Subpopulations of Circulating Cells with Morphological Features of Malignancy Are Preoperatively Detected and Have Differential Prognostic Significance in Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Collection and CTC Enrichment
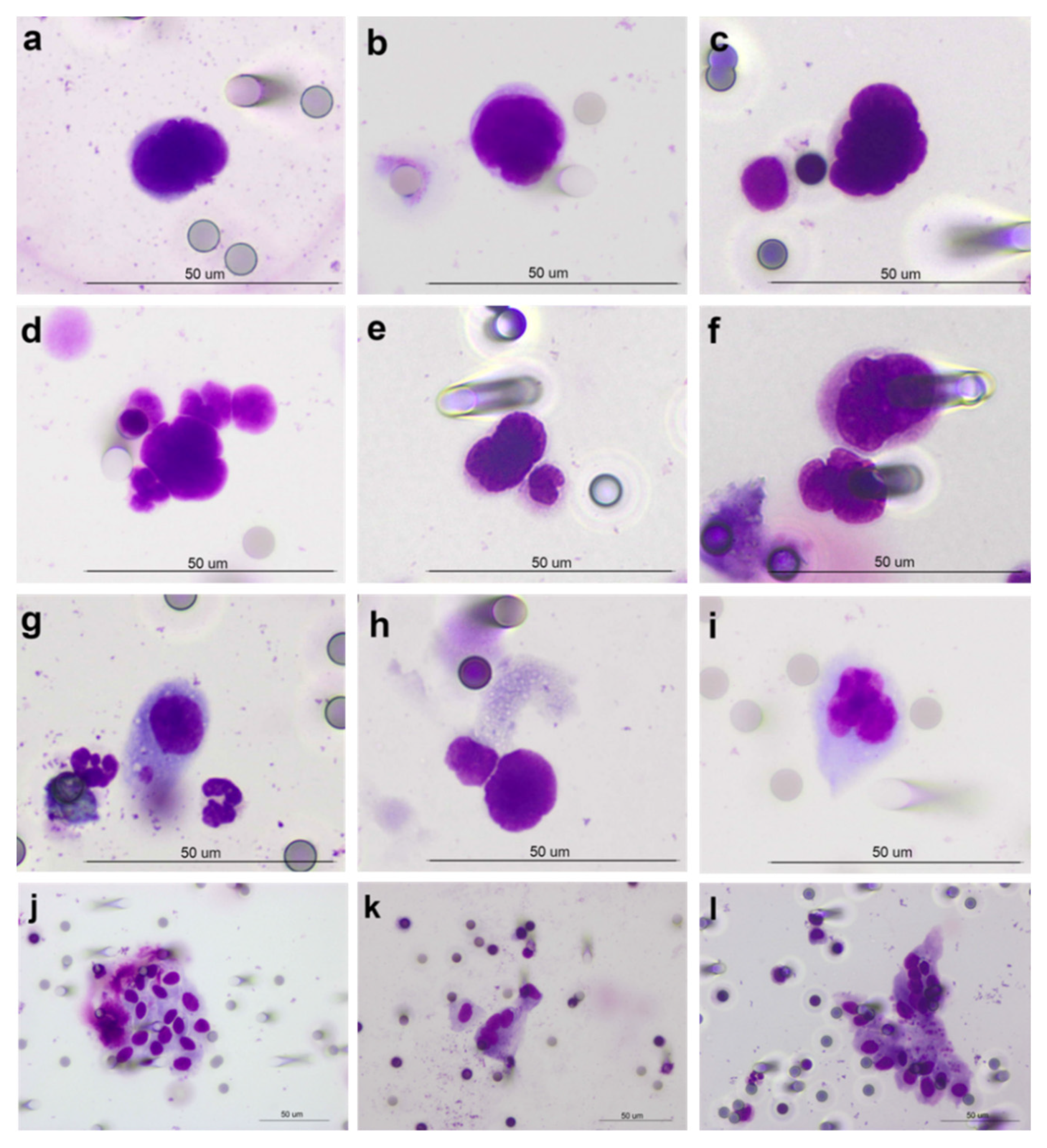
2.3. CTC Detection Method and Identification Criteria
2.4. Spike-in Experiments
2.5. Statistical Analysis
3. Results
3.1. Cancer Cell Hematogenous Dissemination Is a Frequent Event in NSCLC Patients without Clinical Evidence of Metastasis
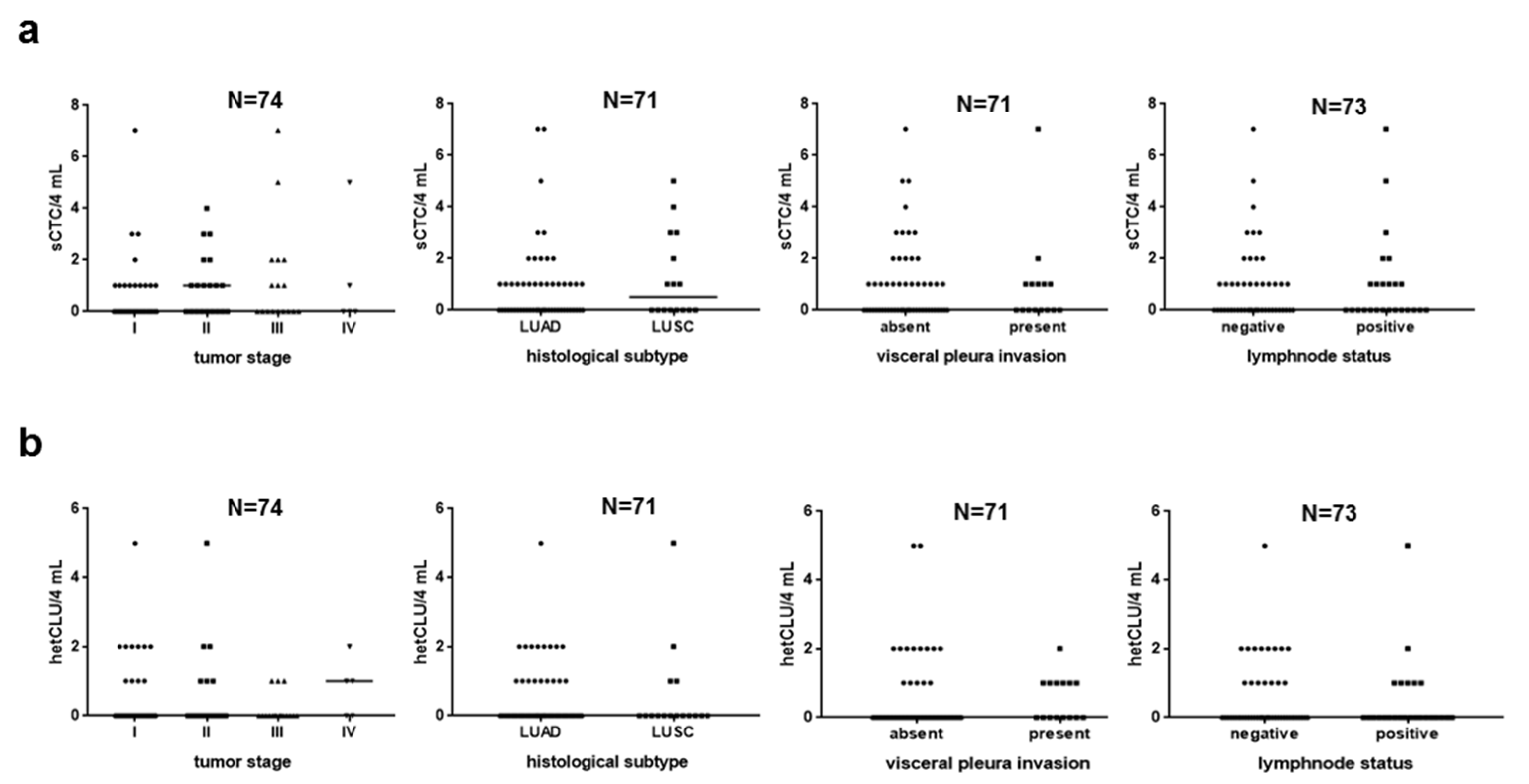
3.2. Subpopulations of Circulating Atypical Cells Differentiate Operable NSCLC Patients from Lung Cancer-Free Individuals
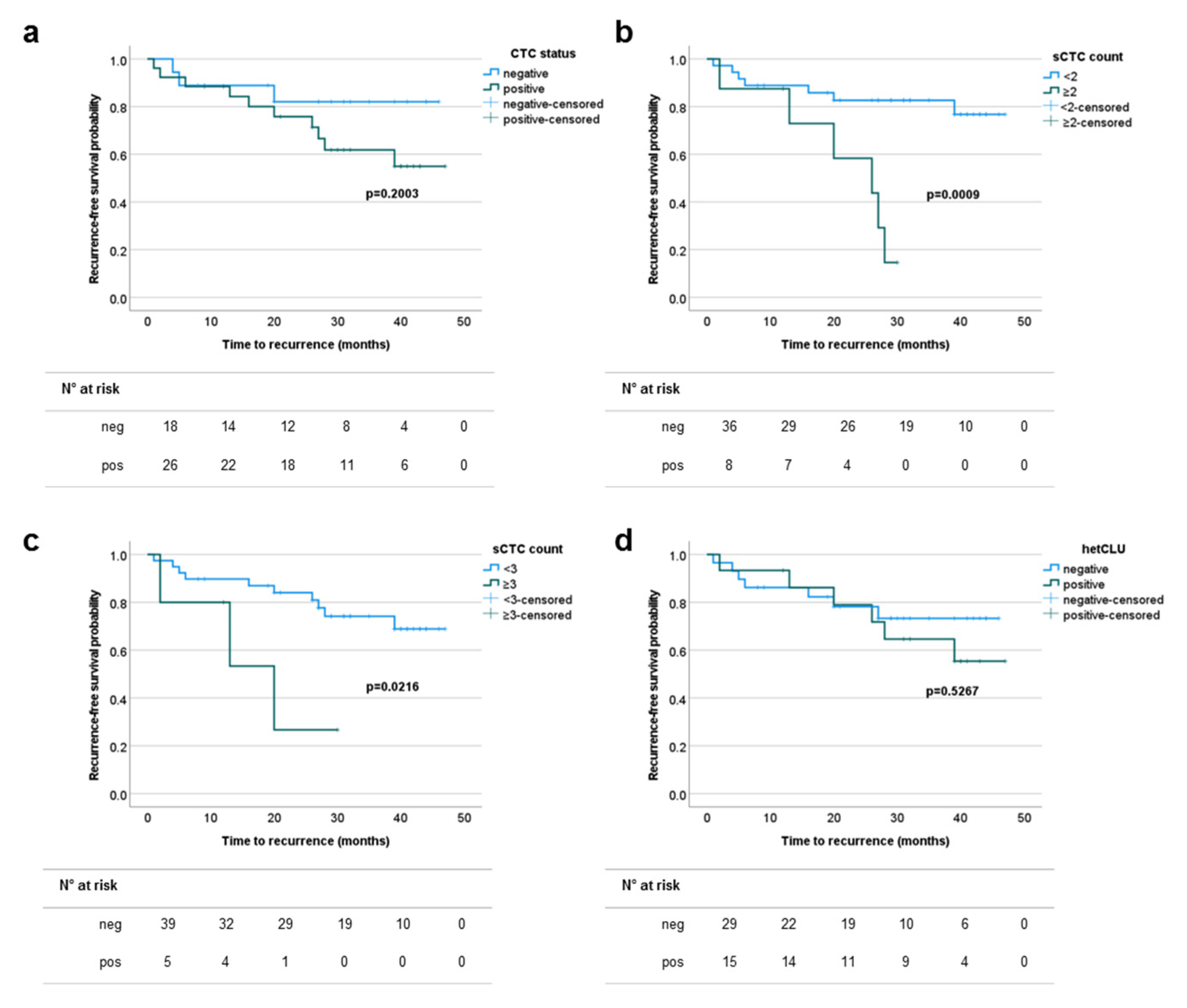
3.3. The Prevalence of Single Circulating Tumor Cells Predicts the Risk of Recurrence in Patients with Surgically Treated Stage I–II NSCLCs
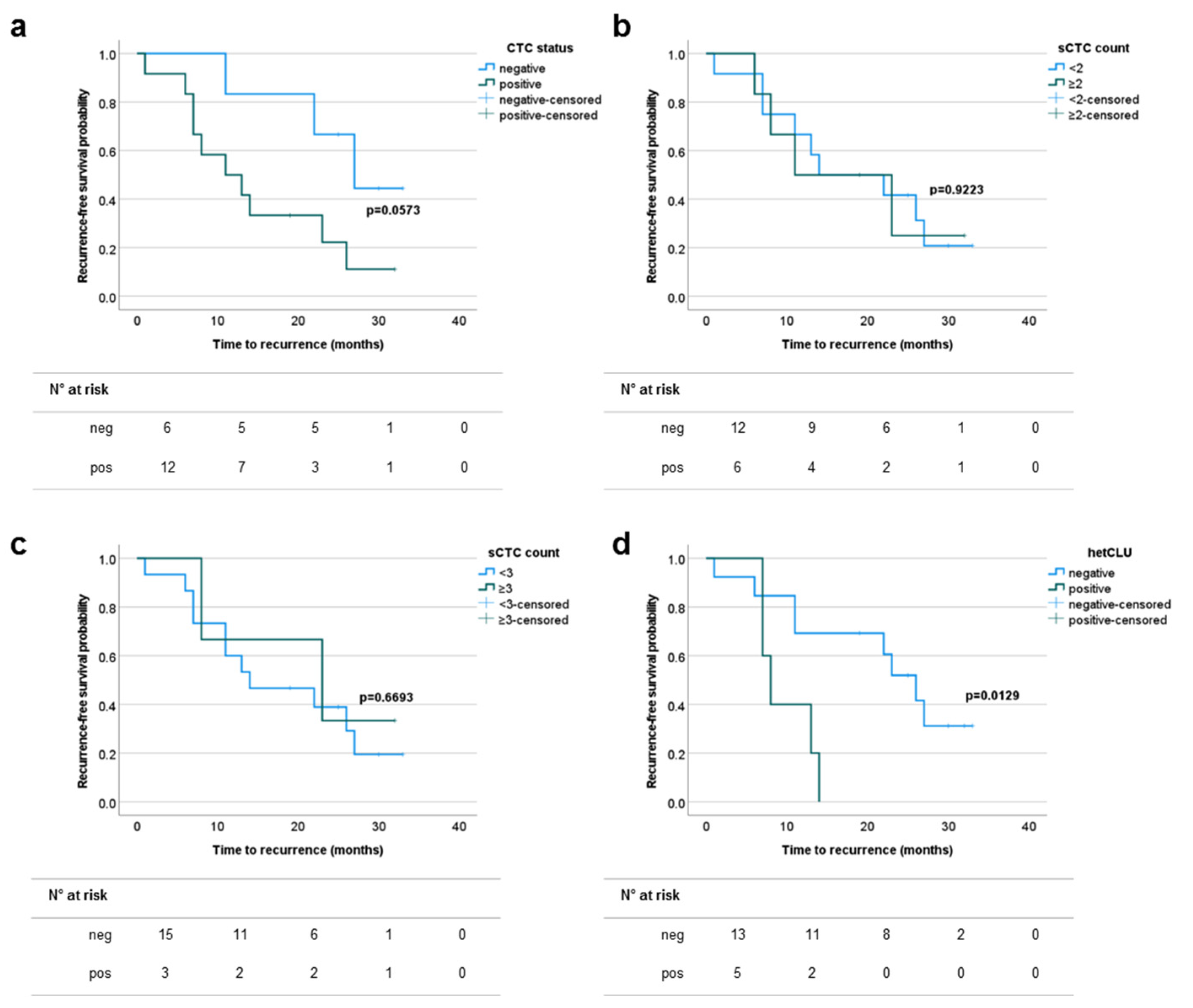
3.4. The Presence of Heterotypic Clusters of CTCs Predicts the Risk of Recurrence in Patients with Surgically Treated Stage III–IVA NSCLCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Campbell, J.D.; Alexandrov, A.; Kim, J.; Wala, J.; Berger, A.H.; Pedamallu, C.S.; Shukla, S.A.; Guo, G.; Brooks, A.N.; Murray, B.A.; et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 2016, 48, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.; Maringe, C.; Coleman, M.; Peake, M.D.; Butler, J.; Young, N.; Bergström, S.; Hanna, L.; Jakobsen, E.; Kölbeck, K.; et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007. Thorax 2013, 68, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Henschke, C.I.; Boffetta, P.; Gorlova, O.; Yip, R.; DeLancey, J.O.; Foy, M. Assessment of lung-cancer mortality reduction from CT Screening. Lung Cancer 2011, 71, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Puggina, A.; Broumas, A.; Ricciardi, W.; Boccia, S. Cost-effectiveness of screening for lung cancer with low-dose computed tomography: A systematic literature review. Eur. J. Public Health 2016, 26, 168–175. [Google Scholar] [CrossRef]
