Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Specimens
2.2. Immunohistochemistry
2.3. Immunohistochemical Staining Quantification
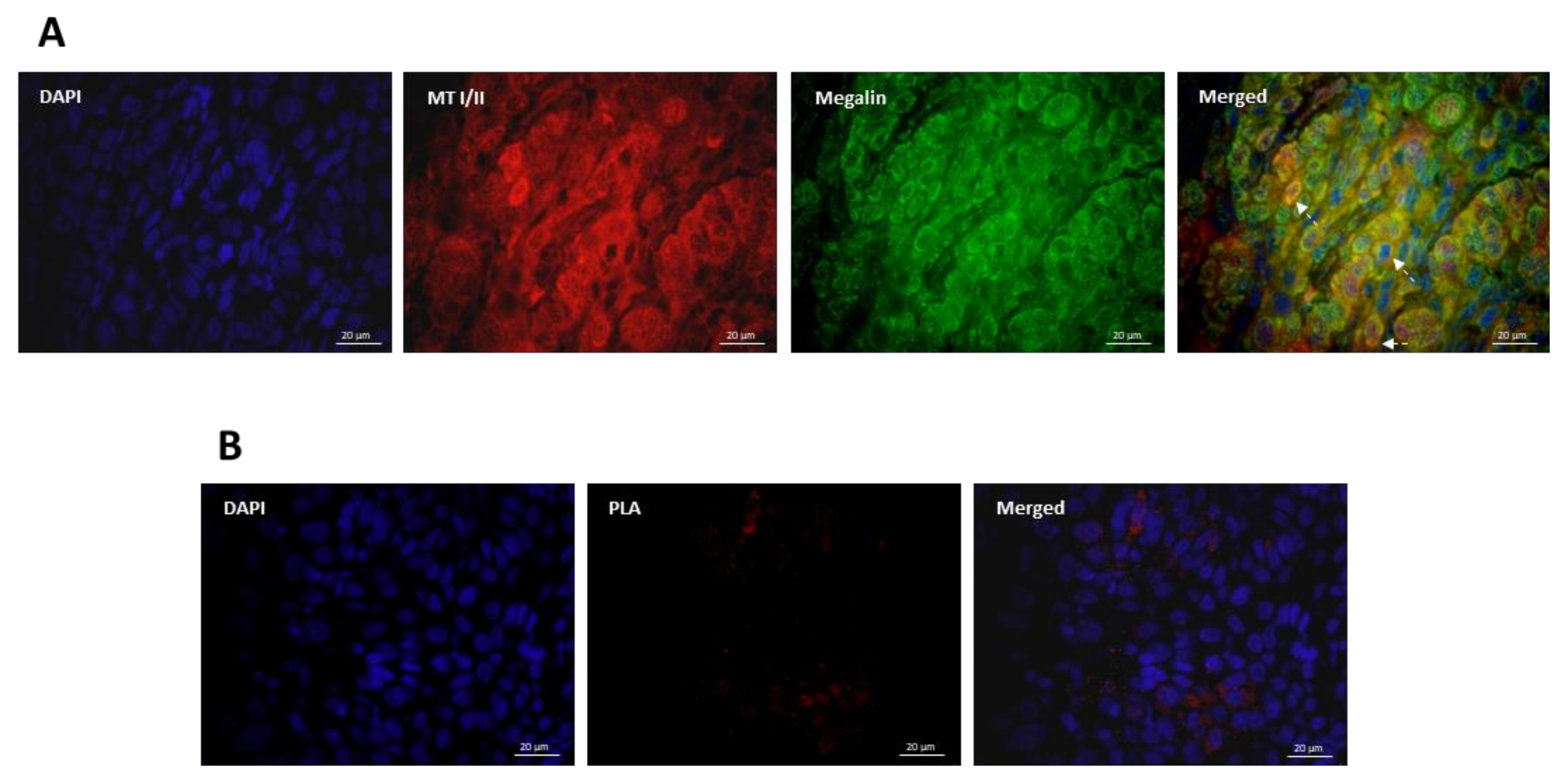
2.4. Immunofluorescence
2.5. Proximity Ligation Assay
2.6. Statistical Analysis
2.7. Ethical Statement
3. Results
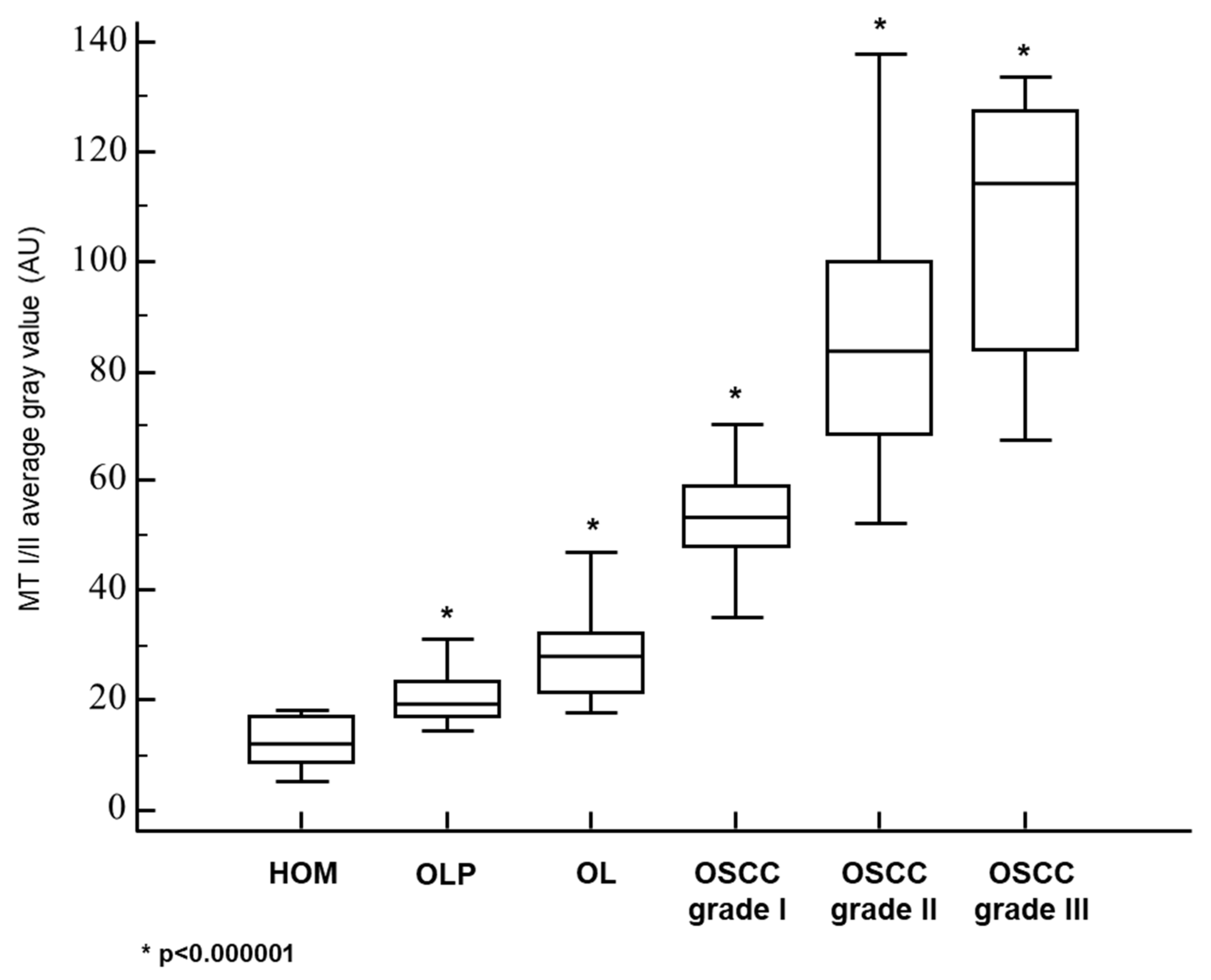
3.1. Metallothionein I/II Expression in OLP, OL and Different Grades of OSCC
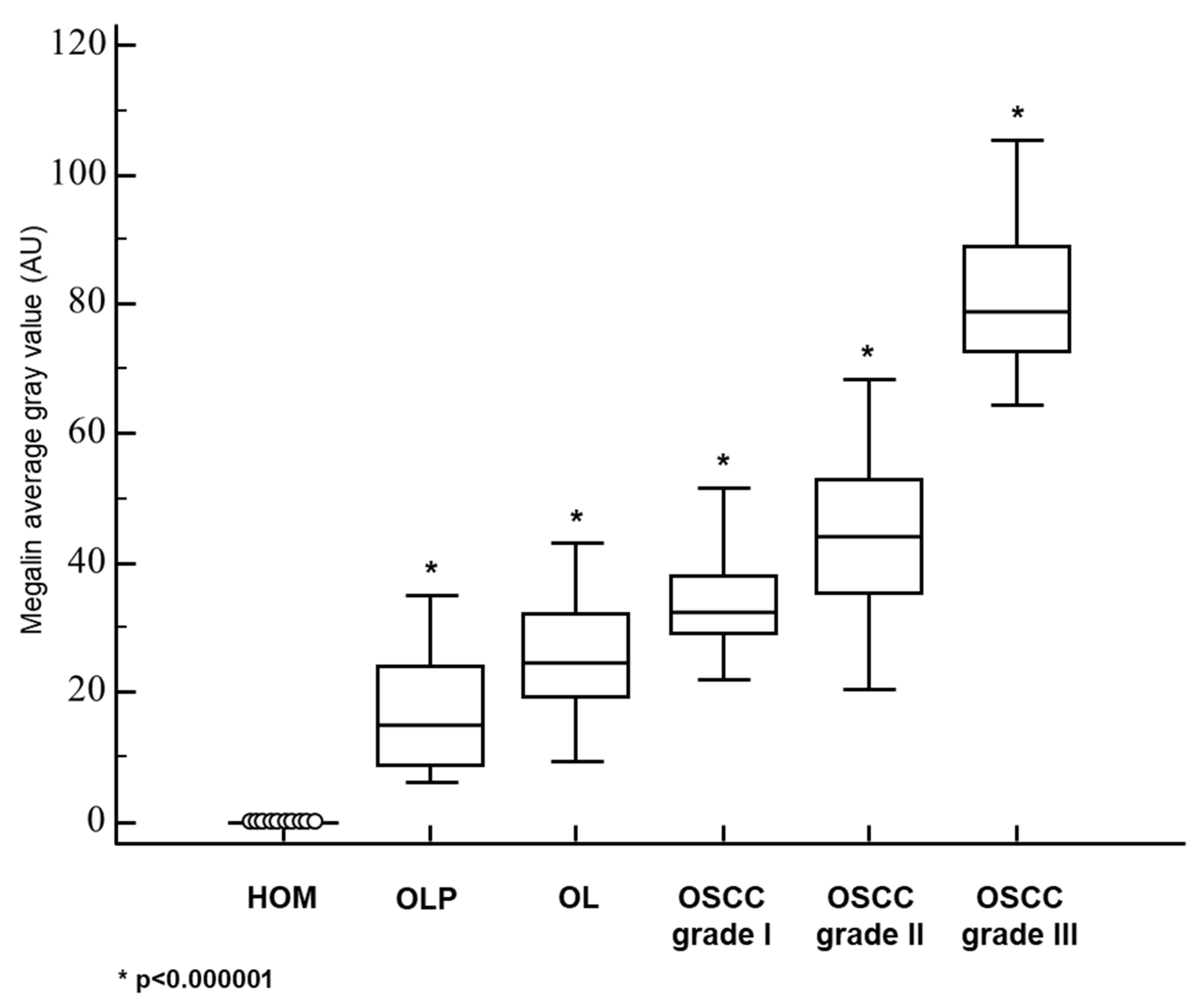
3.2. Megalin Expression in OLP, OL and Different Grades of OSCC
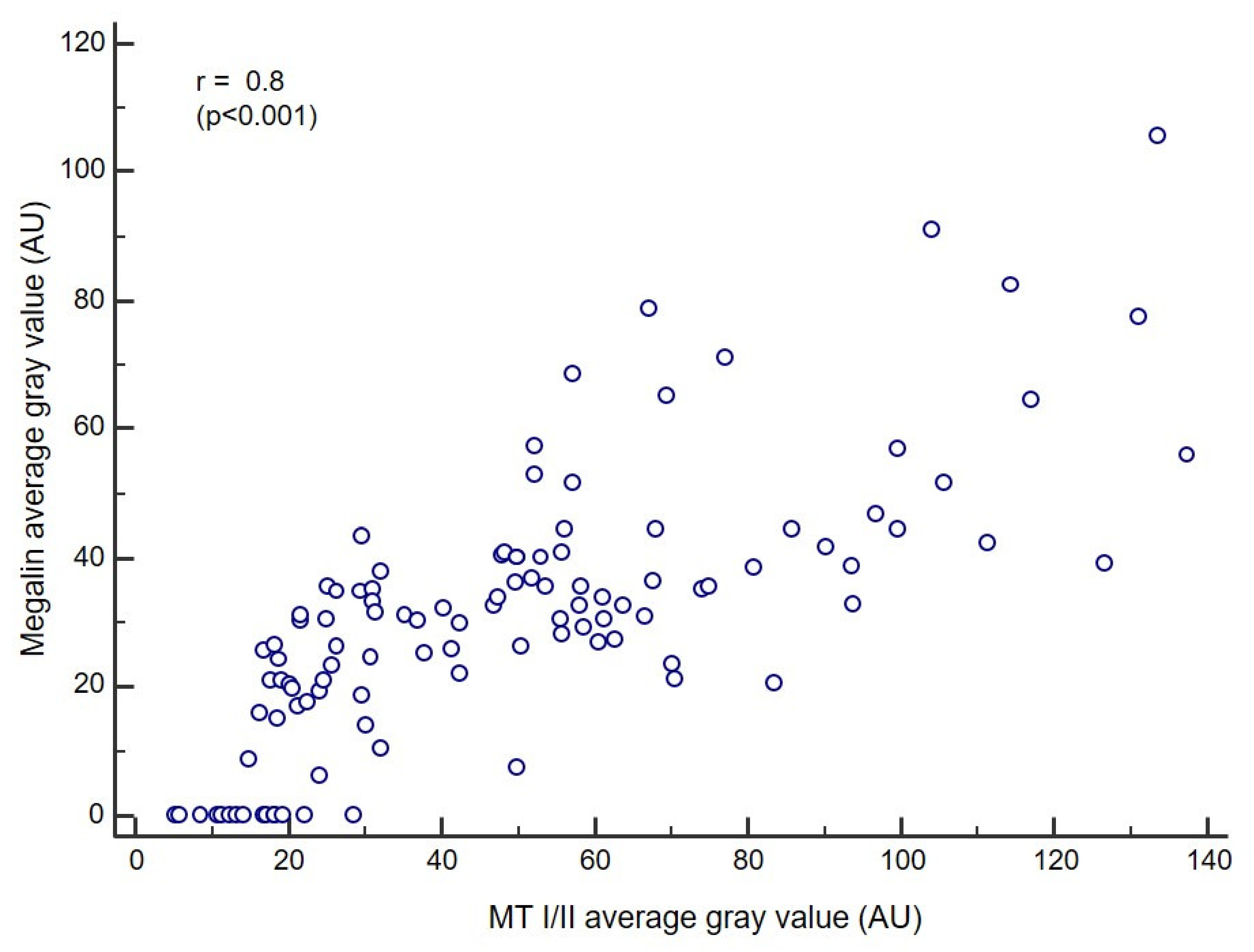
3.3. Co-Expression and Interaction Studies
3.4. Correlation Analysis
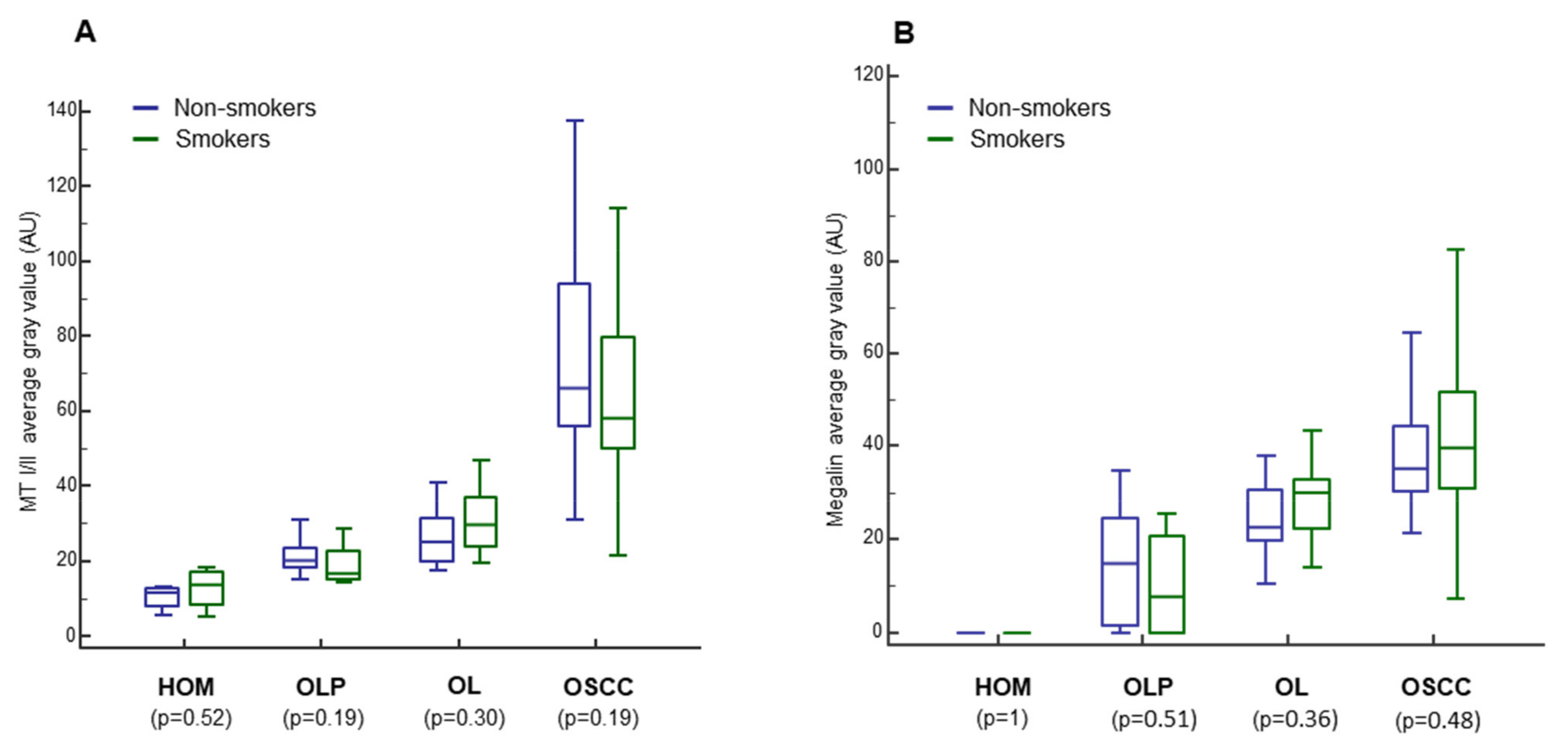
3.5. Impact of Smoking, Sex, and Age on MT I/II and Megalin Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merlos Rodrigo, M.A.; Jimenez Jimemez, A.M.; Haddad, Y.; Bodoor, K.; Adam, P.; Krizkova, S.; Heger, Z.; Adam, V. Metallothionein isoforms as double agents—Their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist. Updat. 2020, 52, 100691. [Google Scholar] [CrossRef] [PubMed]
- Ziller, A.; Fraissinet-Tachet, L. Metallothionein diversity and distribution in the tree of life: A multifunctional protein. Metallomics 2018, 10, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.S.; Irvine, G.W.; Stillman, M.J. Unravelling the mechanistic details of metal binding to mammalian metallothioneins from stoichiometric, kinetic, and binding affinity data. Dalton Trans. 2018, 47, 3613–3637. [Google Scholar] [CrossRef] [Green Version]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Levaot, N.; Hershfinkel, M. How cellular Zn2+ signaling drives physiological functions. Cell Calcium 2018, 75, 53–63. [Google Scholar] [CrossRef]
- Socha-Banasiak, A.; Sputa-Grzegrzółka, P.; Grzegrzółka, J.; Pacześ, K.; Dzięgiel, P.; Sordyl, B.; Romanowicz, H.; Czkwianianc, E. Metallothioneins in Inflammatory Bowel Diseases: Importance in Pathogenesis and Potential Therapy Target. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6665697. [Google Scholar] [CrossRef]
- Aziz, J.; Rahman, M.T.; Vaithilingam, R.D. Dysregulation of metallothionein and zinc aggravates periodontal diseases. J. Trace Elem. Med. Biol. 2021, 66, 126754. [Google Scholar] [CrossRef]
- Álvarez-Barrios, A.; Álvarez, L.; García, M.; Artime, E.; Pereiro, R.; González-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Isani, G.; Carpenè, E. Metallothioneins, unconventional proteins from unconventional animals: A long journey from nematodes to mammals. Biomolecules 2014, 4, 435–457. [Google Scholar] [CrossRef] [Green Version]
