Technical Challenges for CTC Implementation in Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Trials with CTC-Based Treatment Decisions
3. Technical and Biological Challenges for Isolation and Characterization CTCs
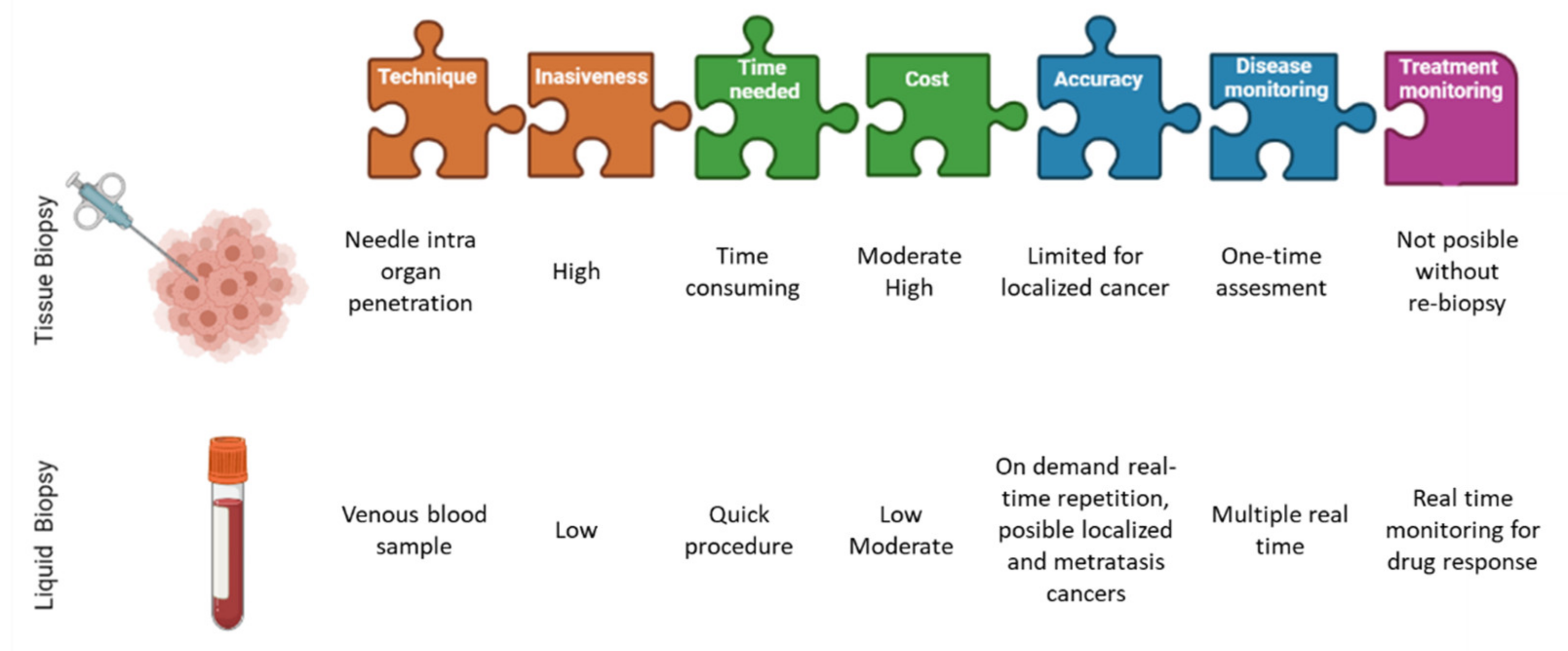
3.1. Pre-Analytical Blood Sample Handling
3.2. Genotype and Phenotype of CTCs in Breast Cancer (BC)
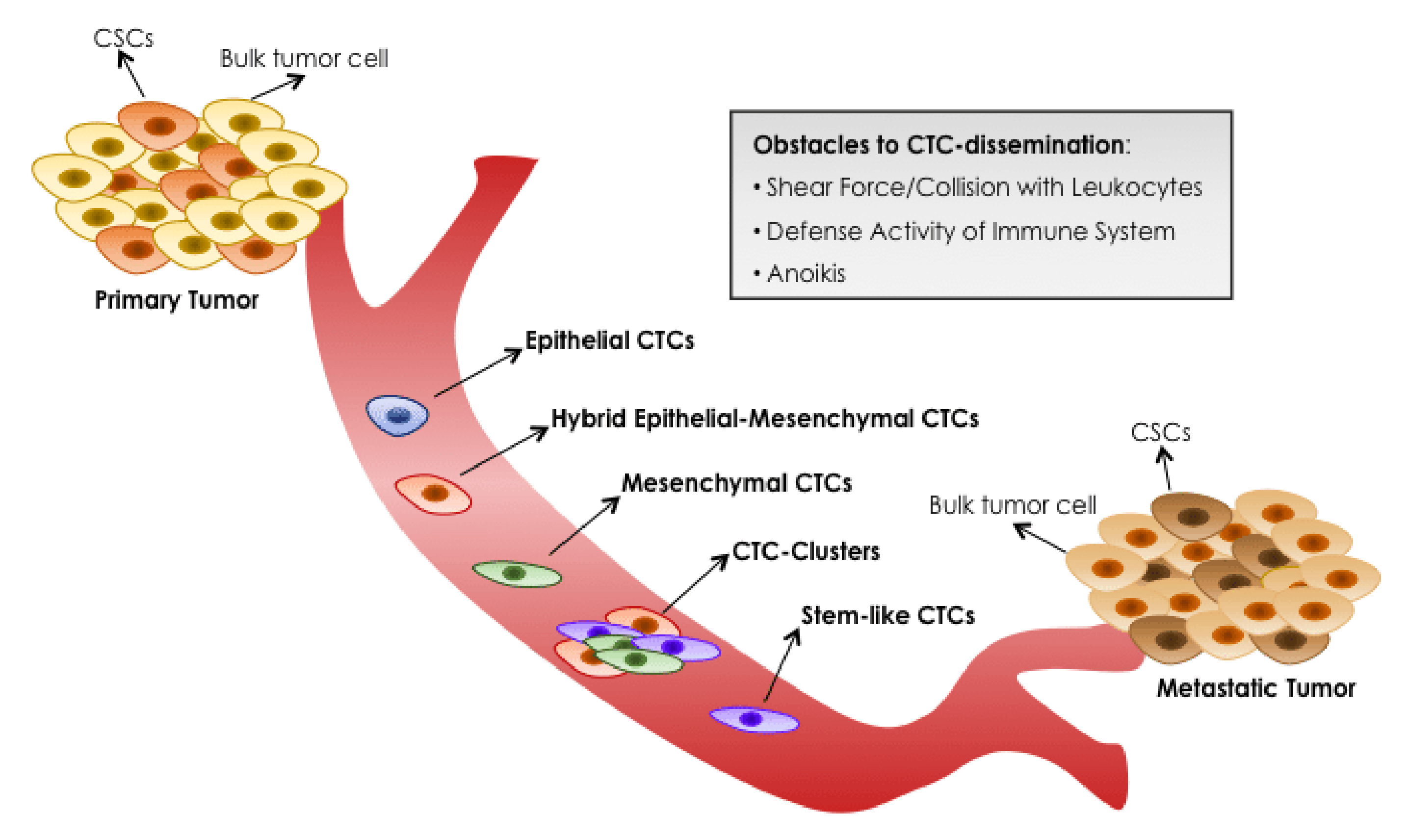
3.2.1. CTC and Epithelial-Mesenchymal Transition
3.2.2. Hybrid Epithelial-Mesenchymal (E/M) Phenotype
3.2.3. ER/PR and HER2 Characterization of CTCs
3.3. Cryopreservation and CTCs Isolation
4. New Approaches
4.1. Optimize the Standardization of Protocols
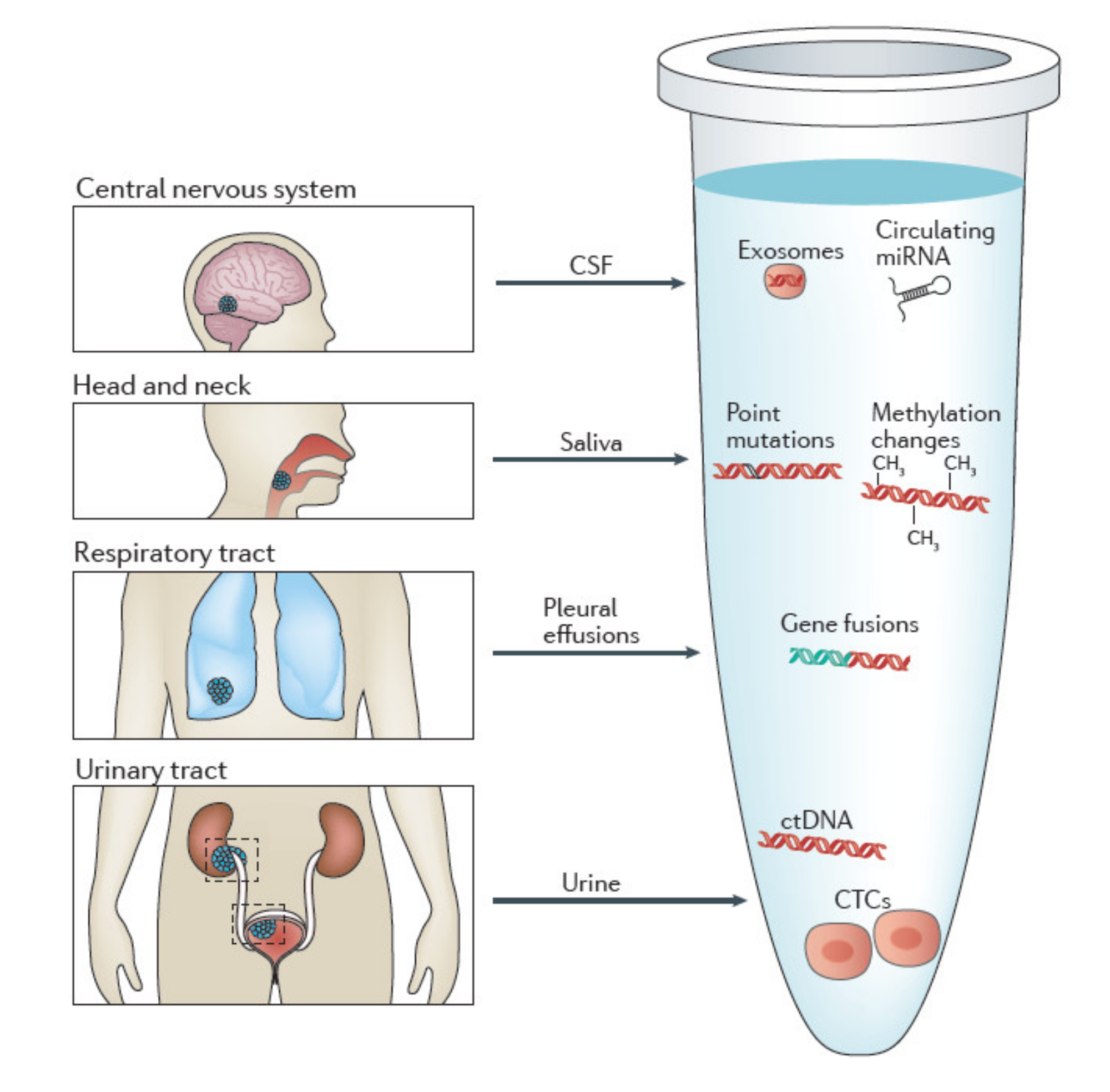
4.2. Isolation of CTCs in Other Body Fluids
- Urine
- Saliva
- CSF
- Other bodily fluids
4.3. Promising Role of CTC Clusters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Antoniou, A.C.; Ivanus, U.; Esserman, L.J.; Easton, D.F.; French, D.; Sroczynski, G.; Hall, P.; Cuzick, J.; Evans, D.G.; et al. Personalized Early Detection and Prevention of Breast Cancer: ENVISION Consensus Statement. Nat. Rev. Clin. Oncol. 2020, 17, 687–705. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid Biopsy in Breast Cancer: A Comprehensive Review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef]
- Hall, C.; Valad, L.; Lucci, A. Circulating Tumor Cells in Breast Cancer Patients. Crit. Rev. Oncog. 2016, 21, 125–139. [Google Scholar] [CrossRef]
- Martelotto, L.G.; Ng, C.K.; Piscuoglio, S.; Weigelt, B.; Reis-Filho, J.S. Breast Cancer Intra-Tumor Heterogeneity. Breast Cancer Res. 2014, 16, 210. [Google Scholar] [CrossRef] [Green Version]
- Januškevičienė, I.; Petrikaitė, V. Heterogeneity of Breast Cancer: The Importance of Interaction between Different Tumor Cell Populations. Life Sci. 2019, 239, 117009. [Google Scholar] [CrossRef]
- Sharma, S.; Zhuang, R.; Long, M.; Pavlovic, M.; Kang, Y.; Ilyas, A.; Asghar, W. Circulating Tumor Cell Isolation, Culture, and Downstream Molecular Analysis. Biotechnol. Adv. 2018, 36, 1063–1078. [Google Scholar] [CrossRef]
- Gaforio, J.-J.; Serrano, M.-J.; Sanchez-Rovira, P.; Sirvent, A.; Delgado-Rodriguez, M.; Campos, M.; de la Torre, N.; Algarra, I.; Dueñas, R.; Lozano, A. Detection of Breast Cancer Cells in the Peripheral Blood Is Positively Correlated with Estrogen-Receptor Status and Predicts for Poor Prognosis: Circulating Breast Tumor Cells. Int. J. Cancer 2003, 107, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B. Liquid Biopsy versus Tumor Biopsy for Clinical-Trial Recruitment. Nat. Med. 2020, 26, 1815–1816. [Google Scholar] [CrossRef]
