Paving the Way for Immunotherapy in Pediatric Acute Myeloid Leukemia: Current Knowledge and the Way Forward
Abstract
:Simple Summary
Abstract
1. Introduction
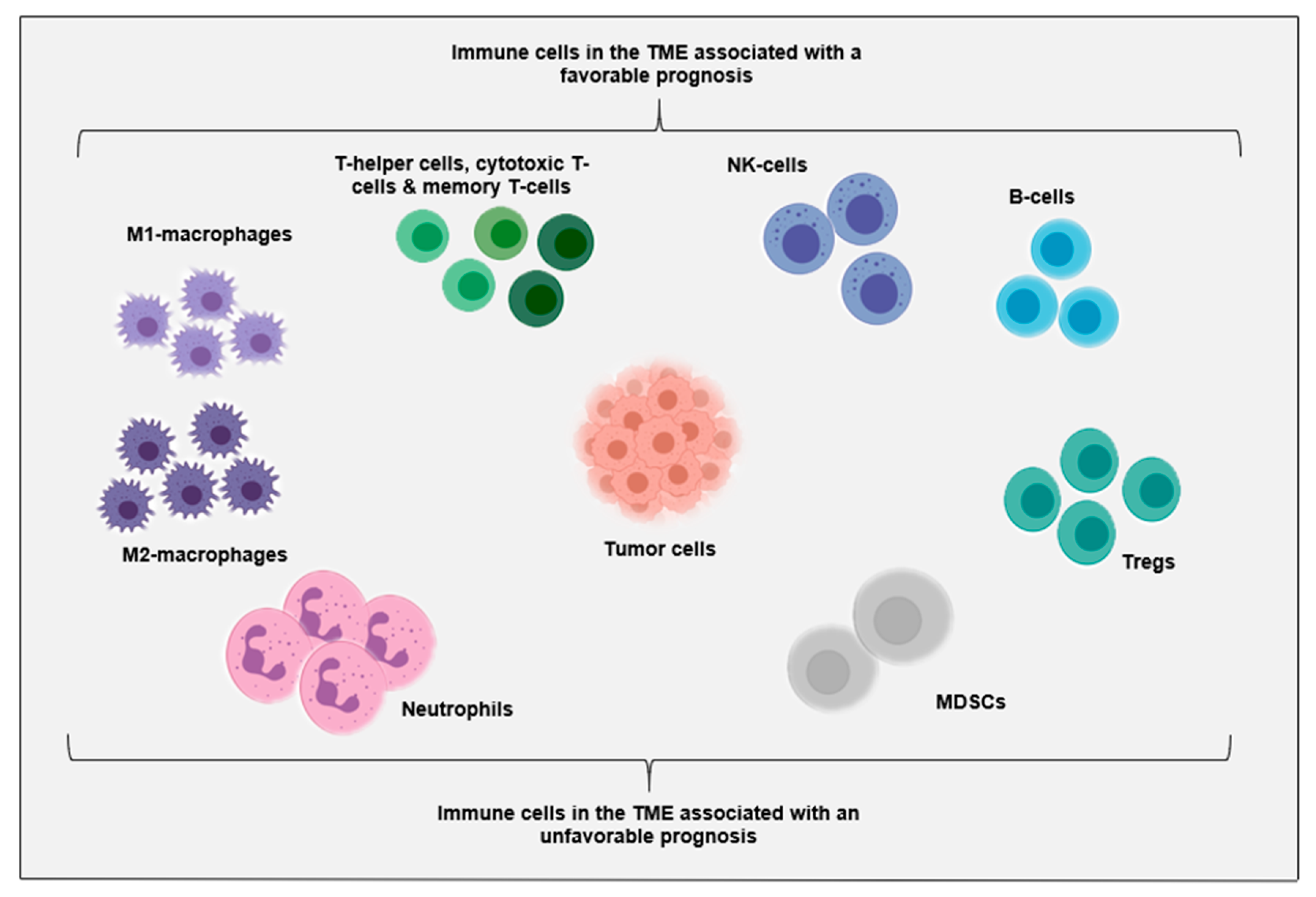
2. Immune Cells in the TME
2.1. Immune Cells in the TME of AML at Diagnosis
2.2. Immune Cells in the TME in Relapsed and Refractory Disease
2.3. Immune Cells in the TME during and after Therapy
2.4. Genetic Alterations That Affect the Immune Cell Abundance in the TME
3. The Relevance of Systemic Immunity for Immunotherapy Responses
4. Target Antigen Expression in Pediatric AML
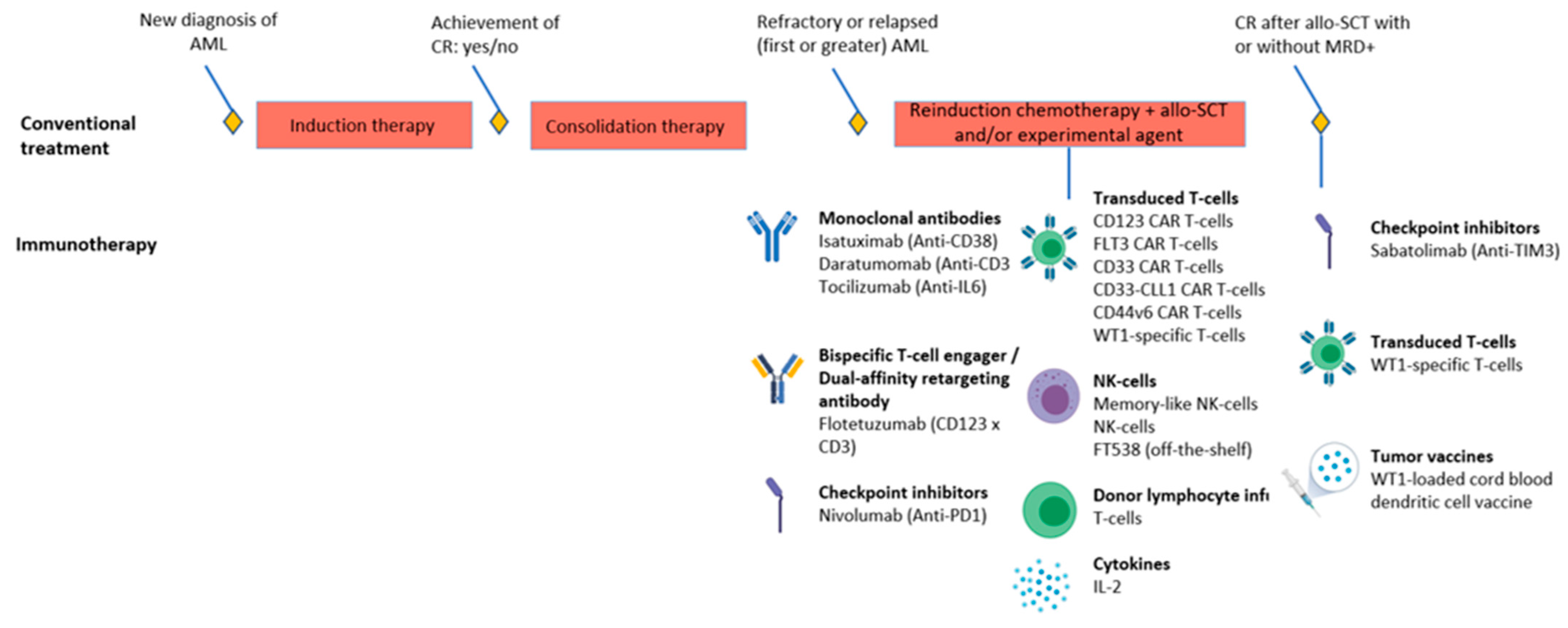
5. Immunotherapy Trials in Pediatric AML
6. The Way Forward in Pediatric AML: The Need for Immune Profiling
7. Discussions
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwaan, C.M.; Kolb, E.A.; Reinhardt, D.; Abrahamsson, J.; Adachi, S.; Aplenc, R.; De Bont, E.S.; De Moerloose, B.; Dworzak, M.; Gibson, B.E.; et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 2949–2962. [Google Scholar] [CrossRef] [Green Version]
- Reedijk, A.M.J.; Klein, K.; Coebergh, J.W.W.; Kremer, L.C.; Dinmohamed, A.G.; de Haas, V.; Versluijs, A.B.; Ossenkoppele, G.J.; Beverloo, H.B.; Pieters, R.; et al. Improved survival for children and young adolescents with acute myeloid leukemia: A Dutch study on incidence, survival and mortality. Leukemia 2019, 33, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Khaldoyanidi, S.; Nagorsen, D.; Stein, A.; Ossenkoppele, G.; Subklewe, M. Immune Biology of Acute Myeloid Leukemia: Implications for Immunotherapy. J. Clin. Oncol. 2021, 39, 419–432. [Google Scholar] [CrossRef]
- Sendker, S.; Reinhardt, D.; Niktoreh, N. Redirecting the Immune Microenvironment in Acute Myeloid Leukemia. Cancers 2021, 13, 1423. [Google Scholar] [CrossRef]
- Majzner, R.G.; Heitzeneder, S.; Mackall, C.L. Harnessing the Immunotherapy Revolution for the Treatment of Childhood Cancers. Cancer Cell 2017, 31, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Orti, G.; Barba, P.; Fox, L.; Salamero, O.; Bosch, F.; Valcarcel, D. Donor lymphocyte infusions in AML and MDS: Enhancing the graft-versus-leukemia effect. Exp. Hematol. 2017, 48, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, C.; Vyas, P. The Graft-Versus-Leukemia Effect in AML. Front. Oncol. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Disis, M.L. Mechanism of action of immunotherapy. Semin. Oncol. 2014, 41 (Suppl. 5), S3–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, R.L.; Hawkins, N.J.; Smith, G.M. Unconjugated antibodies for cancer therapy: Lessons from the clinic. Cancer Treat. Rev. 1997, 23, 305–319. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Innao, V.; Rizzo, V.; Allegra, A.G.; Musolino, C.; Allegra, A. Oncolytic Viruses and Hematological Malignancies: A New Class of Immunotherapy Drugs. Curr. Oncol. 2020, 28, 19. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Blaeschke, F.; Stenger, D.; Kaeuferle, T.; Willier, S.; Lotfi, R.; Kaiser, A.D.; Assenmacher, M.; Döring, M.; Feucht, J.; Feuchtinger, T. Induction of a central memory and stem cell memory phenotype in functionally active CD4+ and CD8+ CAR T cells produced in an automated good manufacturing practice system for the treatment of CD19+ acute lymphoblastic leukemia. Cancer Immunol. Immunother. 2018, 67, 1053–1066. [Google Scholar] [CrossRef]
