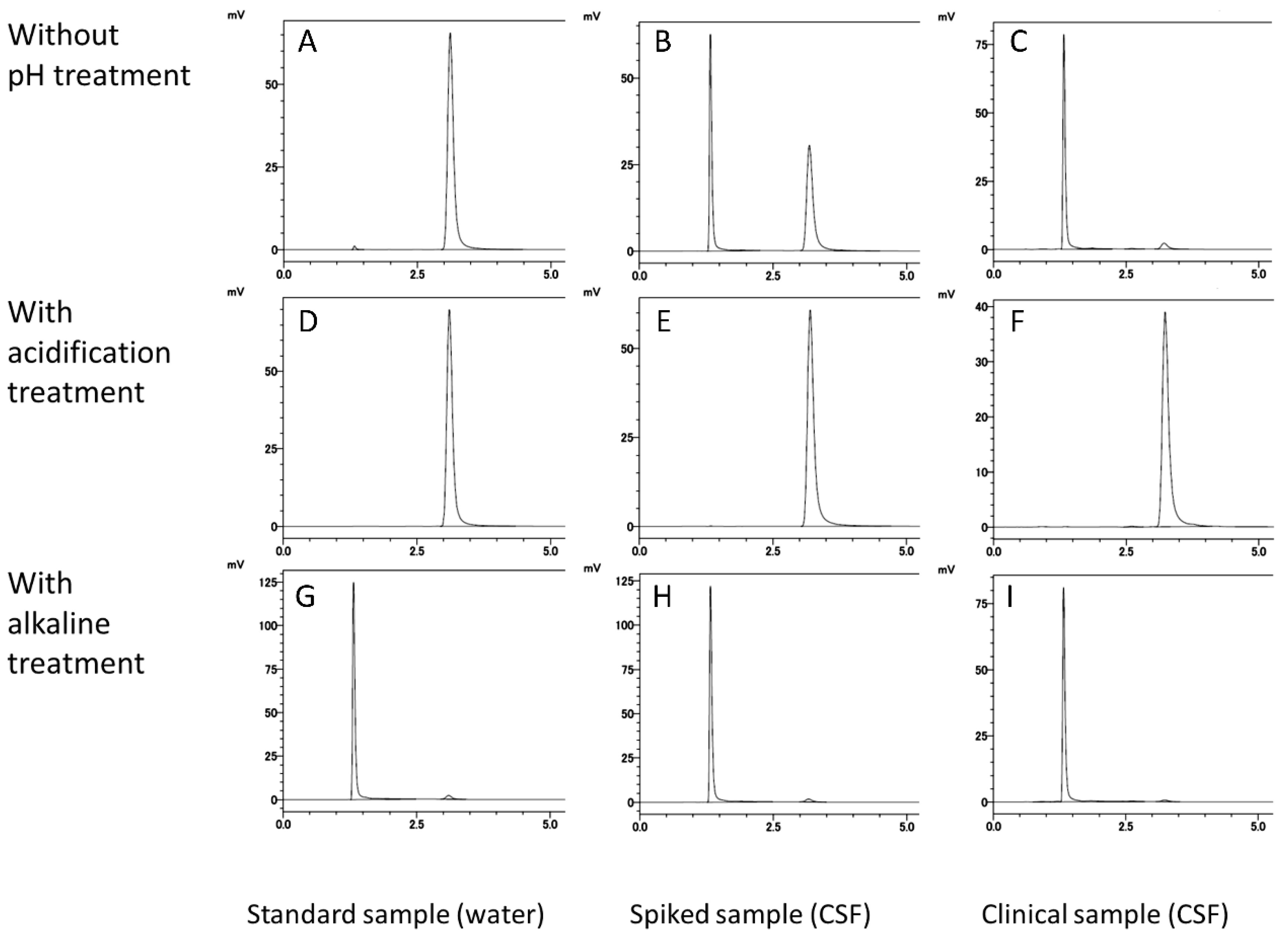
3.1.2. Selectivity and LLOQ
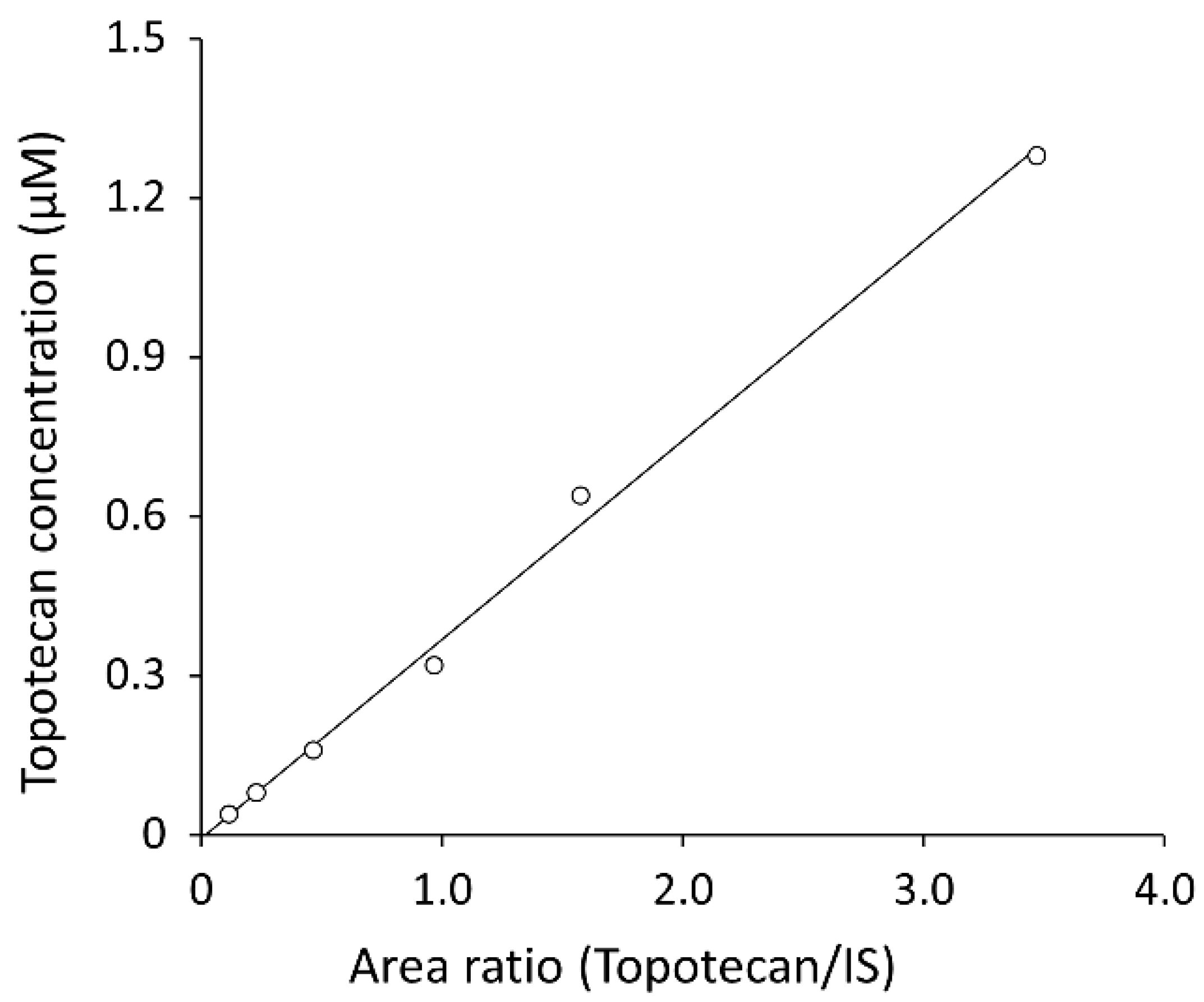
3.1.3. Carry-Over and Linearity
3.1.4. Accuracy and Precision
3.1.5. Recovery Rate
3.1.6. Stability
3.2. Clinical Monitoring of Topotecan
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleischhack, G.; Jaehde, U.; Bode, U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin. Pharmacokinet. 2005, 44, 1–31. [Google Scholar] [CrossRef]
- LeBras, M.; Chow, I.; Mabasa, V.H.; Ensom, M.H.H. Systematic review of efficacy, pharmacokinetics, and administration of intraventricular aminoglycosides in adults. Neurocrit. Care 2016, 25, 492–507. [Google Scholar] [CrossRef]
- Roberts, M.S.; Selvo, N.S.; Roberts, J.K.; Daryani, V.M.; Owens, T.S.; Harstead, K.E.; Gajjar, A.; Stewart, C.F. Determination of methotrexate, 7-hydroxymethotrexate, and 2,4-diamino-n10-methylpteroic acid by LC–MS/MS in plasma and cerebrospinal fluid and application in a pharmacokinetic analysis of high-dose methotrexate. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, N.; Yamada, A.; Yokota, T.; Moritake, H.; Hirabara, Y.; Ikeda, R. Measurement of methotrexate in human cerebrospinal fluid using a chemiluminescence immunoassay intended for serum and plasma matrices. J. Clin. Lab. Anal. 2021, 35, e23661. [Google Scholar] [CrossRef]
- Thomas, A.; Pommier, Y. Targeting topoisomerase I in the era of precision medicine. Clin. Cancer Res. 2019, 25, 6581–6589. [Google Scholar] [CrossRef]
- Stewart, C.F.; Zamboni, W.C.; Crom, W.R.; Gajjar, A.; Heideman, R.L.; Furman, W.L.; Meyer, W.H.; Houghton, P.J.; Pratt, C.B. Topoisomerase I interactive drugs in children with cancer. Investig. New Drugs 1996, 14, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Moritake, H.; Kamimura, S.; Yamashita, S.; Takeshima, H.; Nunoi, H. Proposed strategy for the use of high-dose chemotherapy with stem cell rescue and intrathecal topotecan without whole-brain irradiation for infantile classic medulloblastoma. Pediatr. Blood Cancer 2014, 61, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.F.; Baker, S.D.; Heideman, R.L.; Jones, D.; Crom, W.R.; Pratt, C.B. Clinical pharmacodynamics of continuous infusion topotecan in children: Systemic exposure predicts hematologic toxicity. J. Clin. Oncol. 