- Hirako, N.; Takahashi, S. Upregulation of Metallothionein-1G Accelerates G1/S Transition in the Growth Phase of Acute Promyelocytic Leukemia NB4 Cells. Ann. Clin. Lab. Sci. 2021, 51, 38–43. [Google Scholar]
- Sharma, S.; Ebadi, M. Significance of metallothioneins in aging brain. Neurochem. Int. 2014, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, S.E.; Margeli, A.P.; Klijanienko, J.T.; Kouraklis, G.P. Metallothionein expression in human neoplasia. Histopathology 2004, 45, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Eckschlager, T.; Adam, V.; Hrabeta, J.; Figova, K.; Kizek, R. Metallothioneins and cancer. Curr. Protein Pept. Sci. 2009, 10, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Jakovac, H.; Grebić, D.; Tota, M.; Barac-Latas, V.; Mrakovcić-Sutić, I.; Milin, C.; Radosević-Stasić, B. Time-course expression of metallothioneins and tissue metals in chronic relapsing form of experimental autoimmune encephalomyelitis. Histol. Histopathol. 2011, 26, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Niu, C.; Lv, L.; Ni, M.; Ruan, D.; Chi, L.; Wang, Y.; Yu, Q.; Zhan, K.; Xuan, Y.; et al. Metallothionein Prevents Age-Associated Cardiomyopathy via Inhibiting NF-κB Pathway Activation and Associated Nitrative Damage to 2-OGD. Antioxid. Redox Signal. 2016, 25, 936–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [Green Version]
- Sinduja, P.; Ramani, P.; Gheena, S.; Ramasubramanian, A. Expression of metallothionein in oral squamous cell carcinoma: A systematic review. J. Oral Maxillofac. Pathol. 2020, 24, 143–147. [Google Scholar] [CrossRef]
- Lynes, M.A.; Zaffuto, K.; Unfricht, D.W.; Marusov, G.; Samson, J.S.; Yin, X. The physiological roles of extracellular metallothionein. Exp. Biol Med. 2006, 231, 1548–1554. [Google Scholar] [CrossRef]
- Chung, R.S.; Penkowa, M.; Dittmann, J.; King, C.E.; Bartlett, C.; Asmussen, J.W.; Hidalgo, J.; Carrasco, J.; Leung, Y.K.; Walker, A.K.; et al. Redefining the role of metallothionein within the injured brain: Extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J. Biol. Chem. 2008, 283, 15349–15358. [Google Scholar] [CrossRef] [Green Version]
- Atrian, S.; Capdevila, M. Metallothionein-protein interactions. Biomol. Concepts 2013, 4, 143–160. [Google Scholar] [CrossRef]