- Stenger, D.; Stief, T.A.; Kaeuferle, T.; Willier, S.; Rataj, F.; Schober, K.; Vick, B.; Lotfi, R.; Wagner, B.; Grünewald, T.G.P.; et al. Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood 2020, 136, 1407–1418. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Maio, M.; Robert, C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin. Oncol. 2015, 42, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Stahl, M.; Goldberg, A.D. Immune Checkpoint Inhibitors in Acute Myeloid Leukemia: Novel Combinations and Therapeutic Targets. Curr. Oncol. Rep. 2019, 21, 37. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Zeidan, A.M. Randomized trials with checkpoint inhibitors in acute myeloid leukaemia and myelodysplastic syndromes: What have we learned so far and where are we heading? Best Pract. Res. Clin. Haematol. 2020, 33, 101222. [Google Scholar] [CrossRef]
- Isidori, A.; Daver, N.; Curti, A. Editorial: The Biological Landscape of Immunotherapy in AML. Front. Oncol. 2021, 11, 671252. [Google Scholar] [CrossRef]
- Isidori, A.; Cerchione, C.; Daver, N.; DiNardo, C.; Garcia-Manero, G.; Konopleva, M.; Jabbour, E.; Ravandi, F.; Kadia, T.; Burguera, A.F.; et al. Immunotherapy in Acute Myeloid Leukemia: Where We Stand. Front. Oncol. 2021, 11, 656218. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Bagaev, A.; Kotlov, N.; Nomie, K.; Svekolkin, V.; Gafurov, A.; Isaeva, O.; Osokin, N.; Kozlov, I.; Frenkel, F.; Gancharova, O.; et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 2021, 39, 845–865. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Montoya, F.; Louahed, J.; Dizier, B.; Gruselle, O.; Spiessens, B.; Lehmann, F.F.; Suciu, S.; Kruit, W.H.; Eggermont, A.M.; Vansteenkiste, J.; et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J. Clin. Oncol. 2013, 31, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Lauss, M.; Donia, M.; Harbst, K.; Andersen, R.; Mitra, S.; Rosengren, F.; Salim, M.; Vallon-Christersson, J.; Törngren, T.; Kvist, A.; et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat. Commun. 2017, 8, 1738. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Zhang, L.; Zhang, Y.; Li, Z.; Siemers, N.; Zhang, Z. Insights Gained from Single-Cell Analysis of Immune Cells in the Tumor Microenvironment. Annu. Rev. Immunol. 2021, 39, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Vadakekolathu, J.; Minden, M.D.; Hood, T.; Church, S.E.; Reeder, S.; Altmann, H.; Sullivan, A.H.; Viboch, E.J.; Patel, T.; Ibrahimova, N.; et al. Immune landscapes predict chemotherapy resistance and immunotherapy response in acute myeloid leukemia. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021, 137, 751–762. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Ragoonanan, D.; Khazal, S.J.; Abdel-Azim, H.; McCall, D.; Cuglievan, B.; Tambaro, F.P.; Ahmad, A.H.; Rowan, C.M.; Gutierrez, C.; Schadler, K.; et al. Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer. Nat. Rev. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Adam, T.; Becker, T.M.; Chua, W.; Bray, V.; Roberts, T.L. The Multiple Potential Biomarkers for Predicting Immunotherapy Response-Finding the Needle in the Haystack. Cancers 2021, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.J.; Tasian, S.K. Opportunities for immunotherapy in childhood acute myeloid leukemia. Blood Adv. 2019, 3, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Davidson-Moncada, J.; Viboch, E.; Church, S.E.; Warren, S.E.; Rutella, S. Dissecting the Immune Landscape of Acute Myeloid Leukemia. Biomedicines 2018, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Lamble, A.J.; Lind, E.F. Targeting the Immune Microenvironment in Acute Myeloid Leukemia: A Focus on T Cell Immunity. Front. Oncol. 2018, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Swatler, J.; Turos-Korgul, L.; Kozlowska, E.; Piwocka, K. Immunosuppressive Cell Subsets and Factors in Myeloid Leukemias. Cancers 2021, 13, 1203. [Google Scholar] [CrossRef]
- Bolouri, H.; Farrar, J.E.; Triche, T., Jr.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018, 24, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.H. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2010, 29, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- Lamble, A.J.; Kosaka, Y.; Laderas, T.; Maffit, A.; Kaempf, A.; Brady, L.K.; Wang, W.; Long, N.; Saultz, J.N.; Mori, M.; et al. Reversible suppression of T cell function in the bone marrow microenvironment of acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2020, 117, 14331–14341. [Google Scholar] [CrossRef] [PubMed]
- Brück, O.; Dufva, O.; Hohtari, H.; Blom, S.; Turkki, R.; Ilander, M.; Kovanen, P.; Pallaud, C.; Ramos, P.M.; Lähteenmäki, H.; et al. Immune profiles in acute myeloid leukemia bone marrow associate with patient age, T-cell receptor clonality, and survival. Blood Adv. 2020, 4, 274–286. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Y.Y.; Herold, T.; Nie, R.C.; Zhang, Y.; Gale, R.P.; Metzeler, K.H.; Zeng, Y.; Wang, S.Q.; Pan, X.Y.; et al. An Immune Risk Score Predicts Survival of Patients with Acute Myeloid Leukemia Receiving Chemotherapy. Clin. Cancer Res. 2021, 27, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Dufva, O.; Pölönen, P.; Brück, O.; Keränen, M.A.I.; Klievink, J.; Mehtonen, J.; Huuhtanen, J.; Kumar, A.; Malani, D.; Siitonen, S.; et al. Immunogenomic Landscape of Hematological Malignancies. Cancer Cell 2020, 38, 380–399.e13. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.L.; Sahasrabudhe, K.D.; Sigmund, A.M.; Bhatnagar, B. AML with Myelodysplasia-Related Changes: Development, Challenges, and Treatment Advances. Genes 2020, 11, 845. [Google Scholar] [CrossRef]
- Bailur, J.K.; McCachren, S.S.; Pendleton, K.; Vasquez, J.C.; Lim, H.S.; Duffy, A.; Doxie, D.B.; Kaushal, A.; Foster, C.; DeRyckere, D.; et al. Risk-associated alterations in marrow T cells in pediatric leukemia. JCI Insight 2020, 5, e140179. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.H.; Iorgulescu, J.B.; Braun, D.A.; Keskin, D.B.; Livak, K.J. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 244–256. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Wang, J.; Sahu, A.D.; Cohen, D.; Song, L.; Ouyang, Z.; Fan, J.; Wang, B.; Fu, J.; et al. Immune receptor repertoires in pediatric and adult acute myeloid leukemia. Genome Med. 2019, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Basu, S.; Garcia-Manero, G.; Hourigan, C.S.; Oetjen, K.A.; Cortes, J.E.; Ravandi, F.; Jabbour, E.J.; Al-Hamal, Z.; Konopleva, M.; et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2019, 125, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noviello, M.; Manfredi, F.; Ruggiero, E.; Perini, T.; Oliveira, G.; Cortesi, F.; De Simone, P.; Toffalori, C.; Gambacorta, V.; Greco, R.; et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat. Commun. 