Current Immunotherapeutic Strategies for the Treatment of Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
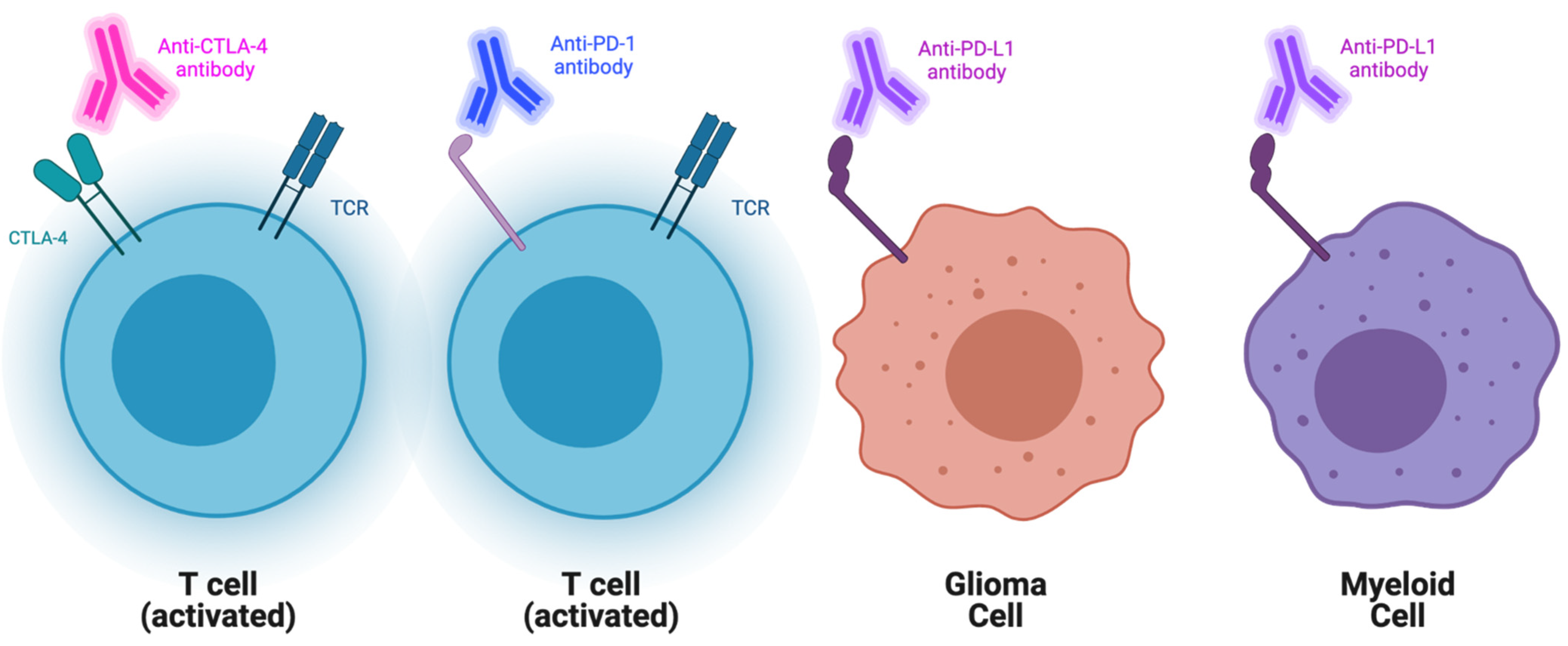
2.1. Checkpoint Blockade
2.2. Peptide-Based Vaccines
2.3. Cell-Based Vaccines
2.4. Oncolytic Viruses
2.5. Cytokine Therapy
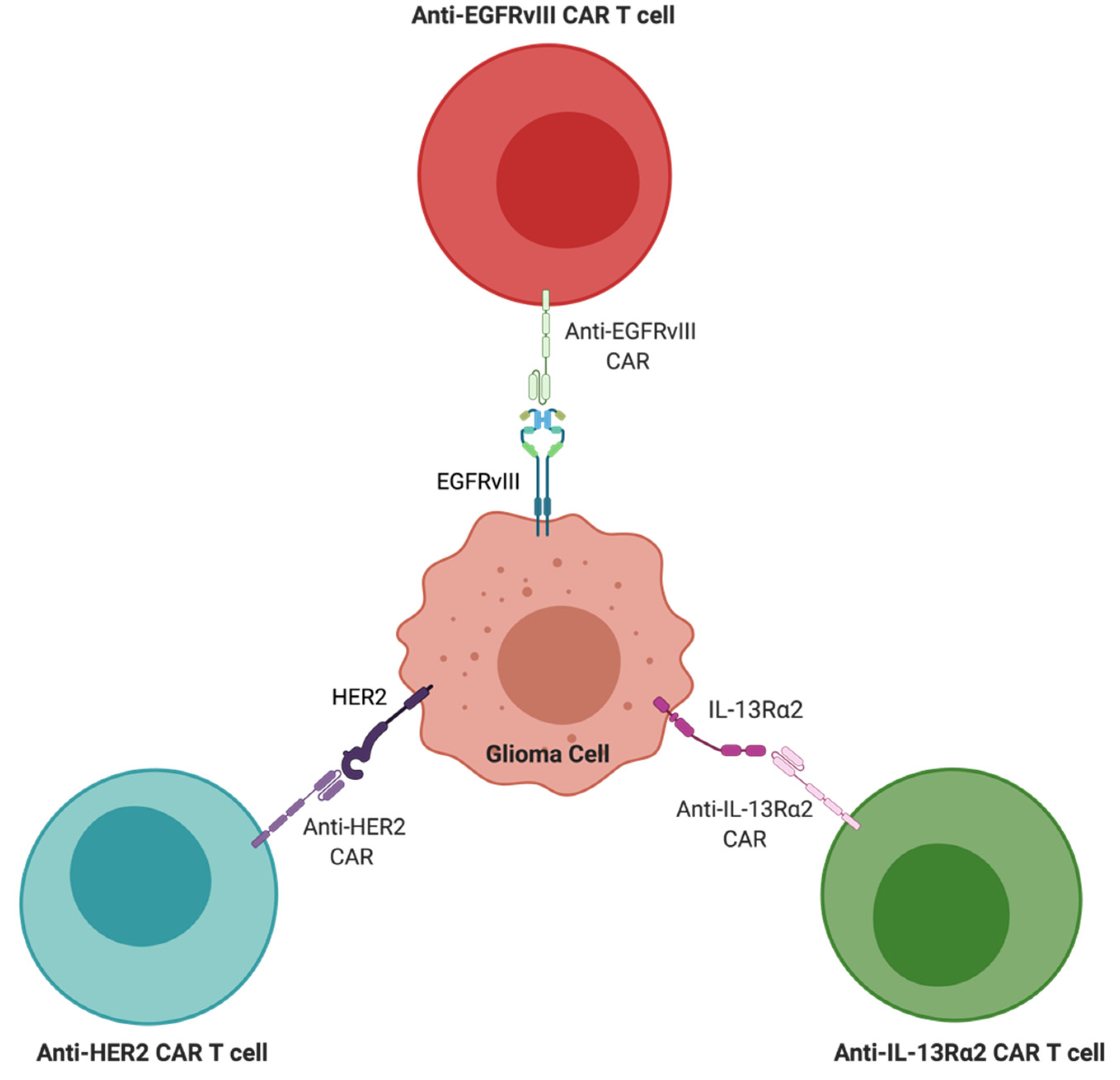
2.6. Chimeric Antigen Receptor (CAR) T-Cell Therapy
3. Conclusions and Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.-J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.-C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef]
- Cui, Y.; Tha, K.K.; Terasaka, S.; Yamaguchi, S.; Wang, J.; Kudo, K.; Xing, L.; Shirato, H.; Li, R. Prognostic Imaging Biomarkers in Glioblastoma: Development and independent validation on the basis of multiregion and quantitative analysis of MR images. Radiology 2015, 278, 546–553. [Google Scholar] [CrossRef]
- Diehn, M.; Nardini, C.; Wang, D.S.; McGovern, S.; Jayaraman, M.; Liang, Y.; Aldape, K.; Cha, S.; Kuo, M.D. Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc. Natl. Acad. Sci. USA 2008, 105, 5213–5218. [Google Scholar] [CrossRef]
- Ellingson, B.M. Radiogenomics and imaging phenotypes in glioblastoma: Novel observations and correlation with molecular characteristics. Curr. Neurol. Neurosci. Rep. 2015, 15, 506. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A Review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Sigaux, F.; Cantell, K.; Falcoff, E.; Boiron, M.; Flandrin, G.; Degos, L. Interferon Alpha in the Treatment of Hairy Cell Leukemia. Cancer 1986, 57, 1681–1684. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Atkins, M.B.; Subedi, P.; Wu, J.; Chambers, J.; Joseph Mattingly, T.; Campbell, J.D.; Allen, J.; Ferris, A.E.; Schilsky, R.L.; et al. The promise of immuno-oncology: Implications for defining the value of cancer treatment. J. ImmunoTherapy Cancer 2019, 7, 129. [Google Scholar] [CrossRef]
- Ventola, C.L. Cancer immunotherapy, Part 2: Efficacy, safety, and other clinical considerations. Pharm. Ther. 2017, 42, 452–463. [Google Scholar]
- Moalem, G.; Leibowitz-Amit, R.; Yoles, E.; Mor, F.; Cohen, I.R.; Schwartz, M. Autoimmune t cells protect neurons from secondary degeneration after central nervous system axotomy. Nat. Med. 1999, 5, 49–55. [Google Scholar] [CrossRef]
- Wraith, D.C.; Nicholson, L.B. The adaptive immune system in diseases of the central nervous system. J. Clin. Investig. 2012, 122, 1172–1179. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Donegá, M.; Giusto, E.; Mallucci, G.; Marchetti, B.; Pluchino, S. The role of the immune system in central nervous system plasticity after acute injury. Neuroscience 2014, 283, 210–221. [Google Scholar] [CrossRef]
- De Luca, C.; Colangelo, A.M.; Alberghina, L.; Papa, M. Neuro-immune hemostasis: Homeostasis and diseases in the central nervous system. Front. Cell. Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Almolda, B.; Gonzalez, B.; Castellano, B. Antigen presentation in EAE: Role of microglia, macrophages and dendritic cells. Front. Biosci. 2011, 16, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, P.M.; Gottfried-Blackmore, A.; Anandasabapathy, N.; Bulloch, K. Brain dendritic cells: Biology and pathology. Acta Neuropathol. 2012, 124, 599–614. [Google Scholar] [CrossRef]
- Yin, J.; Valin, K.L.; Dixon, M.L.; Leavenworth, J.W. The Role of Microglia and Macrophages in CNS Homeostasis, Autoimmunity, and Cancer. Available online: https://www.hindawi.com/journals/jir/2017/5150678/ (accessed on 23 April 2020).
