Clinical Impact of Unexpected Para-Aortic Lymph Node Metastasis in Surgery for Resectable Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
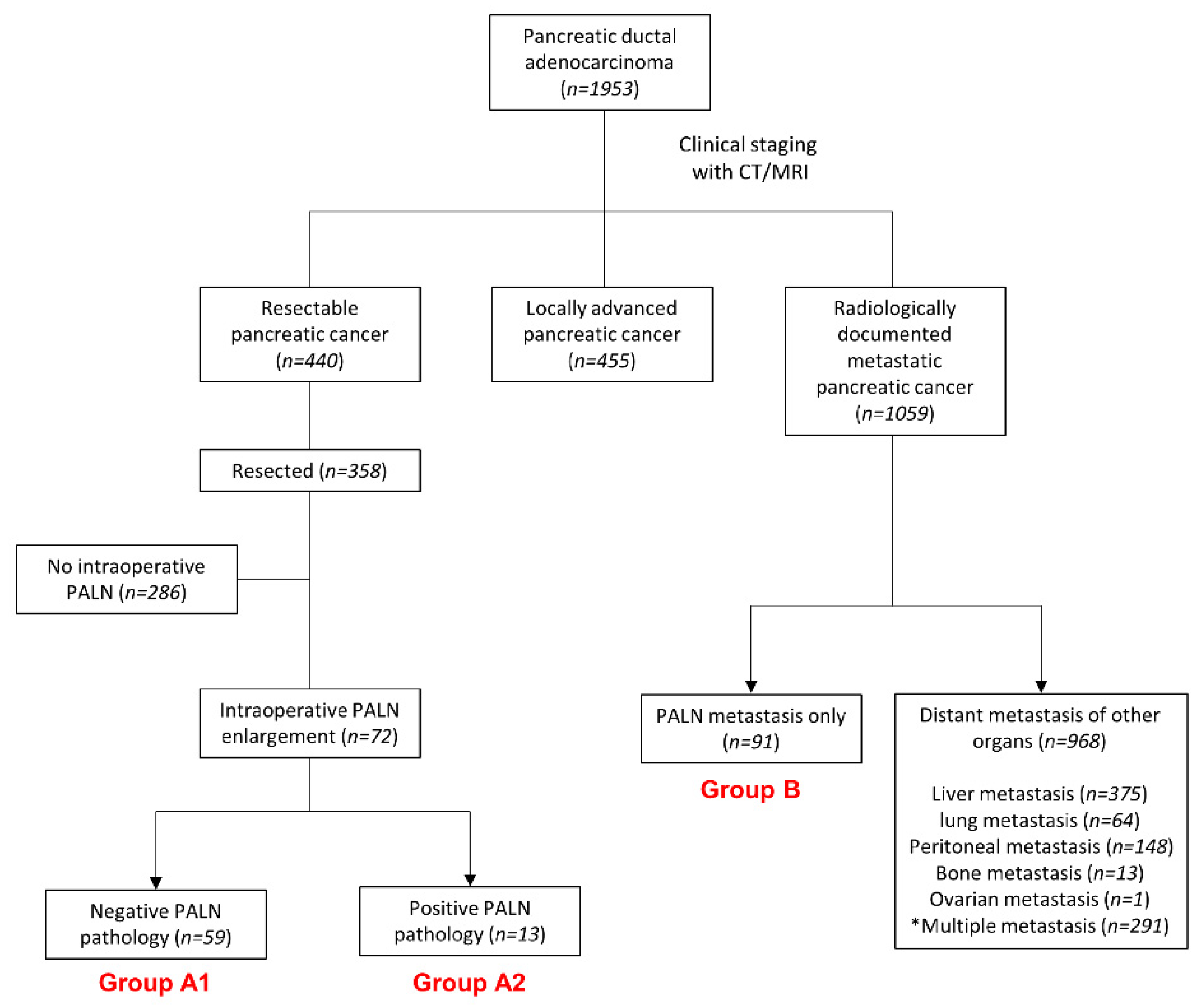
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
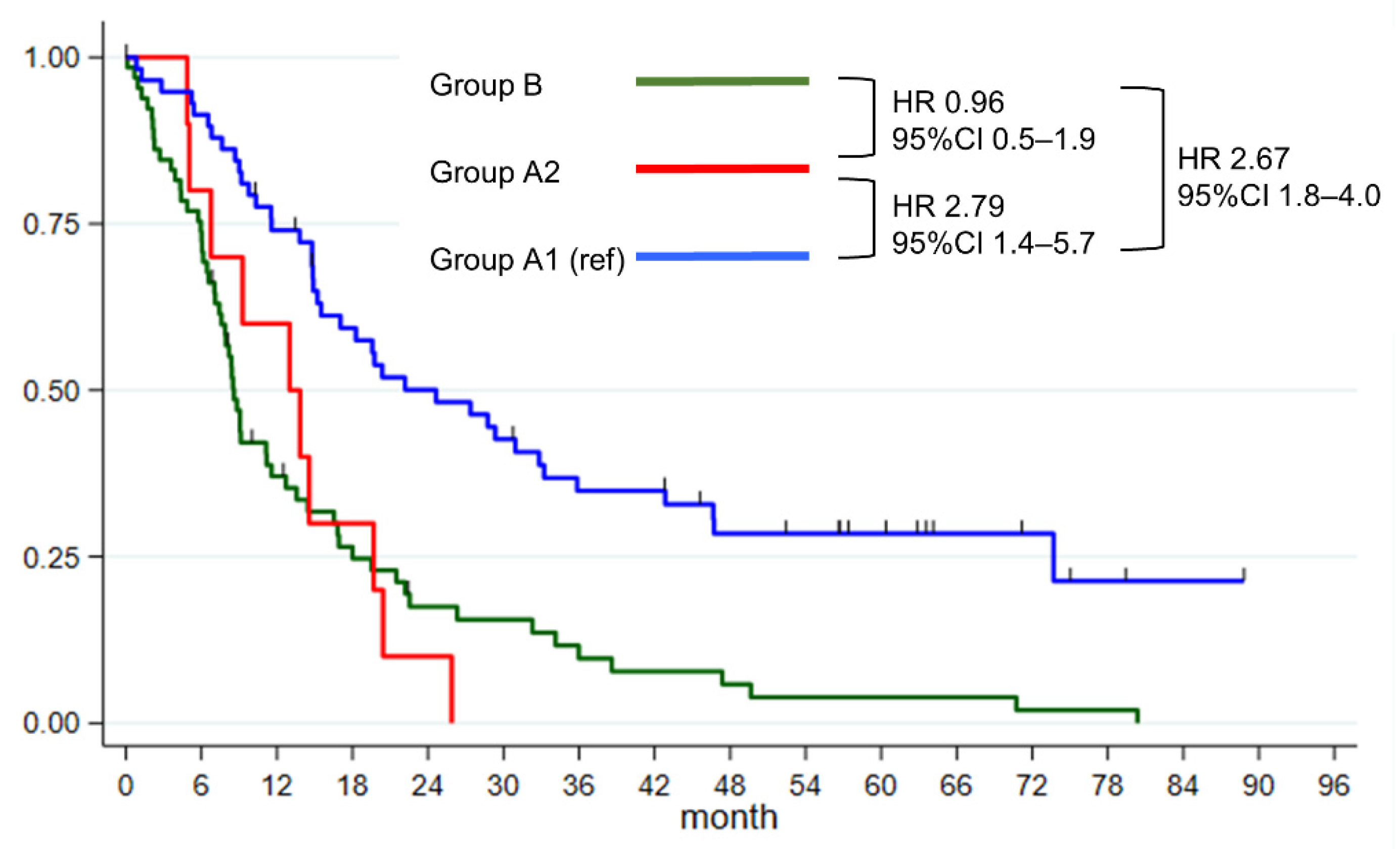
3.2. Survival of Patients with Unexpected PALN Enlargements
3.3. Other Prognostic Factors Affecting Overall Survival
3.4. Surgical Information including Postoperative Complication
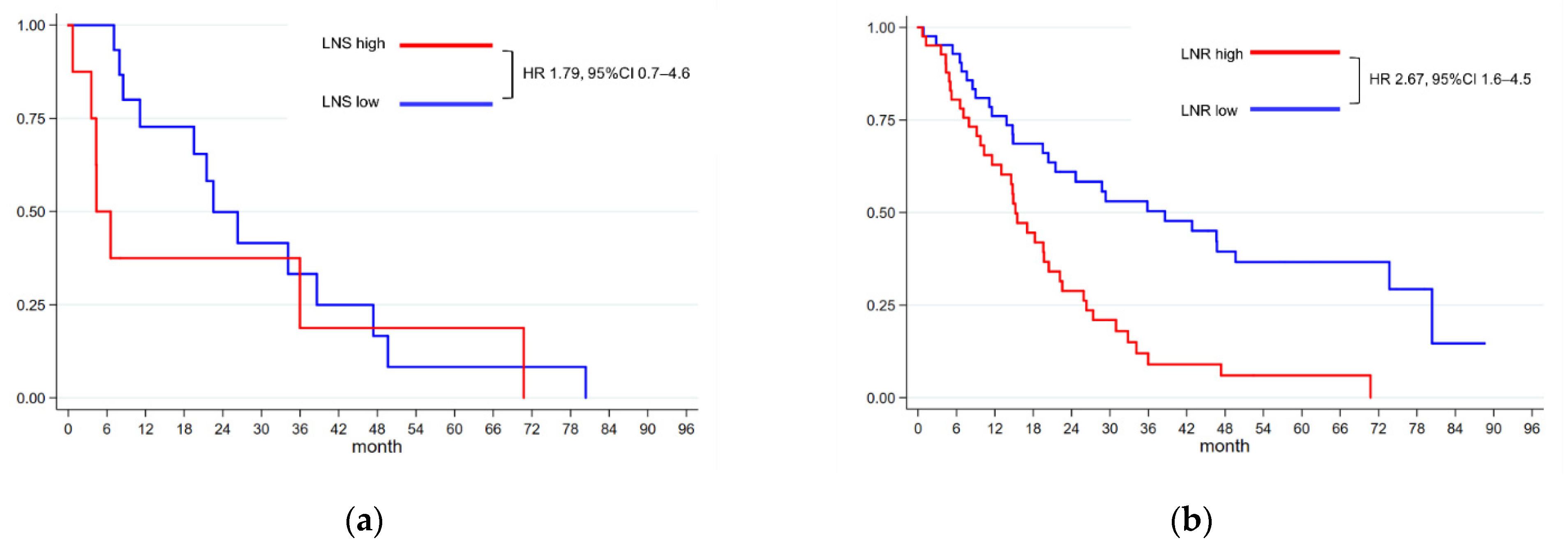
3.5. Effects of Overall Lymph Node Status
3.6. Other Medical Information
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Nitecki, S.S.; Sarr, M.G.; Colby, T.V.; van Heerden, J.A. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann. Surg. 1995, 221, 59–66. [Google Scholar] [CrossRef]
- Conlon, K.C.; Klimstra, D.S.; Brennan, M.F. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann. Surg. 1996, 223, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Riall, T.S.; Coleman, J.; Belcher, K.A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 2006, 244, 10–15. [Google Scholar] [CrossRef]
- Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Arnold, M.A.; Chang, D.C.; Coleman, J.; Hodgin, M.B.; Sauter, P.K.; Hruban, R.H.; Riall, T.S.; et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006, 10, 1199–1210. [Google Scholar] [CrossRef]
- Allema, J.H.; Reinders, M.E.; van Gulik, T.M.; Koelemay, M.J.; Van Leeuwen, D.J.; de Wit, L.T.; Gouma, D.J.; Obertop, H. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer 1995, 75, 2069–2076. [Google Scholar] [CrossRef]
- Lim, J.E.; Chien, M.W.; Earle, C.C. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann. Surg. 2003, 237, 74–85. [Google Scholar] [CrossRef]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Sandini, M.; Gianotti, L.; Butturini, G.; Salvia, R.; Bassi, C. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 616–624. [Google Scholar] [CrossRef]
