Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin
Abstract
Simple Summary
Abstract
1. Introduction
2. CD33 Structure and Expression: Rationale for a Targeted Therapy in AML
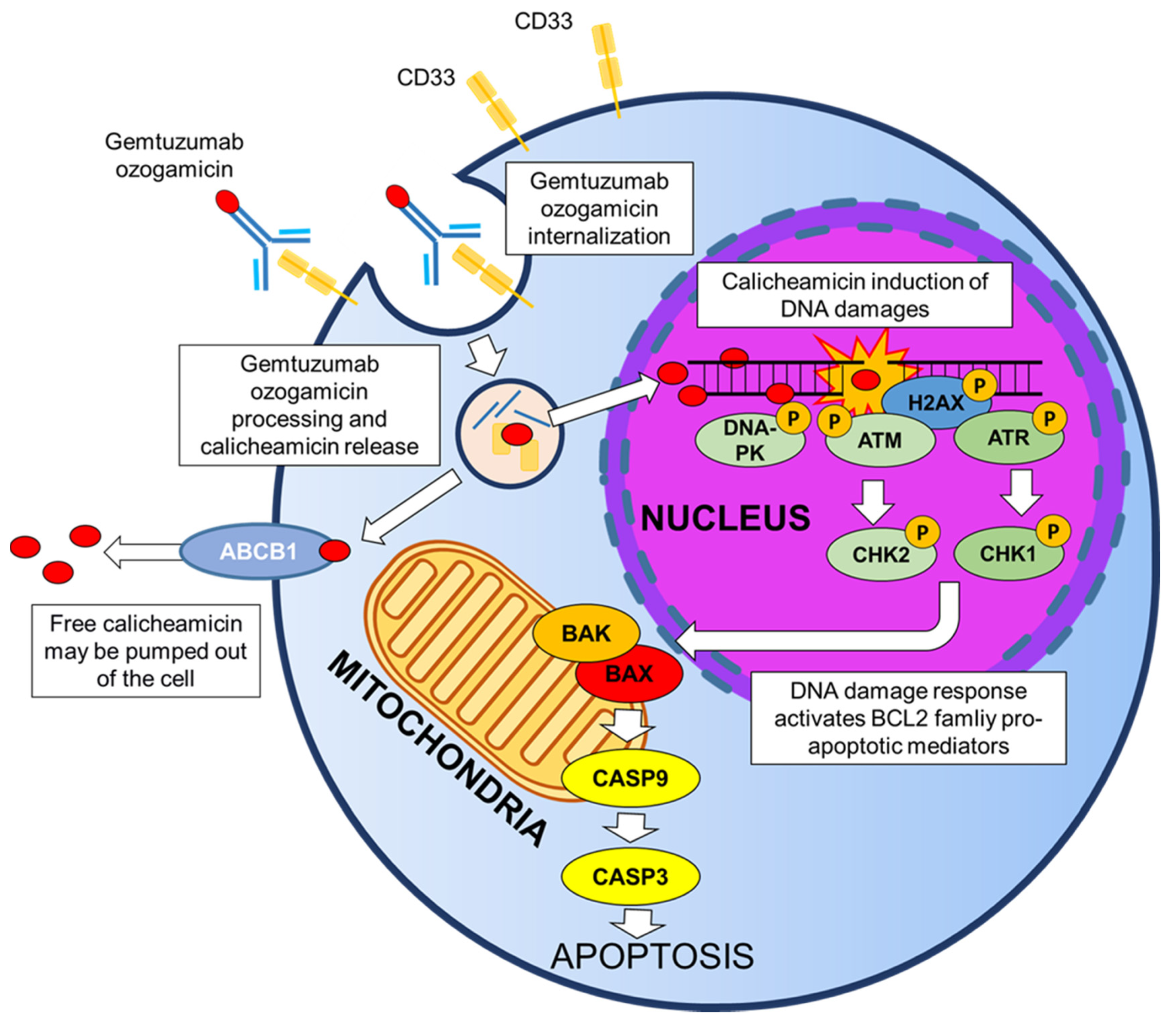
3. Gemtuzumab Ozogamicin: Mechanism of Cytotoxicity
4. Clinical Trials and Clinical Experience of GO in AML
4.1. GO as Monotherapy for Newly Diagnosed or Relapsed/Refractory Adult AML
4.2. GO as Monotherapy for Relapsed/Refractory Pediatric AML
4.3. GO in Combination with Chemotherapy for Newly Diagnosed or Relapsed/Refractory Adult and Pediatric AML
4.4. GO-related Toxicities
4.5. GO Treatment before and after Transplantation in Adult AML Patients
4.6. GO Treatment before and after Transplantation in Pediatric AML
5. Biomarkers of Response to GO Therapy
5.1. CD33 Expression
5.2. CD33 Single Nucleotide Polymorphism
5.3. Cytogenetic Alterations
5.4. Molecular Profile
5.5. Multidrug Resistance
5.6. Minimal Residual Disease
5.7. Stemness Signature
| Biomarker | Main Observations | Study | References |
|---|---|---|---|
| CD33 expression | GO improved OS of patients with >80% CD33+ blasts | EORTC-GIMEMA AML-19 | [37] |
| GO improved EFS and RFS of patients expressing high CD33 surface levels | ALFA-0701 | [80] | |
| Low percentage of CD33+ blasts associated with higher risk of relapse after GO | MRC AML15, NCRI AML16 | [81] | |
| Patients in the 2nd to 4th quartiles of CD33 surface expression had higher CR and lower MRD rates at the end of the first cycle, lower risk of relapse and better DFS | COG AAML0531 | [76] | |
| CD33 SNPs | rs12459419 CC genotype: lower risk of relapse and better EFS and DFS in the GO arm | COG AAML0531 | [82] |
| rs12459419 C > T SNP: no GO benefit | COG AAML0531 | [82] | |
| NPM1-mut patients with the rs12459419 CC genotype showed a superior RFS in the GO arm | AMLSG 09-09 | [83] | |
| Patients carrying rs1803254 GG, rs35112940 GG, rs2455069 GG, rs1736475 TT and rs201074739 CCGG/CCGG had reduced RR to GO | COG AAML0531 | [88] | |
| CD33_PGx6_Score >0 associated with high CD33 expression, better RFS and lower RR in the GO arm | COG AAML0531 | [88] | |
| Cytogenetic alterations | GO provided a survival benefit in patients with good and intermediate cytogenetic risks, but not in the adverse cytogenetic group | Various | [37,43,47,52,89,91] |
| The addition of GO to standard chemotherapy reduced the risk of relapse in CBF cases carrying KIT mutations | FLAG-GO | [93] | |
| The addition of GO to standard chemotherapy induced higher EFS in KMT2A-rearranged AML | COG AAML0531 | [100] | |
| Molecular profile | GO provided a survival benefit in patients from favorable and intermediate, but not adverse molecular risk categories (ELN 2017) | ALFA-0701 | [49,91] |
| GO provided EFS, RFS and OS benefit in NPM1-mut AML and reduced the incidence of relapse in NPM1-mut patients achieving CR/CRi | ALFA-0701, AMLSG 09-09 | [50,107] | |
| GO improved EFS of FLT3-ITD-wt, but not FLT3-ITD-mut patients | AMLSG 09-09, MRC AML15, NCRI AML16 | [52,107] | |
| GO improved OS, EFS and RFS and reduced the RR in adult FLT3-ITD-mut patients | COG AAML03P1, COG AAML0531 | [50,92,107,108] | |
| GO provided clinical benefit to patients with activating signaling mutations | ALFA-0701 | [104] | |
| The mutational status of seven genes identified a group characterized by NPM1-mut, FLT3-ITD-wt or biallelic CEBPA-mut that displayed the best outcome in the GO arm | GOELAMS/FILO AML 2006-IR | [112] | |
| Multidrug resistance | ABCB1 expression associated with failure to clear bone marrow blasts and to achieve CR or poos OS and EFS | Various | [115,118,119,121,122,123,124,125] |
| ABCB1 rs1045642 CT or TT genotype associated with better outcomes in GO recipients | COG AAML0531 | [123] | |
| MRD | GO-treated patients frequently achieved negativity for NPM1 mutation MRD and a reduction 1000 of NPM1 mutation transcript combined with MRD negativity was predictive of lower RR | ALFA-0701, AMLSG 09-09 | [130,131] |
| LSC signature | GO addition improved the outcome of patients having low LSC17 score but not those with high signature score | ALFA-0701 | [132] |
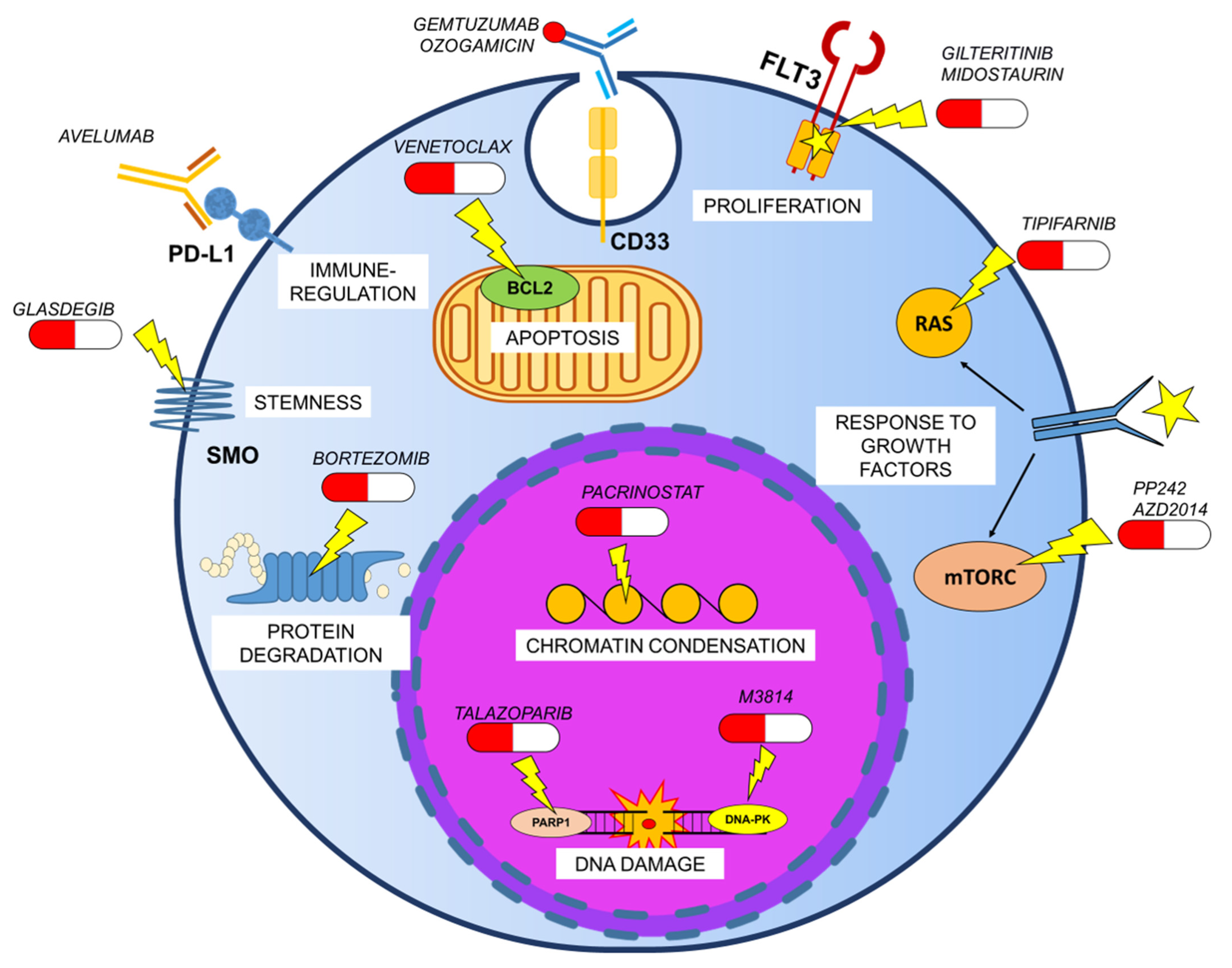
6. Novel Preclinical GO-based Therapeutic Combinations in AML
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Soyano, A.E.; Dholaria, B.; Knutson, K.L.; Lou, Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 1–25. [Google Scholar] [CrossRef]
- Kim, E.G.; Kim, K.M. Strategies and advancement in antibody-drug conjugate optimization for targeted cancer therapeutics. Biomol. Ther. 2015, 23, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Hackenberger, C.P.R.; Leonhardt, H.; Helma, J. Current Status: Site-Specific Antibody Drug Conjugates. J. Clin. Immunol. 2016, 36, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Chari, R.V.J. Antibody conjugate therapeutics: Challenges and potential. Clin. Cancer Res. 2011, 17, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Guo, Y.; Wang, W. Challenges of Antibody Drug Conjugates in Cancer Therapy: Current Understanding of Mechanisms and Future Strategies. Curr. Pharmacol. Rep. 2018, 4, 10–26. [Google Scholar] [CrossRef]
- Von Gunten, S.; Bochner, B.S. Basic and clinical immunology of Siglecs. Ann. N. Y. Acad. Sci. 2008, 1143, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Lajaunias, F.; Dayer, J.M.; Chizzolini, C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur. J. Immunol. 2005, 35, 243–251. [Google Scholar] [CrossRef]
- Orr, S.J.; Morgan, N.M.; Elliott, J.; Burrows, J.F.; Scott, C.J.; McVicar, D.W.; Johnston, J.A. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood 2007, 109, 1061–1068. [Google Scholar] [CrossRef]