- Heidrich, I.; Ačkar, L.; Mossahebi Mohammadi, P.; Pantel, K. Liquid Biopsies: Potential and Challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Belloum, Y.; Wikman, H.; Pantel, K. Clinical Relevance of Blood-Based CtDNA Analysis: Mutation Detection and Beyond. Br. J. Cancer 2021, 124, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B.-R.; Jin, W.-L.; Lou, G. Circulating Tumor Cells in Precision Oncology: Clinical Applications in Liquid Biopsy and 3D Organoid Model. Cancer Cell Int. 2019, 19, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslami-S., Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. The Metastatic Cascade as the Basis for Liquid Biopsy Development. Front. Oncol. 2020, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Agashe, R.; Kurzrock, R. Circulating Tumor Cells: From the Laboratory to the Cancer Clinic. Cancers 2020, 12, 2361. [Google Scholar] [CrossRef]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally Collected CTCs and CTC-Clusters and Clinical Outcomes of Metastatic Breast Cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Gao, W.; Wang, Y.; Jia, C.; Cong, H. A Label-Free Microfluidic Chip for the Highly Selective Isolation of Single and Cluster CTCs from Breast Cancer Patients. Transl. Oncol. 2021, 14, 100959. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Piñeiro, R.; Martínez-Pena, I.; López-López, R. Relevance of CTC Clusters in Breast Cancer Metastasis. Adv. Exp. Med. Biol. 2020, 1220, 93–115. [Google Scholar] [CrossRef]
- Best, M.G.; Wesseling, P.; Wurdinger, T. Tumor-Educated Platelets as a Noninvasive Biomarker Source for Cancer Detection and Progression Monitoring. Cancer Res. 2018, 78, 3407–3412. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.; Kuske, A.; Joosse, S.A.; Yigit, G.; Sflomos, G.; Thaler, S.; Smit, D.J.; Werner, S.; Borgmann, K.; Gärtner, S.; et al. Characterization of Circulating Breast Cancer Cells with Tumorigenic and Metastatic Capacity. EMBO Mol. Med. 2020, 12, e11908. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C. The Future of Liquid Biopsy. Nature 2020, 579, S9. [Google Scholar] [CrossRef] [Green Version]
- Cueva Bañuelos, J.F.; Rodríguez López, C.; Cortegoso Mosquera, A.; Palacios Ozores, P.; Curiel García, T. Clinical Relevance and Therapeutic Application of CTCs in Advanced Breast Cancer. In Circulating Tumor Cells in Breast Cancer Metastatic Disease; Advances in Experimental Medicine and Biology; Piñeiro, R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1220, pp. 147–164. ISBN 978-3-030-35804-4. [Google Scholar]