- Agalianos, C.; Gouvas, N.; Papaparaskeva, K.; Dervenis, C. Positive para-aortic lymph nodes following pancreatectomy for pancreatic cancer. Systematic review and meta-analysis of impact on short term survival and association with clinicopathologic features. HPB 2016, 18, 633–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komo, T.; Murakami, Y.; Kondo, N.; Uemura, K.; Hashimoto, Y.; Nakagawa, N.; Urabe, K.; Takahashi, S.; Sueda, T. Prognostic Impact of Para-Aortic Lymph Node Micrometastasis in Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2016, 23, 2019–2027. [Google Scholar] [CrossRef]
- Sho, M.; Murakami, Y.; Motoi, F.; Satoi, S.; Matsumoto, I.; Kawai, M.; Honda, G.; Uemura, K.; Yanagimoto, H.; Kurata, M.; et al. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: A multicenter study on 822 patients. J. Gastroenterol. 2015, 50, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Redaelli, C.; Lietz, M.; Seiler, C.A.; Friess, H.; Büchler, M.W. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br. J. Surg. 2004, 91, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Niedergethmann, M.; Sturm, J.W.; Lorenz, D.; Post, S.; Trede, M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J. Surg. 2003, 27, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Coupland, V.H.; Kocher, H.M.; Berry, D.P.; Allum, W.; Linklater, K.M.; Konfortion, J.; Møller, H.; Davies, E.A. Incidence and survival for hepatic, pancreatic and biliary cancers in England between 1998 and 2007. Cancer Epidemiol. 