Genetic Alterations and Resectability Predict Outcome in Patients with Neuroblastoma Assigned to High-Risk Solely by MYCN Amplification
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Results
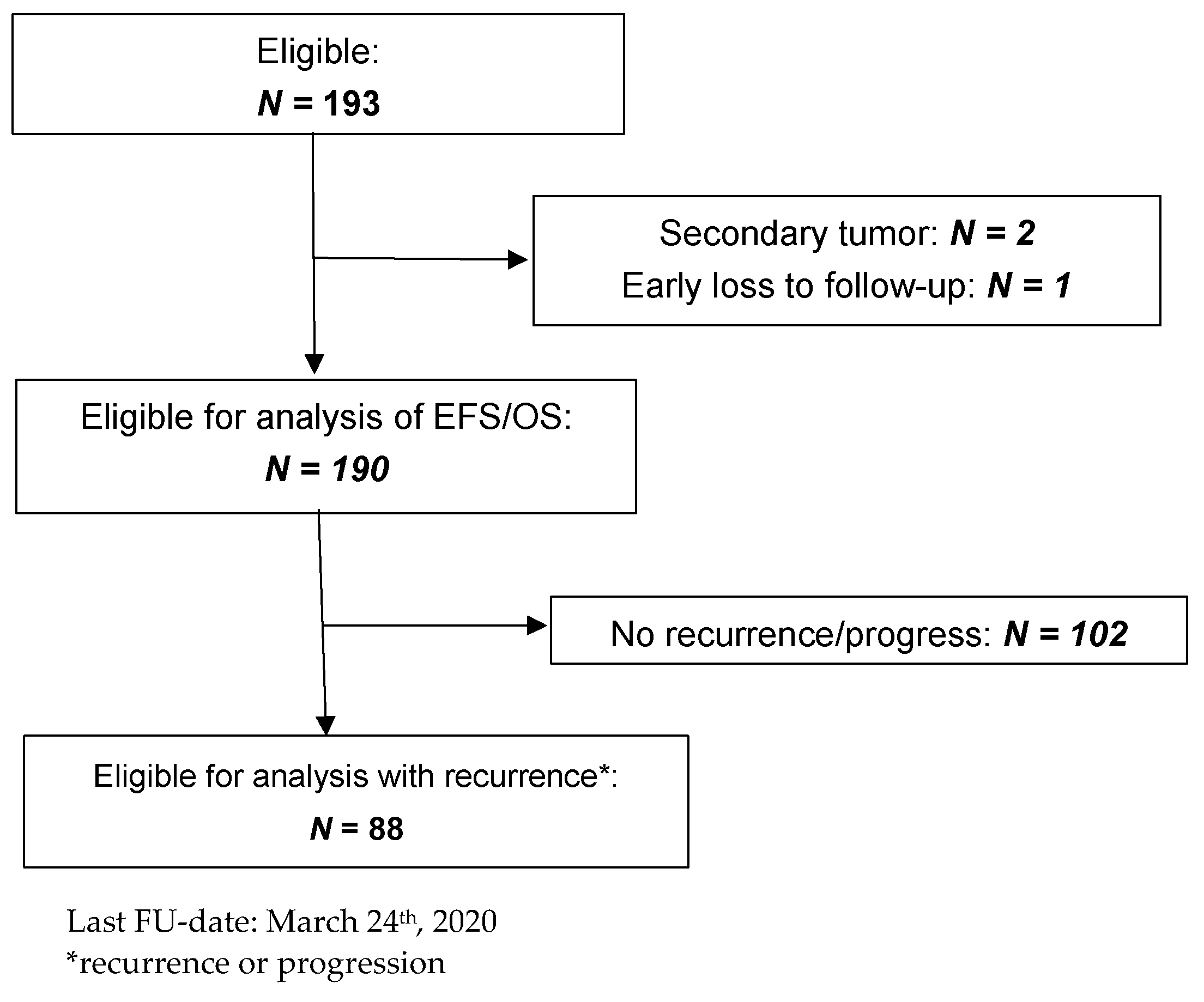
3.1. Characteristics of the Patients and Tumors
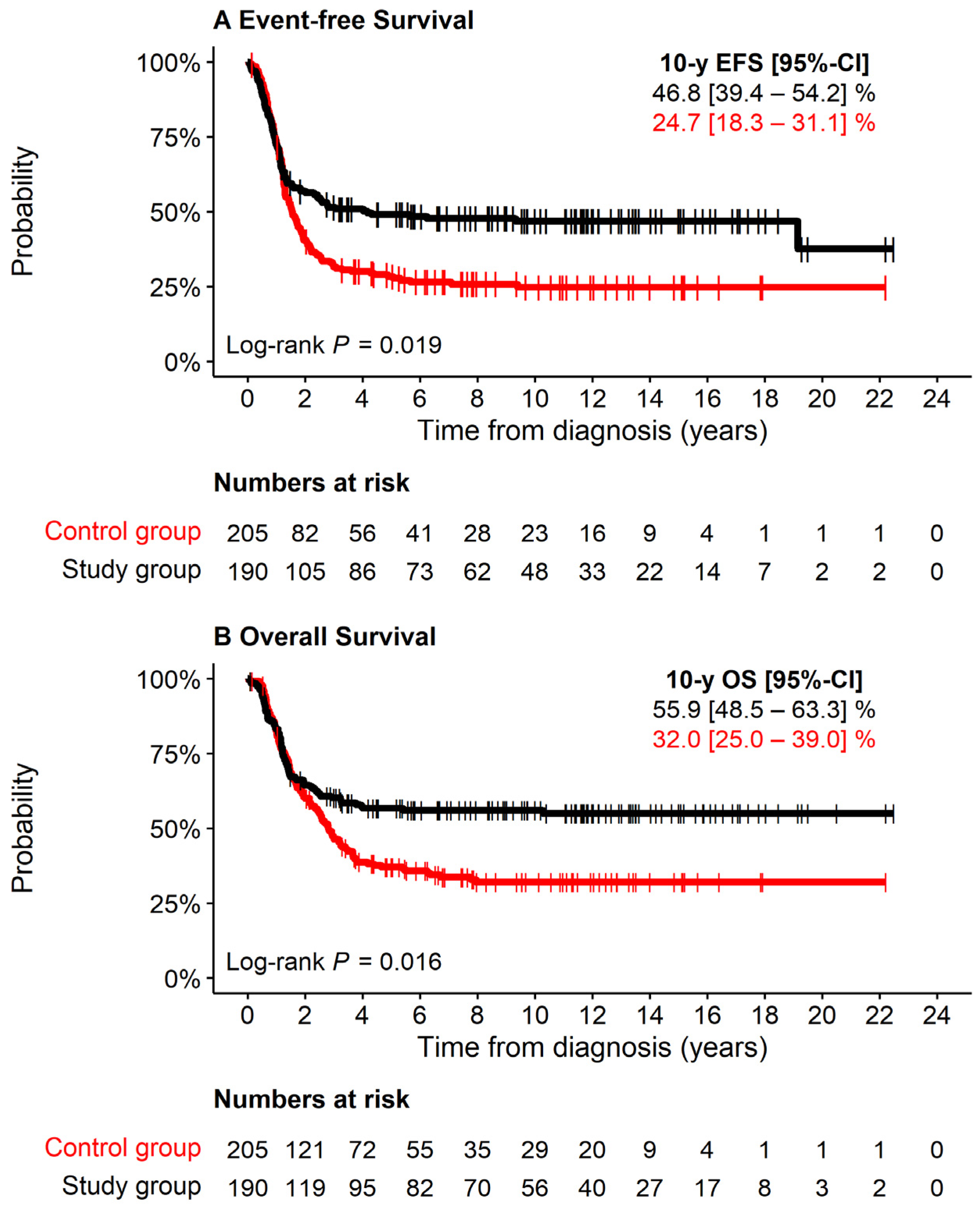
3.2. Treatment and Overall Outcome
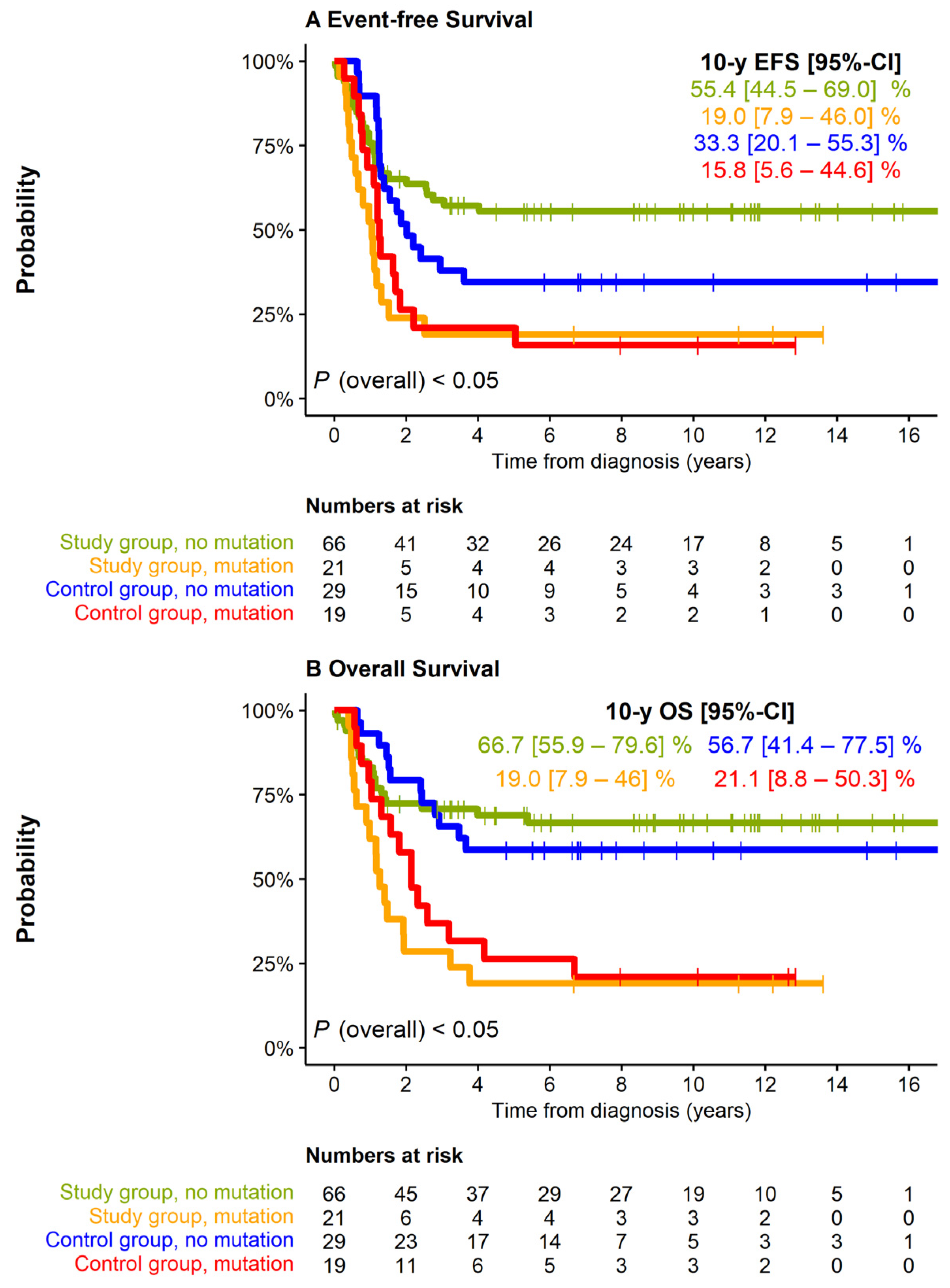
3.3. Impact of Clinical and Biological Characteristics on Outcome in the Study Group
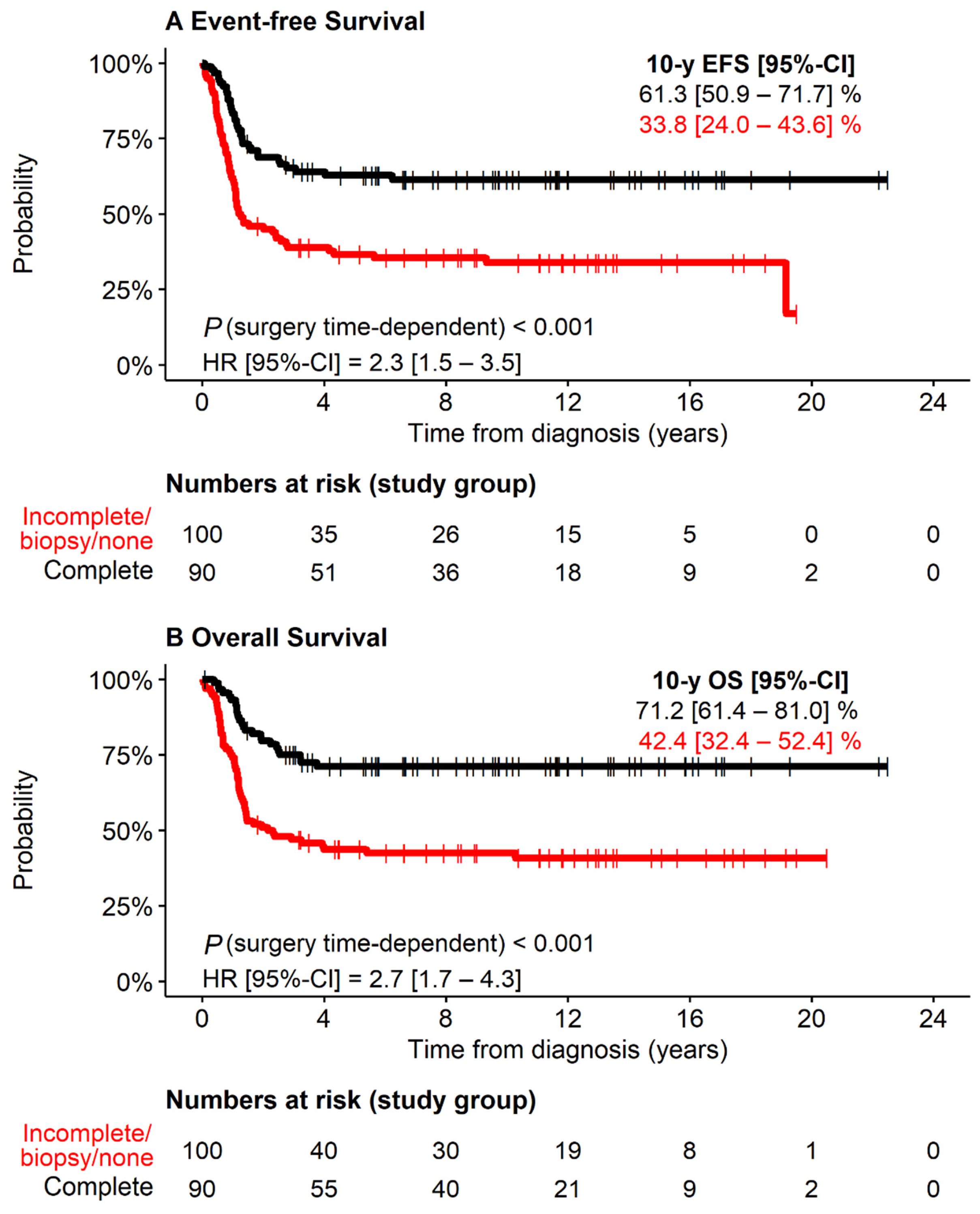
3.4. Therapy-Related Risk Factors
3.5. Definition of Risk Groups
3.6. Tumor Recurrences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Bagatell, R.; Beck-Popovic, M.; London, W.B.; Zhang, Y.; Pearson, A.D.; Matthay, K.K.; Monclair, T.; Ambros, P.F.; Cohn, S.L. Significance of MYCN amplification in international neuroblastoma staging system stage 1 and 2 neuroblastoma: A report from the International Neuroblastoma Risk Group database. J. Clin. Oncol. 2009, 27, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Meany, H.J.; London, W.B.; Ambros, P.F.; Matthay, K.K.; Monclair, T.; Simon, T.; Garaventa, A.; Berthold, F.; Nakagawara, A.; Cohn, S.L.; et al. Significance of clinical and biologic features in Stage 3 neuroblastoma: A report from the International Neuroblastoma Risk Group project. Pediatr. Blood Cancer 2014, 61, 1932–1939. [Google Scholar] [CrossRef]
- Modak, S.; Kushner, B.H.; LaQuaglia, M.P.; Kramer, K.; Cheung, N.K. Management and outcome of stage 3 neuroblastoma. Eur. J. Cancer 2009, 45, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Park, J.R.; Villablanca, J.G.; London, W.B.; Gerbing, R.B.; Haas-Kogan, D.; Adkins, E.S.; Attiyeh, E.F.; Maris, J.M.; Seeger, R.C.; Reynolds, C.P.; et al. Outcome of high-risk stage 3 neuroblastoma with myeloablative therapy and 13-cis-retinoic acid: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2009, 52, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iehara, T.; Hamazaki, M.; Tajiri, T.; Kawano, Y.; Kaneko, M.; Ikeda, H.; Hosoi, H.; Sugimoto, T.; Sawada, T.; Japanese Infantile Neuroblastoma Cooperative Study Group. Successful treatment of infants with localized neuroblastoma based on their MYCN status. Int. J. Clin. Oncol. 