- Fabisiewicz, A.; Szostakowska-Rodzos, M.; Zaczek, A.J.; Grzybowska, E.A. Circulating Tumor Cells in Early and Advanced Breast Cancer; Biology and Prognostic Value. Int. J. Mol. Sci. 2020, 21, 1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janni, W.; Rack, B.K.; Fasching, P.A.; Haeberle, L.; Tesch, H.; Lorenz, R.; Schochter, F.; Tzschaschel, M.; De Gregorio, A.; Fehm, T.N.; et al. Persistence of Circulating Tumor Cells in High Risk Early Breast Cancer Patients Five Years after Adjuvant Chemotherapy and Late Recurrence: Results from the Adjuvant SUCCESS A Trial. J. Clin. Oncol. 2018, 36, 515. [Google Scholar] [CrossRef]
- Sparano, J.; O’Neill, A.; Alpaugh, K.; Wolff, A.C.; Northfelt, D.W.; Dang, C.T.; Sledge, G.W.; Miller, K.D. Association of Circulating Tumor Cells with Late Recurrence of Estrogen Receptor-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1700–1706. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Cristofanilli, M.; Pierga, J.-Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The Clinical Use of Circulating Tumor Cells (CTCs) Enumeration for Staging of Metastatic Breast Cancer (MBC): International Expert Consensus Paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.A.; Lew, D.L.; et al. Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500. J. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Schochter, F.; Friedl, T.W.P.; deGregorio, A.; Krause, S.; Huober, J.; Rack, B.; Janni, W. Are Circulating Tumor Cells (CTCs) Ready for Clinical Use in Breast Cancer? An Overview of Completed and Ongoing Trials Using CTCs for Clinical Treatment Decisions. Cells 2019, 8, 1412. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.J. Circulating Tumor Cells: Quintessential Precision Oncology Presenting Challenges for Biology. NPJ Precis. Oncol. 2017, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.H.D.; Bender, S.; Krahn, T.; Schlange, T. CtDNA and CTCs in Liquid Biopsy—Current Status and Where We Need to Progress. Comput. Struct. Biotechnol. J. 2018, 16, 190–195. [Google Scholar] [CrossRef]
- Page, K.; Shaw, J.A.; Guttery, D.S. The Liquid Biopsy: Towards Standardisation in Preparation for Prime Time. Lancet Oncol. 2019, 20, 758–760. [Google Scholar] [CrossRef] [Green Version]
- Oelmueller, U. Standardization of Generic Pre-Analytical Procedures for in Vitro Diagnostics for Personalized Medicine. New Biotechnol. 2021, 60, 1. [Google Scholar] [CrossRef]