- Gbadamosi, M.O.; Shastri, V.M.; Hylkema, T.; Papageorgiou, I.; Pardo, L.; Cogle, C.R.; Doty, A.; Loken, M.R.; Meshinchi, S.; Lamba, J.K. Novel CD33 antibodies unravel localization, biology and therapeutic implications of CD33 isoforms. Future Oncol. 2021, 17, 263–277. [Google Scholar] [CrossRef]
- Hernández-Caselles, T.; Martínez-Esparza, M.; Pérez-Oliva, A.B.; Quintanilla-Cecconi, A.M.; García-Alonso, A.; Alvarez-López, D.M.R.; García-Peñarrubia, P. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: Two isoforms of CD33 are generated by alternative splicing. J. Leukoc. Biol. 2006, 79, 46–58. [Google Scholar] [CrossRef]
- Pérez-Oliva, A.B.; Martínez-Esparza, M.; Vicente-Fernández, J.J.; Corral-San Miguel, R.; García-Peñarrubia, P.; Hernández-Caselles, T. Epitope mapping, expression and post-translational modifications of two isoforms of CD33 (CD33M and CD33m) on lymphoid and myeloid human cells. Glycobiology 2011, 21, 757–770. [Google Scholar] [CrossRef]
- Laszlo, G.S.; Harrington, K.H.; Gudgeon, C.J.; Beddoe, M.E.; Fitzgibbon, M.P.; Ries, R.E.; Lamba, J.K.; McIntosh, M.W.; Meshinchi, S.; Walter, R.B. Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia. Oncotarget 2016, 7, 43281–43294. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.D.; Linch, D.; Sabbath, K.; Larcom, P.; Schlossman, S.F. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk. Res. 1984, 8, 521–534. [Google Scholar] [CrossRef]
- Andrews, R.G.; Singer, J.W.; Bernstein, I.D. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J. Exp. Med. 1989, 169, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Hamann, P.R.; Hinman, L.M.; Hollander, I.; Beyer, C.F.; Lindh, D.; Holcomb, R.; Hallett, W.; Tsou, H.R.; Upeslacis, J.; Shochat, D.; et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-Calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 2002, 13, 47–58. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Buijsse, A.O.; Van Den Bremer, E.T.J.; Verwilligen, A.Y.W.; Bleeker, W.K.; Thorpe, S.J.; Killestein, J.; Polman, C.H.; Aalberse, R.C.; Schuurman, J.; et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat. Biotechnol. 2009, 27, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Amico, D.; Barbui, A.M.; Erba, E.; Rambaldi, A.; Introna, M.; Golay, J. Differential response of human acute myeloid leukemia cells to gemtuzumab ozogamicin in vitro: Role of Chk1 and Chk2 phosphorylation and caspase 3. Blood 2003, 101, 4589–4597. [Google Scholar] [CrossRef]
- Mårtensson, S.; Nygren, J.; Osheroff, N.; Hammarsten, O. Activation of the DNA-dependent protein kinase by drug-induced and radiation-induced DNA strand breaks. Radiat. Res. 2003, 160, 291–301. [Google Scholar] [CrossRef]
- Elmroth, K.; Nygren, J.; Mårtensson, S.; Ismail, I.H.; Hammarsten, O. Cleavage of cellular DNA by calicheamicin γ1. DNA Repair 2003, 2, 363–374. [Google Scholar] [CrossRef]
- Sullivan, N.; Lyne, L. Sensitivity of fibroblasts derived from ataxia-telangiectasia patients to calicheamicin γ1I. Mutat. Res. Lett. 1990, 245, 171–175. [Google Scholar] [CrossRef]
- Prokop, A.; Wrasidlo, W.; Lode, H.; Herold, R.; Lang, F.; Henze, G.; Dörken, B.; Wieder, T.; Daniel, P.T. Induction of apoptosis by enediyne antibiotic calicheamicin θII proceeds through a caspase-mediated mitochondrial amplification loop in an entirely Bax-dependent manner. Oncogene 2003, 22, 9107–9120. [Google Scholar] [CrossRef]
- Haag, P.; Viktorsson, K.; Lindberg, M.L.; Kanter, L.; Lewensohn, R.; Stenke, L. Deficient activation of Bak and Bax confers resistance to gemtuzumab ozogamicin-induced apoptotic cell death in AML. Exp. Hematol. 2009, 37, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Seiter, K.; Kolitz, J.; Stock, W.; Giles, F.; Kalaycio, M.; Zenk, D.; Marcucci, G. A Phase II study of Bcl-2 antisense (oblimersen sodium) combined with gemtuzumab ozogamicin in older patients with acute myeloid leukemia in first relapse. Leuk. Res. 2006, 30, 777–783. [Google Scholar] [CrossRef] [PubMed]
- McGavin, J.K.; Spencer, C.M. Gemtuzumab ozogamicin. Drugs 2001, 61, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Laszlo, G.S.; Estey, E.H.; Walter, R.B. Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin. Front. Biosci. 2013, 18, 1311–1334. [Google Scholar] [CrossRef]
- Goemans, B.F.; Zwaan, C.M.; Vijverberg, S.J.H.; Loonen, A.H.; Creutzig, U.; Hählen, K.; Reinhardt, D.; Gibson, B.E.S.; Cloos, J.; Kaspers, G.J.L. Large interindividual differences in cellular sensitivity to calicheamicin may influence gemtuzumab ozogamicin response in acute myeloid leukemia. Leukemia 2008, 22, 2284–2285. [Google Scholar] [CrossRef]
- Pagano, L.; Fianchi, L.; Caira, M.; Rutella, S.; Leone, G. The role of Gemtuzumab Ozogamicin in the treatment of acute myeloid leukemia patients. Oncogene 2007, 26, 3679–3690. [Google Scholar] [CrossRef][Green Version]
- Breccia, M.; Lo-Coco, F. Gemtuzumab ozogamicin for the treatment of acute promyelocytic leukemia: Mechanisms of action and resistance, safety and efficacy. Expert Opin. Biol. Ther. 2011, 11, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Htter, M.L.; Schlenk, R.F. Gemtuzumab ozogamicin in non-acute promyelocytic acute myeloid leukemia. Expert Opin. Biol. Ther. 2011, 11, 1369–1380. [Google Scholar] [CrossRef]
- Takeshita, A. Efficacy and resistance of gemtuzumab ozogamicin for acute myeloid leukemia. Int. J. Hematol. 2013, 97, 703–716. [Google Scholar] [CrossRef]
