Risk Score Model for Microvascular Invasion in Hepatocellular Carcinoma: The Role of Tumor Burden and Alpha-Fetoprotein
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Strategy and Liver Resection
2.3. Clinicopathological Profiles and Definitions
2.4. Random Split of Patients and Construct the Risk Score Model
2.5. Biostatistics
3. Results
3.1. Prognostic Value of Microvascular Invasion
3.2. Characteristics of the Patients
3.3. Prognostic Factors for Microvascular Invasion in Training Dataset
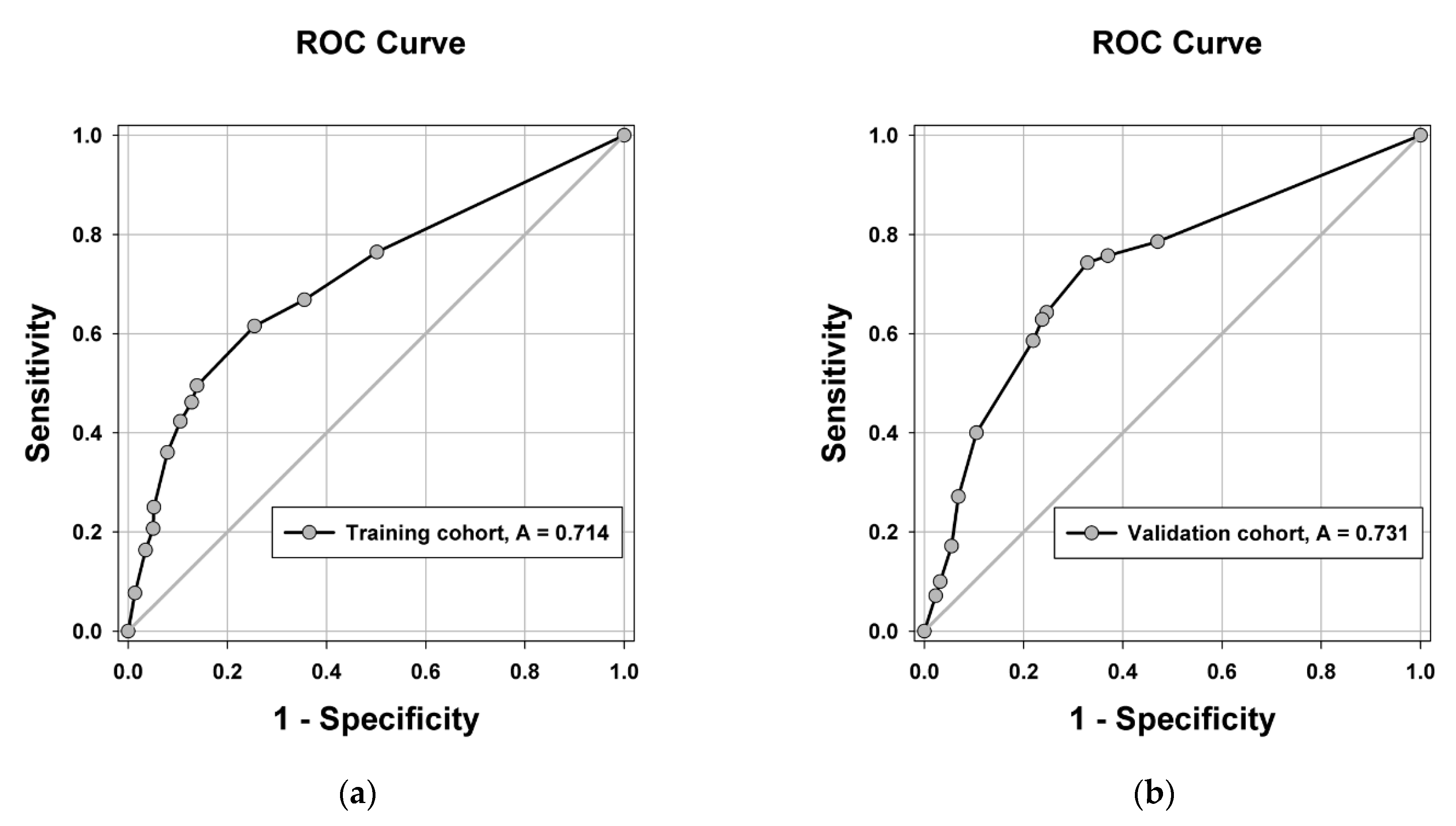
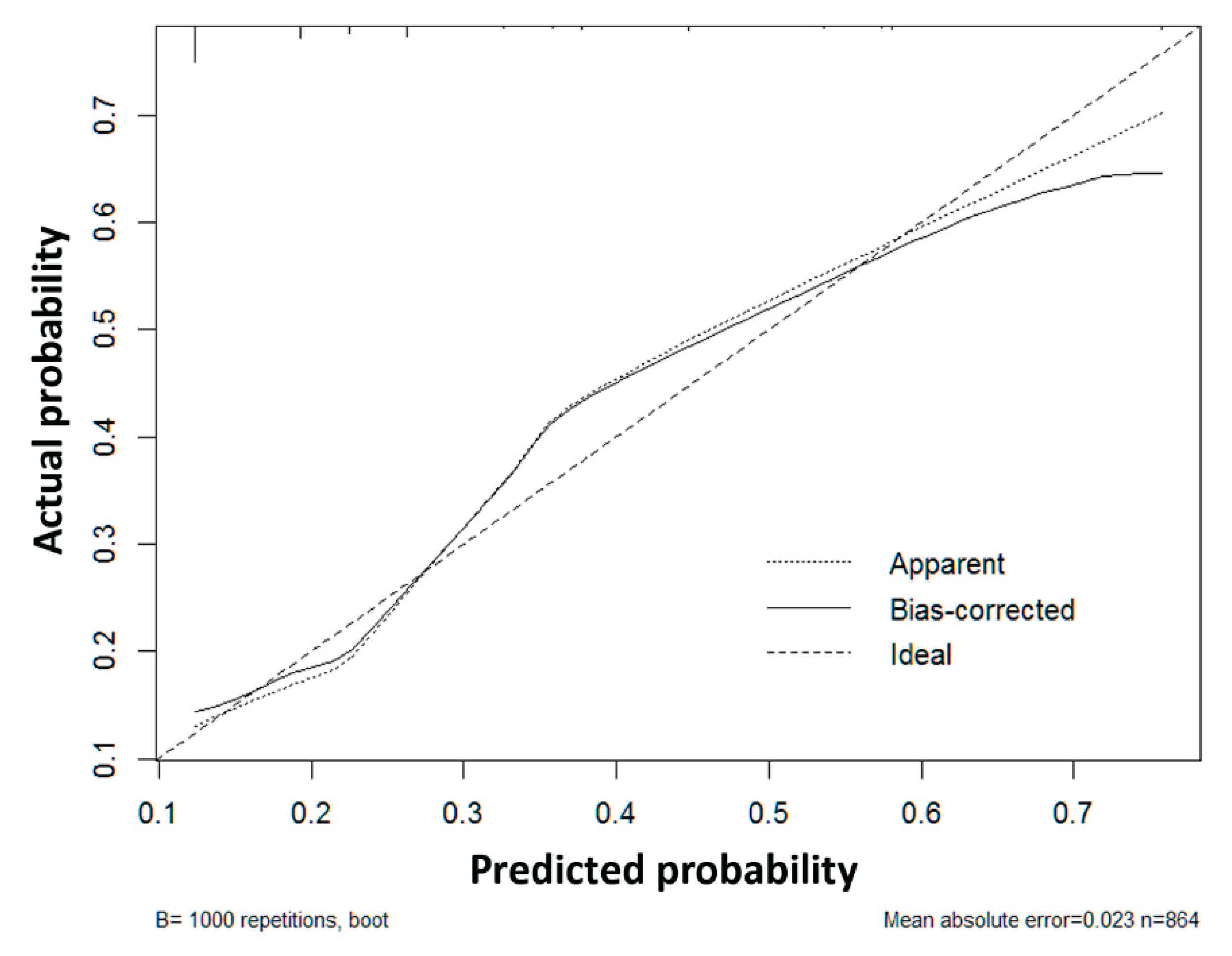
3.4. Construction and Validation of the Risk Score Model for Microvascular Invasion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Cheng, C.H.; Wang, Y.C.; Wu, T.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Chan, K.M.; Lee, W.C. Clinical relevance of alpha-fetoprotein in determining resection margin for hepatocellular carcinoma. Medicine 2019, 98, e14827. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.T.; Mau Lo, C.; Poon, R.T.; Yeung, C.; Leung Liu, C.; Yuen, W.K.; Ming Lam, C.; Ng, K.K.; Ching Chan, S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: A 20-year experience. Ann. Surg. 2011, 253, 745–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nara, S.; Shimada, K.; Sakamoto, Y.; Esaki, M.; Kishi, Y.; Kosuge, T.; Ojima, H. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: Evidence from 570 hepatectomies. Surgery 2012, 151, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Tung-Ping Poon, R.; Fan, S.T.; Wong, J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann. Surg. 2000, 232, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Hanazaki, K.; Kajikawa, S.; Shimozawa, N.; Mihara, M.; Shimada, K.; Hiraguri, M.; Koide, N.; Adachi, W.; Amano, J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J. Am. Coll. Surg. 2000, 191, 381–388. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; Practice Guidelines Committee. AAftSoLD: Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef]