2012, 36, e207–e214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Hwang, H.K.; Lee, W.J.; Kang, C.M. Unexpected Para-aortic Lymph Node Metastasis in Pancreatic Ductal Adenocarcinoma: A Contraindication to Resection? J. Gastrointest. Surg. 2020, 24, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Gruppo, M.; Blandamura, S.; Valmasoni, M.; Pozza, G.; Passuello, N.; Beltrame, V.; Moletta, L. Para-aortic node involvement is not an independent predictor of survival after resection for pancreatic cancer. World J. Gastroenterol. 2017, 23, 4399–4406. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzis, G.; Groot, V.P.; Blair, A.B.; Laheru, D.A.; Zheng, L.; Narang, A.K.; Fishman, E.K.; Hruban, R.H.; Yu, J.; Burkhart, R.A.; et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann. Surg. 2019, 270, 340–347. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.C.; Lee, J.; Kim, J.W.; Kim, J.; Hwang, J.H. Early versus delayed initiation of adjuvant treatment for pancreatic cancer. PLoS ONE 2017, 12, e0173960. [Google Scholar] [CrossRef]
- Fischer, L.K.; Katz, M.H.; Lee, S.M.; Liu, L.; Wang, H.; Varadhachary, G.R.; Wolff, R.A.; Lee, J.E.; Maitra, A.; Roland, C.L.; et al. The number and ratio of positive lymph nodes affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology 2016, 68, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, K.A.; Hollenbeak, C.S.; Wong, J. Greater lymph node retrieval and lymph node ratio impacts survival in resected pancreatic cancer. J. Surg. Res. 2017, 220, 12–24. [Google Scholar] [CrossRef]
- Ashfaq, A.; Pockaj, B.A.; Gray, R.J.; Halfdanarson, T.R.; Wasif, N. Nodal counts and lymph node ratio impact survival after distal pancreatectomy for pancreatic adenocarcinoma. J. Gastrointest. Surg. 2014, 18, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Warschkow, R.; Widmann, B.; Beutner, U.; Marti, L.; Steffen, T.; Schiesser, M.; Schmied, B.M. The More the Better-Lower Rate of Stage Migration and Better Survival in Patients with Retrieval of 20 or More Regional Lymph Nodes in Pancreatic Cancer: A Population-Based Propensity Score Matched and Trend SEER Analysis. Pancreas 2017, 46, 648–657. [Google Scholar] [CrossRef]