- Malo, C.S.; Huggins, M.A.; Goddery, E.N.; Tolcher, H.M.A.; Renner, D.N.; Jin, F.; Hansen, M.J.; Pease, L.R.; Pavelko, K.D.; Johnson, A.J. Non-equivalent antigen presenting capabilities of dendritic cells and macrophages in generating brain-infiltrating CD8 + T Cell responses. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell 2018, 33, 581–598. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of checkmate 143. Neuro-Oncology 2018, 20, 674–686. [Google Scholar] [CrossRef]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.A.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. JCO 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef]
- Reardon, D.A.; Omuro, A.; Brandes, A.A.; Rieger, J.; Wick, A.; Sepulveda, J.; Phuphanich, S.; de Souza, P.; Ahluwalia, M.S.; Lim, M.; et al. OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: CheckMate 143. Neuro. Oncol. 2017, 19, iii21. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.-T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Tremelimumab and Durvalumab in Combination or Alone in Treating Patients with Recurrent Malignant Glioma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02794883 (accessed on 22 April 2020).
- Sanborn, R.E.; Pishvaian, M.J.; Kluger, H.M.; Callahan, M.K.; Weise, A.M.; Lutzky, J.; Yellin, M.J.; Rawls, T.; Vitale, L.; Halim, A.; et al. Clinical results with combination of anti-CD27 agonist antibody, varlilumab, with anti-PD1 antibody nivolumab in advanced cancer patients. JCO 2017, 35, 3007. [Google Scholar] [CrossRef]
- Carter, T.; Shaw, H.; Cohn-Brown, D.; Chester, K.; Mulholland, P. Ipilimumab and bevacizumab in glioblastoma. Clin. Oncol. 2016, 28, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Lukas, R.V.; Rodon, J.; Becker, K.; Wong, E.T.; Shih, K.; Touat, M.; Fassò, M.; Osborne, S.; Molinero, L.; O’Hear, C.; et al. Clinical Activity and safety of atezolizumab in patients with recurrent glioblastoma. J. Neurooncol. 2018, 140, 317–328. [Google Scholar] [CrossRef]
- Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; López-Janeiro, A.; Porciuncula, A.; Idoate, M.A.; Inogés, S.; de Andrea, C.; López-Diaz de Cerio, A.; Tejada, S.; et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 2019, 25, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Liu, K.; Bizargity, P.; Hancock, W.W.; Visner, G.A. Reduced cytotoxic function of effector CD8+ t cells is responsible for indoleamine 2,3-dioxygenase-dependent immune suppression. J. Immunol. 2009, 183, 1022–1031. [Google Scholar] [CrossRef]
- Baban, B.; Chandler, P.R.; Sharma, M.D.; Pihkala, J.; Koni, P.A.; Munn, D.H.; Mellor, A.L. IDO activates regulatory t cells and blocks their conversion into TH17-like t cells. J. Immunol. 2009, 183, 2475–2483. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Balyasnikova, I.V.; Chang, A.L.; Ahmed, A.U.; Moon, K.-S.; Auffinger, B.; Tobias, A.L.; Han, Y.; Lesniak, M.S. IDO Expression in brain tumors increases the recruitment of regulatory t cells and negatively impacts survival. Clin. Cancer Res 2012, 18, 6110–6121. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, S.; Li, T.; Liu, Y.-J.; Chen, W.; Chen, J. Targeting immune checkpoints in malignant glioma. Oncotarget 2016, 8, 7157–7174. [Google Scholar] [CrossRef]
- Jochems, C.; Fantini, M.; Fernando, R.I.; Kwilas, A.R.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Kim, Y.-S.; Brechbiel, M.W.; Gulley, J.L.; et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific t cells. Oncotarget 2016, 7, 37762–37772. [Google Scholar] [CrossRef]
- Zakharia, Y.; Johnson, T.S.; Colman, H.; Vahanian, N.N.; Link, C.J.; Kennedy, E.; Sadek, R.F.; Kong, F.M.; Vender, J.; Munn, D.; et al. A Phase I/II study of the combination of indoximod and temozolomide for adult patients with temozolomide-refractory primary malignant brain tumors. JCO 2014, 32, 2107. [Google Scholar] [CrossRef]
- A Study of the Safety, Tolerability, and Efficacy of Epacadostat Administered in Combination with Nivolumab in Select Advanced Cancers (ECHO-204)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02327078 (accessed on 22 April 2020).