- Rodriguez-Peralvarez, M.; Luong, T.V.; Andreana, L.; Meyer, T.; Dhillon, A.P.; Burroughs, A.K. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann. Surg. Oncol. 2013, 20, 325–339. [Google Scholar] [CrossRef]
- Lauwers, G.Y.; Terris, B.; Balis, U.J.; Batts, K.P.; Regimbeau, J.M.; Chang, Y.; Graeme-Cook, F.; Yamabe, H.; Ikai, I.; Cleary, K.R.; et al. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: A multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am. J. Surg. Pathol. 2002, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Lee, J.C.; Cheng, C.H.; Wu, T.H.; Wang, Y.C.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Chan, K.M.; Lee, W.C. Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection. J. Hepatobiliary Pancreat. Sci. 2017, 24, 559–569. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tsuijita, E.; Takeishi, K.; Fujiwara, M.; Kira, S.; Mori, M.; Aishima, S.; Taketomi, A.; Shirabe, K.; Ishida, T.; et al. Predictors for microinvasion of small hepatocellular carcinoma ≤ 2 cm. Ann. Surg. Oncol. 2012, 19, 2027–2034. [Google Scholar] [CrossRef]
- Shen, J.; Wen, J.; Li, C.; Wen, T.; Yan, L.; Li, B.; Yang, J.; Lu, C. The prognostic value of microvascular invasion in early-intermediate stage hepatocelluar carcinoma: A propensity score matching analysis. BMC Cancer 2018, 18, 278. [Google Scholar] [CrossRef]
- Bertuzzo, V.R.; Cescon, M.; Ravaioli, M.; Grazi, G.L.; Ercolani, G.; Del Gaudio, M.; Cucchetti, A.; D’Errico-Grigioni, A.; Golfieri, R.; Pinna, A.D. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation 2011, 91, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Hemming, A.W.; Cattral, M.S.; Reed, A.I.; Van Der Werf, W.J.; Greig, P.D.; Howard, R.J. Liver transplantation for hepatocellular carcinoma. Ann. Surg. 2001, 233, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwatsuki, S.; Dvorchik, I.; Marsh, J.W.; Madariaga, J.R.; Carr, B.; Fung, J.J.; Starzl, T.E. Liver transplantation for hepatocellular carcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 2000, 191, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Esnaola, N.F.; Lauwers, G.Y.; Mirza, N.Q.; Nagorney, D.M.; Doherty, D.; Ikai, I.; Yamaoka, Y.; Regimbeau, J.M.; Belghiti, J.; Curley, S.A.; et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J. Gastrointest. Surg. 2002, 6, 224–232. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Delman, K.A.; Vauthey, J.N.; Nagorney, D.M.; Ng, I.O.; Ikai, I.; Yamaoka, Y.; Belghiti, J.; Lauwers, G.Y.; Poon, R.T.; et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005, 11, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.C.; Fan, L.F.; Yang, N.; Zhang, H.B.; Chen, B.D.; Yang, G.S. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur. J. Surg. Oncol. 2013, 39, 858–864. [Google Scholar] [CrossRef]
- Lei, Z.; Li, J.; Wu, D.; Xia, Y.; Wang, Q.; Si, A.; Wang, K.; Wan, X.; Lau, W.Y.; Wu, M.; et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma within the Milan Criteria. JAMA Surg. 2016, 151, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wei, J.; Gu, D.; Zhu, Y.; Feng, B.; Liang, M.; Wang, S.; Zhao, X.; Tian, J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur. Radiol. 2019. [Google Scholar] [CrossRef]
- Euhus, D.M.; Hudd, C.; LaRegina, M.C.; Johnson, F.E. Tumor measurement in the nude mouse. J. Surg. Oncol. 1986, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Imura, S.; Teraoku, H.; Yoshikawa, M.; Ishikawa, D.; Yamada, S.; Saito, Y.; Iwahashi, S.; Ikemoto, T.; Morine, Y.; Shimada, M. Potential predictive factors for microvascular invasion in hepatocellular carcinoma classified within the Milan criteria. Int. J. Clin. Oncol. 2018, 23, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H. The prediction of microvascular invasion of hepatocellular carcinoma using multiple imaging modalities. Hepatoma Res. 2018, 4, 75. [Google Scholar] [CrossRef] [Green Version]
- Cuccurullo, V.; Di Stasio, G.D.; Mazzarella, G.; Cascini, G.L. Microvascular Invasion in HCC: The Molecular Imaging Perspective. Contrast. Media Mol. Imaging 2018, 2018, 9487938. [Google Scholar] [CrossRef] [Green Version]