2019, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Löwenberg, B. Towards precision medicine for AML. Nat. Rev. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Michelozzi, I.M.; Kirtsios, E.; Giustacchini, A. Driving CAR T Stem Cell Targeting in Acute Myeloid Leukemia: The Roads to Success. Cancers 2021, 13, 2816. [Google Scholar] [CrossRef]
- Topp, M.S.; Kufer, P.; Gökbuget, N.; Goebeler, M.; Klinger, M.; Neumann, S.; Horst, H.A.; Raff, T.; Viardot, A.; Schmid, M.; et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 2011, 29, 2493–2498. [Google Scholar] [CrossRef]
- Lussana, F.; Gritti, G.; Rambaldi, A. Immunotherapy of Acute Lymphoblastic Leukemia and Lymphoma With T Cell-Redirected Bispecific Antibodies. J. Clin. Oncol. 2021, 39, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Saudemont, A.; Quesnel, B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7.1 expression and resist CTL-mediated lysis. Blood 2004, 104, 2124–2133. [Google Scholar] [CrossRef]
- Kadia, T.M.; Cortes, J.E.; Ghorab, A.; Ravandi, F.; Jabbour, E.; Daver, N.G.; Alvarado, Y.; Ohanian, M.; Konopleva, M.; Kantarjian, H.M. Nivolumb (Nivo) maintenance (maint) in high-risk (HR) acute myeloid leukemia (AML) patients. J. Clin. Oncol. 2018, 36 (Suppl. 15), 7014. [Google Scholar] [CrossRef]
- Petit, A.; Ducassou, S.; Leblanc, T.; Pasquet, M.; Rousseau, A.; Ragu, C.; Cachanado, M.; Nelken, B.; Bertrand, Y.; Michel, G.; et al. Maintenance Therapy With Interleukin-2 for Childhood AML: Results of ELAM02 Phase III Randomized Trial. Hemasphere 2018, 2, e159. [Google Scholar] [CrossRef]
- Nguyen, R.; Wu, H.; Pounds, S.; Inaba, H.; Ribeiro, R.C.; Cullins, D.; Rooney, B.; Bell, T.; Lacayo, N.J.; Heym, K.; et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J. Immunother. Cancer 2019, 7, 81. [Google Scholar] [CrossRef]
- Gómez García, L.M.; Escudero, A.; Mestre, C.; Fuster, S.J.L.; Martínez, A.P.; Vagace Valero, J.M.; Vela, M.; Ruz, B.; Navarro, A.; Fernández, L.; et al. Phase 2 Clinical Trial of Infusing Haploidentical K562-mb15-41BBL-Activated and Expanded Natural Killer Cells as Consolidation Therapy for Pediatric Acute Myeloblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2021, 21, 328–337.e1. [Google Scholar] [CrossRef]
- Knaus, H.A.; Berglund, S.; Hackl, H.; Blackford, A.L.; Zeidner, J.F.; Montiel-Esparza, R.; Mukhopadhyay, R.; Vanura, K.; Blazar, B.R.; Karp, J.E.; et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight 2018, 3, e120974. [Google Scholar] [CrossRef] [Green Version]