2013, 18, 389–395. [Google Scholar] [CrossRef] [PubMed]
- De Bernardi, B.; Di Cataldo, A.; Garaventa, A.; Massirio, P.; Viscardi, E.; Podda, M.G.; Castellano, A.; D’Angelo, P.; Tirtei, E.; Melchionda, F.; et al. Stage 4 s neuroblastoma: Features, management and outcome of 268 cases from the Italian Neuroblastoma Registry. Ital. J. Pediatr. 2019, 45, 8. [Google Scholar] [CrossRef] [PubMed]
- Schleiermacher, G.; Rubie, H.; Hartmann, O.; Bergeron, C.; Chastagner, P.; Mechinaud, F.; Michon, J.; Neuroblastoma Study Group of the French Society of Paediatric Oncology. Treatment of stage 4s neuroblastoma—Report of 10 years’ experience of the French Society of Paediatric Oncology (SFOP). Br. J. Cancer 2003, 89, 470–476. [Google Scholar] [CrossRef]
- Bagatell, R.; Rumcheva, P.; London, W.B.; Cohn, S.L.; Look, A.T.; Brodeur, G.M.; Frantz, C.; Joshi, V.; Thorner, P.; Rao, P.V.; et al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J. Clin. Oncol. 2005, 23, 8819–8827. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Lukens, J.N.; Seeger, R.C.; Brodeur, G.M.; Shimada, H.; Gerbing, R.B.; Stram, D.O.; Perez, C.; Haase, G.M.; Matthay, K.K. Biologic factors determine prognosis in infants with stage IV neuroblastoma: A prospective Children’s Cancer Group study. J. Clin. Oncol. 2000, 18, 1260–1268. [Google Scholar] [CrossRef]
- Berthold, F.; Spix, C.; Kaatsch, P.; Lampert, F. Incidence, Survival, and Treatment of Localized and Metastatic Neuroblastoma in Germany 1979–2015. Paediatr. Drugs 2017, 19, 577–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, K.; Potschger, U.; Pearson, A.D.J.; Sarnacki, S.; Cecchetto, G.; Gomez-Chacon, J.; Squire, R.; Freud, E.; Bysiek, A.; Matthyssens, L.E.; et al. Influence of Surgical Excision on the Survival of Patients with Stage 4 High-Risk Neuroblastoma: A Report From the HR-NBL1/SIOPEN Study. J. Clin. Oncol. 2020, 38, 2902–2915. [Google Scholar] [CrossRef]