- West, A.K.; Leung, J.Y.; Chung, R.S. Neuroprotection and regeneration by extracellular metallothionein via lipoprotein-receptor-related proteins. J. Biol. Inorg. Chem. 2011, 16, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.Ø.; Jensen, R.; Pedersen, D.S.; Skjolding, A.D.; Hempel, C.; Maretty, L.; Penkowa, M. Metallothionein-I+II in neuroprotection. Biofactors 2009, 35, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Hidalgo, J.; West, A.K. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J. Neurochem. 2008, 104, 14–20. [Google Scholar] [CrossRef]
- Li, Y.; Cam, J.; Bu, G. Low-density lipoprotein receptor family: Endocytosis and signal transduction. Mol. Neurobiol. 2001, 23, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Spuch, C.; Ortolano, S.; Navarro, C. LRP-1 and LRP-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer’s disease. Front. Physiol. 2012, 3, 269. [Google Scholar] [CrossRef] [Green Version]
- Jakovac, H.; Grubić Kezele, T.; Radošević-Stašić, B. Expression Profiles of Metallothionein I/II and Megalin in Cuprizone Model of De- and Remyelination. Neuroscience 2018, 388, 69–86. [Google Scholar] [CrossRef]
- Gomes, J.R.; Lobo, A.; Nogueira, R.; Terceiro, A.F.; Costelha, S.; Lopes, I.M.; Magalhães, A.; Summavielle, T.; Saraiva, M.J. Neuronal megalin mediates synaptic plasticity-a novel mechanism underlying intellectual disabilities in megalin gene pathologies. Brain Commun. 2020, 2, fcaa135. [Google Scholar] [CrossRef]
- Li, Y.; Cong, R.; Biemesderfer, D. The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am. J. Physiol. Cell Physiol. 2008, 295, C529–C537. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Baterina, O.Y., Jr.; Taupin, V.; Farquhar, M.G. ARH directs megalin to the endocytic recycling compartment to regulate its proteolysis and gene expression. J. Cell Biol. 2013, 202, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xin, F.; Lin, N.; Wang, Y.; Liu, X.; Liu, J. Metallothioneins may be a potential prognostic biomarker for tumors: A Prisma-compliant meta-analysis. Medicine 2018, 97, e13786. [Google Scholar] [CrossRef]
- Krizkova, S.; Kepinska, M.; Emri, G.; Eckschlager, T.; Stiborova, M.; Pokorna, P.; Heger, Z.; Adam, V. An insight into the complex roles of metallothioneins in malignant diseases with emphasis on (sub)isoforms/isoforms and epigenetics phenomena. Pharmacol. Ther. 2018, 183, 90–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio, F.A.; Martins, L.M.; Dourado, C.S.M.E.; Revoredo, C.M.S.; Costa-Silva, D.R.; Oliveira, V.A.; Alves-Ribeiro, F.A.; Silva, B.B.D. A case-control study of Metallothionein-1 expression in breast cancer and breast fibroadenoma. Sci. Rep. 2019, 9, 7407. [Google Scholar] [CrossRef]