- Ocadlikova, D.; Lecciso, M.; Isidori, A.; Loscocco, F.; Visani, G.; Amadori, S.; Cavo, M.; Curti, A. Chemotherapy-Induced Tumor Cell Death at the Crossroads Between Immunogenicity and Immunotolerance: Focus on Acute Myeloid Leukemia. Front. Oncol. 2019, 9, 1004. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Hess, A.D.; Gocke, C.D.; Thoburn, C.; Kos, F.; Meyer, C.; Briel, J.; Luznik, L.; Smith, B.D.; Levitsky, H.; et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: Peripheral oligoclonal expansion of regulatory T cells. Blood 2011, 117, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Xu, Y. Clinical significance of Treg cell frequency in acute myeloid leukemia. Int. J. Hematol. 2013, 98, 558–562. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Tian, T.; Zhang, T.; Wang, R.; Han, F.; Zhong, C.; Hua, M.; Ma, D. Increased Regulatory T Cells in Peripheral Blood of Acute Myeloid Leukemia Patients Rely on Tumor Necrosis Factor (TNF)-α-TNF Receptor-2 Pathway. Front. Immunol. 2018, 9, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef]
- Yan, Y.; Kumar, A.B.; Finnes, H.; Markovic, S.N.; Park, S.; Dronca, R.S.; Dong, H. Combining Immune Checkpoint Inhibitors With Conventional Cancer Therapy. Front. Immunol. 2018, 9, 1739. [Google Scholar] [CrossRef] [Green Version]
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol. Immunother. 2016, 65, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Käsmann, L.; Eze, C.; Dantes, M.; Roengvoraphoj, O.; Niyazi, M.; Belka, C.; Manapov, F. State of clinical research of radiotherapy/chemoradiotherapy and immune checkpoint inhibitor therapy combinations in solid tumours-a German radiation oncology survey. Eur. J. Cancer 2019, 108, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.M.; Posey, R.R.; Pandolfi, P.P. The Interplay Between the Genetic and Immune Landscapes of AML: Mechanisms and Implications for Risk Stratification and Therapy. Front. Oncol. 2019, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Wellenstein, M.D.; de Visser, K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018, 48, 399–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhury, S.; O’Connor, C.; Cañete, A.; Bittencourt-Silvestre, J.; Sarrou, E.; Prendergast, Á.; Choi, J.; Johnston, P.; Wells, C.A.; Gibson, B.; et al. Age-specific biological and molecular profiling distinguishes paediatric from adult acute myeloid leukaemias. Nat. Commun. 2018, 9, 5280. [Google Scholar] [CrossRef] [Green Version]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Yost, K.E.; Satpathy, A.T.; Wells, D.K.; Qi, Y.; Wang, C.; Kageyama, R.; McNamara, K.L.; Granja, J.M.; Sarin, K.Y.; Brown, R.A.; et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019, 25, 1251–1259. [Google Scholar] [CrossRef]
- Wu, T.D.; Madireddi, S.; de Almeida, P.E.; Banchereau, R.; Chen, Y.J.; Chitre, A.S.; Chiang, E.Y.; Iftikhar, H.; O’Gorman, W.E.; Au-Yeung, A.; et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 2020, 579, 274–278. [Google Scholar] [CrossRef]
- Valpione, S.; Galvani, E.; Tweedy, J.; Mundra, P.A.; Banyard, A.; Middlehurst, P.; Barry, J.; Mills, S.; Salih, Z.; Weightman, J.; et al. Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat. Cancer 2020, 1, 210–221. [Google Scholar] [CrossRef]
- Bansal, A.K.; Sharawat, S.K.; Gupta, R.; Vishnubhatla, S.; Dhawan, D.; Bakhshi, S. Regulatory T cells in pediatric AML are associated with disease load and their serial assessment suggests role in leukemogenesis. Am. J. Blood Res. 2020, 10, 90–96. [Google Scholar]