- Grölz, D.; Hauch, S.; Schlumpberger, M.; Guenther, K.; Voss, T.; Sprenger-Haussels, M.; Oelmüller, U. Liquid Biopsy Preservation Solutions for Standardized Pre-Analytical Workflows—Venous Whole Blood and Plasma. Curr. Pathobiol. Rep. 2018, 6, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, S.; Chemi, F.; Brady, G. Challenges and Unanswered Questions for the next Decade of Circulating Tumour Cell Research in Lung Cancer. Transl. Lung Cancer Res. 2017, 6, 454–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidard, F.-C.; Proudhon, C.; Pierga, J.-Y. Circulating Tumor Cells in Breast Cancer. Mol. Oncol. 2016, 10, 418–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.J.; Ortega, F.G.; Alvarez-Cubero, M.J.; Nadal, R.; Sanchez-Rovira, P.; Salido, M.; Rodríguez, M.; García-Puche, J.L.; Delgado-Rodriguez, M.; Solé, F.; et al. EMT and EGFR in CTCs Cytokeratin Negative Non-Metastatic Breast Cancer. Oncotarget 2014, 5, 7486–7497. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Balic, M.; El-Ashry, D.; Cote, R.J. Circulating Tumor Cells: Strategies for Capture, Analyses, and Propagation. Cancer J. 2018, 24, 70–77. [Google Scholar] [CrossRef]
- Correnti, M.; Raggi, C. Stem-like Plasticity and Heterogeneity of Circulating Tumor Cells: Current Status and Prospect Challenges in Liver Cancer. Oncotarget 2017, 8, 7094–7115. [Google Scholar] [CrossRef] [Green Version]
- Mego, M.; Karaba, M.; Minarik, G.; Benca, J.; Silvia, J.; Sedlackova, T.; Manasova, D.; Kalavska, K.; Pindak, D.; Cristofanilli, M.; et al. Circulating Tumor Cells With Epithelial–to–Mesenchymal Transition Phenotypes Associated with Inferior Outcomes in Primary Breast Cancer. Anticancer Res. 2019, 39, 1829–1837. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Cadilha, B.L.; Markota, A.; Voigt, C.; Huang, Z.; Lin, P.P.; Wang, D.D.; Dai, J.; Kranz, G.; et al. Epithelial-Type Systemic Breast Carcinoma Cells with a Restricted Mesenchymal Transition Are a Major Source of Metastasis. Sci. Adv. 2019, 5, eaav4275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Magbanua, M.J.M.; Park, J.W. Circulating Tumor Cells in Breast Cancer: Applications in Personalized Medicine. Breast Cancer Res. Treat. 2016, 160, 411–424. [Google Scholar] [CrossRef]
- De Dueñas, E.M.; Hernández, A.L.; Zotano, Á.G.; Carrión, R.M.P.; López-Muñiz, J.I.C.; Novoa, S.A.; Rodríguez, Á.L.; Fidalgo, J.A.P.; Lozano, J.F.; Gasión, O.B.; et al. Prospective Evaluation of the Conversion Rate in the Receptor Status between Primary Breast Cancer and Metastasis: Results from the GEICAM 2009-03 ConvertHER Study. Breast Cancer Res. Treat. 2014, 143, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Cejalvo, J.M.; Martínez de Dueñas, E.; Galván, P.; García-Recio, S.; Burgués Gasión, O.; Paré, L.; Antolín, S.; Martinello, R.; Blancas, I.; Adamo, B.; et al. Intrinsic Subtypes and Gene Expression Profiles in Primary and Metastatic Breast Cancer. Cancer Res. 2017, 77, 2213–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadal, R.; Fernandez, A.; Sanchez-Rovira, P.; Salido, M.; Rodríguez, M.; García-Puche, J.L.; Macià, M.; Corominas, J.M.; Delgado-Rodriguez, M.; Gonzalez, L.; et al. Biomarkers Characterization of Circulating Tumour Cells in Breast Cancer Patients. Breast Cancer Res. 2012, 14, R71. [Google Scholar] [CrossRef]