- Centonze, L.; Di Sandro, S.; Lauterio, A.; De Carlis, R.; Frassoni, S.; Rampoldi, A.; Tuscano, B.; Bagnardi, V.; Vanzulli, A.; De Carlis, L. Surgical Resection vs. Percutaneous Ablation for Single Hepatocellular Carcinoma: Exploring the Impact of Li-RADS Classification on Oncological Outcomes. Cancers 2021, 13, 1671. [Google Scholar] [CrossRef]
- Lin, S.; Ye, F.; Rong, W.; Song, Y.; Wu, F.; Liu, Y.; Zheng, Y.; Siqin, T.; Zhang, K.; Wang, L.; et al. Nomogram to Assist in Surgical Plan for Hepatocellular Carcinoma: A Prediction Model for Microvascular Invasion. J. Gastrointest. Surg. 2019, 23, 2372–2382. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, J.; Zhang, Q.; Xu, Y.; Zhou, J.; Liu, L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn. Interv. Radiol. 2018, 24, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Cucchetti, A.; Piscaglia, F.; Grigioni, A.D.; Ravaioli, M.; Cescon, M.; Zanello, M.; Grazi, G.L.; Golfieri, R.; Grigioni, W.F.; Pinna, A.D. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: A pilot study. J. Hepatol. 2010, 52, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Zhao, H.; Bi, X.Y.; Li, Z.Y.; Huang, Z.; Han, Y.; Zhou, J.G.; Zhao, J.J.; Zhang, Y.F.; Wei, W.Q.; et al. Total tumor volume predicts survival following liver resection in patients with hepatocellular carcinoma. Tumour. Biol. 2016, 37, 9301–9310. [Google Scholar] [CrossRef]
- Toso, C.; Trotter, J.; Wei, A.; Bigam, D.L.; Shah, S.; Lancaster, J.; Grant, D.R.; Greig, P.D.; Shapiro, A.M.; Kneteman, N.M. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008, 14, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Chen, W.J.; Lai, P.L.; Jeng, Y.M.; Sheu, J.C.; Hsu, H.C. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: Significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int. J. Cancer 2004, 112, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.; Ioannou, G.N. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013, 19, 634–645. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994.e983, quiz e914–e985. [Google Scholar] [CrossRef] [PubMed]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.F.