- Lima, L.A.O.; Bittencourt, L.O.; Puty, B.; Fernandes, R.M.; Nascimento, P.C.; Silva, M.C.F.; Alves-Junior, S.M.; Pinheiro, J.J.V.; Lima, R.R. Methylmercury Intoxication Promotes Metallothionein Response and Cell Damage in Salivary Glands of Rats. Biol. Trace Elem. Res. 2018, 185, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Grebić, D.; Jakovac, H.; Mrakovcić-Sutić, I.; Tomac, J.; Bulog, A.; Micović, V.; Radosević-Stasić, B. Short-term exposure of mice to gasoline vapor increases the metallothionein expression in the brain, lungs and kidney. Histol. Histopathol. 2007, 22, 593–601. [Google Scholar] [CrossRef]
- Yadav, V.S.; Mir, R.A.; Bhatia, A.; Yadav, R.; Shadang, M.; Chauhan, S.S.; Dhingra, K.; Kharbanda, O.P.; Yadav, R.; Garg, R. Metallothionein levels in gingival crevicular fluid, saliva and serum of smokers and non-smokers with chronic periodontitis. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Satoh, M. Protective role of metallothionein in chemical and radiation carcinogenesis. Curr. Pharm. Biotechnol. 2013, 14, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Nishimura, N.; Suzuki, J.S.; Tohyama, C.; Naganuma, A.; Satoh, M. Role of metallothionein as a protective factor against radiation carcinogenesis. J. Toxicol. Sci. 2008, 33, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Biemesderfer, D. Regulated intramembrane proteolysis of megalin: Linking urinary protein and gene regulation in proximal tubule? Kidney Int. 2006, 69, 1717–1721. [Google Scholar] [CrossRef] [Green Version]
- Fridrich, S.; Karmilin, K.; Stöcker, W. Handling Metalloproteinases. Curr. Protoc. Protein Sci. 2016, 83, 21.16.1–21.16.20. [Google Scholar] [CrossRef]
- Jakovac, H.; Stašić, N.; Krašević, M.; Jonjić, N.; Radošević-Stašić, B. Expression profiles of metallothionein-I/II and megalin/LRP-2 in uterine cervical squamous lesions. Virchows Arch. 2021, 478, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Ghayvat, H.; Krishnan Pandarathodiyil, A.; Nabillah Ghani, W.M.; Ramanathan, A.; Pandya, S.; Walter, N.; Saad, M.N.; Zain, R.B.; Faye, I. Healthcare Professional in the Loop (HPIL): Classification of Standard and Oral Cancer-Causing Anomalous Regions of Oral Cavity Using Textural Analysis Technique in Autofluorescence Imaging. Sensors 2020, 20, 5780. [Google Scholar] [CrossRef] [PubMed]