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E.; McLendon, R.E.; Mitchell, D.A.; et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 4722–4729. [Google Scholar] [CrossRef]
- Sampson, J.H.; Aldape, K.D.; Archer, G.E.; Coan, A.; Desjardins, A.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E.; McLendon, R.E.; et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011, 13, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A Phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro-Oncology 2015, 17, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Oji, Y.; Ogawa, H.; Tamaki, H.; Oka, Y.; Tsuboi, A.; Kim, E.H.; Soma, T.; Tatekawa, T.; Kawakami, M.; Asada, M.; et al. Expression of the wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn. J. Cancer Res. 1999, 90, 194–204. [Google Scholar] [CrossRef]
- Menssen, H.D.; Bertelmann, E.; Bartelt, S.; Schmidt, R.A.; Pecher, G.; Schramm, K.; Thiel, E. Wilms’ Tumor Gene (WT1) Expression in lung cancer, colon cancer and glioblastoma cell lines compared to freshly isolated tumor specimens. J. Cancer Res. Clin. Oncol. 2000, 126, 226–232. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Ando, A.; Egawa, C.; Taguchi, T.; Tamaki, Y.; Tamaki, H.; Sugiyama, H.; Noguchi, S. High expression of wilms’ tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin. Cancer Res. 2002, 8, 1167–1171. [Google Scholar] [PubMed]
- Rauscher, J.; Beschorner, R.; Gierke, M.; Bisdas, S.; Braun, C.; Ebner, F.H.; Schittenhelm, J. WT1 Expression increases with malignancy and indicates unfavourable outcome in astrocytoma. J. Clin. Pathol. 2014, 67, 556–561. [Google Scholar] [CrossRef]
- Izumoto, S.; Tsuboi, A.; Oka, Y.; Suzuki, T.; Hashiba, T.; Kagawa, N.; Hashimoto, N.; Maruno, M.; Elisseeva, O.A.; Shirakata, T.; et al. Phase II clinical trial of wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J. Neurosurg. 2008, 108, 963–971. [Google Scholar] [CrossRef]
- Hashimoto, N.; Tsuboi, A.; Kagawa, N.; Chiba, Y.; Izumoto, S.; Kinoshita, M.; Kijima, N.; Oka, Y.; Morimoto, S.; Nakajima, H.; et al. Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: Safety and impact on immunological response. Cancer Immunol. Immunother. 2015, 64, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yang, P.; Wang, K.; Liu, Y.; Liu, X.; Shan, X.; Huang, R.; Zhang, K.; Wang, J. Survivin is a prognostic indicator in glioblastoma and may be a target of MicroRNA-218. Oncol. Lett. 2019, 18, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, M.J.; Apfel, L.; Barone, T.A.; Castro, C.A.; Weiss, T.C.; Fenstermaker, R.A. Antitumor effects of a xenogeneic survivin bone marrow derived dendritic cell vaccine against murine GL261 gliomas. Cancer Immunol. Immunother. 2006, 55, 1491–1503. [Google Scholar] [CrossRef]
- Fenstermaker, R.A.; Figel, S.A.; Qiu, J.; Barone, T.A.; Dharma, S.S.; Winograd, E.K.; Galbo, P.M.; Wiltsie, L.M.; Ciesielski, M.J. Survivin monoclonal antibodies detect survivin cell surface expression and inhibit tumor growth in vivo. Clin. Cancer Res. 2018, 24, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- SurVaxM Vaccine Therapy and Temozolomide in Treating Patients with Newly Diagnosed Glioblastoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02455557 (accessed on 7 August 2020).
- Platten, M.; Schilling, D.; Bunse, L.; Wick, A.; Bunse, T.; Riehl, D.; Green, E.; Sanghvi, K.; Karapanagiotou-Schenkel, I.; Harting, I.; et al. ATIM-33. NOA-16: A first-in-man multicenter phase i clinical trial of the german neurooncology working group evaluating a mutation-specific peptide vaccine targeting Idh1r132h in patients with newly diagnosed malignant astrocytomas. Neuro Oncol. 2018, 20, vi8–vi9. [Google Scholar] [CrossRef]
- IDH1 Peptide Vaccine for Recurrent Grade II Glioma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02193347 (accessed on 22 April 2020).
- Dutoit, V.; Herold-Mende, C.; Hilf, N.; Schoor, O.; Beckhove, P.; Bucher, J.; Dorsch, K.; Flohr, S.; Fritsche, J.; Lewandrowski, P.; et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012, 135, 1042–1054. [Google Scholar] [CrossRef]
- Rampling, R.; Peoples, S.; Mulholland, P.J.; James, A.; Al-Salihi, O.; Twelves, C.J.; McBain, C.; Jefferies, S.; Jackson, A.; Stewart, W.; et al. A cancer research UK first time in human phase i trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin. Cancer Res. 2016, 22, 4776–4785. [Google Scholar] [CrossRef]
- Peptide-Based Glioma Vaccine IMA950 in Patients with Glioblastoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01403285 (accessed on 7 August 2020).
- Stemline Therapeutics, Inc. A Phase 1/2 Study of SL-701, a Subcutaneously Injected Multivalent Glioma-Associated Antigen Vaccine, in Adult Patients with Recurrent Glioblastoma Multiforme (GBM); clinicaltrials.gov. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02078648 (accessed on 7 August 2020).
- Ampie, L.; Choy, W.; Lamano, J.B.; Fakurnejad, S.; Bloch, O.; Parsa, A.T. Heat shock protein vaccines against glioblastoma: From bench to bedside. J. Neurooncol. 2015, 123, 441–448. [Google Scholar] [CrossRef]
- Crane, C.A.; Han, S.J.; Ahn, B.; Oehlke, J.; Kivett, V.; Fedoroff, A.; Butowski, N.; Chang, S.M.; Clarke, J.; Berger, M.S.; et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin. Cancer Res. 2013, 19, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Crane, C.A.; Fuks, Y.; Kaur, R.; Aghi, M.K.; Berger, M.S.; Butowski, N.A.; Chang, S.M.; Clarke, J.L.; McDermott, M.W.; et al. Heat-shock protein peptide complex–96 vaccination for recurrent glioblastoma: A phase II, single-arm trial. Neuro Oncol. 2014, 16, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for melanoma patients. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; van der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral t cell responses in phase ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human dendritic cells: Their heterogeneity and clinical application potential in cancer immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- Rutkowski, S.; De Vleeschouwer, S.; Kaempgen, E.; Wolff, J.E.A.; Kühl, J.; Demaerel, P.; Warmuth-Metz, M.; Flamen, P.; Van Calenbergh, F.; Plets, C.; et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br. J. Cancer 2004, 91, 1656–1662. [Google Scholar] [CrossRef]
- Liau, L.M.; Prins, R.M.; Kiertscher, S.M.; Odesa, S.K.; Kremen, T.J.; Giovannone, A.J.; Lin, J.-W.; Chute, D.J.; Mischel, P.S.; Cloughesy, T.F.; et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial t-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 2005, 11, 5515–5525. [Google Scholar] [CrossRef]
- Wheeler, C.J.; Black, K.L.; Liu, G.; Mazer, M.; Zhang, X.; Pepkowitz, S.; Goldfinger, D.; Ng, H.; Irvin, D.; Yu, J.S. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008, 68, 5955–5964. [Google Scholar] [CrossRef]
- Yamanaka, R.; Homma, J.; Yajima, N.; Tsuchiya, N.; Sano, M.; Kobayashi, T.; Yoshida, S.; Abe, T.; Narita, M.; Takahashi, M.; et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: Results of a clinical phase I/II trial. Clin. Cancer Res. 2005, 11, 4160–4167. [Google Scholar] [CrossRef]
- Fadul, C.E.; Fisher, J.L.; Hampton, T.H.; Lallana, E.C.; Li, Z.; Gui, J.; Szczepiorkowski, Z.M.; Tosteson, T.D.; Rhodes, C.H.; Wishart, H.A.; et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J. Immunother 2011, 34, 382–389. [Google Scholar] [CrossRef]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic vaccination against autologous cancer stem cells with MRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef]
- Phuphanich, S.; Wheeler, C.J.; Rudnick, J.D.; Mazer, M.; Wang, H.; Nuño, M.A.; Richardson, J.E.; Fan, X.; Ji, J.; Chu, R.M.; et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2013, 62, 125–135. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.-J.; Glantz, M.; Peereboom, D.M.; et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef] [PubMed]
- Phase 3 Randomized, Double-Blind, Controlled Study of ICT-107 in Glioblastoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02546102 (accessed on 23 April 2020).