- Admiraal, R.; van Kesteren, C.; Jol-van der Zijde, C.M.; Lankester, A.C.; Bierings, M.B.; Egberts, T.C.; van Tol, M.J.; Knibbe, C.A.; Bredius, R.G.; Boelens, J.J. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: A multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015, 2, e194–e203. [Google Scholar] [CrossRef]
- de Koning, C.; Admiraal, R.; Nierkens, S.; Boelens, J.J. Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem. Cell Investig. 2017, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, R.; Gilmour, K.; Qasim, W.; Adams, S.; Worth, A.J.; Zhan, H.; Montiel-Equihua, C.A.; Derniame, S.; Cale, C.; Rao, K.; et al. Omission of in vivo T-cell depletion promotes rapid expansion of naïve CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br. J. Haematol. 2012, 156, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, R.; Lindemans, C.A.; van Kesteren, C.; Bierings, M.B.; Versluijs, A.B.; Nierkens, S.; Boelens, J.J. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood 2016, 128, 2734–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soiffer, R.J.; Davids, M.S.; Chen, Y.B. Tyrosine kinase inhibitors and immune checkpoint blockade in allogeneic hematopoietic cell transplantation. Blood 2018, 131, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Kline, J.; Stock, W.; Kosuri, S.; Artz, A.; Larson, R.A.; Riedell, P.A.; Bishop, M.; Liu, H. Unexpected Toxicities When Nivolumab Was Given as Maintenance Therapy following Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2020, 26, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Alotaibi, A.S.; Bücklein, V.; Subklewe, M. T-cell-based immunotherapy of acute myeloid leukemia: Current concepts and future developments. Leukemia 2021. [Google Scholar] [CrossRef]
- De Moerloose, B. CAR-T treatment of pediatric AML: A long and winding road. Blood 2021, 137, 1004–1006. [Google Scholar] [CrossRef]
- Willier, S.; Rothämel, P.; Hastreiter, M.; Wilhelm, J.; Stenger, D.; Blaeschke, F.; Rohlfs, M.; Kaeuferle, T.; Schmid, I.; Albert, M.H.; et al. CLEC12A and CD33 coexpression as a preferential target for pediatric AML combinatorial immunotherapy. Blood 2021, 137, 1037–1049. [Google Scholar] [CrossRef]
- Bras, A.E.; de Haas, V.; van Stigt, A.; Jongen-Lavrencic, M.; Beverloo, H.B.; Te Marvelde, J.G.; Zwaan, C.M.; van Dongen, J.J.M.; Leusen, J.H.W.; van der Velden, V.H.J. CD123 expression levels in 846 acute leukemia patients based on standardized immunophenotyping. Cytom. B Clin. Cytom. 2019, 96, 134–142. [Google Scholar] [CrossRef]
- Shah, N.N.; Maatman, T.; Hari, P.; Johnson, B. Multi Targeted CAR-T Cell Therapies for B-Cell Malignancies. Front. Oncol. 2019, 9, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloss, C.C.; Condomines, M.; Cartellieri, M.; Bachmann, M.; Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013, 31, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cao, Y.; Pinz, K.; Ma, Y.; Wada, M.; Chen, K.; Ma, G.; Shen, J.; Tse, C.O.; Su, Y.; et al. First-in-human CLL1-CD33 compount CAR T cell therapy induces complete remission in patients with refractory acute myeloid leukemia: Update on phase 1 clinical trial. Blood 2018, 132, 901. [Google Scholar] [CrossRef]