- Thol, F.; Schlenk, R.F. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin. Biol. Ther. 2014, 14, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, M.; Sperotto, A.; Di Rorà, A.G.L.; Padella, A.; Cangini, D.; Giannini, M.B.; Simonetti, G.; Martinelli, G.; Cerchione, C. Gemtuzumab ozogamicin in acute myeloid leukemia: Past, present and future. Minerva Med. 2020, 111, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval Summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496. [Google Scholar] [PubMed]
- Laszlo, G.S.; Estey, E.H.; Walter, R.B. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014, 28, 143–153. [Google Scholar] [CrossRef]
- Amadori, S.; Suciu, S.; Selleslag, D.; Stasi, R.; Alimena, G.; Baila, L.; Rizzoli, V.; Borlenghi, E.; Gaidano, G.; Magro, D.; et al. Randomized trial of two schedules of low-dose gemtuzumab ozogamicin as induction monotherapy for newly diagnosed acute myeloid leukaemia in older patients not considered candidates for intensive chemotherapy. A phase II study of the EORTC and GIMEMA leuka. Br. J. Haematol. 2010, 149, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Venditti, A.; Voso, M.T.; Annino, L.; De Fabritiis, P.; Alimena, G.; Mancini, M.; Paoloni, F.; Vignetti, M.; Fazi, P.; et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC-GIMEMA AML-19 Trial. J. Clin. Oncol. 2016, 34, 972–979. [Google Scholar] [CrossRef]
- Zwaan, C.M.; Reinhardt, D.; Corbacioglu, S.; Van Wering, E.R.; Bökkerink, J.P.M.; Tissing, W.J.E.; Samuelsson, U.; Feingold, J.; Creutzig, U.; Kaspers, G.J.L. Gemtuzumab ozogamicin: First clinical experiences in children with relapsed/refractory acute myeloid leukemia treated on compassionate-use basis. Blood 2003, 101, 3868–3871. [Google Scholar] [CrossRef]
- Arceci, R.J.; Sande, J.; Lange, B.; Shannon, K.; Franklin, J.; Hutchinson, R.; Vik, T.A.; Flowers, D.; Aplenc, R.; Berger, M.S.; et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood 2005, 106, 1183–1188. [Google Scholar] [CrossRef]
- Zwaan, C.M.; Reinhardt, D.; Zimmerman, M.; Hasle, H.; Stary, J.; Stark, B.; Dworzak, M.; Creutzig, U.; Kaspers, G.J.L. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: Results of a phase II study. Br. J. Haematol. 2010, 148, 768–776. [Google Scholar] [CrossRef]
- Malfuson, J.V.; Konopacki, J.; Thepenier, C.; Eddou, H.; Foissaud, V.; De Revel, T. Fractionated doses of gemtuzumab ozogamicin combined with 3+7 induction chemotherapy as salvage treatment for young patients with acute myeloid leukemia in first relapse. Ann. Hematol. 2012, 91, 1871–1877. [Google Scholar] [CrossRef]
- Kell, W.J.; Burnett, A.K.; Chopra, R.; Yin, J.A.L.; Clark, R.E.; Rohatiner, A.; Culligan, D.; Hunter, A.; Prentice, A.G.; Milligan, D.W. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood 2003, 102, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Hills, R.K.; Milligan, D.; Kjeldsen, L.; Kell, J.; Russell, N.H.; Yin, J.A.L.; Hunter, A.; Goldstone, A.H.; Wheatley, K. Identification of Patients with Acute Myeloblastic Leukemia Who Benefit from the Addition of Gemtuzumab Ozogamicin: Results of the MRC AML15 Trial. J. Clin. Oncol. 2011, 29, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Kell, J.; Freeman, S.; Kjeldsen, L.; Hunter, A.E.; Yin, J.; Craddock, C.F.; Dufva, I.H.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Farhat, H.; Reman, O.; Raffoux, E.; Berthon, C.; Pautas, C.; Kammoun, L.; Chantepie, S.; Gardin, C.; Rousselot, P.; Chevret, S.; et al. Fractionated doses of gemtuzumab ozogamicin with escalated doses of daunorubicin and cytarabine as first acute myeloid leukemia salvage in patients aged 50–70-year old: A phase 1/2 study of the acute leukemia French association. Am. J. Hematol. 2012, 87, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Pilorge, S.; Rigaudeau, S.; Rabian, F.; Sarkozy, C.; Taksin, A.L.; Farhat, H.; Merabet, F.; Ghez, S.; Raggueneau, V.; Terré, C.; et al. Fractionated gemtuzumab ozogamicin and standard dose cytarabine produced prolonged second remissions in patients over the age of 55 years with acute myeloid leukemia in late first relapse. Am. J. Hematol. 2014, 89, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. The addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Burnett, A.; Cavenagh, J.; Russell, N.; Hills, R.; Kell, J.; Jones, G.; Nielsen, O.J.; Khwaja, A.; Thomas, I.; Clark, R. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: A comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 trial. Haematologica 2016, 101, 724–731. [Google Scholar] [CrossRef]
- Maniecki, M.B.; Hasle, H.; Friis-Hansen, L.; Lausen, B.; Nielsen, O.J.; Bendix, K.; Moestrup, S.K.; Møller, H.J. Impaired CD163-mediated hemoglobin-scavenging and severe toxic symptoms in patients treated with gemtuzumab ozogamicin. Blood 2008, 112, 1510–1514. [Google Scholar] [CrossRef][Green Version]
- Tsimberidou, A.; Estey, E.; Cortes, J.; Thomas, D.; Faderl, S.; Verstovsek, S.; Garcia-Manero, G.; Keating, M.; Albitar, M.; O’Brien, S.; et al. Gemtuzumab, fludarabine, cytarabine, and cyclosporine in patients with newly diagnosed acute myelogenous leukemia or high-risk myelodysplastic syndromes. Cancer 2003, 97, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Sievers, E.L.; Stadtmauer, E.A.; Löwenberg, B.; Estey, E.H.; Dombret, H.; Theobald, M.; Voliotis, D.; Bennett, J.M.; Richte, M.; et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005, 104, 1442–1452. [Google Scholar] [CrossRef]