- Rapp, M.; Grauer, O.M.; Kamp, M.; Sevens, N.; Zotz, N.; Sabel, M.; Sorg, R.V. A randomized controlled Phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (gliovax): Study protocol for a randomized controlled trial. Trials 2018, 19, 1–14. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. first results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Q.; Wang, Q.R.; Zhao, X.; Yang, L.; Tian, L.; Lu, Y.; Kang, B.; Lu, C.J.; Huang, M.J.; Lou, Y.Y.; et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat. Med. 2000, 6, 1160–1166. [Google Scholar] [CrossRef]
- Zhou, L.; Si, C.; Li, D.; Lu, M.; Zhong, W.; Xie, Z.; Guo, L.; Zhang, S.; Xu, M. Assessment of in vivo anti-tumor activity of human umbilical vein endothelial cell vaccines prepared by various antigen forms. Eur. J. Pharm. Sci. 2018, 114, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Zhang, W.; Zhang, W.; Wu, S.; Bi, F.; Su, Y.-J.; Tan, X.-Y.; Liu, J.-N.; Zhang, J. Vaccination with viable human umbilical vein endothelial cells prevents metastatic tumors by attack on tumor vasculature with both cellular and humoral immunity. Clin. Cancer Res. 2006, 12, 5834–5840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, M.; Tsuno, N.H.; Fujii, T.; Todo, T.; Saito, N.; Takahashi, K. Human umbilical vein endothelial cell vaccine therapy in patients with recurrent glioblastoma. Cancer Sci. 2013, 104, 200–205. [Google Scholar] [CrossRef]
- Okaji, Y.; Tsuno, N.H.; Tanaka, M.; Yoneyama, S.; Matsuhashi, M.; Kitayama, J.; Saito, S.; Nagura, Y.; Tsuchiya, T.; Yamada, J.; et al. Pilot study of anti-angiogenic vaccine using fixed whole endothelium in patients with progressive malignancy after failure of conventional therapy. Eur. J. Cancer 2008, 44, 383–390. [Google Scholar] [CrossRef]
- Ishikawa, E.; Tsuboi, K.; Yamamoto, T.; Muroi, A.; Takano, S.; Enomoto, T.; Matsumura, A.; Ohno, T. clinical trial of autologous formalin-fixed tumor vaccine for glioblastoma multiforme patients. Cancer Sci. 2007, 98, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Muragaki, Y.; Maruyama, T.; Iseki, H.; Tanaka, M.; Shinohara, C.; Takakura, K.; Tsuboi, K.; Yamamoto, T.; Matsumura, A.; Matsutani, M.; et al. Phase I/IIa trial of autologous formalin-fixed tumor vaccine concomitant with fractionated radiotherapy for newly diagnosed glioblastoma. clinical article. J. Neurosurg. 2011, 115, 248–255. [Google Scholar] [CrossRef]
- Muragaki, Y.; Maruyama, T.; Ishikawa, E.; Nitta, M.; Ikuta, S.; Yamamoto, T.; Tsuboi, K.; Matsumura, A.; Nakamura, H.; Kuroda, J.; et al. OS09.8 Randomized Placebo-Controlled Trial of Autologous Formalin-Fixed Tumor Vaccine for Newly Diagnosed Glioblastoma. Neuro Oncol. 2017, 19, iii20. [Google Scholar] [CrossRef]
- Cobbs, C.S.; Harkins, L.; Samanta, M.; Gillespie, G.Y.; Bharara, S.; King, P.H.; Nabors, L.B.; Cobbs, C.G.; Britt, W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar] [PubMed]
- Mitchell, D.A.; Xie, W.; Schmittling, R.; Learn, C.; Friedman, A.; McLendon, R.E.; Sampson, J.H. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology 2008, 10, 10–18. [Google Scholar] [CrossRef]
- Prins, R.M.; Cloughesy, T.F.; Liau, L.M. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N. Engl. J. Med. 2008, 359, 539–541. [Google Scholar] [CrossRef]
- Dziurzynski, K.; Chang, S.M.; Heimberger, A.B.; Kalejta, R.F.; McGregor Dallas, S.R.; Smit, M.; Soroceanu, L.; Cobbs, C.S. HCMV and gliomas symposium consensus on the role of human cytomegalovirus in glioblastoma. Neuro-Oncology 2012, 14, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Clark, P.A.; Kuo, J.S.; Salamat, M.S.; Kalejta, R.F. Significant association of multiple human cytomegalovirus genomic loci with glioblastoma multiforme samples. J. Virol. 2012, 86, 854–864. [Google Scholar] [CrossRef]
- Nair, S.K.; De Leon, G.; Boczkowski, D.; Schmittling, R.; Xie, W.; Staats, J.; Liu, R.; Johnson, L.A.; Weinhold, K.; Archer, G.E.; et al. Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus Pp65-specific cytotoxic t cells. Clin. Cancer Res. 2014, 20, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E.; Healy, P.; et al. Long-term survival in glioblastoma with cytomegalovirus Pp65-targeted vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology 2018, 20, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Tocagen Inc. A Phase 2/3 Randomized, Open-Label Study of Toca 511, a Retroviral Replicating Vector, Combined with Toca FC Versus Standard of Care in Subjects Undergoing Planned Resection for Recurrent Glioblastoma or Anaplastic Astrocytoma; clinicaltrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02414165 (accessed on 23 April 2020).
- Cavanagh, T.T.; Holle, L.M. Potential new gene therapy option with sitimagene ceradenovec for newly diagnosed patients with glioblastoma multiforme. Cancer Biol. 2014, 15, 263–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Floros, T.; Tarhini, A.A. Anticancer cytokines: Biology and clinical effects of IFN-A2, IL-2, IL-15, IL-21, and IL-12. Semin. Oncol. 2015, 42, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2018, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef]
- Rini, B.I.; Halabi, S.; Rosenberg, J.E.; Stadler, W.M.; Vaena, D.A.; Archer, L.; Atkins, J.N.; Picus, J.; Czaykowski, P.; Dutcher, J.; et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J. Clin. Oncol. 2010, 28, 2137–2143. [Google Scholar] [CrossRef]
- Escudier, B.; Bellmunt, J.; Négrier, S.; Bajetta, E.; Melichar, B.; Bracarda, S.; Ravaud, A.; Golding, S.; Jethwa, S.; Sneller, V. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J. Clin. Oncol. 2010, 28, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Barzon, L.; Franchin, E.; Pacenti, M.; Pinna, V.; Danieli, D.; Zanusso, M.; Palù, G. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: Biological and clinical results. Cancer Gene Ther. 2005, 12, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Lieberman, F.S.; Walter, K.A.; Lunsford, L.D.; Kondziolka, D.S.; Bejjani, G.K.; Hamilton, R.L.; Torres-Trejo, A.; Kalinski, P.; Cai, Q.; et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J. Transl. Med. 2007, 5, 67. [Google Scholar] [CrossRef]
- Weber, F.; Asher, A.; Bucholz, R.; Berger, M.; Prados, M.; Chang, S.; Bruce, J.; Hall, W.; Rainov, N.G.; Westphal, M.; et al. Safety, tolerability, and tumor response of il4-pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 2003, 64, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: Presentation of interim findings from ongoing phase 1 studies. Acta Neurochir. Suppl. 2003, 88, 105–111. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs gliadel wafers for recurrent glioblastoma. Neuro-Oncology 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.D.; Puduvalli, V.K.; Gilbert, M.R.; Levin, V.A.; Conrad, C.A.; Liu, V.H.; Hunter, K.; Meyers, C.; Hess, K.R.; Alfred Yung, W.K. Two phase II Trials of temozolomide with interferon-alpha2b (pegylated and non-pegylated) in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2009, 101, 615–620. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Natsume, A.; Hashizume, Y.; Fujii, M.; Mizuno, M.; Yoshida, J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: Novel findings from gene expression profiling and autopsy. J. Gene Med. 2008, 10, 329–339. [Google Scholar] [CrossRef]