- Veronesi, G.; Novellis, P.; Voulaz, E.; Alloisio, M. Early detection and early treatment of lung cancer: Risks and benefits. J. Thorac. Dis. 2016, 8, E1060–E1062. [Google Scholar] [CrossRef]
- Veronesi, G.; Baldwin, D.; Henschke, C.; Ghislandi, S.; Iavicoli, S.; Oudkerk, M.; De Koning, H.; Shemesh, J.; Field, J.; Zulueta, J.; et al. Recommendations for Implementing Lung Cancer Screening with Low-Dose Computed Tomography in Europe. Cancers 2020, 12, 1672. [Google Scholar] [CrossRef]
- Veronesi, G.; Bellomi, M.; Scanagatta, P.; Preda, L.; Rampinelli, C.; Guarize, J.; Pelosi, G.; Maisonneuve, P.; Leo, F.; Solli, P.; et al. Difficulties encountered managing nodules detected during a computed tomography lung cancer screening program. J. Thorac. Cardiovasc. Surg. 2008, 136, 611–617. [Google Scholar] [CrossRef]
- Shah, R.; Sabanathan, S.; Richardson, J.; Mearns, A.J.; Goulden, C. Results of surgical treatment of stage I and II lung cancer. J. Cardiovasc. Surg. 1996, 37, 169–172. [Google Scholar]
- Nesbitt, J.C.; Putnam, J.B.; Walsh, G.L.; Roth, J.A.; Mountain, C.F. Survival in early-stage non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 466–472. [Google Scholar] [CrossRef]
- Al-Kattan, K.; Sepsas, E.; Fountain, S.W.; Townsend, E.R. Disease recurrence after resection for stage I lung cancer. Eur. J. Cardio-Thorac. Surg. 1997, 12, 380–384. [Google Scholar] [CrossRef]
- Hoffman, P.C.; Mauer, A.M.; Vokes, E.E. Lung cancer. Lancet 2000, 355, 479–485. [Google Scholar] [CrossRef]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Blanchon, F.; Grivaux, M.; Asselain, B.; Lebas, F.-X.; Orlando, J.-P.; Piquet, J.; Zureik, M. 4-year mortality in patients with non-small-cell lung cancer: Development and validation of a prognostic index. Lancet Oncol. 2006, 7, 829–836. [Google Scholar] [CrossRef]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Mok, T.S.K. Personalized medicine in lung cancer: What we need to know. Nat. Rev. Clin. Oncol. 