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Z.L.; Xing, H.; Wu, H.; Zhu, P.; Lau, W.Y.; Zhou, Y.H.; Gu, W.M.; Wang, H.; Chen, T.H.; et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: A multi-institutional study. HPB 2019, 21, 962–971. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Z.; Shi, X.L.; He, X.W.; Chen, J.; Song, J.H. Anatomic versus non-anatomic resection of hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Asian J. Surg. 2021, 44, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
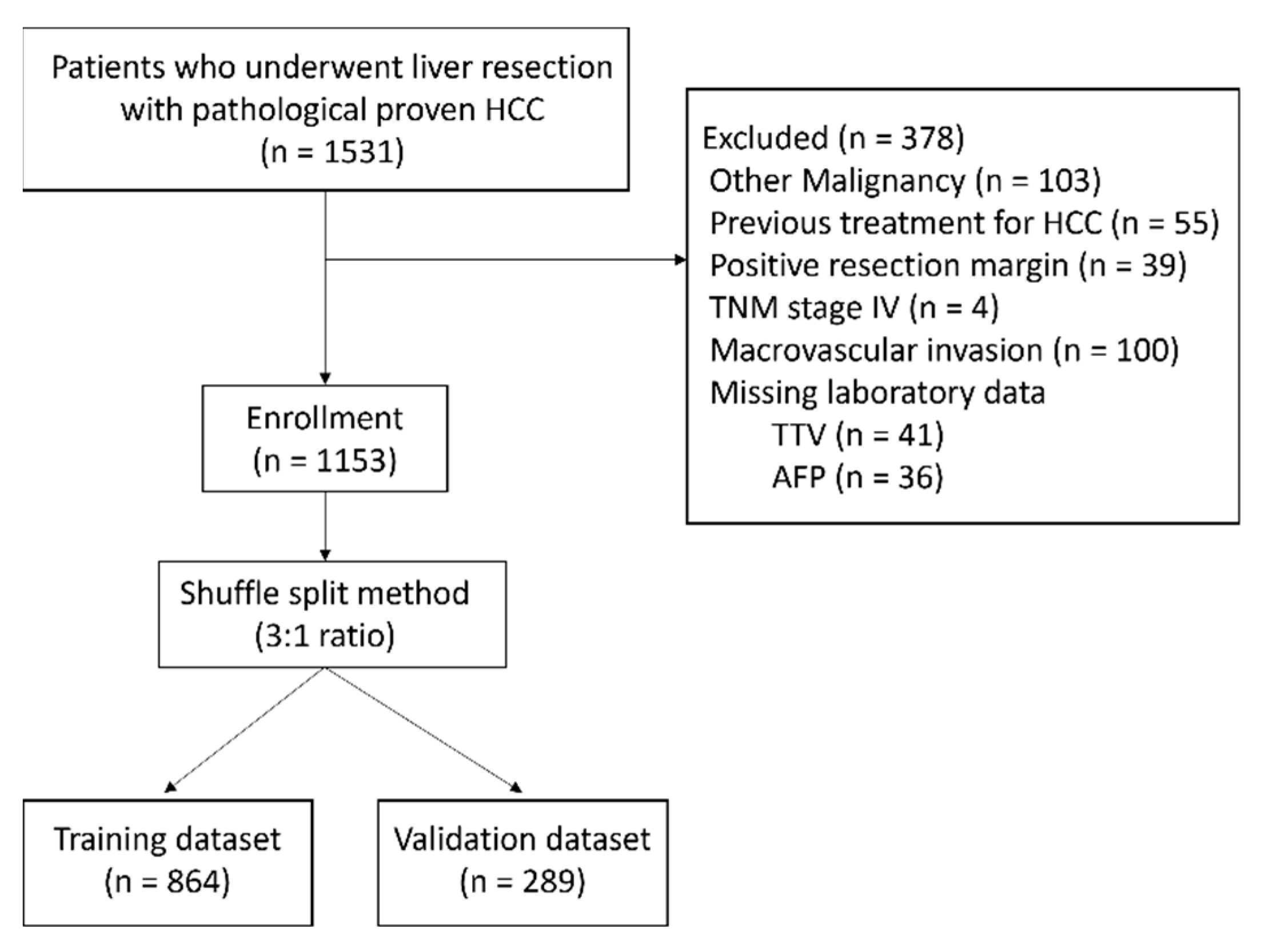

| Factors | Total (n = 1153) | Training Dataset (n = 864) | Validation Dataset (n = 289) | p-Value |
|---|---|---|---|---|
| Age (years) | 58.7 ± 12.8 | 58.4 ± 13.1 | 59.8 ± 11.5 | 0.101 |
| Sex | 0.761 | |||
| Male | 905 (78.5) | 680 (78.7) | 225 (77.9) | |
| Female | 248 (21.5) | 184 (21.3) | 64 (22.1) | |
| Viral hepatitis status | 0.349 | |||
| HBV | 606 (52.6) | 458 (53.0) | 148 (51.2) | |
| HCV | 278 (24.1) | 207 (24.0) | 71 (24.6) | |
| HBV + HCV | 75 (6.5) | 61 (7.0) | 14 (4.8) | |
| NBNC | 194 (16.8) | 138 (16.0) | 56 (19.4) | |
| Albumin (g/mL) | 4.15 ± 0.45 | 4.15 ± 0.45 | 4.14 ± 0.45 | 0.940 |
| Platelet count (1000/uL) | 177.8 ± 73.9 | 176.8 ± 71.0 | 180.7 ± 82.1 | 0.450 |
| Bilirubin (mg/dL) | 0.82 ± 0.97 | 0.85 ± 1.10 | 0.76 ± 0.40 | 0.199 |
| INR | 1.08 ± 0.09 | 1.08 ± 0.09 | 1.08 ± 0.10 | 0.560 |
| ICG (%) | 0.138 | |||
| ≤10 | 728 (66.7) | 556 (67.9) | 172 (63.0) | |
| >10 | 364 (33.3) | 263 (32.1) | 101 (37.0) | |
| Child classification | 0.469 | |||
| A | 1125 (98.7) | 842 (98.8) | 283 (98.3) | |
| B | 15 (1.3) | 10 (1.2) | 5 (1.7) | |
| AFP (ng/mL) * | 19.3 (5.5–276.6) | 19.4 (5.6–318.7) | 18.9 (5.1–168.6) | 0.212 |
| ≤160 | 805 (69.8) | 595 (68.9) | 210 (72.7) | 0.473 |
| >160, ≤2000 | 231 (20.0) | 179 (20.7) | 52 (18.0) | |
| >2000 | 117 (10.2) | 90 (10.4) | 27 (9.3) | |
| Total tumor volume (cm3) * | 17.6 (5.6–78.6) | 17.3 (5.5–77.2) | 18.6 (5.8–90.3) | 0.448 |
| ≤30 | 681 (59.1) | 515 (59.6) | 166 (57.4) | 0.917 |
| >30, ≤60 | 135 (11.7) | 101 (11.7) | 34 (11.8) | |
| >60, ≤300 | 221 (19.2) | 163 (18.9) | 58 (20.1) | |
| >300 | 116 (10.1) | 85 (9.8) | 31 (10.7) | |
| Tumor number | 0.346 | |||