- Doussot, A.; Bouvier, A.; Santucci, N.; Lequeu, J.B.; Cheynel, N.; Ortega-Deballon, P.; Rat, P.; Facy, O. Pancreatic ductal adenocarcinoma and paraaortic lymph nodes metastases: The accuracy of intraoperative frozen section. Pancreatology 2019, 19, 710–715. [Google Scholar] [CrossRef]
- Schwarz, L.; Lupinacci, R.M.; Svrcek, M.; Lesurtel, M.; Bubenheim, M.; Vuarnesson, H.; Balladur, P.; Paye, F. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br. J. Surg. 2014, 101, 530–538. [Google Scholar] [CrossRef]
- Imai, H.; Doi, R.; Kanazawa, H.; Kamo, N.; Koizumi, M.; Masui, T.; Iwanaga, Y.; Kawaguchi, Y.; Takada, Y.; Isoda, H.; et al. Preoperative assessment of para-aortic lymph node metastasis in patients with pancreatic cancer. Int. J. Clin. Oncol. 2010, 15, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Karabagli, P.; Ugras, S.; Yilmaz, B.S.; Celik, C. The evaluation of reliability and contribution of frozen section pathology to staging endometrioid adenocarcinomas. Arch. Gynecol. Obstet. 2015, 292, 391–397. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group A1 (n = 59) | Group A2 (n = 13) | Group B (n = 91) | Total Patients (n = 163) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 63 (53–72) | 60 (57–66) | 67 (60–76) | 65 (58–74) | 0.321 |
| Sex | 0.790 | ||||
| Male | 29 (49.1) | 5 (38.5) | 45 (49.5) | 79 (48.5) | |
| Female | 30 (50.8) | 8 (61.5) | 46 (50.6) | 84 (51.5) | |
| BMI | 22.1 (20.5–23.9) | 21.30 (19.7–23.5) | 22.58 (20.7–25.0) | 22.33 (20.5–24.8) | 0.331 |
| Initial tumor markers | |||||
| CA 19-9 | 101.8 (38.2–430) | 191.9 (41–1653.75) | 240 (74–731.3) | 180.9 (49–607.5) | 0.081 |
| CEA | 2.9 (1.4–5.15) | 4.9 (3.6–5.4) | 2.55 (1.9– 5) | 2.8 (1.65–5.1) | 0.302 |
| Tumor location | 0.175 | ||||