- Du, L.; Liu, L.; Zhang, C.; Cai, W.; Wu, Y.; Wang, J.; Lv, F. Role of surgery in the treatment of patients with high-risk neuroblastoma who have a poor response to induction chemotherapy. J. Pediatr. Surg. 2014, 49, 528–533. [Google Scholar] [CrossRef]
- Simon, T.; Haberle, B.; Hero, B.; von Schweinitz, D.; Berthold, F. Role of surgery in the treatment of patients with stage 4 neuroblastoma age 18 months or older at diagnosis. J. Clin. Oncol. 2013, 31, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Mullassery, D.; Farrelly, P.; Losty, P.D. Does aggressive surgical resection improve survival in advanced stage 3 and 4 neuroblastoma? A systematic review and meta-analysis. Pediatr. Hematol. Oncol. 2014, 31, 703–716. [Google Scholar] [CrossRef]
- McGregor, L.M.; Rao, B.N.; Davidoff, A.M.; Billups, C.A.; Hongeng, S.; Santana, V.M.; Hill, D.A.; Fuller, C.; Furman, W.L. The impact of early resection of primary neuroblastoma on the survival of children older than 1 year of age with stage 4 disease: The St. Jude Children’s Research Hospital Experience. Cancer 2005, 104, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, S.; Cartolano, M.; Hero, B.; Welte, A.; Kahlert, Y.; Roderwieser, A.; Bartenhagen, C.; Walter, E.; Gecht, J.; Kerschke, L.; et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science 2018, 362, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Ney, G.M.; McKay, L.; Koschmann, C.; Mody, R.; Li, Q. The Emerging Role of Ras Pathway Signaling in Pediatric Cancer. Cancer Res. 2020, 80, 5155–5163. [Google Scholar] [CrossRef]