- Dionne, K.R.; Warnakulasuriya, S.; Zain, R.B.; Cheong, S.C. Potentially malignant disorders of the oral cavity: Current practice and future directions in the clinic and laboratory. Int. J. Cancer 2015, 136, 503–515. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; IARC Press: Lyon, France, 2017; pp. 105–115. [Google Scholar]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; pp. 79–94. [Google Scholar]
- Ferenčić, A.; Cuculić, D.; Stemberga, V.; Šešo, B.; Arbanas, S.; Jakovac, H. Left ventricular hypertrophy is associated with overexpression of HSP60, TLR2, and TLR4 in the myocardium. Scand. J. Clin. Lab. Invest. 2020, 80, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.G.; Servato, J.P.; Borges, F.C.; Rosa, R.R.; Siqueira, C.S.; de Faria, P.R.; Loyola, A.M.; Cardoso, S.V. Differential metallothionein expression in oral lichen planus and amalgam-associated oral lichenoid lesions. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e262–e268. [Google Scholar] [CrossRef] [PubMed]
- Allon, I.; Ofir, M.; Vered, H.; Hirshberg, A. Metallothionein, a marker of antiapoptosis, is associated with clinical forms of oral lichen planus. J. Oral Pathol. Med. 2014, 43, 728–733. [Google Scholar] [CrossRef]
- Johann, A.C.; da Silveira-Júnior, J.B.; Souto, G.R.; Horta, M.C.; Aguiar, M.C.; Mesquita, R.A. Metallothionein immunoexpression in oral leukoplakia. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, E156–E160. [Google Scholar]
- Pontes, H.A.; de Aquino Xavier, F.C.; da Silva, T.S.; Fonseca, F.P.; Paiva, H.B.; Pontes, F.S.; dos Santos Pinto, D., Jr. Metallothionein and p-Akt proteins in oral dysplasia and in oral squamous cell carcinoma: An immunohistochemical study. J. Oral Pathol. Med. 2009, 38, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Brazão-Silva, M.T.; Rodrigues, M.F.; Eisenberg, A.L.; Dias, F.L.; de Castro, L.M.; Nunes, F.D.; Faria, P.R.; Cardoso, S.V.; Loyola, A.M.; de Sousa, S.C. Metallothionein gene expression is altered in oral cancer and may predict metastasis and patient outcomes. Histopathology 2015, 67, 358–367. [Google Scholar] [CrossRef]
- Theocharis, S.; Klijanienko, J.; Giaginis, C.; Rodriguez, J.; Jouffroy, T.; Girod, A.; Point, D.; Tsourouflis, G.; Sastre-Garau, X. Metallothionein expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patient survival. Histopathology 2011, 59, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Szelachowska, J.; Dziegiel, P.; Jelen-Krzeszewska, J.; Jelen, M.; Tarkowski, R.; Spytkowska, B.; Matkowski, R.; Kornafel, J. Correlation of metallothionein expression with clinical progression of cancer in the oral cavity. Anticancer Res. 2009, 29, 589–595. [Google Scholar]