| Head | 49 (83.1) | 13 (100) | 62 (68.1) | 124 (76.1) | |
| Body | 7 (11.9) | 0 | 15 (16.5) | 22 (13.5) | |
| Tail | 2 (3.3) | 0 | 11 (12.1) | 13 (8.0) | |
| Multiple | 1 (1.7) | 0 | 3 (3.3) | 4 (2.4) | |
| Tumor size (cm) | 2.7 (2–3.3) | 2.7 (2.1–3.3) | 3.2 (2.5–4.2) | 3.00 (2.4–3.9) | 0.102 |
| Concomitant regional lymph node * | 5 (8.5) | 1 (7.7) | 39 (42.9) | 45 (27.6) | <0.001 |
| Variable (n) | Median Survival (month) | 95% CI | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| Overall patients | 14.6 | 11.1–17.1 | ||||||
| Sex | ||||||||
| Male (67) | 16.9 | 11.5–20.4 | – | |||||
| Female (67) | 13.0 | 8.8–15.5 | 0.92 | 0.6–1.3 | 0.680 | |||
| Age | ||||||||
| Age ≤ 65 (65) | 19.6 | 14.9–27.4 | – | – | ||||
| Age > 65 (69) | 8.7 | 6.6–13.0 | 1.98 | 1.4–2.9 | <0.001 | 1.73 | 1.1–2.7 | 0.012 |
| BMI | ||||||||
| BMI ≤ 22.33 (61) | 14.8 | 10.3–17.1 | – | |||||
| BMI > 22.33 (54) | 21.5 | 11.1–29.3 | 0.70 | 0.5–1.1 | 0.089 | |||
| Group (ref A1) | – | |||||||
| Group A1 | 24.6 | 15.2–33.2 | – | – | ||||
| Group A2 | 13.0 | 4.9–19.7 | 2.79 | 1.4–5.7 | 0.005 | 2.57 | 1.2–5.3 | 0.010 |
| Group B | 8.6 | 7.4–11.6 | 2.67 | 1.8–4.0 | <0.001 | 1.72 | 1.0–2.9 | 0.041 |
| Group (ref A2) * | ||||||||
| Group B | 24.6 | 15.2–33.2 | 0.96 | 0.5–1.9 | 0.905 | |||
| Regional lymph node | ||||||||
| No metastasis | 14.9 | 11.2–20.4 | ||||||
| Metastasis | 11.6 | 7.9–16.5 | 1.69 | 1.1–2.5 | 0.010 | 1.32 | 0.8–2.3 | 0.315 |
| CA19–9 | ||||||||
| CA19–9 ≤ 180 (60) | 21.5 | 14.5–28.8 | – | – | ||||
| CA19–9 > 180 (56) | 11.6 | 9.2–15.5 | 1.70 | 1.1–2.6 | 0.011 | 1.49 | 0.97–2.3 | 0.067 |
| CEA | ||||||||
| CEA ≤ 2.8 (39) | 15.5 | 11.2–21.5 | – | |||||
| CEA > 2.8 (51) | 13.0 | 8.0–18.3 | 1.16 | 0.7–1.8 | 0.543 | |||
| Tumor size | ||||||||
| Tumor size ≤ 3.0 (59) | 14.8 | 11.2–24.6 | ||||||
| Tumor size > 3.0 (75) | 13.0 | 8.5–18.0 | 1.43 | 1.0–2.1 | 0.069 | |||