- Lu, D.; Graf, R.P.; Harvey, M.; Madan, R.A.; Heery, C.; Marte, J.; Beasley, S.; Tsang, K.Y.; Krupa, R.; Louw, J.; et al. Detection and Characterization of Circulating Tumour Cells from Frozen Peripheral Blood Mononuclear Cells. J. Circ. Biomark. 2015, 4, 4. [Google Scholar] [CrossRef]
- Nejlund, S.; Smith, J.; Kraan, J.; Stender, H.; Van, M.N.; Langkjer, S.T.; Nielsen, M.T.; Sölétormos, G.; Hillig, T. Cryopreservation of Circulating Tumor Cells for Enumeration and Characterization. Biopreserv. Biobank. 2016, 14, 330–337. [Google Scholar] [CrossRef]
- Zhu, P.; Stanton, M.L.; Castle, E.P.; Joseph, R.W.; Adams, D.L.; Li, S.; Amstutz, P.; Tang, C.-M.; Ho, T.H. Detection of Tumor-Associated Cells in Cryopreserved Peripheral Blood Mononuclear Cell Samples for Retrospective Analysis. J. Transl. Med. 2016, 14, 198. [Google Scholar] [CrossRef] [Green Version]
- Brungs, D.; Lynch, D.; Luk, A.W.; Minaei, E.; Ranson, M.; Aghmesheh, M.; Vine, K.L.; Carolan, M.; Jaber, M.; de Souza, P.; et al. Cryopreservation for Delayed Circulating Tumor Cell Isolation Is a Valid Strategy for Prognostic Association of Circulating Tumor Cells in Gastroesophageal Cancer. World J. Gastroenterol. 2018, 24, 810–818. [Google Scholar] [CrossRef]
- Friedlander, T.W.; Ngo, V.T.; Dong, H.; Premasekharan, G.; Weinberg, V.; Doty, S.; Zhao, Q.; Gilbert, E.G.; Ryan, C.J.; Chen, W.-T.; et al. Detection and Characterization of Invasive Circulating Tumor Cells Derived from Men with Metastatic Castration-Resistant Prostate Cancer. Int. J. Cancer 2014, 134, 2284–2293. [Google Scholar] [CrossRef] [Green Version]
- Alba-Bernal, A.; Lavado-Valenzuela, R.; Domínguez-Recio, M.E.; Jiménez-Rodriguez, B.; Queipo-Ortuño, M.I.; Alba, E.; Comino-Méndez, I. Challenges and Achievements of Liquid Biopsy Technologies Employed in Early Breast Cancer. EBioMedicine 2020, 62, 103100. [Google Scholar] [CrossRef]
- Kowalik, A.; Kowalewska, M.; Góźdź, S. Current Approaches for Avoiding the Limitations of Circulating Tumor Cells Detection Methods—Implications for Diagnosis and Treatment of Patients with Solid Tumors. Transl. Res. 2017, 185, 58–84.e15. [Google Scholar] [CrossRef] [Green Version]