- Cardoso, S.V.; Barbosa, H.M.; Candellori, I.M.; Loyola, A.M.; Aguiar, M.C. Prognostic impact of metallothionein on oral squamous cell carcinoma. Virchows Arch. 2002, 441, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, G.M.D.R.; Pontes, F.S.C.; Conte Neto, N.; Nascimento, L.S.D.; Souza, L.L.; Pinto Junior, D.D.S.; Pontes, H.A.R. Nuclear metallothionein in oral squamous cell carcinoma: Clinicopathological parameters and patient survival. Braz. Oral Res. 2018, 32, e105. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.V.; Silveira-Júnior, J.B.; De Carvalho Machado, V.; De-Paula, A.M.; Loyola, A.M.; De Aguiar, M.C. Expression of metallothionein and p53 antigens are correlated in oral squamous cell carcinoma. Anticancer Res. 2009, 29, 1189–1193. [Google Scholar]
- Szelachowska, J.; Dziegiel, P.; Jelen-Krzeszewska, J.; Jelen, M.; Tarkowski, R.; Wlodarska, I.; Spytkowska, B.; Gisterek, I.; Matkowski, R.; Kornafel, J. Prognostic significance of nuclear and cytoplasmic expression of metallothioneins as related to proliferative activity in squamous cell carcinomas of oral cavity. Histol. Histopathol. 2008, 23, 843–851. [Google Scholar] [CrossRef]
- Pedersen, M.Ø.; Hansen, P.B.; Nielsen, S.L.; Penkowa, M. Metallothionein-I + II and receptor megalin are altered in relation to oxidative stress in cerebral lymphomas. Leuk. Lymphoma 2010, 51, 314–328. [Google Scholar] [CrossRef]
- Andersen, R.K.; Hammer, K.; Hager, H.; Christensen, J.N.; Ludvigsen, M.; Honoré, B.; Thomsen, M.B.; Madsen, M. Melanoma tumors frequently acquire LRP2/megalin expression, which modulates melanoma cell proliferation and survival rates. Pigment. Cell Melanoma Res. 2015, 28, 267–280. [Google Scholar] [CrossRef]
- Holt, S.K.; Karyadi, D.M.; Kwon, E.M.; Stanford, J.L.; Nelson, P.S.; Ostrander, E.A. Association of megalin genetic polymorphisms with prostate cancer risk and prognosis. Clin. Cancer Res. 2008, 14, 3823–3831. [Google Scholar] [CrossRef] [Green Version]
- Cabezas, F.; Farfán, P.; Marzolo, M.P. Participation of the SMAD2/3 signalling pathway in the down regulation of megalin/LRP2 by transforming growth factor beta (TGF-ß1). PLoS ONE 2019, 14, e0213127. [Google Scholar] [CrossRef] [Green Version]
- Nagaraj, N.S.; Datta, P.K. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin. Investig. Drugs 2010, 19, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Perez Bay, A.E.; Schreiner, R.; Benedicto, I.; Paz Marzolo, M.; Banfelder, J.; Weinstein, A.M.; Rodriguez-Boulan, E.J. The fast-recycling receptor Megalin defines the apical recycling pathway of epithelial cells. Nat. Commun. 2016, 7, 11550. [Google Scholar] [CrossRef] [PubMed]