| Patient Group (n, %) | A1 (59, 100%) | A2 (13, 100%) | p-Value |
|---|---|---|---|
| OP type | 0.477 | ||
| Pancreaticoduodenectomy * | 47 (80%) | 11 (85%) | |
| Distal pancreatectomy | 5 (8.5%) | 0 (0%) | |
| Total pancreatectomy | 5 (8.5%) | 1 (7.5%) | |
| Others † | 2 (3.4%) | 1 (7.5%) | |
| Resection margin | 0.272 | ||
| Negative | 41 (69%) | 6 (46%) | |
| Positive | 14 (24%) | 5 (38%) | |
| Overall number of dissected LN (median, IQR) | 24 (16–33) | 25 (15–32) | 0.915 |
| No of harvested PALN | 6 (2–9) | 3 (1–5) | 0.117 |
| No of harvested regional LN | 18 (11–25) | 22 (12–31) | 0.476 |
| Pathologic T staging | 0.505 | ||
| Tx | 0 (0%) | 2 (15%) | |
| T1–T2 | 47 (80%) | 10 (77%) | |
| T3–T4 | 12 (20%) | 1 (8%) | |
| Pathologic N staging | 0.191 | ||
| Nx | 2 (3%) | 2 (15%) | |
| N0 | 27 (46%) | 0 (0%) | |
| N1 | 33 (56%) | 3 (23%) | |
| N2 | 7 (12%) | 8 (62%) | |
| Ratio of positive regional LN (%) | |||
| 8.1 (0–30.7) | 26.67 (0–51.9) | 0.0002 | |
| Pathologic M staging ‡ | |||
| M0 | 58 (98%) | 0 (0%) | |
| M1 | 1 (2%) | 11 (100%) | |
| Moderate to severe surgical complication | |||
| All complication | 32 (54%) | 4 (31%) | 0.163 |
| Surgical site infection | 4 (7%) | 1 (8%) | 0.826 |
| Postoperative hemorrhage | 4 (7%) | 0 (0%) | 0.831 |
| Pancreas fistula | 9 (15%) | 0 (0%) | 0.344 |
| Liver abscess | 1 (2%) | 0 (0%) | 0.647 |
| Others § | 14 (24%) | 3 (23%) |
| Patient Group (n, %) | A1 (59) | A2 (13) | B (91) | p-Value |
|---|---|---|---|---|
| First-line palliative chemotherapy | 0.918 | |||
| FOLFIRINOX | 2 (3.4%) | 2 (15.4%) | 25 (27.5%) | |
| Gemcitabine with nab-paclitaxel | 0 (0%) | 0 (0%) | 5 (5.5%) | |
| Gemcitabine monotherapy | 1 (1.7%) | 1 (7.7%) | 6 (6.6%) | |
| Other gemcitabine-based chemotherapy | 3 (5.1%) | 1 (7.7%) | 12 (13.2%) | |
| Adjuvant chemotherapy * | 0.012 | |||