- Arechederra, M.; Ávila, M.A.; Berasain, C. Liquid Biopsy for Cancer Management: A Revolutionary but Still Limited New Tool for Precision Medicine. Adv. Lab. Med. Av. Med. Lab. 2020, 1, 20200009. [Google Scholar] [CrossRef]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for Circulating Tumor Cell Separation from Whole Blood. J. Hematol. Oncol. 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riethdorf, S.; Müller, V.; Loibl, S.; Nekljudova, V.; Weber, K.; Huober, J.; Fehm, T.; Schrader, I.; Hilfrich, J.; Holms, F.; et al. Prognostic Impact of Circulating Tumor Cells for Breast Cancer Patients Treated in the Neoadjuvant “Geparquattro” Trial. Clin. Cancer Res. 2017, 23, 5384–5393. [Google Scholar] [CrossRef] [Green Version]
- Trapp, E.; Janni, W.; Schindlbeck, C.; Jückstock, J.; Andergassen, U.; de Gregorio, A.; Alunni-Fabbroni, M.; Tzschaschel, M.; Polasik, A.; Koch, J.G.; et al. Presence of Circulating Tumor Cells in High-Risk Early Breast Cancer During Follow-Up and Prognosis. J. Natl. Cancer Inst. 2019, 111, 380–387. [Google Scholar] [CrossRef]
- Goodman, C.R.; Seagle, B.-L.L.; Friedl, T.W.P.; Rack, B.; Lato, K.; Fink, V.; Cristofanilli, M.; Donnelly, E.D.; Janni, W.; Shahabi, S.; et al. Association of Circulating Tumor Cell Status with Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol. 2018, 4, e180163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, T.T.; Bardia, A.; Spring, L.M.; Giobbie-Hurder, A.; Kalinich, M.; Dubash, T.; Sundaresan, T.; Hong, X.; LiCausi, J.A.; Ho, U.; et al. A Digital RNA Signature of Circulating Tumor Cells Predicting Early Therapeutic Response in Localized and Metastatic Breast Cancer. Cancer Discov. 2018, 8, 1286–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bünger, S.; Zimmermann, M.; Habermann, J.K. Diversity of Assessing Circulating Tumor Cells (CTCs) Emphasizes Need for Standardization: A CTC Guide to Design and Report Trials. Cancer Metastasis Rev. 2015, 34, 527–545. [Google Scholar] [CrossRef]
- De Rubis, G.; Rajeev Krishnan, S.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef]
- Oshi, M.; Murthy, V.; Takahashi, H.; Huyser, M.; Okano, M.; Tokumaru, Y.; Rashid, O.M.; Matsuyama, R.; Endo, I.; Takabe, K. Urine as a Source of Liquid Biopsy for Cancer. Cancers 2021, 13, 2652. [Google Scholar] [CrossRef] [PubMed]
- Husain, H.; Nykin, D.; Bui, N.; Quan, D.; Gomez, G.; Woodward, B.; Venkatapathy, S.; Duttagupta, R.; Fung, E.; Lippman, S.M.; et al. Cell-Free DNA from Ascites and Pleural Effusions: Molecular Insights into Genomic Aberrations and Disease Biology. Mol. Cancer Ther. 2017, 16, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-H.; Wang, M.; Brenner, D.E.; Norton, P.A.; Block, T.M. Detection of Mutated K- Ras DNA in Urine, Plasma, and Serum of Patients with Colorectal Carcinoma or Adenomatous Polyps. Ann. N. Y. Acad. Sci. 2008, 1137, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, H.; Shimura, T.; Yamada, T.; Okuda, Y.; Natsume, M.; Kitagawa, M.; Horike, S.; Kataoka, H. A Novel Urinary MicroRNA Biomarker Panel for Detecting Gastric Cancer. J. Gastroenterol. 