- Färkkilä, M.; Jääskeläinen, J.; Kallio, M.; Blomstedt, G.; Raininko, R.; Virkkunen, P.; Paetau, A.; Sarelin, H.; Mäntylä, M. Randomised, controlled study of intratumoral recombinant gamma-interferon treatment in newly diagnosed glioblastoma. Br. J. Cancer 1994, 70, 138–141. [Google Scholar] [CrossRef][Green Version]
- Wong, A.J.; Ruppert, J.M.; Bigner, S.H.; Grzeschik, C.H.; Humphrey, P.A.; Bigner, D.S.; Vogelstein, B. structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc. Natl Acad Sci USA 1992, 89, 2965–2969. [Google Scholar] [CrossRef]
- Moscatello, D.K.; Ramirez, G.; Wong, A.J. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997, 57, 1419–1424. [Google Scholar] [PubMed]
- Gan, H.K.; Kaye, A.H.; Luwor, R.B. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 2009, 16, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Scholler, J.; Ohkuri, T.; Kosaka, A.; Patel, P.R.; McGettigan, S.E.; Nace, A.K.; Dentchev, T.; Thekkat, P.; Loew, A.; et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor t cells for glioblastoma. Sci. Transl. Med. 2015, 7, 275ra22. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR t cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Obiri, N.I.; Powers, S.K.; Pastan, I.; Puri, R.K. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin. Cancer Res. 1995, 1, 1253–1258. [Google Scholar] [PubMed]
- Sengupta, S.; Thaci, B.; Crawford, A.C.; Sampath, P. Interleukin-13 receptor alpha 2-targeted glioblastoma immunotherapy. Biomed. Res. Int. 2014, 2014, 952128. [Google Scholar] [CrossRef]
- Rahaman, S.O.; Sharma, P.; Harbor, P.C.; Aman, M.J.; Vogelbaum, M.A.; Haque, S.J. IL-13Rα2, a decoy receptor for IL-13 acts as an inhibitor of il-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002, 62, 1103–1109. [Google Scholar]
- Newman, J.P.; Wang, G.Y.; Arima, K.; Guan, S.P.; Waters, M.R.; Cavenee, W.K.; Pan, E.; Aliwarga, E.; Chong, S.T.; Kok, C.Y.L.; et al. Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat. Commun 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Jarboe, J.S.; Johnson, K.R.; Choi, Y.; Lonser, R.R.; Park, J.K. Expression of interleukin-13 receptor α2 in glioblastoma multiforme: Implications for targeted therapies. Cancer Res. 2007, 67, 7983–7986. [Google Scholar] [CrossRef]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of interleukin-13 receptor alpha 2–targeted glioblastoma therapy. Neuro. Oncol. 2014, 16, 1304–1312. [Google Scholar] [CrossRef]
- Hershey, G.K.K. IL-13 receptors and signaling pathways: An evolving web. J. Allergy Clin. Immunol. 2003, 111, 677–690. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Clements, V.; Dissanayake, S.I.; Miller, S.; Davis, C.; Danna, E. signal transducer and activator of transcription 6 (Stat6) and CD1: Inhibitors of immunosurveillance against primary tumors and metastatic disease. Cancer Immunol. Immunother. 2004, 53, 86–91. [Google Scholar] [CrossRef]
- Akhavan, D.; Alizadeh, D.; Wang, D.; Weist, M.R.; Shepphird, J.K.; Brown, C.E. CAR T Cells for brain tumors: Lessons learned and road ahead. Immunol. Rev. 2019, 290, 60–84. [Google Scholar] [CrossRef] [PubMed]
- IL13Ralpha2-Targeted Chimeric Antigen Receptor (CAR) T Cells with or without Nivolumab and Ipilimumab in Treating Patients With Recurrent or Refractory Glioblastoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04003649 (accessed on 18 May 2020).
- Liu, G.; Ying, H.; Zeng, G.; Wheeler, C.J.; Black, K.L.; Yu, J.S. HER-2, Gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic t cells. Cancer Res. 2004, 64, 4980–4986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Kruse, C.A.; Driggers, L.; Hoa, N.; Wisoff, J.; Allen, J.C.; Zagzag, D.; Newcomb, E.W.; Jadus, M.R. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J. Neurooncol. 2008, 88, 65–76. [Google Scholar] [CrossRef]
- Haynik, D.M.; Roma, A.A.; Prayson, R.A. HER-2/neu expression in glioblastoma multiforme. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 56–58. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-specific chimeric antigen receptor-modified virus-specific t cells for progressive glioblastoma: A phase 1 dose-escalation trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.H.; et al. Tandem CAR T Cells Targeting HER2 and IL13Rα2 Mitigate Tumor Antigen Escape. J. Clin. Investig. 2016, 126, 3036–3052. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, K.; Fousek, K.; Byrd, T.T.; Samaha, H.; Mukherjee, M.; Aware, N.; Wu, M.-F.; Orange, J.S.; Sumazin, P.; Man, T.-K.; et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro-Oncology 2018, 20, 506–518. [Google Scholar] [CrossRef]
- CytoVac A/S An Open Label, Randomised, Phase II Study to Investigate the Efficacy and Safety of ALECSAT Treatment as an Add-on Therapy to Radiotherapy and Temozolomide in Patients with Newly Diagnosed Glioblastoma. clinicaltrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02799238 (accessed on 21 April 2020).
- Wenger, A.; Werlenius, K.; Hallner, A.; Bergh Thorén, F.; Farahmand, D.; Tisell, M.; Smits, A.; Rydenhag, B.; Jakola, A.S.; Carén, H. Determinants for effective ALECSAT immunotherapy treatment on autologous patient-derived glioblastoma stem cells. Neoplasia 2017, 20, 25–31. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, T.-S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef]
- Sangiolo, D.; Martinuzzi, E.; Todorovic, M.; Vitaggio, K.; Vallario, A.; Jordaney, N.; Carnevale-Schianca, F.; Capaldi, A.; Geuna, M.; Casorzo, L.; et al. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: Implications for their infusion across major HLA barriers. Int. Immunol. 2008, 20, 841–848. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.G.; Negrin, R.S.; Kiem, H.P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef]
- Garofano, F.; Gonzalez-Carmona, M.A.; Skowasch, D.; Schmidt-Wolf, R.; Abramian, A.; Hauser, S.; Strassburg, C.P.; Schmidt-Wolf, I.G.H. Clinical trials with combination of cytokine-induced killer cells and dendritic cells for cancer therapy. Int. J. Mol. Sci. 2019, 20, 4307. [Google Scholar] [CrossRef]
- Mesiano, G.; Todorovic, M.; Gammaitoni, L.; Leuci, V.; Diego, L.G.; Carnevale-Schianca, F.; Fagioli, F.; Piacibello, W.; Aglietta, M.; Sangiolo, D. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin. Biol. Ther. 2012, 12, 673–684. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.-H.; Lim, Y.-S.; Yeon, J.E.; Song, T.-J.; Yu, S.J.; Gwak, G.-Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015, 148, 1383–1391.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, L.; Zhang, Y.; Wang, Y.; Lu, X.; Shi, F.; Liu, Y.; Chen, M.; Feng, K.; Zhang, W.; et al. Analysis of adverse events following the treatment of autologous cytokine-induced killer cells for adoptive immunotherapy in malignant tumour sufferers. Expert Opin. Biol. Ther. 2015, 15, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-S.; Nam, D.-H.; Kang, S.-H.; Lee, J.W.; Chang, J.-H.; Kim, J.-H.; Lim, Y.-J.; Koh, Y.-C.; Chung, Y.-G.; Kim, J.-M.; et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget 2016, 8, 7003–7013. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb Announces Phase 3 CheckMate-498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (Nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme|BMS Newsroom. Available online: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did (accessed on 21 April 2020).