- Ho, V.T.; Martin, A.S.; Pérez, W.S.; Steinert, P.; Zhang, M.J.; Chirnomas, D.; Hoang, C.J.; Loberiza, F.R.; Saber, W. Prior Gemtuzumab Ozogamicin Exposure in Adults with Acute Myeloid Leukemia Does Not Increase Hepatic Veno-Occlusive Disease Risk after Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Analysis. Biol. Blood Marrow Transplant. 2020, 26, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Selby, C.; Yacko, L.R.; Glode, A.E. Gemtuzumab Ozogamicin: Back Again. J. Adv. Pract. Oncol. 2019, 10, 68–82. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.; Suciu, S.; Meert, L.; Halkes, C.; Selleslag, D.; Bron, D.; Amadori, S.; Willemze, R.; Muus, P.; Baron, F. Idarubicin and cytarabine in combination with gemtuzumab ozogamicin (IAGO) for untreated patients with high-risk MDS or AML evolved from MDS: A phase II study from the EORTC and GIMEMA Leukemia Groups (protocol 06013). Ann. Hematol. 2015, 94, 1981–1989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wynne, J.; Wright, D.; Stock, W. Inotuzumab: From preclinical development to success in B-cell acute lymphoblastic leukemia. Blood Adv. 2019, 3, 96–104. [Google Scholar] [CrossRef]
- Richardson, P.G.; Corbacioglu, S. Veno-occlusive disease/sinusoidal obstruction syndrome in patients with prior gemtuzumab ozogamicin: Literature analysis of survival after defibrotide treatment. Blood Cancer J. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Van Der Reijden, B.A.; Simons, A.; Luiten, E.; Van Der Poel, S.C.; Hogenbirk, P.E.; Tönnissen, E.; Valk, P.J.M.; Löwenberg, B.; De Greef, G.E.; Breuning, M.H.; et al. Minimal residual disease quantification in patients with acute myeloid leukaemia and inv(16)/CBFB-MYH11 gene fusion. Br. J. Haematol. 2002, 118, 411–418. [Google Scholar] [CrossRef]
- Pautas, C.; Raffoux, E.; Lambert, J.; Legrand, O.; Chantepie, S.; Gastaud, L.; Marolleau, J.P.; Thomas, X.; Turlure, P.; Benner, R.J.; et al. Outcomes following hematopoietic stem cell transplantation in patients treated with standard chemotherapy with or without gemtuzumab ozogamicin for acute myeloid leukemia. Bone Marrow Transplant. 2021, 56, 1474–1477. [Google Scholar] [CrossRef]
- Debureaux, P.E.; Labopin, M.; Mamez, A.C.; Lapusan, S.; Isnard, F.; Adaeva, R.; Bonnin, A.; Hirsch, P.; Delhommeau, F.; Battipaglia, G.; et al. Fractionated gemtuzumab ozogamicin in association with high dose chemotherapy: A bridge to allogeneic stem cell transplantation in refractory and relapsed acute myeloid leukemia. Bone Marrow Transplant. 2020, 55, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Paubelle, E.; Ducastelle-Leprêtre, S.; Labussière-Wallet, H.; Nicolini, F.E.; Barraco, F.; Plesa, A.; Salles, G.; Wattel, E.; Thomas, X. Fractionated gemtuzumab ozogamicin combined with intermediate-dose cytarabine and daunorubicin as salvage therapy in very high-risk AML patients: A bridge to reduced intensity conditioning transplant? Ann. Hematol. 2017, 96, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, R.; Tashiro, H.; Saito, S.; Matsuo, T.; Yamamoto, T.; Matsumoto, K.; Ooi, J.; Shirafuji, N. Gemtuzumab ozogamicin monotherapy prior to stem cell infusion induces sustained remission in a relapsed acute myeloid leukemia patient after allogeneic stem cell transplantation: A case report. Medicine 2020, 99, e22064. [Google Scholar] [CrossRef] [PubMed]
- Mosna, F.; Papayannidis, C.; Martinelli, G.; Di Bona, E.; Bonalumi, A.; Tecchio, C.; Candoni, A.; Capelli, D.; Piccin, A.; Forghieri, F.; et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up. Am. J. Hematol. 2015, 90, 515–523. [Google Scholar] [CrossRef]
- Terwijn, M.; Kelder, A.; Huijgens, P.C.; Dräger, A.M.; Oussoren, Y.J.M.; Scholten, W.J.; Snel, A.N.; Ossenkoppele, G.J.; Schuurhuis, G.J.; Biemond, B.J.; et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: Data from the HOVON/SAKK AML 42A study. J. Clin. Oncol. 2013, 31, 3889–3897. [Google Scholar] [CrossRef]
- Walter, R.B.; Gooley, T.A.; Wood, B.L.; Milano, F.; Fang, M.; Sorror, M.L.; Estey, E.H.; Salter, A.I.; Lansverk, E.; Chien, J.W.; et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 1190–1197. [Google Scholar] [CrossRef]
- Pollard, J.A.; Alonzo, T.A.; Loken, M.; Gerbing, R.B.; Ho, P.A.; Bernstein, I.D.; Raimondi, S.C.; Hirsch, B.; Franklin, J.; Walter, R.B.; et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood 2012, 119, 3705–3711. [Google Scholar] [CrossRef]
- Genthon, A.; Brissot, E.; Malard, F.; Van de Wyngaert, Z.; Bonnin, A.; Banet, A.; Marjanovic, Z.; Ikhlef, S.; Lapusan, S.; Sestili, S.; et al. Gemtuzumab Ozogamicin Combined With Intensive Chemotherapy in Patients With Acute Myeloid Leukemia Relapsing After Allogenic Stem Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2020, 20, 791–796. [Google Scholar] [CrossRef]
- Satwani, P.; Bhatia, M.; Garvin, J.H.; George, D.; Dela Cruz, F.; Le Gall, J.; Jin, Z.; Schwartz, J.; Duffy, D.; Van de Ven, C.; et al. A Phase I Study of Gemtuzumab Ozogamicin (GO) in Combination with Busulfan and Cyclophosphamide (Bu/Cy) and Allogeneic Stem Cell Transplantation in Children with Poor-Risk CD33+ AML: A New Targeted Immunochemotherapy Myeloablative Conditioning (MAC) Regim. Biol. Blood Marrow Transplant. 2012, 18, 324–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penel-Page, M.; Plesa, A.; Girard, S.; Marceau-Renaut, A.; Renard, C.; Bertrand, Y. Association of fludarabin, cytarabine, and fractioned gemtuzumab followed by hematopoietic stem cell transplantation for first-line refractory acute myeloid leukemia in children: A single-center experience. Pediatr. Blood Cancer 2020, 67, e28305. [Google Scholar] [CrossRef] [PubMed]
- Toyama, D.; Matsuno, R.; Sugishita, Y.; Kaneko, R.; Okamoto, N.; Koganesawa, M.; Fujita, S.; Akiyama, K.; Isoyama, K.; Yamamoto, S. Successful Treatment of Pediatric Refractory/Relapsed AML with KIR-Ligand-Mismatched Cord Blood Transplant after FLAG-IDA Reinduction Therapy with or without the GO Regimen. Case Rep. Hematol. 2020. [Google Scholar] [CrossRef]