1994, 12, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Rowinsky, E.; Slichenmyer, W.; Donehower, R.C.; Forastiere, A.; Ettinger, D.; Chen, T.L.; Sartorius, S.; Bowling, K.; Smith, J.; et al. Phase I and pharmacologic studies of topotecan in patients with impaired hepatic function. J. Natl. Cancer Inst. 1996, 88, 817–824. [Google Scholar] [CrossRef]
- Blaney, S.M.; Heideman, R.; Berg, S.; Adamson, P.; Gillespie, A.; Geyer, J.R.; Packer, R.; Matthay, K.; Jaeckle, K.; Cole, D.; et al. Phase I clinical trial of intrathecal topotecan in patients with neoplastic meningitis. J. Clin. Oncol. 2003, 21, 143–147. [Google Scholar] [CrossRef]
- Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 28 January 2020).
- Ye, L.; Shi, J.; Wan, S.; Yang, X.; Wang, Y.; Zhang, J.; Zheng, D.; Liu, Z. Development and validation of a liquid chromatography-tandem mass spectrometry method for topotecan determination in beagle dog plasma and its application in a bioequivalence study. Biomed. Chromatogr. 2013, 27, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A. Review of chromatographic methods coupled with modern detection techniques applied in the therapeutic drugs monitoring (TDM). Molecules 2020, 25, 4026. [Google Scholar] [CrossRef]
- Rosing, H.; Doyle, E.; Davies, B.E.; Beijnen, J.H. High-performance liquid chromatographic determination of the novel antitumour drug topotecan and topotecan as the total of the lactone plus carboxylate forms, in human plasma. J. Chromatogr. B Biomed. Appl. 1995, 668, 107–115. [Google Scholar] [CrossRef]
- Underberg, W.J.; Goossen, R.M.; Smith, B.R.; Beijnen, J.H. Equilibrium kinetics of the new experimental anti-tumour compound SK&F 104864-A in aqueous solution. J. Pharm. Biomed. Anal. 1990, 8, 681–683. [Google Scholar] [CrossRef]
- Fassberg, J.; Stella, V.J. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J. Pharm. Sci. 1992, 81, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Arimori, K.; Kuroki, N.; Hidaka, M.; Iwakiri, T.; Yamsaki, K.; Okumura, M.; Ono, H.; Takamura, N.; Kikuchi, M.; Nakano, M. Effect of P-glycoprotein modulator, cyclosporin a, on the gastrointestinal excretion of irinotecan and its metabolite SN-38 in rats. Pharm. Res. 2003, 20, 910–917. [Google Scholar] [CrossRef]