- Peifer, M.; Hertwig, F.; Roels, F.; Dreidax, D.; Gartlgruber, M.; Menon, R.; Kramer, A.; Roncaioli, J.L.; Sand, F.; Heuckmann, J.M.; et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 2015, 526, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F.; et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Ambros, P.F.; Ambros, I.M.; SIOP Europe Neuroblastoma Pathology, Biology, and Bone Marrow Group. Pathology and biology guidelines for resectable and unresectable neuroblastic tumors and bone marrow examination guidelines. Med. Pediatr. Oncol. 2001, 37, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Berthold, F.; Faldum, A.; Ernst, A.; Boos, J.; Dilloo, D.; Eggert, A.; Fischer, M.; Fruhwald, M.; Henze, G.; Klingebiel, T.; et al. Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: Results of the randomized open-label GPOH trial NB2004-HR. Ann. Oncol. 2020, 31, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Berthold, F.; Ernst, A.; Hero, B.; Klingebiel, T.; Kremens, B.; Schilling, F.H.; Simon, T. Long-term outcomes of the GPOH NB97 trial for children with high-risk neuroblastoma comparing high-dose chemotherapy with autologous stem cell transplantation and oral chemotherapy as consolidation. Br. J. Cancer 2018, 119, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ambros, P.F.; Ambros, I.M.; Brodeur, G.M.; Haber, M.; Khan, J.; Nakagawara, A.; Schleiermacher, G.; Speleman, F.; Spitz, R.; London, W.B.; et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br. J. Cancer 2009, 100, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Mac, S.M.; D’Cunha, C.A.; Farnham, P.J. Direct recruitment of N-myc to target gene promoters. Mol. Carcinog. 2000, 29, 76–86. [Google Scholar] [CrossRef]
- Shimada, H.; Ambros, I.M.; Dehner, L.P.; Hata, J.; Joshi, V.V.; Roald, B. Terminology and morphologic criteria of neuroblastic tumors: Recommendations by the International Neuroblastoma Pathology Committee. Cancer 1999, 86, 349–363. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Schulte, J.H.; Schulte, S.; Heukamp, L.C.; Astrahantseff, K.; Stephan, H.; Fischer, M.; Schramm, A.; Eggert, A. Targeted Therapy for Neuroblastoma: ALK Inhibitors. Klin. Padiatr. 2013, 225, 303–308. [Google Scholar] [CrossRef]