- Niktoreh, N.; Lerius, B.; Zimmermann, M.; Gruhn, B.; Escherich, G.; Bourquin, J.P.; Dworzak, M.; Sramkova, L.; Rossig, C.; Creutzig, U.; et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: A report by Berlin-Frankfurt-Münster study group. Haematologica 2019, 104, 120–127. [Google Scholar] [CrossRef]
- O’Hear, C.; Inaba, H.; Pounds, S.; Shi, L.; Dahl, G.; Paul Bowman, W.; Taub, J.W.; Pui, C.H.; Ribeiro, R.C.; Coustan-Smith, E.; et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer 2013, 119, 4036–4043. [Google Scholar] [CrossRef]
- Roman, E.; Cooney, E.; Harrison, L.; Militano, O.; Wolownik, K.; Hawks, R.; Foley, S.; Satwani, P.; Unal, E.; Bhatia, M.; et al. Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia. Clin. Cancer Res. 2005, 11, 7164s–7170s. [Google Scholar] [CrossRef][Green Version]
- Zahler, S.; Bhatia, M.; Ricci, A.; Roy, S.; Morris, E.; Harrison, L.; van de Ven, C.; Fabricatore, S.; Wolownik, K.; Cooney-Qualter, E.; et al. A Phase I Study of Reduced-Intensity Conditioning and Allogeneic Stem Cell Transplantation Followed by Dose Escalation of Targeted Consolidation Immunotherapy with Gemtuzumab Ozogamicin in Children and Adolescents with CD33+ Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.A.; Loken, M.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Aplenc, R.; Bernstein, I.D.; Gamis, A.S.; Alonzo, T.A.; Meshinchi, S. CD33 expression and its association with gemtuzumab ozogamicin response: Results from the randomized phase III children’s oncology group trial AAML0531. J. Clin. Oncol. 2016, 34, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velden, V.H.J.; Te Marvelde, J.G.; Hoogeveen, P.G.; Bernstein, I.D.; Houtsmuller, A.B.; Berger, M.S.; Van Dongen, J.J.M. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: In vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 2001, 97, 3197–3204. [Google Scholar] [CrossRef]
- Jager, E.; Van der Velden, V.H.J.; Te Marvelde, J.G.; Walter, R.B.; Agur, Z.; Vainstein, V. Targeted drug delivery by gemtuzumab ozogamicin: Mechanism-Based mathematical model for treatment strategy improvement and therapy individualization. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Raden, B.W.; Kamikura, D.M.; Cooper, J.A.; Bernstein, I.D. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood 2005, 105, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Olombel, G.; Guerin, E.; Guy, J.; Perrot, J.Y.; Dumezy, F.; de Labarthe, A.; Bastie, J.N.; Legrand, O.; Raffoux, E.; Plesa, A.; et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Blood 2016, 127, 2157–2160. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Hills, R.K.; Virgo, P.; Couzens, S.; Clark, N.; Gilkes, A.; Richardson, P.; Knapper, S.; Grimwade, D.; Russell, N.H.; et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia 2017, 31, 1059–1068. [Google Scholar] [CrossRef]
- Lamba, J.K.; Chauhan, L.; Shin, M.; Loken, M.R.; Pollard, J.A.; Wang, Y.C.; Ries, R.E.; Aplenc, R.; Hirsch, B.A.; Raimondi, S.C.; et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: Report from randomized phase III children’s oncology group trial AAML0531. J. Clin. Oncol. 2017, 35, 674–2682. [Google Scholar] [CrossRef]
- Teich, K.; Krzykalla, J.; Kapp-Schwoerer, S.; Gaidzik, V.I.; Schlenk, R.F.; Paschka, P.; Weber, D.; Fiedler, W.; Kühn, M.W.M.; Schroeder, T.; et al. Cluster of differentiation 33 single nucleotide polymorphism rs12459419 is a predictive factor in patients with nucleophosmin1 mutated acute myeloid leukemia receiving gemtuzumab ozogamicin. Haematologica 2021. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Ryan, K.J.; Replogle, J.M.; Chibnik, L.B.; Rosenkrantz, L.; Tang, A.; Rothamel, K.; Stranger, B.E.; Bennett, D.A.; Evans, D.A.; et al. CD33: Increased inclusion of exon 2 implicates the Ig V-set domain in Alzheimer’s disease susceptibility. Hum. Mol. Genet. 2014, 23, 2729–2736. [Google Scholar] [CrossRef]
- Mortland, L.; Alonzo, T.A.; Walter, R.B.; Gerbing, R.B.; Mitra, A.K.; Pollard, J.A.; Loken, M.R.; Hirsch, B.; Raimondi, S.; Franklin, J.; et al. Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clin. Cancer Res. 2013, 19, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Lamba, J.K.; Voigt, A.P.; Chauhan, L.; Shin, M.; Aplenc, R.; Eidenschink Brodersen, L.; Gamis, A.S.; Meshinchi, S.; Loken, M.R. CD33 splicing SNP regulates expression levels of CD33 in normal regenerating monocytes in AML patients. Leuk. Lymphoma 2018, 59, 2250–2253. [Google Scholar] [CrossRef]
- Malik, M.; Simpson, J.F.; Parikh, I.; Wilfred, B.R.; Fardo, D.W.; Nelson, P.T.; Estus, S. CD33 Alzheimer’s risk-altering polymorphism, CD33 expression, and exon 2 splicing. J. Neurosci. 2013, 33, 13320–13325. [Google Scholar] [CrossRef]
- Chauhan, L.; Shin, M.; Wang, Y.-C.; Loken, M.; Pollard, J.; Aplenc, R.; Hirsch, B.A.; Raimondi, S.; Ries, R.E.; Bernstein, I.D.; et al. CD33_PGx6_Score Predicts Gemtuzumab Ozogamicin Response in Childhood Acute Myeloid Leukemia: A Report From the Children’s Oncology Group. JCO Precis. Oncol. 2019, 3, 1–15. [Google Scholar] [CrossRef]