- DeVries, N.A.; Zhao, J.; Kroon, E.; Buckle, T.; Beijnen, J.H.; Van Tellingen, O. P-Glycoprotein and breast cancer resistance protein: Two dominant transporters working together in limiting the brain penetration of topotecan. Clin. Cancer Res. 2007, 13, 6440–6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Kang, J.; Kwon, N.Y.; Sivaraman, A.; Naik, R.; Jin, S.Y.; Oh, A.R.; Shin, J.H.; Na, Y.; Lee, K.; et al. Dual inhibition of P-gp and BCRP improves oral topotecan bioavailability in rodents. Pharmaceutics 2021, 13, 559. [Google Scholar] [CrossRef]
- Shen, J.; Carcaboso, A.M.; Hubbard, K.E.; Tagen, M.; Wynn, H.G.; Panetta, J.C.; Waters, C.M.; Elmeliegy, M.A.; Stewart, C.F. Compartment-specific roles of ATP-binding cassette transporters define differential topotecan distribution in brain parenchyma and cerebrospinal fluid. Cancer Res. 2009, 69, 5885–5892. [Google Scholar] [CrossRef] [Green Version]
- Motl, S.; Zhuang, Y.; Waters, C.M.; Stewart, C.F. Pharmacokinetic considerations in the treatment of CNS tumours. Clin. Pharmacokinet. 2006, 45, 871–903. [Google Scholar] [CrossRef]
- Zhuang, Y.; Fraga, C.H.; Hubbard, K.E.; Hagedorn, N.; Panetta, J.C.; Waters, C.M.; Stewart, C.F. Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor. Cancer Res. 2006, 66, 11305–11313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacus, M.O.; Daryani, V.M.; Harstead, K.E.; Patel, Y.T.; Throm, S.L.; Stewart, C.F. Pharmacokinetic properties of anticancer agents for the treatment of central nervous system tumors: Update of the literature. Clin. Pharmacokinet. 2016, 55, 297–311. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Yang, G.; Cui, J.; Li, W.; Li, Y.; Gao, P.; Jiang, T.; Sun, Y.; Dong, L.; Song, Y.; et al. A pilot phase 1 study of intrathecal pemetrexed for refractory leptomeningeal metastases from non-small-cell lung cancer. Front. Oncol. 2019, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.; Angeli, E.; Bousquet, G. The pharmacology of xenobiotics after intracerebro spinal fluid administration: Implications for the treatment of brain tumors. Int. J. Mol. Sci. 2021, 22, 1281. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, C.A.; Houghton, P.J.; Stewart, C.F.; Cheshire, P.J.; Richmond, L.B.; Danks, M.K. Effective schedules of exposure of medulloblastoma and rhabdomyosarcoma xenografts to topotecan correlate with in vitro assays. Clin. Cancer Res. 1998, 4, 1995–2002. [Google Scholar]


| Concentration (µM) | |||
|---|---|---|---|
| Spiked | Measured (Mean ± SD) | CV (%) | Relative Error (%) |
| 0.04 | 0.038 ± 0.000 | 0.00 | 5.0 |
| 0.10 | 0.086 ± 0.001 | 1.51 | 13.8 |
| 0.50 | 0.469 ± 0.006 | 1.28 | 6.2 |
| 1.00 | 0.921 ± 0.005 | 0.52 | 7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, N.; Yamada, A.; Yokota, T.; Yamada, Y.; Kinoshita, M.; Moritake, H.; Ikeda, R. Development and Validation of an HPLC Method for Analysis of Topotecan in Human Cerebrospinal Fluid and Its Application in Elimination Evaluation of Topotecan after Intraventricular Injection. Cancers 2021, 13, 4643. https://doi.org/10.3390/cancers13184643
Yoshikawa N, Yamada A, Yokota T, Yamada Y, Kinoshita M, Moritake H, Ikeda R. Development and Validation of an HPLC Method for Analysis of Topotecan in Human Cerebrospinal Fluid and Its Application in Elimination Evaluation of Topotecan after Intraventricular Injection. Cancers. 2021; 13(18):4643. https://doi.org/10.3390/cancers13184643
Chicago/Turabian StyleYoshikawa, Naoki, Ai Yamada, Tsubasa Yokota, Yusei Yamada, Mariko Kinoshita, Hiroshi Moritake, and Ryuji Ikeda. 2021. "Development and Validation of an HPLC Method for Analysis of Topotecan in Human Cerebrospinal Fluid and Its Application in Elimination Evaluation of Topotecan after Intraventricular Injection" Cancers 13, no. 18: 4643. https://doi.org/10.3390/cancers13184643
APA StyleYoshikawa, N., Yamada, A., Yokota, T., Yamada, Y., Kinoshita, M., Moritake, H., & Ikeda, R. (2021). Development and Validation of an HPLC Method for Analysis of Topotecan in Human Cerebrospinal Fluid and Its Application in Elimination Evaluation of Topotecan after Intraventricular Injection. Cancers, 13(18), 4643. https://doi.org/10.3390/cancers13184643