- Cortés, M.; Sanchez-Moral, L.; de Barrios, O.; Fernández-Aceñero, M.J.; Martínez-Campanario, M.C.; Esteve-Codina, A.; Darling, D.S.; Győrffy, B.; Lawrence, T.; Dean, D.C.; et al. Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J. 2017, 36, 3336–3355. [Google Scholar] [CrossRef]
- Xu, C.; Yan, T.; Yang, J. OVOL1 inhibits oral squamous cell carcinoma growth and metastasis by suppressing zinc finger E-box binding homeobox 1. Int. J. Clin. Exp. Pathol. 2019, 12, 2801–2808. [Google Scholar]
- Wang, H.; Deng, X.; Zhang, J.; Ou, Z.; Mai, J.; Ding, S.; Huo, S. Elevated Expression of Zinc Finger Protein 703 Promotes Cell Proliferation and Metastasis through PI3K/AKT/GSK-3β Signalling in Oral Squamous Cell Carcinoma. Cell Physiol. Biochem. 2017, 44, 920–934. [Google Scholar] [CrossRef] [Green Version]
- Ayinampudi, B.K.; Narsimhan, M. Salivary copper and zinc levels in oral pre-malignant and malignant lesions. J. Oral Maxillofac. Pathol. 2012, 16, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Milin, C.; Tota, M.; Domitrovic, R.; Giacometti, J.; Pantovic, R.; Cuk, M.; Mrakovcic-Sutic, I.; Jakovac, H.; Radosevic-Stasic, B. Metal tissue kinetics in regenerating liver, thymus, spleen, and submandibular gland after partial hepatectomy in mice. Biol. Trace Elem. Res. 2005, 108, 225–243. [Google Scholar] [CrossRef]
- Kantarci, S.; Al-Gazali, L.; Hill, R.S.; Donnai, D.; Black, G.C.; Bieth, E.; Chassaing, N.; Lacombe, D.; Devriendt, K.; Teebi, A.; et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat. Genet. 2007, 39, 957–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, O.; Al-Sahlawi, Z.; Imtiaz, F.; Ramzan, K.; Allam, R.; Al-Mostafa, A.; Abdel-Fattah, M.; Abuharb, G.; Nester, M.; Verloes, A.; et al. Variable expression pattern in Donnai-Barrow syndrome: Report of two novel LRP2 mutations and review of the literature. Eur. J. Med. Genet. 2015, 58, 293–299. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 256–266. [Google Scholar] [CrossRef]
- Ljubojević, M.; Orct, T.; Micek, V.; Karaica, D.; Jurasović, J.; Breljak, D.; Madunić, I.V.; Rašić, D.; Jovanović, I.N.; Peraica, M.; et al. Sex-dependent expression of metallothioneins MT1 and MT2 and concentrations of trace elements in rat liver and kidney tissues: Effect of gonadectomy. J. Trace Elem. Med. Biol. 2019, 53, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, T.; Xu, Y.Q.; Lu, Y.F.; Wu, Q.; Zhang, Y.K.; Liu, J. Diurnal-and