| 1 | 1009 (87.5) | 756 (87.5) | 253 (87.5) | |
| 2 | 116 (10.1) | 90 (10.4) | 26 (9.0) | |
| ≥3 | 28 (2.4) | 18 (2.1) | 10 (3.5) | |
| Microvascular invasion | 0.960 | |||
| Yes | 278 (24.1) | 208 (24.1) | 70 (24.2) | |
| No | 875 (75.9) | 656 (75.9) | 219 (75.8) | |
| Disease-free survival (%) | 0.501 | |||
| 1 year | 75.3 | 74.9 | 76.6 | |
| 5 years | 42.2 | 42.9 | 40.4 | |
| 10 years | 29.7 | 29.9 | 29.2 | |
| Overall survival (%) | 0.403 | |||
| 1 year | 94.0 | 93.3 | 96.2 | |
| 5 years | 70.2 | 71.0 | 67.7 | |
| 10 years | 51.2 | 51.7 | 49.8 | |
| Recurrence pattern | 1.000 | |||
| Intrahepatic recurrence only | 691 (91.3) | 513 (91.3) | 178 (91.3) | |
| Extrahepatic recurrence | 66 (8.7) | 49 (8.7) | 17 (8.7) |
| Factors | Odds Ratio | 95% C.I. | p-Value | Odds Ratio | 95% C.I. | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| ≤60/>60 | 1.333 | 0.972–1.826 | 0.074 | |||
| Gender | ||||||
| Male/Female | 1.181 | 0.799–1.747 | 0.404 | |||
| Hepatitis | ||||||
| HBV/NBNC | 0.958 | 0.632–1.474 | 0.846 | |||
| HCV/HBNC | 0.654 | 0.392–1.090 | 0.103 | |||
| HBV and HCV/NBNC | 0.669 | 0.321–1.394 | 0.283 | |||
| Albumin (g/dL) | ||||||
| ≤3.5/>3.5 | 1.146 | 0.661–1.986 | 0.627 | |||
| Platelet count (1000/uL) | ||||||
| >150/≤150 | 1.281 | 0.923–1.779 | 0.139 | |||
| ICG (R15) | ||||||
| >10/≤10 | 1.131 | 0.798–1.601 | 0.490 | |||
| Child classification | ||||||
| B/A | 3.189 | 0.914–11.127 | 0.069 | |||
| AFP (ng/mL) | ||||||
| >160, <2000/≤160 | 1.700 | 1.152–2.508 | 0.008 | 1.658 | 1.103–2.491 | 0.015 |
| >2000/≤160 | 5.897 | 3.700–9.398 | <0.001 | 4.030 | 2.452–6.625 | <0.001 |
| Total tumor volume (cm3) | ||||||
| >30, ≤60/≤30 | 2.075 | 1.255–3.432 | 0.004 | 1.983 | 1.181–3.329 | 0.010 |
| >60, ≤300/≤30 | 2.977 | 1.988–4.459 | <0.001 | 2.404 | 1.573–3.673 | <0.001 |
| >300/≤30 | 7.379 | 4.510–12.075 | <0.001 | 5.335 | 3.177–8.960 | <0.001 |
| Tumor number | ||||||
| 2/1 | 0.996 | 0.594–1.669 | 0.987 | 0.908 | 0.518–1.590 | 0.735 |
| ≥3/1 | 4.089 | 1.590–10.518 | 0.003 | 2.505 | 0.917–6.844 | 0.073 |
| Predictor Variables | Regression Coefficients (β) | Categories | Point | ||||