- Ladenstein, R.; Potschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef]
| Characteristic | Study Group | Control Group | p |
|---|---|---|---|
| Stages 1, 2, 3, 4S, 4 < 18 m | Stage 4 ≥ 18 m and MNA | ||
| N (%) | N (%) | ||
| All | 190 (100) | 205 (100) | |
| Sex | 190 (100) | 205 (100) | |
| Male | 111 (58) | 124 (60.5) | 0.683 |
| Female | 79 (42) | 81 (39.5) | |
| Age at diagnosis (m) | 190 (100) | 205 (100) | |
| Median (inter-quartile range) | 14.3 (9.0–26.0) | 30.5 (24.1–43.9) | <0.001 ** |
| Primary tumor site | 190 (100) | 205 (100) | |
| Abdominal adrenal | 132 (70) | 143 (70) | >0.999 |
| Abdominal non-adrenal | 53 (28) | 59 (29) | 0.911 |
| Thoracic | 12 (6) | 18 (9) | 0.448 |
| Cervical | 1 (1) | 2 (1) | N.A. |
| Unknown | 0 (0) | 0 (0) | N.A. |
| >1 site (combined regions) | 8 (4) | 14 (7) | 0.280 |
| >1 site (multilocular) | 8 (4) | 3 (2) | 0.128 |
| Stage (INSS) | 190 (100) | 205 (100) | |
| 1, 2, 3 or 4S | 119 (63) | 0 (0) | N.A. |
| 1 | 5 (3) | 0 (0) | N.A. |
| 2 | 20 (10) | 0 (0) | N.A. |
| 3 | 68 (36) | 0 (0) | N.A. |
| 4S | 26 (14) | 0 (0) | N.A. |
| 4 < 18 m | 71 (37) | 0 (0) | N.A. |
| 4 ≥18 m | 0 (0) | 205 (100) | N.A. |
| Sites of initial metastasis stage 4 | Stage 4 < 18 m 71 (100) | Stage 4 ≥ 18 m and MNA 205 (100) | |
| Bone marrow (cytology) | 65 (92) | 189 (92) | 0.804 |
| Osteomedullary (mIBG scintigraphy) | 67 (94) | 196 (96) | 0.746 |
| Lymph nodes | 20 (28) | 55 (27) | 0.877 |
| Liver | 20 (28) | 24 (12) | 0.002 |
| Brain/spinal cord | 2 (3) | 9 (4) | 0.734 |
| Lung/pleura | 8 (11) | 16 (8) | 0.463 |
| Skin | 2 (3) | 0 (0) | N.A. |
| Soft tissue | 5 (7) | 3 (2) | 0.029 |
| Other | 2 (3) | 2 (1) | 0.273 |
| Osteomedullary only | 30 (42) | 117 (57) | 0.038 |
| Sites of initial metastasis stage 4S | Stage 4S 26 (100) | Stage 4 ≥ 18 m and MNA 205 (100) | |
| Bone marrow (cytology) | 17 (14) | 189 (92) | <0.001 |