- Danaher, P.; Warren, S.; Lu, R.; Samayoa, J.; Sullivan, A.; Pekker, I.; Wallden, B.; Marincola, F.M.; Cesano, A. Pan-cancer adaptive immune resistance as defined by the Tumor Inflammation Signature (TIS): Results from The Cancer Genome Atlas (TCGA). J. Immunother. Cancer 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaher, P.; Warren, S.; Dennis, L.; D’Amico, L.; White, A.; Disis, M.L.; Geller, M.A.; Odunsi, K.; Beechem, J.; Fling, S.P. Gene expression markers of Tumor Infiltrating Leukocytes. J. Immunother. Cancer 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Terry, R.L.; Meyran, D.; Ziegler, D.S.; Haber, M.; Ekert, P.G.; Trapani, J.A.; Neeson, P.J. Immune profiling of pediatric solid tumors. J. Clin. Investig. 2020, 130, 3391–3402. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S.; Bhaumik, S.; Dhodapkar, K.; Grivel, J.J.B.; Gupta, S.; Hanks, B.A.; Janetzki, S.; Kleen, T.O.; Koguchi, Y.; Lund, A.W.; et al. SITC cancer immunotherapy resource document: A compass in the land of biomarker discovery. J. Immunother. Cancer 2020, 8, e000705. [Google Scholar] [CrossRef]
- Zafeiris, D.; Vadakekolathu, J.; Wagner, S.; Pockley, A.G.; Ball, G.R.; Rutella, S. Discovery and application of immune biomarkers for hematological malignancies. Expert Rev. Mol. Diagn. 2017, 17, 983–1000. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
| Class of Immunotherapy | Main Mechanism of Action | Example for AML | Corresponding Phase(s) of Clinical Trials for Adult AML * |
|---|---|---|---|
| Immune checkpoint inhibitors | Bind to immune checkpoints or their ligands and block their immunosuppressive signal [9] | Nivolumab (anti-PD1) Durvalumab (anti-PDL1) | I–II I–II |
| Unconjugated antibodies | Bind to tumor specific- or associated antigens and consequently facilitate recruitment of immune effector cells [10] | Daratumumab (anti-CD38) | I–II |
| Bispecific antibodies | Redirect T- or NK-cells to tumor-specific or -associated antigens [11] | Flotetuzumab (CD3 × CD123) AMG330 (CD3 × CD33) | I–II I |
| Adoptive cell therapy | Ex vivo transduced or expanded tumor-specific or -associated T- or NK-cells for direct lysis of tumor cells [9] | CAR T-cells directed at CD33 or CD123 | I–II I–III |
| Cytokines and other soluble immune-modulating factors | Growth and activation of T- and NK-cells [9] | IL-2 | IV |
| Vaccines | Increase presentation of tumor specific- or associated antigens [9] | DCP-001 (dendritic cell vaccine) | I–II |
| Oncolytic viruses | Viral oncolysis of cancer cells and consequent induction of anti-tumor immunity [12] | Vesicular stomatitis virus | I † |
| Disease Stage | Phase | Drug & Target | Concomitant Therapy | Indication | Ages | Patients | Measures Target Antigen Expression before Enrollment | Performs Immune Characterization | NCT/EUDRACT & Acronym If Applicable | Expected Completion |
|---|---|---|---|---|---|---|---|---|---|---|
| Relapse/Refractory | II | Isatuximab Anti-CD38 | Chemotherapy | R/R | <18 y | 96 | No | No | NCT03860844 (ISAKIDS) | 2022 |
| Relapse/Refractory | II | Memory-like NK-cells | Chemotherapy | R/R | 1–21 y | 48 | NA | No | NCT04354025 | 2026 |
| Relapse/Refractory | I/II | Nivolumab Anti-PD1 | Azacytidine | R/R | 1–30 y | 26 | No | No | NCT03825367 | 2024 |
| Relapse/Refractory | I/II | Memory-like NK-cells and DLI | Chemotherapy | Relapse after allo-SCT | >0 y | 90 (also adults) | NA | No | NCT03068819 | 2026 |