2011, 8, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Bubendorf, L.; Edelman, M.; Marchetti, A.; Mok, T.; Novello, S.; O’Byrne, K.; Stahel, R.; Peters, S.; Felip, E.; et al. Second ESMO consensus conference on lung cancer: Pathology and molecular biomarkers for non-small-cell lung cancer. Ann. Oncol. 2014, 25, 1681–1690. [Google Scholar] [CrossRef]
- Hofman, P. Liquid biopsy for early detection of lung cancer. Curr. Opin. Oncol. 2017, 29, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mathai, R.A.; Vidya, R.V.S.; Reddy, B.S.; Thomas, L.; Udupa, K.; Kolesar, J.; Rao, M. Potential Utility of Liquid Biopsy as a Diagnostic and Prognostic Tool for the Assessment of Solid Tumors: Implications in the Precision Oncology. J. Clin. Med. 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Klein, C.A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B. Circulating Tumor Cells in the Parallel Invasion Model Supporting Early Metastasis. Oncomedicine 2018, 3, 15–27. [Google Scholar] [CrossRef]
- Ilie, M.; Hofman, V.; Long, E.; Selva, E.; Vignaud, J.-M.; Padovani, B.; Mouroux, J.; Marquette, C.H.; Hofman, P. “Sentinel” Circulating Tumor Cells Allow Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e111597. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.P.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by Size of Epithelial Tumor Cells: A New Method for the Immunomorphological and Molecular Characterization of Circulating Tumor Cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Laget, S.; Broncy, L.; Hormigos, K.; Dhingra, D.M.; Benmohamed, F.; Capiod, T.; Osteras, M.; Farinelli, L.; Jackson, S.; Paterlini-Bréchot, P. Technical Insights into Highly Sensitive Isolation and Molecular Characterization of Fixed and Live Circulating Tumor Cells for Early Detection of Tumor Invasion. PLoS ONE 2017, 12, e0169427. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Bonnetaud, C.; Ilié, M.; Vielh, P.; Vignaud, J.M.; Fléjou, J.F.; Lantuejoul, S.; Piaton, E.; Mourad, N.; Butori, C.; et al. Preoperative Circulating Tumor Cell Detection Using the Isolation by Size of Epithelial Tumor Cell Method for Patients with Lung Cancer Is a New Prognostic Biomarker. Clin. Cancer Res. 2011, 17, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Ilie, M.I.; Long, E.; Selva, E.; Bonnetaud, C.; Molina, T.; Venissac, N.; Mouroux, J.; Vielh, P.; Hofman, P. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: Comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int. J. Cancer 2010, 129, 1651–1660. [Google Scholar] [CrossRef]
- Marquette, C.-H.; Boutros, J.; Benzaquen, J.; Ferreira, M.; Pastre, J.; Pison, C.; Padovani, B.; Bettayeb, F.; Fallet, V.; Guibert, N.; et al. Circulating tumour cells as a potential biomarker for lung cancer screening: A prospective cohort study. Lancet Respir. Med. 2020, 8, 709–716. [Google Scholar] [CrossRef]
- Hofman, V.; Long, E.; Ilié, M.; Bonnetaud, C.; Vignaud, J.M.; Fléjou, J.F.; Lantuejoul, S.; Piaton, E.; Mourad, N.; Butori, C.; et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 2011, 23, 30–38. [Google Scholar] [CrossRef]
- Wechsler, J. Circulating Tumor Cells from Solid Cancers; Sauramps Medical Press: Montpellier, France, 2015. [Google Scholar]
- Alix-Panabières, C.; Pantel, K. Circulating Tumor Cells: Liquid Biopsy of Cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Schehr, J.L.; Schultz, Z.D.; Warrick, J.W.; Guckenberger, D.J.; Pezzi, H.M.; Sperger, J.M.; Heninger, E.; Saeed, A.; Leal, T.; Mattox, K.; et al. High Specificity in Circulating Tumor Cell Identification Is Required for Accurate Evaluation of Programmed Death-Ligand 1. PLoS ONE 2016, 11, e0159397. [Google Scholar] [CrossRef]
- Krebs, M.G.; Hou, J.-M.; Sloane, R.S.; Lancashire, L.; Priest, L.; Nonaka, D.; Ward, T.H.; Backen, A.; Clack, G.; Hughes, A.; et al. Analysis of Circulating Tumor Cells in Patients with Non-small Cell Lung Cancer Using Epithelial Marker-Dependent and -Independent Approaches. J. Thorac. Oncol. 2012, 7, 306–315. [Google Scholar] [CrossRef]