- Bristol-Myers Squibb Provides Update on Phase 3 Opdivo (Nivolumab) CheckMate -548 Trial in Patients with Newly Diagnosed MGMT-Methylated Glioblastoma Multiforme|BMS Newsroom. Available online: https://news.bms.com/news/corporate-financial/2019/Bristol-Myers-Squibb-Provides-Update-on-Phase-3-Opdivo-nivolumab-CheckMate--548-Trial-in-Patients-with-Newly-Diagnosed-MGMT-Methylated-Glioblastoma-Multiforme/default.aspx (accessed on 21 April 2020).
- Reardon, D.A.; Kaley, T.J.; Dietrich, J.; Clarke, J.L.; Dunn, G.; Lim, M.; Cloughesy, T.F.; Gan, H.K.; Park, A.J.; Schwarzenberger, P.; et al. Phase II study to evaluate safety and efficacy of MEDI4736 (Durvalumab) + radiotherapy in patients with newly diagnosed unmethylated MGMT glioblastoma (new unmeth GBM). JCO 2019, 37, 2032. [Google Scholar] [CrossRef]
- An Expanded Access Program of Ipilimumab for Patients with Glioblastomas and Gliomas—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03460782 (accessed on 23 April 2020).
- Study of IDO Inhibitor and Temozolomide for Adult Patients with Primary Malignant Brain Tumors—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02052648 (accessed on 23 April 2020).
- Ipilimumab and/or Nivolumab in Combination with Temozolomide in Treating Patients with Newly Diagnosed Glioblastoma or Gliosarcoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02311920 (accessed on 23 April 2020).
- Celldex Therapeutics a Phase l/Ll Dose Escalation and Cohort Expansion Study of the Safety, Tolerability and Efficacy of Anti-CD27 Antibody (Varlilumab) Administered in Combination with Anti-PD-1 (Nivolumab) in Advanced Refractory Solid Tumors; clinicaltrials.gov. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT02335918 (accessed on 22 April 2020).
- Anti-LAG-3 Alone & in Combination w/ Nivolumab Treating Patients w/ Recurrent GBM (Anti-CD137 Arm Closed 10/16/18)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02658981 (accessed on 22 April 2020).
- Alliance for Clinical Trials in Oncology a Phase II Randomized Trial Comparing the Efficacy of Heat Shock Protein-Peptide Complex-96 (HSPPC-96) (NSC #725085, ALLIANCE IND # 15380) Vaccine Given with Bevacizumab Versus Bevacizumab Alone in the Treatment of Surgically Resectable Recurrent Glioblastoma Multiforme (GBM); clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT01814813 (accessed on 22 April 2020).
- Burzynski, S.R.; Janicki, T.J.; Burzynski, G.S. A phase II study of antineoplastons A10 and AS2-1 in adult patients with recurrent glioblastoma multiforme: Final report (protocol BT-21). J. Cancer Ther. 2014, 2014. [Google Scholar] [CrossRef]
- A Study of Ad-RTS-HIL-12 with Veledimex in Subjects with Glioblastoma or Malignant Glioma | Brain Tumor Center. Available online: https://braintumorcenter.ucsf.edu/clinical-trial/study-ad-rts-hil-12-veledimex-subjects-glioblastoma-or-malignant-glioma (accessed on 18 May 2020).
- Curry, W.T.; Gorrepati, R.; Piesche, M.; Sasada, T.; Agarwalla, P.; Jones, P.S.; Gerstner, E.R.; Golby, A.J.; Batchelor, T.T.; Wen, P.Y.; et al. Vaccination with irradiated autologous tumor cells mixed with irradiated GM-K562 cells stimulates antitumor immunity and t lymphocyte activation in patients with recurrent malignant glioma. Clin. Cancer Res. 2016, 22, 2885–2896. [Google Scholar] [CrossRef]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ t cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- Krenciute, G.; Prinzing, B.L.; Yi, Z.; Wu, M.-F.; Liu, H.; Dotti, G.; Balyasnikova, I.V.; Gottschalk, S. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR t cells but results in antigen loss variants. Cancer Immunol. Res. 2017, 5, 571–581. [Google Scholar] [CrossRef]
- Pombo Antunes, A.R.; Scheyltjens, I.; Duerinck, J.; Neyns, B.; Movahedi, K.; Van Ginderachter, J.A. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. eLife 2020, 9, e52176. [Google Scholar] [CrossRef] [PubMed]
- Ugel, S.; De Sanctis, F.; Mandruzzato, S.; Bronte, V. Tumor-induced myeloid deviation: When myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Invest. 2015, 125, 3365–3376. [Google Scholar] [CrossRef]
- Gieryng, A.; Kaminska, B. Myeloid-derived suppressor cells in gliomas. Contemp Oncol. (Pozn.) 2016, 20, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Won, W.-J.; Deshane, J.S.; Leavenworth, J.W.; Oliva, C.R.; Griguer, C.E. Metabolic and functional reprogramming of myeloid-derived suppressor cells and their therapeutic control in glioblastoma. Cell Stress 2019, 3, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, R.; Liu, M.; Feng, J.; Chen, J.; Hu, K. Remodeling the blood–brain barrier microenvironment by natural products for brain tumor therapy. Acta Pharm. Sin. B 2017, 7, 541–553. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef]
- Feng, X.; Szulzewsky, F.; Yerevanian, A.; Chen, Z.; Heinzmann, D.; Rasmussen, R.D.; Alvarez-Garcia, V.; Kim, Y.; Wang, B.; Tamagno, I.; et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget 2015, 6, 15077–15094. [Google Scholar] [CrossRef]
- Held-Feindt, J.; Hattermann, K.; Müerköster, S.S.; Wedderkopp, H.; Knerlich-Lukoschus, F.; Ungefroren, H.; Mehdorn, H.M.; Mentlein, R. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Exp. Cell Res. 2010, 316, 1553–1566. [Google Scholar] [CrossRef]
- Lee, S.; Jilani, S.M.; Nikolova, G.V.; Carpizo, D.; Iruela-Arispe, M.L. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 2005, 169, 681–691. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, A.; diPierro, C.G.; Carpenter, J.E.; Abdel-Fattah, R.; Redpath, G.T.; Lopes, M.-B.S.; Hussaini, I.M. An extensive invasive intracranial human glioblastoma xenograft model: Role of high level matrix metalloproteinase 9. Am. J. Pathol. 2010, 176, 3032–3049. [Google Scholar] [CrossRef]
- Xue, Q.; Cao, L.; Chen, X.-Y.; Zhao, J.; Gao, L.; Li, S.-Z.; Fei, Z. High expression of MMP9 in glioma affects cell proliferation and is associated with patient survival rates. Oncol. Lett. 2017, 13, 1325–1330. [Google Scholar] [CrossRef]
- Ye, X.; Xu, S.; Xin, Y.; Yu, S.; Ping, Y.; Chen, L.; Xiao, H.; Wang, B.; Yi, L.; Wang, Q.; et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-Β1 signaling pathway. J. Immunol. 2012, 189, 444–453. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, F.; Zheng, X.; Katakowski, M.; Buller, B.; To, S.-S.T.; Chopp, M. TGF-Β1 promotes motility and invasiveness of glioma cells through activation of ADAM17. Oncol. Rep. 2011, 25, 1329–1335. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chadalavada, K.; Wilshire, J.; Kowalik, U.; Hovinga, K.E.; Geber, A.; Fligelman, B.; Leversha, M.; Brennan, C.; Tabar, V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010, 468, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Zhang, D.; Wang, C.; Tao, J.; Tie, X.; Qiao, Y.; Xu, K.; Wang, Y.; Wu, A. IL-10 and TGF-Β2 are overexpressed in tumor spheres cultured from human gliomas. Mol. Biol. Rep. 2011, 38, 3585–3591. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ma, L.; Yi, D.; Yoon, J.; Diercks, A.; Foltz, G.; Price, N.D.; Hood, L.E.; Tian, Q. A CD133-related gene expression signature identifies an aggressive glioblastoma subtype with excessive mutations. Proc. Natl. Acad. Sci. USA 2011, 108, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- McCord, A.M.; Jamal, M.; Williams, E.S.; Camphausen, K.; Tofilon, P.J. CD133+ glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin. Cancer Res. 2009, 15, 5145–5153. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Aoyagi, M.; Wakimoto, H.; Ando, N.; Nariai, T.; Yamamoto, M.; Ohno, K. Accumulation of CD133-positive glioma cells after high-dose irradiation by gamma knife surgery plus external beam radiation. J. Neurosurg. 2010, 113, 310–318. [Google Scholar] [CrossRef]