| Relapse/Refractory | I | Memory-like NK-cells and IL-2 | Chemotherapy | R/R | 2–17 y | Unclear for pediatric cohort | NA | No | NCT01898793 | 2028 |
| Relapse/Refractory | I | NK-cells | Chemotherapy | R/R | 1–30 y | 10 (also adults) | NA | Yes | NCT04327037 | 2021 |
| Relapse/Refractory | I | CD123 CAR T-cells | Chemotherapy | R/R | >11 y | 42 (also adults) | Yes | No | NCT02159495 | 2021 |
| Relapse/Refractory | I | CD123 CAR T-cells | Chemotherapy | R/R | 1–29 y | 12 | No | No | NCT04678336 | 2036 |
| Relapse/Refractory | I | CD123 CAR T-cells | Chemotherapy | R/R | <22 y | 32 | No | Yes | NCT04318678 (CATCHAML) | 2025 |
| Relapse/Refractory | I | FLT3 CAR T-cells | - | R/R | >11 y | 40 (also adults) | Yes | No | NCT03904069 | 2029 |
| Relapse/Refractory | I/II | CD33 CAR T-cells | - | R/R | 1–35 y | 34 (also adults) | Yes | No | NCT03971799 | 2039 |
| Relapse/Refractory | I | CD33-CLL1 CAR T-cells | - | HR disease | All | 20 (also adults) | No | No | NCT03795779 | 2022 |
| Relapse/Refractory | I/II | CD44v6CAR T-cells | Chemotherapy | R/R | 1–17 y for pediatric cohort | 58 (also adults) | Yes | No | NCT04097301 | 2023 |
| Relapse/Refractory | I | Flotetuzumab CD123 × CD3 BiTE | Chemotherapy | R/R | <21 y | 47 | No | Yes | NCT04158739 (ADVL1812) | 2021 |
| Relapse/Refractory | I | Daratumomab Anti-CD38 & FT538 (NK-cell product) | Chemotherapy | R/R after 2 lines of therapy | >11 y | 50 (also adults) | Yes | No | NCT04714372 | 2025 |
| Relapse and post-SCT | I/II | WT1-sensitized allogeneic T-cells and IL-2 | Relapse-arm: chemotherapy Post-SCT arm: - | Relapsed after SCT or high risk of relapse post-SCT | Unclear, but weight should be at least 15 kg | 45 (also adults) | No | No | NCT01640301 | 2029 |
| Post-SCT | I/II | Sabatolimab Anti-TIM3 | With/without Azacytidine | Post-SCT with CR but MRD+ | 12–99 y | 59 (also adults) | No | No | NCT04623216 | 2026 |
| Post-SCT | I/II | WT1-loaded cord blood dendritic cell vaccine | - | Post-SCT with CR | 0–18 y | 54 | Yes | Yes | 2015-000827-94 (U-DANCE) | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koedijk, J.B.; van der Werf, I.; Calkoen, F.G.; Nierkens, S.; Kaspers, G.J.L.; Zwaan, C.M.; Heidenreich, O. Paving the Way for Immunotherapy in Pediatric Acute Myeloid Leukemia: Current Knowledge and the Way Forward. Cancers 2021, 13, 4364. https://doi.org/10.3390/cancers13174364
Koedijk JB, van der Werf I, Calkoen FG, Nierkens S, Kaspers GJL, Zwaan CM, Heidenreich O. Paving the Way for Immunotherapy in Pediatric Acute Myeloid Leukemia: Current Knowledge and the Way Forward. Cancers. 2021; 13(17):4364. https://doi.org/10.3390/cancers13174364
Chicago/Turabian StyleKoedijk, Joost B., Inge van der Werf, Friso G. Calkoen, Stefan Nierkens, Gertjan J. L. Kaspers, Christian Michel Zwaan, and Olaf Heidenreich. 2021. "Paving the Way for Immunotherapy in Pediatric Acute Myeloid Leukemia: Current Knowledge and the Way Forward" Cancers 13, no. 17: 4364. https://doi.org/10.3390/cancers13174364
APA StyleKoedijk, J. B., van der Werf, I., Calkoen, F. G., Nierkens, S., Kaspers, G. J. L., Zwaan, C. M., & Heidenreich, O. (2021). Paving the Way for Immunotherapy in Pediatric Acute Myeloid Leukemia: Current Knowledge and the Way Forward. Cancers, 13(17), 4364. https://doi.org/10.3390/cancers13174364