sex-related difference of metallothionein expression in mice. J. Circadian Rhythms 2012, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santarelli, A.; Mascitti, M.; Rubini, C.; Bambini, F.; Giannatempo, G.; Lo Russo, L.; Sartini, D.; Emanuelli, M.; Procaccini, M.; Lo Muzio, L. Nuclear Survivin as a Prognostic Factor in Squamous-Cell Carcinoma of the Oral Cavity. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Shu, Y.; Guo, P.; Ho, S.M. Application of phi29 motor pRNA for targeted therapeutic delivery of siRNA silencing metallothionein-IIA and survivin in ovarian cancers. Mol. Ther. 2011, 19, 386–394. [Google Scholar] [CrossRef] [PubMed]
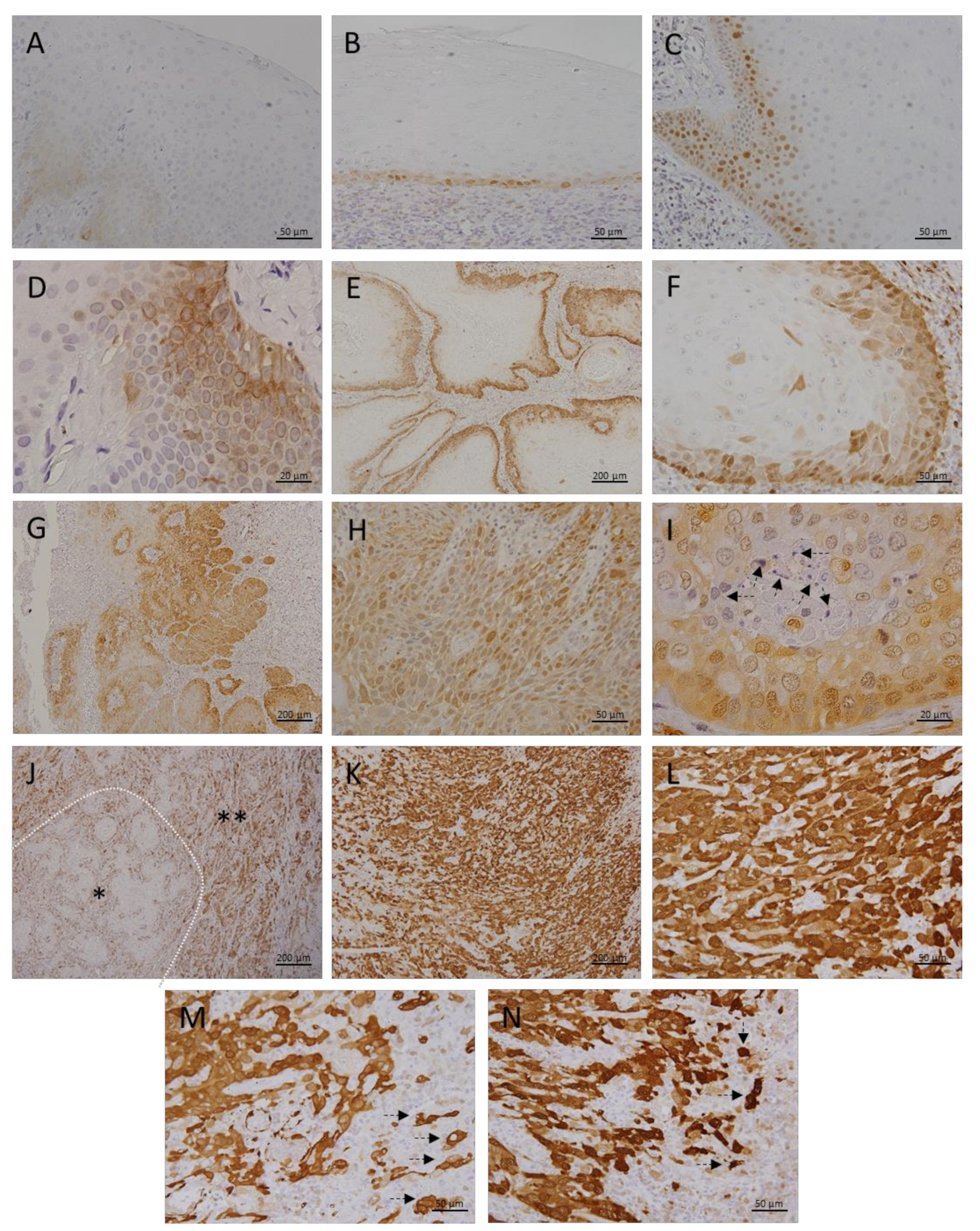
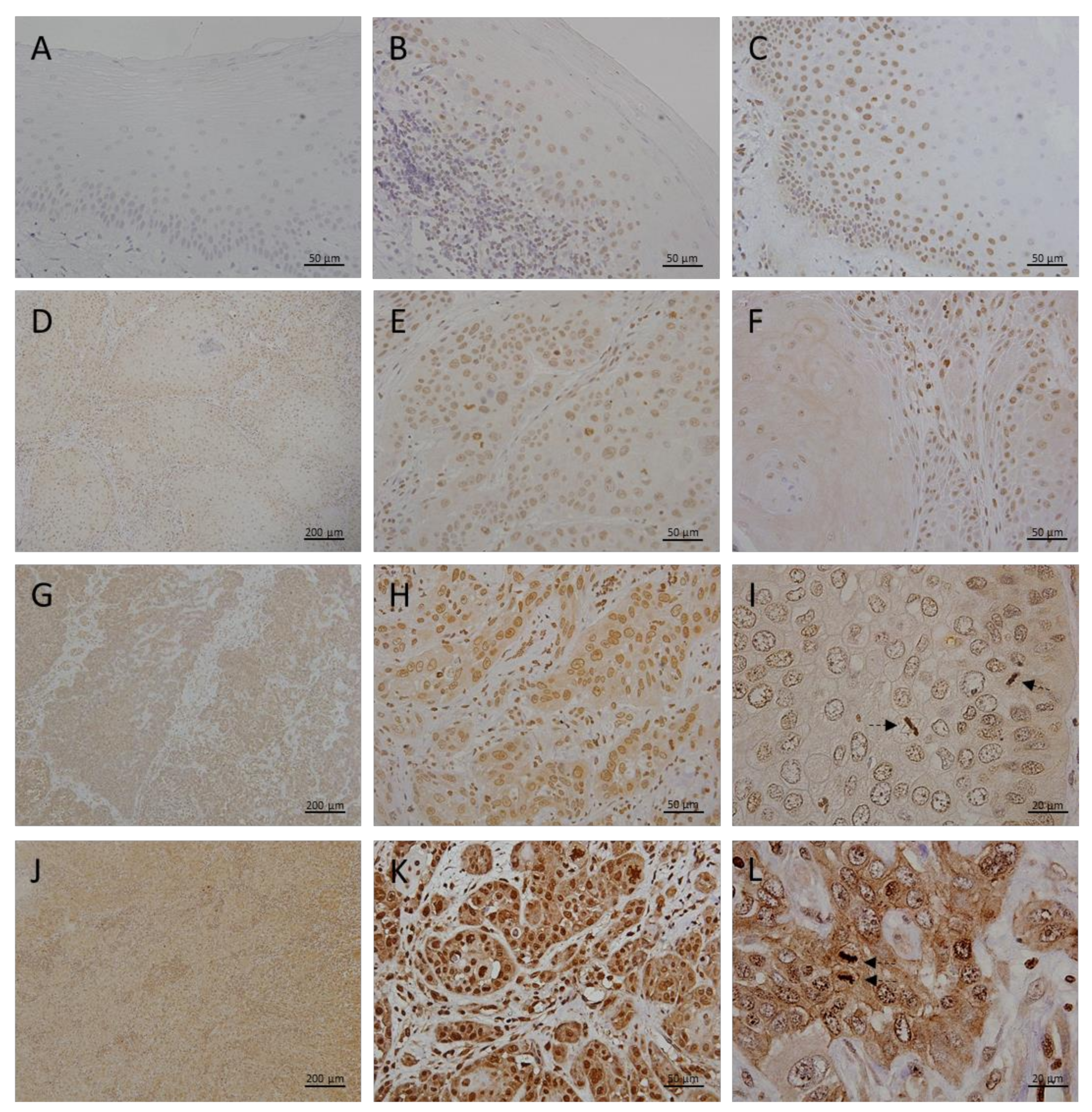
| HEALTHY ORAL MUCOSA | ORAL LICHEN PLANUS | ORAL LEUKOPLAKIA | ORAL SQUAMOS CELL CANCER | |||
|---|---|---|---|---|---|---|
| GRADE I | GRADE II | GRADE III | ||||
| N | 10 | 15 | 26 | 28 | 23 | 12 |
| SEX (M:F) | 7:3 | 10:5 | 16:10 | 14:14 | 15:8 | 10:2 |
| AGE AT DIAGNOSIS | 51.1 ± 15.8 | 63.9 ± 9.8 | 61.4 ± 9.6 | 63.9 ± 8.4 | 65.4 ± 7 | 65.8 ± 10.6 |
| SMOKING (Y:N) | 6:4 | 4:11 | 13:13 | 16:12 | 13:10 | 5:7 |
| MT—AVERAGE GRAY VALUE: MEAN±SD | 12.0 ± 4.6 | 20.8 ± 4.9 | 28.4 ± 7.6 | 53.7 ± 12.4 | 85.2 ± 23.6 | 106.4 ± 25.4 |
| MT—AVERAGE GRAY VALUE: MEDIAN (RANGE) | 11.9 (5.3−18.4) | 19.4 (14.2−31.1) | 27.9 (17.8−46.9) | 53.5 (21.7−93.7) | 83.5 (52.2−137.6) | 114.3 (67.2−133.6) |
| MEGALIN—AVERAGE GRAY VALUE: MEAN±SD | 0 | 16.1 ± 8.8 | 25.6 ± 8.5 | 32.8 ± 7.8 | 44.1 ± 12.3 | 81.5 ± 13.4 |
| MEGALIN—AVERAGE GRAY VALUE: MEDIAN (RANGE) | 0 | 14.8 (6.1−35.0) | 24.7 (9.6−43.2) | 32.5 (7.1−51.5) | 44.2 (20.4−68.3) | 78.8 (64.6−105.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulijani, A.; Dekanić, A.; Ćabov, T.; Jakovac, H. Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions. Cancers 2021, 13, 4530. https://doi.org/10.3390/cancers13184530
Zulijani A, Dekanić A, Ćabov T, Jakovac H. Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions. Cancers. 2021; 13(18):4530. https://doi.org/10.3390/cancers13184530
Chicago/Turabian StyleZulijani, Ana, Andrea Dekanić, Tomislav Ćabov, and Hrvoje Jakovac. 2021. "Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions" Cancers 13, no. 18: 4530. https://doi.org/10.3390/cancers13184530
APA StyleZulijani, A., Dekanić, A., Ćabov, T., & Jakovac, H. (2021). Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions. Cancers, 13(18), 4530. https://doi.org/10.3390/cancers13184530