- Sonn, C.-H.; Cho, J.H.; Kim, J.-W.; Kang, M.S.; Lee, J.; Kim, J. Detection of circulating tumor cells in patients with non-small cell lung cancer using a size-based platform. Oncol. Lett. 2017, 13, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Nair, V.S.; Luttgen, M.S.; Keu, K.V.; Horng, G.; Vasanawala, M.; Kolatkar, A.; Jamali, M.; Iagaru, A.H.; Kuschner, W.; et al. Circulating Tumor Microemboli Diagnostics for Patients with Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 1111–1119. [Google Scholar] [CrossRef]
- Mascalchi, M.; Maddau, C.; Sali, L.; Bertelli, E.; Salvianti, F.; Zuccherelli, S.; Matucci, M.; Borgheresi, A.; Raspanti, C.; Lanzetta, M.M.; et al. Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions. J. Cancer 2017, 8, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, Y.; Upparahalli, S.V.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G.; Kaifi, J.T. Circulating tumor cell clusters are a potential biomarker for detection of non-small cell lung cancer. Lung Cancer 2019, 134, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N. Bring along your friends: Homotypic and heterotypic circulating tumor cell clustering to accelerate metastasis. Biomed. J. 2020, 43, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.-M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef]
- Manjunath, Y.; Mitchem, J.B.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Deroche, C.B.; Pantel, K.; Li, G.; Kaifi, J.T. Circulating Giant Tumor-Macrophage Fusion Cells Are Independent Prognosticators in Patients With NSCLC. J. Thorac. Oncol. 2020, 15, 1460–1471. [Google Scholar] [CrossRef]
- Zeinali, M.; Lee, M.; Nadhan, A.; Mathur, A.; Hedman, C.; Lin, E.; Harouaka, R.; Wicha, M.; Zhao, L.; Palanisamy, N.; et al. High-Throughput Label-Free Isolation of Heterogeneous Circulating Tumor Cells and CTC Clusters from Non-Small-Cell Lung Cancer Patients. Cancers 2020, 12, 127. [Google Scholar] [CrossRef]
- Lee, M.; Kim, E.J.; Cho, Y.; Kim, S.; Chung, H.H.; Park, N.H.; Song, Y.-S. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 361–365. [Google Scholar] [CrossRef]