2019, 54, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Shen, S. Treatment and Relapse in Breast Cancer Show Significant Correlations to Noninvasive Testing Using Urinary and Plasma DNA. Future Oncol. 2020, 16, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, B.; Van Deun, J.; Vermaerke, S.; de Marco, A.; Lumen, N.; De Wever, O.; Hendrix, A. Urinary Extracellular Vesicle Biomarkers in Urological Cancers: From Discovery towards Clinical Implementation. Int. J. Biochem. Cell Biol. 2018, 99, 236–256. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chang, C.-H.; Wu, H.-C.; Lin, C.-C.; Chang, K.-P.; Yang, C.-R.; Huang, C.-P.; Hsu, W.-H.; Chang, C.-T.; Chen, C.-J. Proteome Profiling of Urinary Exosomes Identifies Alpha 1-Antitrypsin and H2B1K as Diagnostic and Prognostic Biomarkers for Urothelial Carcinoma. Sci. Rep. 2016, 6, 34446. [Google Scholar] [CrossRef]
- Streckfus, C.; Bigler, L. The Use of Soluble, Salivary c- ErbB-2 for the Detection and Post-Operative Follow-up of Breast Cancer in Women: The Results of a Five-Year Translational Research Study. Adv. Dent. Res. 2005, 18, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Wong, D.T.W. Breast Cancer Exosome-like Microvesicles and Salivary Gland Cells Interplay Alters Salivary Gland Cell-Derived Exosome-like Microvesicles In Vitro. PLoS ONE 2012, 7, e33037. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Karlan, S.; Zhou, H.; Gross, J.; Elashoff, D.; Akin, D.; Yan, X.; Chia, D.; Karlan, B.; et al. Discovery and Preclinical Validation of Salivary Transcriptomic and Proteomic Biomarkers for the Non-Invasive Detection of Breast Cancer. PLoS ONE 2010, 5, e15573. [Google Scholar] [CrossRef] [PubMed]
- Seoane, J.; De Mattos-Arruda, L.; Le Rhun, E.; Bardelli, A.; Weller, M. Cerebrospinal Fluid Cell-Free Tumour DNA as a Liquid Biopsy for Primary Brain Tumours and Central Nervous System Metastases. Ann. Oncol. 2019, 30, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Menyailo, M.E.; Tretyakova, M.S.; Denisov, E.V. Heterogeneity of Circulating Tumor Cells in Breast Cancer: Identifying Metastatic Seeds. Int. J. Mol. Sci. 2020, 21, 1696. [Google Scholar] [CrossRef] [Green Version]
- Padmanaban, V.; Krol, I.; Suhail, Y.; Szczerba, B.M.; Aceto, N.; Bader, J.S.; Ewald, A.J. E-Cadherin Is Required for Metastasis in Multiple Models of Breast Cancer. Nature 2019, 573, 439–444. [Google Scholar] [CrossRef]