| Osteomedullary (mIBG scintigraphy) | 17 (62) | 196 (96) | <0.001 |
| Lymph nodes | 0 (0) | 55 (27) | <0.001 |
| Liver | 22 (19) | 24 (12) | 0.101 |
| Brain/spinal cord | 0 (0) | 9 (4) | N.A. |
| Lung/pleura | 1 (1) | 16 (8) | 0.008 |
| Skin | 2 (3) | 0 (0) | N.A. |
| Soft tissue | 0 (0) | 3 (2) | N.A. |
| Other | 0 (0) | 2 (1) | N.A. |
| Osteomedullary only | 3 (10) | 117 (57) | <0.001 |
| Tumor marker (NSE) | 167 (100) | 188 (100) | |
| Normal | 4 (2) | 1 (1) | 0.192 |
| abnormal | 163 (98) | 187 (99) | |
| Tumor marker (VMA/HVA) | 190 (100) | 205 (100) | |
| normal | 59 (31) | 39 (19) | 0.007 |
| Abnormal | 131 (69) | 166 (81) | |
| Tumor marker LDH | 185 (100) | 202 (100) | |
| Normal | 8 (4) | 1 (0) | 0.016 |
| abnormal | 177 (96) | 201 (100) | |
| Tumor marker ferritin | 142 (100) | 166 | |
| Normal | 76 (53) | 59 (35) | 0.002 |
| Abnormal | 66 (47) | 107 (65) | |
| Histology (Shimada) | 163 (100) | 164 (100) | |
| Favorable | 42 (26) | 7 (4) | <0.001 |
| Unfavorable | 121 (74) | 157 (96) | |
| Chromosome 1p aberration | 175 (100) | 188 (100) | |
| Heterozygosity | 25 (14) | 32 (17) | 0.155 |
| Imbalance | 25 (14) | 39 (21) | |
| Deletion | 125 (71) | 117 (62) | |
| Mutation RAS/p53 | 87 (100) | 49 (100) | 0.081 |
| yes | 21 (24) | 19 (39) | |
| Treatment protocol | 190 (100) | 205 (100) | |
| NB 97 | 57 (30) | 47 (23) | 0.137 |
| NB 2004 | 133 (70) | 158 (77) | |
| Surgery * | 190 (100) | 205 (100) | |
| Complete resection | 90 (47) | 95 (46) | 0.960 |
| Incomplete resection | 66 (35) | 71 (35) | |
| Biopsy only | 19 (10) | 21 (10) | |
| No surgery | 15 (8) | 18 (9) | |
| Radiotherapy | 190 (100) | 205 (100) | |
| Not given | 177 (93) | 184 (90) | 0.282 |
| Given | 13 (7) | 21 (10) | |
| mIBG therapy | 190 (100) | 205 (100) | |
| Not given | 171 (90) | 43 (21) | 0.003 |
| Given | 19 (10) | 162 (79) | |
| ASCT | 190 (100) | 205 (100) | |
| Given | 151 (79.5) | 172 (84) | 0.243 |
| Not given | 39 (20.5) | 33 (16) | |