- Bithi, S.S.; Vanapalli, S.A. Microfluidic cell isolation technology for drug testing of single tumor cells and their clusters. Sci. Rep. 2017, 7, 41707. [Google Scholar] [CrossRef]
- Sprouse, M.L.; Welte, T.; Boral, D.; Liu, H.N.; Yin, W.; Vishnoi, M.; Goswami-Sewell, D.; Li, L.; Pei, G.; Jia, P.; et al. PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling. Int. J. Mol. Sci. 2019, 20, 1916. [Google Scholar] [CrossRef]
- Akolkar, D.; Patil, D.; Crook, T.; Limaye, S.; Page, R.; Datta, V.; Patil, R.; Sims, C.; Ranade, A.; Fulmali, P.; et al. Circulating ensembles of tumor-associated cells: A redoubtable new systemic hallmark of cancer. Int. J. Cancer 2020, 146, 3485–3494. [Google Scholar] [CrossRef]
- Bayarri-Lara, C.; Ortega, F.G.; De Guevara, A.C.L.; Puche, J.L.; Zafra, J.R.; De Miguel-Pérez, D.; Ramos, A.S.-P.; Giraldo-Ospina, C.F.; Gómez, J.A.N.; Delgado-Rodríguez, M.; et al. Circulating Tumor Cells Identify Early Recurrence in Patients with Non-Small Cell Lung Cancer Undergoing Radical Resection. PLoS ONE 2016, 11, e0148659. [Google Scholar] [CrossRef]
- Chemi, F.; Rothwell, D.; McGranahan, N.; Gulati, S.; Abbosh, C.; Pearce, S.P.; Zhou, C.; Wilson, G.A.; Jamal-Hanjani, M.; Birkbak, N.; et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat. Med. 2019, 25, 1534–1539. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lee, C.-L.; Fu, J.-Y.; Yang, C.-T.; Wen, C.-T.; Liu, Y.-H.; Liu, H.-P.; Hsieh, J.C.-H.; Wu, C.-F. Circulating Tumor Cells as a Tool of Minimal Residual Disease Can Predict Lung Cancer Recurrence: A longitudinal, Prospective Trial. Diagnostics 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Lecharpentier, A.; Vielh, P.; Perez-Moreno, P.; Planchard, D.; Soria, J.C.; Farace, F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer 2011, 105, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.J.; Ilié, M.; Bonnetaud, C.; Selva, E.; Long, E.; Molina, T.; Vignaud, J.M.; Fléjou, J.F.; Lantuejoul, S.; Piaton, E.; et al. Cytopathologic Detection of Circulating Tumor Cells Using the Isolation by Size of Epithelial Tumor Cell Method. Am. J. Clin. Pathol. 2011, 135, 146–156. [Google Scholar] [CrossRef]