- Renneville, A.; Abdelali, R.B.; Chevret, S.; Nibourel, O.; Cheok, M.; Pautas, C.; Duléry, R.; Boyer, T.; Cayuela, J.M.; Hayette, S.; et al. Clinical impact of gene mutations and lesions detected by SNP-array karyotyping in acute myeloid leukemia patients in the context of gemtuzumab ozogamicin treatment: Results of the ALFA-0701 trial. Oncotarget 2014, 5, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Krupka, C.; Kufer, P.; Kischel, R.; Zugmaier, G.; Bögeholz, J.; Köhnke, T.; Lichtenegger, F.S.; Schneider, S.; Metzeler, K.H.; Fiegl, M.; et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood 2014, 123, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Appelbaum, F.R.; Estey, E.H.; Bernstein, I.D. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 2012, 119, 6198–6208. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Cortes, J.E.; Estey, E.E.; Jabbour, E.; Faderl, S.; O’Brien, S.; Garcia-Manero, G.; Kadia, T.M.; Wang, X.; Patel, K.; et al. Gemtuzumab ozogamicin with fludarabine, cytarabine, and granulocyte colony stimulating factor (FLAG-GO) as front-line regimen in patients with core binding factor acute myelogenous leukemia. Am. J. Hematol. 2014, 89, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Tarlock, K.; Alonzo, T.A.; Wang, Y.C.; Gerbing, R.B.; Ries, R.; Loken, M.R.; Pardo, L.; Hylkema, T.; Joaquin, J.; Sarukkai, L.; et al. Functional properties of KIT mutations are associated with differential clinical outcomes and response to targeted therapeutics in CBF acute myeloid leukemia. Clin. Cancer Res. 2019, 25, 5038–5048. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.M.; Cortes, J.E.; Ravandi, F.; Garcia-Manero, G.; Kadia, T.M.; Jabbour, E.; Patel, K.; Issa, G.C.; Daver, N.G.; Ohanian, M.N.; et al. Fludarabine, Cytarabine, G-CSF and Gemtuzumab Ozogamicin (FLAG-GO) Regimen Results in Better Molecular Response and Relapse-Free Survival in Core Binding Factor Acute Myeloid Leukemia Than FLAG and Idarubicin (FLAG-Ida). Blood 2019, 134. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Balgobind, B.V.; Zwaan, C.M.; Pieters, R.; Van Den Heuvel-Eibrink, M.M. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia 2011, 25, 1239–1248. [Google Scholar] [CrossRef]
- Muñoz, L.; Nomdedéu, J.F.; Villamor, N.; Guardia, R.; Colomer, D.; Ribera, J.M.; Torres, J.P.; Berlanga, J.J.; Fernández, C.; Llorente, A.; et al. Acute myeloid leukemia with MLL rearrangements: Clinicobiological features, prognostic impact and value of flow cytometry in the detection of residual leukemic cells. Leukemia 2003, 17, 76–82. [Google Scholar] [CrossRef]
- Tamai, H.; Shioi, Y.; Yamaguchi, H.; Okabe, M.; Wakita, S.; Mizuki, T.; Nakayama, K.; Inokuchi, K.; Tajika, K.; Dan, K. Treatment of relapsed acute myeloid leukemia with MLL/AF6 fusion after allogeneic hematopoietic stem cell transplantation with gemtuzumab ozogamicin with a long interval followed by donor lymphocyte infusion. Leukemia 2008, 22, 1273–1274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asano, H.; Yamamoto, G.; Hosoi, M.; Takahashi, T.; Hangaishi, A.; Kurokawa, M. Complete molecular remission in refractory acute myeloid leukemia with MLL/AF9 treated with gemtuzumab ozogamicin. Leuk. Res. 2010, 34. [Google Scholar] [CrossRef]
- Pollard, J.A.; Guest, E.; Alonzo, T.A.; Gerbing, R.B.; Loken, M.R.; Brodersen, L.E.; Kolb, E.A.; Aplenc, R.; Meshinchi, S.; Raimondi, S.C.; et al. Gemtuzumab Ozogamicin Improves Event-Free Survival and Reduces Relapse in Pediatric KMT2A-Rearranged AML: Results From the Phase III Children’s Oncology Group Trial AAML0531. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Legrand, O.; Zompi, S.; Perrot, J.Y.; Faussat, A.M.; Benderra, Z.; Chaoui, D.; Marie, J.P. P-glycoprotein and multidrug resistance associated pr otein-1 activity in 132 acute myeloid leukemias according to FAB subtypes and cytogenetic risk groups. Haematologica 2004, 89, 34–41. [Google Scholar] [PubMed]
- Seedhouse, C.H.; Grundy, M.; White, P.; Li, Y.; Fisher, J.; Yakunina, D.; Moorman, A.V.; Hoy, T.; Russell, N.; Burnett, A.; et al. Sequential influences of leukemia-specific and genetic factors on P-glycoprotein expression in blasts from 817 patients entered into the National Cancer Research Network acute myeloid leukemia 14 and 15 trials. Clin. Cancer Res. 2007, 13, 7059–7066. [Google Scholar] [CrossRef] [PubMed]
- Candoni, A.; Papayannidis, C.; Martinelli, G.; Simeone, E.; Gottardi, M.; Iacobucci, I.; Gherlinzoni, F.; Visani, G.; Baccarani, M.; Fanin, R. Flai (fludarabine, cytarabine, idarubicin) plus low-dose Gemtuzumab Ozogamicin as induction therapy in CD33-positive AML: Final results and long term outcome of a phase II multicenter clinical trial. Am. J. Hematol. 2018, 93, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Duployez, N.; Ducourneau, B.; Raffoux, E.; Turlure, P.; Caillot, D.; Thomas, X.; Marceau-Renaut, A.; Chantepie, S.; Malfuson, J.V.; et al. Mutational profile and benefit of gemtuzumab ozogamicin in acute myeloid leukemia. Blood 2020, 135, 542–546. [Google Scholar] [CrossRef]
- De Propris, M.S.; Raponi, S.; Diverio, D.; Milani, M.L.; Meloni, G.; Falini, B.; Foà, R.; Guarini, A. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica 2011, 96, 1548–1551. [Google Scholar] [CrossRef]
- Ehninger, A.; Kramer, M.; Röllig, C.; Thiede, C.; Bornhäuser, M.; Von Bonin, M.; Wermke, M.; Feldmann, A.; Bachmann, M.; Ehninger, G.; et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014, 4. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Paschka, P.; Krzykalla, J.; Weber, D.; Kapp-Schwoerer, S.; Gaidzik, V.I.; Leis, C.; Fiedler, W.; Kindler, T.; Schroeder, T.; et al. Gemtuzumab ozogamicin in NPM1-mutated acute myeloid leukemia: Early results from the prospective randomized AMLSG 09-09 Phase III study. J. Clin. Oncol. 2020, 38, 623–632. [Google Scholar] [CrossRef]
- Tarlock, K.; Alonzo, T.A.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Sung, L.; Pollard, J.A.; Aplenc, R.; Loken, M.R.; Gamis, A.S.; et al. Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: A report from the children’s oncology group. Clin. Cancer Res. 2016, 22, 1951–1957. [Google Scholar] [CrossRef]