| Antibody therapy | 190 (100) | 205 (100) | |
| Given | 30 (16) | 24 (12) | 0.245 |
| Not given | 160 (84) | 181 (88) | |
| Treatment modalities | 190 (100) | 205 (100) | |
| Neither surgery nor CT | 1 (1) | 0 (0) | 0.294 |
| Surgery only | 1 (1) | 0 (0) | |
| CT only | 14 (7) | 18 (9) | |
| Surgery and CT | 174 (92) | 187 (91) | |
| Follow-up (years) | |||
| Median [inter-quartile range; min–max] | 4.1 [1.2–11.4; 0.0–22.5] | 2.7 [1.2–6.3; 0.0–22.2] | 0.018 |
| Cause of death | 83 (100) | 133 (100) | |
| Tumor | 72 (87) | 116 (87) | 0.333 |
| Toxicity | 11 (13) | 14 (11) | |
| Tumor or toxicity | 0 (0) | 3 (2) |
| Gene | Study Group | Control Group |
|---|---|---|
| ALK mutations | 8 * | 7 # |
| ALK amplifications | 4 | 3 |
| HRAS | 1 | 1 # |
| NRAS | 1 | 0 |
| KRAS | 0 | 1 |
| NF1 | 1 | 1# |
| LIN28B | 1 | 0 |
| CCND1 | 1 | 0 |
| FGFR1 | 0 | 1 |
| PTPN11 | 0 | 1 |
| TP53 | 3 * | 0 |
| ATM | 1 | 0 |
| MDM2 | 1 | 2 |
| MDM4 | 0 | 2 # |
| CDKN2A | 0 | 2 # |
| CREBBP | 0 | 1 |
| Variable | N (No Event) | N (Event) | HR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Model 1: Accounting for mutation status (‘mutation status available’) | ||||||
| Event-free survival (EFS) proportional hazard assumption p = 0.274 | ||||||
| Mutation | No mutation | 37 | 29 | 1 | ||
| Mutation | 4 | 17 | 2.904 | 1.577–5.348 | 0.001 | |
| Age | <18 months | 54 | 70 | 1 | ||
| ≥18 months | 36 | 30 | 2.197 | 0.927–5.203 | 0.074 | |
| ASCT | No ASCT | 10 | 29 | 1 | ||
| ASCT | 80 | 71 | 2.697 | 0.858–8.482 | 0.090 | |
| Overall survival (OS) proportional hazard assumption p = 0.229 | ||||||
| Mutation | No mutation | 45 | 21 | 1 | ||
| Mutation | 4 | 17 | 3.214 | 1.678–6.156 | <0.001 | |
| Best surgery | Complete | 65 | 25 | 1 | ||
| Incomplete/biopsy/none | 42 | 58 | 2.564 | 1.240–5.304 | 0.011 | |
| Model 2: Mutation status not included into multivariable modelling (‘mutation status not available’) | ||||||
| Event-free survival (EFS) proportional hazard assumption p = 0.557 | ||||||