| Modified FOLFIRINOX | 1 (1.7%) | 1 (7.7%) | 3 (3.3%) | |
| Other 5-FU-based regimen | 1 (1.7%) | 0 (0%) | 0 (0%) | |
| Gemcitabine monotherapy | 28 (47.5%) | 3 (23.1%) | 6 (6.6%) | |
| Other gemcitabine-based chemotherapy | 5 (8.5%) | 1 (7.7%) | 3 (3.3%) | |
| Pre-operative chemotherapy | 0.230 | |||
| FOLFIRINOX | 2 (3.4%) | 1 (7.7%) | 0 (0%) | |
| Gemcitabine monotherapy | 0 (0%) | 0 (0%) | 0 (0%) | |
| Gemcitabine + Erlotinib | 2 (3.4%) | 0 (0%) | 0 (0%) | |
| Radiation therapy | <0.001 | |||
| Yes | 19 (32.2%) | 1 (7.7%) | 4 (4.4%) | |
| No | 40 (67.8%) | 12 (92.3%) | 87 (95.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-K.; Yoon, Y.-S.; Han, H.-S.; Lee, J.S.; Na, H.Y.; Ahn, S.; Park, J.; Jung, K.; Jung, J.H.; Kim, J.; et al. Clinical Impact of Unexpected Para-Aortic Lymph Node Metastasis in Surgery for Resectable Pancreatic Cancer. Cancers 2021, 13, 4454. https://doi.org/10.3390/cancers13174454
Lee H-K, Yoon Y-S, Han H-S, Lee JS, Na HY, Ahn S, Park J, Jung K, Jung JH, Kim J, et al. Clinical Impact of Unexpected Para-Aortic Lymph Node Metastasis in Surgery for Resectable Pancreatic Cancer. Cancers. 2021; 13(17):4454. https://doi.org/10.3390/cancers13174454
Chicago/Turabian StyleLee, Ho-Kyoung, Yoo-Seok Yoon, Ho-Seong Han, Jun Suh Lee, Hee Young Na, Soomin Ahn, Jaewoo Park, Kwangrok Jung, Jae Hyup Jung, Jaihwan Kim, and et al. 2021. "Clinical Impact of Unexpected Para-Aortic Lymph Node Metastasis in Surgery for Resectable Pancreatic Cancer" Cancers 13, no. 17: 4454. https://doi.org/10.3390/cancers13174454
APA StyleLee, H.-K., Yoon, Y.-S., Han, H.-S., Lee, J. S., Na, H. Y., Ahn, S., Park, J., Jung, K., Jung, J. H., Kim, J., Hwang, J.-H., & Lee, J.-C. (2021). Clinical Impact of Unexpected Para-Aortic Lymph Node Metastasis in Surgery for Resectable Pancreatic Cancer. Cancers, 13(17), 4454. https://doi.org/10.3390/cancers13174454