- Zhang, H.; Nakauchi, Y.; Köhnke, T.; Stafford, M.; Bottomly, D.; Thomas, R.; Wilmot, B.; McWeeney, S.K.; Majeti, R.; Tyner, J.W. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat. Cancer 2020, 1, 826–839. [Google Scholar] [CrossRef]
- Itzykson, R.; Duployez, N.; Fasan, A.; Decool, G.; Marceau-Renaut, A.; Meggendorfer, M.; Jourdan, E.; Petit, A.; Lapillonne, H.; Micol, J.B.; et al. Clonal interference of signaling mutations worsens prognosis in core-binding factor acute myeloid leukemia. Blood 2018, 132, 187–196. [Google Scholar] [CrossRef]
- Balaian, L.; Zhong, R.K.; Ball, E.D. The inhibitory effect of anti-CD33 monoclonal antibodies on AML cell growth correlates with Syk and/or ZAP-70 expression. Exp. Hematol. 2003, 31, 363–371. [Google Scholar] [CrossRef]
- Bouvier, A.; Hamel, J.; Delaunay, J.; Delabesse, E.; Dumas, P.; Ledoux, M.; Peterlin, P.; Luquet, I.; Roth Guepin, G.; Bulabois, C.E.; et al. Molecular classification and prognosis in younger adults with acute myeloid leukemia and intermediate-risk cytogenetics treated or not by gemtuzumab ozogamycin: Final results of the GOELAMS/FILO acute myeloid leukemia 2006-intermediate-risk trial. Eur. J. Haematol. 2021. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Zhang, P.; Lou, S.; Chen, Y.; Li, H.; Zeng, H.; Shen, Y.; Deng, J. Overexpression of annexin A5 might guide the gemtuzumab ozogamicin treatment choice in patients with pediatric acute myeloid leukemia. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef]
- Matsumoto, T.; Jimi, S.; Hara, S.; Takamatsu, Y.; Suzumiya, J.; Tamura, K. Importance of inducible multidrug resistance 1 expression in HL-60 cells resistant to gemtuzumab ozogamicin. Leuk. Lymphoma 2012, 53, 1399–1405. [Google Scholar] [CrossRef]
- Walter, R.B.; Raden, B.W.; Hong, T.C.; Flowers, D.A.; Bernstein, I.D.; Linenberger, M.L. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood 2003, 102, 1466–1473. [Google Scholar] [CrossRef]
- Matsui, H.; Takeshita, A.; Naito, K.; Shinjo, K.; Shigeno, K.; Maekawa, M.; Yamakawa, Y.; Tanimoto, M.; Kobayashi, M.; Ohnishi, K.; et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia 2002, 16, 813–819. [Google Scholar] [CrossRef]
- Walter, R.B.; Raden, B.W.; Thompson, J.; Flowers, D.A.; Kiem, H.P.; Bernstein, I.D.; Linenberger, M.L. Breast cancer resistance protein (BCRP/ABCG2) does not confer resistance to gemtuzumab ozogamicin and calicheamicin-γ1 in acute myeloid leukemia cells. Leukemia 2004, 18, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Gooley, T.A.; Van Der Velden, V.H.J.; Loken, M.R.; Van Dongen, J.J.M.; Flowers, D.A.; Bernstein, I.D.; Appelbaum, F.R. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 2007, 109, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel-Eibrink, M.M.; Van Der Holt, B.; Te Boekhorst, P.A.W.; Pieters, R.; Schoester, M.; Löwenberg, B.; Sonneveld, P. MDR 1 expression is an independent prognostic factor for response and survival in de novo acute myeloid leukaemia. Br. J. Haematol. 1997, 99, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Leith, C.P.; Kopecky, K.J.; Chen, I.M.; Eijdems, L.; Slovak, M.L.; McConnell, T.S.; Head, D.R.; Weick, J.; Grever, M.R.; Appelbaum, F.R.; et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia. A Southwest Oncology Group study. Blood 1999, 94, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Linenberger, M.L.; Hong, T.; Flowers, D.; Sievers, E.L.; Gooley, T.A.; Bennett, J.M.; Berger, M.S.; Leopold, L.H.; Appelbaum, F.R.; Bernstein, I.D. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood 2001, 98, 988–994. [Google Scholar] [CrossRef]
- Del Poeta, G.; Venditti, A.; Aronica, G.; Stasi, R.; Cox, M.C.; Buccisano, F.; Bruno, A.; Tamburini, A.; Suppo, G.; Simone, M.D.; et al. P-glycoprotein expression in de novo acute myeloid leukemia. Leuk. Lymphoma 1997, 27, 257–274. [Google Scholar] [CrossRef]
- Rafiee, R.; Chauhan, L.; Alonzo, T.A.; Wang, Y.C.; Elmasry, A.; Loken, M.R.; Pollard, J.; Aplenc, R.; Raimondi, S.; Hirsch, B.A.; et al. ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with Gemtuzumab ozogamicin: A report from Children’s Oncology Group AAML0531 Trial. Blood Cancer J. 2019, 9. [Google Scholar] [CrossRef]
- Michieli, M.; Damiani, D.; Ermacora, A.; Masolini, P.; Raspadori, D.; Visani, G.; Scheper, R.J.; Baccarani, M. P-glycoprotein, lung resistance-related protein and multidrug resistance associated protein in de novo acute non-lymphocytic leukaemias: Biological and clinical implications. Br. J. Haematol. 1999, 104, 328–335. [Google Scholar] [CrossRef]
- Boyer, T.; Gonzales, F.; Barthélémy, A.; Marceau-Renaut, A.; Peyrouze, P.; Guihard, S.; Lepelley, P.; Plesa, A.; Nibourel, O.; Delattre, C.; et al. Clinical significance of ABCB1 in acute myeloid leukemia: A comprehensive study. Cancers 2019, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Balsat, M.; Renneville, A.; Thomas, X.; De Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.P.; Nibourel, O.; Berthon, C.; et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: A study by the acute leukemia French association group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.; Hills, R.; Freeman, S.; Potter, N.; Jovanovic, J.; Ivey, A.; Kanda, A.S.; Runglall, M.; Foot, N.; Valganon, M.; et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood 2020, 135, 680–688. [Google Scholar] [CrossRef]