|---|---|---|---|---|---|---|---|
| Total tumor volume | 1.730 | >300 | 3 | ||||
| 0.913 | >60, ≤300 | 2 | |||||
| 0.708 | >30, ≤60 | 1 | |||||
| ≤30 * | 0 | ||||||
| AFP | 1.369 | >2000 | 3 | ||||
| 0.526 | >160, ≤2000 | 1 | |||||
| ≤160 * | 0 | ||||||
| Total Score | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Probability of MVI | 13.0% (49/376) | 16.1% (31/193) | 27.8% (32/115) | 46.8% (44/94) | 62.1% (18/29) | 56.3% (18/32) | 64.0% (16/25) |
| Risk | Low risk | Intermediate risk | High risk | ||||
| Probability of MVI | 14.1% | 36.4% | 60.5% | ||||
| Model | Overall Performance Measure | Discrimination | Calibration |
|---|---|---|---|
| Nagelkerke R2 | C Statistic | Hosmer–Lemeshow Test | |
| Training cohort | 0.174 | 0.714 | 0.314 |
| Validation cohort | 0.193 | 0.731 | 0.342 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-C.; Hung, H.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Risk Score Model for Microvascular Invasion in Hepatocellular Carcinoma: The Role of Tumor Burden and Alpha-Fetoprotein. Cancers 2021, 13, 4403. https://doi.org/10.3390/cancers13174403
Lee J-C, Hung H-C, Wang Y-C, Cheng C-H, Wu T-H, Lee C-F, Wu T-J, Chou H-S, Chan K-M, Lee W-C. Risk Score Model for Microvascular Invasion in Hepatocellular Carcinoma: The Role of Tumor Burden and Alpha-Fetoprotein. Cancers. 2021; 13(17):4403. https://doi.org/10.3390/cancers13174403
Chicago/Turabian StyleLee, Jin-Chiao, Hao-Chien Hung, Yu-Chao Wang, Chih-Hsien Cheng, Tsung-Han Wu, Chen-Fang Lee, Ting-Jung Wu, Hong-Shiue Chou, Kun-Ming Chan, and Wei-Chen Lee. 2021. "Risk Score Model for Microvascular Invasion in Hepatocellular Carcinoma: The Role of Tumor Burden and Alpha-Fetoprotein" Cancers 13, no. 17: 4403. https://doi.org/10.3390/cancers13174403
APA StyleLee, J.-C., Hung, H.-C., Wang, Y.-C., Cheng, C.-H., Wu, T.-H., Lee, C.-F., Wu, T.-J., Chou, H.-S., Chan, K.-M., & Lee, W.-C. (2021). Risk Score Model for Microvascular Invasion in Hepatocellular Carcinoma: The Role of Tumor Burden and Alpha-Fetoprotein. Cancers, 13(17), 4403. https://doi.org/10.3390/cancers13174403