- Humphries, W.; Wei, J.; Sampson, J.H.; Heimberger, A.B. The Role of tregs in glioma-mediated immunosuppression: Potential target for intervention. Neurosurg. Clin. N. Am. 2010, 21, 125–137. [Google Scholar] [CrossRef]
- Dieckmann, D.; Plottner, H.; Berchtold, S.; Berger, T.; Schuler, G. Ex vivo isolation and characterization of CD4(+)CD25(+) t cells with regulatory properties from human blood. J. Exp. Med. 2001, 193, 1303–1310. [Google Scholar] [CrossRef]
- Fecci, P.E.; Mitchell, D.A.; Whitesides, J.F.; Xie, W.; Friedman, A.H.; Archer, G.E.; Herndon, J.E.; Bigner, D.D.; Dranoff, G.; Sampson, J.H. Increased regulatory t-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006, 66, 3294–3302. [Google Scholar] [CrossRef]
- Han, J.; Alvarez-Breckenridge, C.A.; Wang, Q.-E.; Yu, J. TGF-β signaling and its targeting for glioma treatment. Am. J. Cancer Res. 2015, 5, 945–955. [Google Scholar]
- Andaloussi, A.E.; Lesniak, M.S. An increase in CD4+CD25+FOXP3+ regulatory t cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-Oncology 2006, 8, 234–243. [Google Scholar] [CrossRef]
- Chongsathidkiet, P.; Jackson, C.; Koyama, S.; Loebel, F.; Cui, X.; Farber, S.H.; Woroniecka, K.; Elsamadicy, A.A.; Dechant, C.A.; Kemeny, H.R.; et al. Sequestration of t cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 2018, 24, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Ye, X.; Lesser, G.; Sloan, A.; Carraway, H.; Desideri, S.; Piantadosi, S. Immunosuppression in patients with high grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 2011, 17, 5473–5480. [Google Scholar] [CrossRef]
- Pitter, K.L.; Tamagno, I.; Alikhanyan, K.; Hosni-Ahmed, A.; Pattwell, S.S.; Donnola, S.; Dai, C.; Ozawa, T.; Chang, M.; Chan, T.A.; et al. Corticosteroids compromise survival in glioblastoma. Brain 2016, 139, 1458–1471. [Google Scholar] [CrossRef]
- Woroniecka, K.; Fecci, P.E. T-Cell Exhaustion in glioblastoma. Oncotarget 2018, 9, 35287–35288. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Cox, M.A.; Zajac, A.J. T-Cell Exhaustion: Characteristics, causes and conversion. Immunology 2010, 129, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Friedmann-Morvinski, D. Glioblastoma heterogeneity and cancer cell plasticity. Crit. Rev. Oncog 2014, 19, 327–336. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Mooney, J.H.; Ilyas, A.; Chagoya, G.; Estevez-Ordonez, D.; Ibrahim, A.; Nakano, I. Molecular and cellular intratumoral heterogeneity in primary glioblastoma: Clinical and translational implications. J. Neurosurg. 2019, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Skaga, E.; Kulesskiy, E.; Fayzullin, A.; Sandberg, C.J.; Potdar, S.; Kyttälä, A.; Langmoen, I.A.; Laakso, A.; Gaál-Paavola, E.; Perola, M.; et al. Intertumoral heterogeneity in patient-specific drug sensitivities in treatment-naïve glioblastoma. BMC Cancer 2019, 19, 628. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor antigen escape from CAR T-Cell therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Vandenberk, L.; Woensel, M.; Belmans, J.; Schaaf, M.; Boon, L.; De Vleeschouwer, S.; Agostinis, P. Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma. OncoImmunology 2017, 6, e1295903. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Zamora, A.E.; Crawford, J.C.; Thomas, P.G. Hitting the target: How t cells detect and eliminate tumors. J. Immunol. 2018, 200, 392–399. [Google Scholar] [CrossRef]
- McGranahan, T.; Li, G.; Nagpal, S. History and current state of immunotherapy in glioma and brain metastasis. Adv. Med. Oncol. 2017, 9, 347–368. [Google Scholar] [CrossRef] [PubMed]
| Class | Target | Intervention | Comments | References |
|---|---|---|---|---|
| Checkpoint Inhibitor | PD-1 & CTLA-4 | Treatment Arm 1: nivolumab Treatment Arm 2: nivolumab + ipilimumab | Overall survival (OS) at 6 months was 75% among the 20 treated patients; promising compared to historical controls | Omuro, A; Vlahovic, G; Lim, M; et al. [28] |
| Checkpoint Inhibitor | PD-1 | Nivolumab + RT | Discontinued due to inability to meet primary endpoint | Bristol Meyer Squibb press release [151] |
| Checkpoint Inhibitor | PD-1 | Nivolumab | No statistically significant improvement in PFS noted | Bristol Meyer Squibb press release [152] |
| Checkpoint Inhibitor | PD-1 | Neoadjuvant Nivolumab | Increased immune cell infiltration and T-cell receptor clonal diversity; no clear benefit shown following salvage surgery | Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; et al. [38] |
| Checkpoint Inhibitor | PD-1 | Treatment Arm 1: Neoadjuvant pembrolizumab with continued adjuvant therapy following surgery Treatment Arm 2: Adjuvant pembrolizumab following surgery only | Prolonged overall survival was found to be statically significant in the neoadjuvant group | Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R. et al. [39] |
| Checkpoint Inhibitor | PD-L1 | Durvalumab in addition to the radiotherapy (60 Grays over 30 fractions) followed by durva monotherapy | Median OS was 15.1 months with OS of 12 months seen in 60% of patients from this study | Reardon, D; Kaley, T; Dietrich, J; et al. [153] |
| Checkpoint Inhibitor | CTLA-4 | Adjuvant Ipilimumab | Ongoing | Clinical Trial NCT03460782 [154] |
| Checkpoint Inhibitor | IDO | Indoximod + standard of care | Ongoing | Clinical Trial NCT02052648 [155] |
| Checkpoint Inhibitor | PD-1 & CTLA-4 | Treatment Arm 1: Ipilimumab Treatment Arm 2: Nivolumab Treatment Arm 3: Ipilimumab + Nivolumab | Ongoing | Clinical Trial NCT02311920 [156] |
| Checkpoint Inhibitor | PD1 & CTLA-4 | Treatment Arm 1: Adjuvant trememlimumab Treatment Arm 2: Adjuvant durvalumab Treatment Arm 3: Adjuvant trememlimumab + durvalumab | Ongoing | Clinical Trial NCT02794883 [34] |
| Checkpoint Inhibitor | PD-1 & anti-CD27 | Varlilumab + nivolumab | Ongoing | Clinical Trial NCT02335918 [157] |
| Checkpoint Inhibitor | IDO-1 | Epacadostat + Nivolumab | Ongoing | Clinical Trial NCT02327078 [46] |
| Checkpoint Inhibitor | Anti-LAG-3 & anti-CD137 & PD-1 | Treatment Arm 1: BMS-986016 Treatment Arm 2: Urelumab Treatment Arm 3: BMS-986016 + Nivolumab Treatment Arm 4: Urelumab + Nivolumab | Ongoing | Clinical Trial NCT02658981 [158] |