- Krol, I.; Schwab, F.D.; Carbone, R.; Ritter, M.; Picocci, S.; De Marni, M.L.; Stepien, G.; Franchi, G.M.; Zanardi, A.; Rissoglio, M.D.; et al. Detection of Clustered Circulating Tumour Cells in Early Breast Cancer. Br. J. Cancer 2021, 125, 23–27. [Google Scholar] [CrossRef]
- Au, S.H.; Edd, J.; Stoddard, A.E.; Wong, K.H.K.; Fachin, F.; Maheswaran, S.; Haber, D.A.; Stott, S.L.; Kapur, R.; Toner, M. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci. Rep. 2017, 7, 2433. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer Therapy. Ex Vivo Culture of Circulating Breast Tumor Cells for Individualized Testing of Drug Susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef] [Green Version]
| Trail | Condition | Intervention | Primary End Points |
|---|---|---|---|
| Treat-CTC NCT01548677 (phase II) | HER2-neg EBC with CTCs after CT | Trastuzumab iv 6 cycles vs. observation | CTC detection rate at week 18 |
| SWOG S0500 NCT00382018 (phase III) | CT-resistant, CTC-posMBC | Early switch in therapy vs. treatment until progression | OS, PFS |
| CirCe01, NCT01349842 (phase III) | CT-resistant, CTC-posMBC | Early switch in therapy vs. treatment until progression | OS |
| STIC-CTC, NCT01710605 (phase III) | HR-pos and HER2-neg MBC | Decision CT or ER by clinical choice vs. CTC count | PFS, economic value |
| DETECT III NCT01619111 (phase III) | HER2-neg MBC and HER2-pos CTCs | Standard treatment vs. Standard treatment + lapatinib | CTC clearance |
| DETECT IV NCT02035813 (phase II) | HER2-neg MBC and HER2-neg CTCs | A: ET + ribociclib or everolimus B: eribulin | A: CTC clearance B: PFS |
| DETECT V NCT02344472 (phase III) | HR-pos, HER2-post MBC | Trastuzumab/pertuzumab + CT or ET with ribociclib | Tolerability, safety and quality of life |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Medina, R.; López-Tarruella, S.; del Monte-Millán, M.; Massarrah, T.; Martín, M. Technical Challenges for CTC Implementation in Breast Cancer. Cancers 2021, 13, 4619. https://doi.org/10.3390/cancers13184619
Ramos-Medina R, López-Tarruella S, del Monte-Millán M, Massarrah T, Martín M. Technical Challenges for CTC Implementation in Breast Cancer. Cancers. 2021; 13(18):4619. https://doi.org/10.3390/cancers13184619
Chicago/Turabian StyleRamos-Medina, Rocío, Sara López-Tarruella, María del Monte-Millán, Tatiana Massarrah, and Miguel Martín. 2021. "Technical Challenges for CTC Implementation in Breast Cancer" Cancers 13, no. 18: 4619. https://doi.org/10.3390/cancers13184619
APA StyleRamos-Medina, R., López-Tarruella, S., del Monte-Millán, M., Massarrah, T., & Martín, M. (2021). Technical Challenges for CTC Implementation in Breast Cancer. Cancers, 13(18), 4619. https://doi.org/10.3390/cancers13184619