- Freeman, S.D.; Virgo, P.; Couzens, S.; Grimwade, D.; Russell, N.; Hills, R.K.; Burnett, A.K. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J. Clin. Oncol. 2013, 31, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Lambert, J.; Nibourel, O.; Pautas, C.; Hayette, S.; Cayuela, J.M.; Terré, C.; Rousselot, P.; Dombret, H.; Chevret, S.; et al. MRD assessed by WT1 and NPM1 transcript levels identifies distinct outcomes in AML patients and is influenced by gemtuzumab ozogamicin. Oncotarget 2014, 5, 6280–6288. [Google Scholar] [CrossRef] [PubMed]
- Kapp-Schwoerer, S.; Weber, D.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; Krönke, J.; Theis, F.; Rücker, F.G.; Teleanu, M.V.; Panina, E.; et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in patients with NPM1-mutated AML: Results from the AMLSG 09-09 trial. Blood 2020, 136, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Majeti, R. Biology and relevance of human acute myeloid leukemia stem cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Maimaitili, Y.; Inase, A.; Miyata, Y.; Kitao, A.; Mizutani, Y.; Kakiuchi, S.; Shimono, Y.; Saito, Y.; Sonoki, T.; Minami, H.; et al. An mTORC1/2 kinase inhibitor enhances the cytotoxicity of gemtuzumab ozogamicin by activation of lysosomal function. Leuk. Res. 2018, 74, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Inase, A.; Maimaitili, Y.; Miyata, Y.; Kitao, A.; Matsumoto, H.; Kawaguchi, K.; Higashime, A.; Goto, H.; Kurata, K.; et al. An mTORC1/2 dual inhibitor, AZD2014, acts as a lysosomal function activator and enhances gemtuzumab ozogamicin-induced apoptosis in primary human leukemia cells. Int. J. Hematol. 2019, 110, 490–499. [Google Scholar] [CrossRef]
- Pan, J.; She, M.; Xu, Z.X.; Sun, L.; Yeung, S.C.J. Farnesyltransferase inhibitors induce DNA damage via reactive oxygen species in human cancer cells. Cancer Res. 2005, 65, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.; Yu, N.; Seedhouse, C.; Tandon, K.; Russell, N.H.; Pallis, M. Targeting of CD34+CD38- cells using Gemtuzumab ozogamicin (Mylotarg) in combination with tipifarnib (Zarnestra) in acute Myeloid Leukaemia. BMC Cancer 2012, 12. [Google Scholar] [CrossRef]
- Carr, M.I.; Zimmermann, A.; Chiu, L.Y.; Zenke, F.T.; Blaukat, A.; Vassilev, L.T. DNA-PK Inhibitor, M3814, as a New Combination Partner of Mylotarg in the Treatment of Acute Myeloid Leukemia. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Uzui, K.; Nishi, R.I.; Shigemi, H.; Ueda, T. Gemtuzumab Ozogamicin and Olaparib Exert Synergistic Cytotoxicity in CD33-positive HL-60 Myeloid Leukemia Cells. Anticancer Res. 2014, 34, 5487–5494. [Google Scholar] [PubMed]
- Portwood, S.M.; Cantella, M.C.; Cronin, T.L.; Wang, E.S. Addition of the PARP Inhibitor, Talazoparib, to Gemtuzumab Ozogamicin Significantly Enhances Anti-Leukemic Activity in Human CD33+ Acute Myeloid Leukemia. Blood 2019, 134, 1371. [Google Scholar] [CrossRef]

| NCT Number | Intervention | Conditions | Age | Phase | Trial Status |
|---|---|---|---|---|---|
| NCT00044733 | GO at relapse after auto or alloSCT | AML | child, adult, older adult | II | Completed |
| NCT02221310 | GO+chemotherapy followed by alloSCT | high-risk AML/MDS | up to 25 years | II | Recruiting |
| NCT00669890 | GO+Busulfan and Cyclophosphamid before alloSCT | high-risk AML/MDS/JMML | up to 30 years | I | Terminated |
| NCT02117297 | GO consolidation after alloSCT | average-risk AML/MDS | up to 25 years | II | Recruiting |
| NCT01020539 | GO consolidation after alloSCT | average-risk AML/MDS/JMML | up to 30 years | I | Active, not recruiting |
| NCT00460447 | GO before alloSCT at relapse | AML | 18–70 years | I/II | Unknown |
| NCT00038831 | GO+Melphalan+Fludarabine before alloSCT in older or medically infirm patients | AML/MDS/CLL | 12–75 years | I/II | Completed |
| NCT00476541 | GO consolidation after SCT | AML | up to 18 years | III | Completed |
| NCT00008151 | GO+fludarabine+total-body irradiation before alloSCT | advanced AML/MDS | child, adult, older adult | II | Completed |
| NCT00038805 | GO+nonmyeloablative preparative regimen before mini-alloSCT in older or medically infirm patients | AML/ALL/CML/MDS | 55–75 years | II/III | Terminated |
| NCT01723657 | GO “in vivo purging” before autoSCT in patients with favorable/intermediate characteristics and without matched related donor | AML | 18–70 years | II | Completed |
| NCT00070174 | GO in remission induction, intensification therapy before alloSCT | AML | child, adult, older adult | II | Completed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottardi, M.; Simonetti, G.; Sperotto, A.; Nappi, D.; Ghelli Luserna di Rorà, A.; Padella, A.; Norata, M.; Giannini, M.B.; Musuraca, G.; Lanza, F.; et al. Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin. Cancers 2021, 13, 4566. https://doi.org/10.3390/cancers13184566
Gottardi M, Simonetti G, Sperotto A, Nappi D, Ghelli Luserna di Rorà A, Padella A, Norata M, Giannini MB, Musuraca G, Lanza F, et al. Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin. Cancers. 2021; 13(18):4566. https://doi.org/10.3390/cancers13184566
Chicago/Turabian StyleGottardi, Michele, Giorgia Simonetti, Alessandra Sperotto, Davide Nappi, Andrea Ghelli Luserna di Rorà, Antonella Padella, Marianna Norata, Maria Benedetta Giannini, Gerardo Musuraca, Francesco Lanza, and et al. 2021. "Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin" Cancers 13, no. 18: 4566. https://doi.org/10.3390/cancers13184566
APA StyleGottardi, M., Simonetti, G., Sperotto, A., Nappi, D., Ghelli Luserna di Rorà, A., Padella, A., Norata, M., Giannini, M. B., Musuraca, G., Lanza, F., Cerchione, C., & Martinelli, G. (2021). Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin. Cancers, 13(18), 4566. https://doi.org/10.3390/cancers13184566