| Peptide-based Vaccine | EGFRvIII | ACTIVATE: Investigated use of rindopepimut alone with standard of care ACT II and ACT III: Investigated use of rindopepimut with adjuvant TMZ ACT IV: rindopepimut + standard of care in patients with minimal residual disease | ACT II and ACT III: PFS of roughly 15 months from time of diagnosis and OS of 24 months compared to cohort that received standard of care ACT IV: No significant difference in OS | Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; et al. [47] Sampson, J.H.; A, K.D.; Archer, G.E.; et al. [48] Schuster, J; Lai, R.K.; Recht, L.D.; et al. [49] Weller, M; Butowski, N; Tran, D.D.; et al. [50] |
| Peptide-based Vaccine | Tumor associated antigens | Treatment Arm 1: ICT-107 Treatment Arm 2: Standard of care | Trial suspended due to lack of funding | Clinical Trial NCT02546102 [84] |
| Peptide-based Vaccine | IDH1 | Safety and feasibility trial | Ongoing | Clinical Trial NCT02454634 [61] Clinical trial NCT02193347 [62] |
| Peptide-based Vaccine | Neoantigen vaccine (APVAC1 & APVAC2) | Safety and feasibility trial | The 15 vaccinated patients had a median OS of 29 months | Keskin, D.B.; Anandappa, A.J.; Sun, J.; et al. [74] |
| Peptide-based Vaccine | Heat Shocked Proteins | HSPPC-96 vaccine Treatment Arm 1: HSPPC-96 with concomitant bevacizumab Treatment Arm 2: HSPPC-96 with administration of bevacizumab at tumor progression | Ongoing | Clinical Trial NCT01814813 [159] |
| Peptide-based Vaccine | Tumor Associated Antigens: Antineoplastons | Treatment Arm 1: Radiation and chemotherapy alone Treatment Arm 2: antineoplaston | In the RGBM population, 6.7% of patients had complete responses; 6.7% had partial responses, with 16.7% PFS seen at 6 months Overall survival for the RGBM group was 34.7% at one year and 3.47% at 2, 5, and 10 years In the ERGBM cohort, complete responses occurred in 8.3% of patients; partial response occurred in 8.3% of patients Progression-free survival at 6 months was 20.8%, overall survival was 39.3% at 1 year and 4.4% at 2, 5, and 10 years | Burzynski, S; Janicki, T; and Burzynski, G. [160] |
| Peptide-based Vaccine | APVAC1 & 2: which targeted (HLA)-A*02:01 or HLA-A*24:02 neoantigens | APVAC1&2 vaccines | APVAC1 vaccines were able to elicit sustained CD8+ T-cell response, while the APVAC2 elicited a CD4+ T-cell response; both vaccines were well tolerated | Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; et al. [73] |
| Peptide-based Vaccine | CMV protein-pp65 antigen | CMV pp65-specific dendritic cells (pp65-DCs) when combined with vaccine site pre-conditioning using tetanus-diphtheria toxoid | Highlighted that this treatment modality enhanced antigen-specific immunity and increased long-term PFS and OS Profound lymphopenia and increased Treg proportions following DI-TMX | Batich, K.A.; Reap, E.A.; Archer, G.E.; et al. [101] |
| Peptide-based Vaccine | Ad-RTS-hiL-12 | Ad-RTS-hiL-12 is a non-pathogenic form of an adenovirus that has been genetically modified to encode the IL-12 protein; Veledimex serves as an oral ligand activator for IL-12. | Ongoing | Clinical Trial NCT03636477 [161] |
| Cell-based Vaccine | Autologous glioma cells | Autologous glioma cells mixed with irradiated GM-K562 cells | T- lymphocyte activation with significant increased expression of PD-1 and 4-1BB by CD8+ cells and CTLA-4, PD-1, 4-1BB, and OX40 by CD4+ cells Vaccination resulted in increased frequency of regulatory CD4+ T-lymphocytes | Curry, W; Gorrepati, R; Piesche, M; et al. [162] |
| Cell-based Vaccine | Autologous glioma cells | DCV vaccine | Statistically significant median overall survival seen in treatment group (480 vs. 400 days) | Yamanaka, R.; Homma, J.; Yajima, N.; et al. [79] |
| Cell-based Vaccine | Autologous glioma cells | Gliovax vaccine | Ongoing | Rapp, M.; Grauer, O.M.; Kamp, M.; et al. [85] |
| Cell-based Vaccine | Autologous glioma cells | DCVax®-L vaccine | Median OS (mOS) for the intent-to-treat (ITT) population was 23.1 months from surgery; an improvement from the typical mOS for the standard of care | Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; et al. [75] |
| Cell-based Vaccine | Autologous glioma cells Target: EGFRvIII | EGFRvIII-directed CAR T-cells were able to activate the immune system resulting in regulation of tumor growth in xenogeneic subcutaneous and orthotopic models of human EGFRIII + GBM | Johnson, L.A.; Scholler, J; Ohkuri, T; et al. [122] | |
| Cell-based Vaccine | Autologous glioma cells Target: IL13Rα2 | Vaccine | Anti-glioma responses observed in 2 patients, 1 had increased tumor necrotic volume on MRI at administration site | Brown, C; Badie, B; Barish, M; et al. [163] |
| Cell-based Vaccine | Autologous glioma cells Target: IL13Rα2 | Vaccine | Ongoing | Clinical Trial NCT004003649 [133] |
| Cell-based Vaccine | Autologous glioma cells Target: IL13Rα2 | Vaccine | IL13Rα2-CAR.IL15 T-cells in vivo had a greater persistence and anti-tumor activity than IL13Rα2-CAR T-cells | Krenciute, G; Prinzing, B; Yi, Z; et al. [164] |
| Cell-based Vaccine | Autologous glioma cells Targets: IL13Rα2 & HER2 | Vaccine | Super-additive effect on T-cell activation | Hegde, M.; Mukherjee, M.; Grada, Z.; et al. [138] |
| Cell-based Vaccine | Autologous glioma cells Targets: IL13Rα2 & HER2 & EphA2 | Vaccine | Improved survival of treated animals highlight the utility of trivalent CAR T-cell therapy | Bielamowicz, K.; Fousek, K.; Byrd, T.T.; et al. [139] |
| Cell-based Vaccine | Autologous glioma cells | Vaccine | ALECSAT exhibited a robust cytotoxic dose-dependent response that preferentially targets GBM CSCs Decreased proliferation of surviving CSC Number of infused cells is important and must be established. | Wenger, A.; Werlenius, K.; Hallner, A.; et al. [141] |
| Cell-based Vaccine | Autologous glioma cells | Vaccine | Intention-to-treat analysis yielded a PFS of 8.1 months in the treatment arm versus 5.4 months in the control arm | Kong, D.S.; Nam, D.H.; Kang, S.H.; et al. [150] |
| Cell-based Vaccine | Autologous glioma cells Target: EGFRvIII | Vaccine | In 7 patients, CAR T-cells were found in the region of active GBM; 5/7 seven patients had decreased EGFRvIII antigens | O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; et al. [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dapash, M.; Castro, B.; Hou, D.; Lee-Chang, C. Current Immunotherapeutic Strategies for the Treatment of Glioblastoma. Cancers 2021, 13, 4548. https://doi.org/10.3390/cancers13184548
Dapash M, Castro B, Hou D, Lee-Chang C. Current Immunotherapeutic Strategies for the Treatment of Glioblastoma. Cancers. 2021; 13(18):4548. https://doi.org/10.3390/cancers13184548
Chicago/Turabian StyleDapash, Mark, Brandyn Castro, David Hou, and Catalina Lee-Chang. 2021. "Current Immunotherapeutic Strategies for the Treatment of Glioblastoma" Cancers 13, no. 18: 4548. https://doi.org/10.3390/cancers13184548
APA StyleDapash, M., Castro, B., Hou, D., & Lee-Chang, C. (2021). Current Immunotherapeutic Strategies for the Treatment of Glioblastoma. Cancers, 13(18), 4548. https://doi.org/10.3390/cancers13184548