- Yang, L.; Yan, X.; Chen, J.; Zhan, Q.; Hua, Y.; Xu, S.; Li, Z.; Wang, Z.; Dong, Y.; Zuo, D.; et al. Hexokinase 2 discerns a novel circulating tumor cell population associated with poor prognosis in lung cancer patients. Proc. Natl. Acad. Sci. USA 2021, 118, 2012228118. [Google Scholar] [CrossRef] [PubMed]
| N (%) | N CTC+ve (%) | p-Value | |
|---|---|---|---|
| Patients with NSCLC | 74 (100) | 44 (59.5) | |
| Median (range) Age (years) | 71 (43–86) | ||
| Sex | |||
| Female | 33 (44.6) | 18 (54.5) | |
| Male | 41 (55.4) | 26 (63.4) | 0.4820 |
| Smoking habits | |||
| Current smoker | 25 (33.8) | 17 (68.0) | |
| Former smoker | 32 (43.2) | 18 (56.3) | |
| Never smoker | 16 (21.6) | 9 (56.3) | |
| Missing | 1 (1.4) | 0 | 0.7764 a |
| Tumor stage | |||
| IA | 27 (36.5) | 15 (55.6) | |
| IB | 5 (6.8) | 2 (40.0) | |
| IIA | 4 (5.4) | 4 (100) | |
| IIB | 16 (21.6) | 9 (56.3) | |
| IIIA | 11 (14.9) | 7 (63.6) | |
| IIIB | 6 (8.1) | 3 (50.0) | |
| IIIC | 0 | 0 | |
| IVA | 5 (6.8) | 4 (80.0) | 0.7964 b |
| Histology | |||
| Adenocarcinoma | 55 (74.3) | 33 (60.0) | |
| Squamous cell carcinoma | 16 (21.6) | 10 (62.5) | |
| Other | 2 (2.7) | 1 (50.0) | |
| Missing | 1 (1.4) | 0 | >0.9999 c |
| Grading | |||
| G1 | 4 (5.4) | 3 (75.0) | |
| G2 | 44 (59.5) | 23 (52.3) | |
| G3 | 24 (32.4) | 17 (70.8) | |
| G4 | 0 | 0 | |
| Missing | 2 (2.7) | 1 (50.0) | 0.2092 d |
| Visceral pleura invasion | |||
| PL0 | 56 (75.7) | 31 (55.4) | |
| PL1 | 8 (10.8) | 6 (75.0) | |
| PL2 | 5 (6.8) | 3 (60.0) | |
| PL3 | 2 (2.7) | 2 (100) | |
| Missing | 3 (4.1) | 2 (66.7) | 0.2494 e |
| Peritumoral neoplastic angioinvasion | |||
| Absent | 66 (89.2) | 42 (63.6) | |
| Present | 6 (8.1) | 1 (16.7) | |
| Missing | 2 (2.7) | 1 (50.0) | 0.0357 |
| Lymph-node status | |||
| Negative | 48 (64.9) | 29 (60.4) | |
| Positive | 25 (33.8) | 14 (56.0) | |
| Missing | 1 (1.4) | 0 | 0.8039 |
| Healthy volunteers | 10 | ||
| Median (range) Age (years) | 35 (33–47) | ||
| Sex | |||
| Female | 7 (70.0) | 0 | - |
| Male | 3 (30.0) | 0 | - |
| Smoking habits | |||
| Smoker | 2 (20.0) | 0 | - |
| Never smoker | 8 (80.0) | 0 | - |
| High-risk subjects | 7 | ||
| Median (range) Age (years) | 63 (53–73) | ||
| Sex | |||
| Female | 3 (42.9) | 0 | - |
| Male | 4 (57.1) | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fina, E.; Federico, D.; Novellis, P.; Dieci, E.; Monterisi, S.; Cioffi, F.; Mangiameli, G.; Finocchiaro, G.; Alloisio, M.; Veronesi, G. Subpopulations of Circulating Cells with Morphological Features of Malignancy Are Preoperatively Detected and Have Differential Prognostic Significance in Non-Small Cell Lung Cancer. Cancers 2021, 13, 4488. https://doi.org/10.3390/cancers13174488
Fina E, Federico D, Novellis P, Dieci E, Monterisi S, Cioffi F, Mangiameli G, Finocchiaro G, Alloisio M, Veronesi G. Subpopulations of Circulating Cells with Morphological Features of Malignancy Are Preoperatively Detected and Have Differential Prognostic Significance in Non-Small Cell Lung Cancer. Cancers. 2021; 13(17):4488. https://doi.org/10.3390/cancers13174488
Chicago/Turabian StyleFina, Emanuela, Davide Federico, Pierluigi Novellis, Elisa Dieci, Simona Monterisi, Federica Cioffi, Giuseppe Mangiameli, Giovanna Finocchiaro, Marco Alloisio, and Giulia Veronesi. 2021. "Subpopulations of Circulating Cells with Morphological Features of Malignancy Are Preoperatively Detected and Have Differential Prognostic Significance in Non-Small Cell Lung Cancer" Cancers 13, no. 17: 4488. https://doi.org/10.3390/cancers13174488
APA StyleFina, E., Federico, D., Novellis, P., Dieci, E., Monterisi, S., Cioffi, F., Mangiameli, G., Finocchiaro, G., Alloisio, M., & Veronesi, G. (2021). Subpopulations of Circulating Cells with Morphological Features of Malignancy Are Preoperatively Detected and Have Differential Prognostic Significance in Non-Small Cell Lung Cancer. Cancers, 13(17), 4488. https://doi.org/10.3390/cancers13174488