| Stage | Stage 1, 2, 3, 4S | 64 | 55 | 1 | ||
| Stage 4 | 26 | 45 | 1.609 | 1.082–2.393 | 0.019 | |
| Best surgery | Complete | 56 | 34 | 1 | ||
| Incomplete/biopsy/none | 34 | 66 | 1.923 | 1.255–2.947 | 0.003 | |
| Overall survival (OS) proportional hazard assumption p = 0.837 | ||||||
| Stage | Stage 1, 2, 3, 4S | 73 | 46 | 1 | ||
| Stage 4 | 34 | 37 | 1.570 | 1.017–2.425 | 0.042 | |
| Best surgery | Complete | 65 | 25 | 1 | ||
| Incomplete/biopsy/none | 42 | 58 | 2.425 | 1.504–3.909 | <0.001 | |
| Parameter | Study Group | Control Group | p |
|---|---|---|---|
| Stages 1, 2, 3, 4S, 4 < 18 m | Stage 4 ≥ 18 m and MNA | ||
| N (%) | N (%) | ||
| Recurrences | 88 (100) | 139 (100) | |
| Primary site | 58 (66) | 84 (60) | 0.036 |
| Osteomedullary * | 31 (35) | 80 (58) | 0.001 |
| Bone marrow | 20 (23) | 62 (45) | 0.001 |
| Lymph nodes | 13 (15) | 11 (8) | 0.124 |
| Liver | 18 (20) | 19 (14) | 0.202 |
| Brain/spinal cord | 18 (20) | 21 (15) | 0.368 |
| Lung/pleura | 6 (7) | 8 (6) | 0.783 |
| Skin | 0 (0) | 0 (0) | N.A. |
| Soft tissue | 0 (0) | 2 (1) | N.A. |
| Other | 4 (5) | 4 (3) | 0.714 |
| Osteomedullary only | 6 (7) | 48 (35) | <0.001 |
| Primary site only | 32 (36) | 29 (21) | 0.014 |
| Number of recurrence sites | |||
| 1 | 49 (55) | 59 (42) | 0.077 |
| >1 | 40 (45) | 80 (58) | |
| Median time ** (d) min–max | 382 28–3407 | 455 60–3427 | 0.003 |
| EFS and OS | |||
| 5y-secEFS *** (%) | 13.0 (5.6–20.4) | 10.2 (5.0–15.4) | 0.005 † |
| 5y-secOS (%) | 14.4 (6.6–22.2) | 9.8 (4.6–15.0) | 0.124 † |
| 10y-secEFS (%) | 13.0 (5.6–20.4) | 10.2 (5.0–15.4) | 0.005 † |
| 10y-secOS (%) | 14.4 (6.6–22.2) | 9.8 (4.6–15.0) | 0.124 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthold, F.; Ernst, A.; Ackermann, S.; Bartenhagen, C.; Christiansen, H.; Hero, B.; Rosswog, C.; von Schweinitz, D.; Klingebiel, T.; Schmid, I.; et al. Genetic Alterations and Resectability Predict Outcome in Patients with Neuroblastoma Assigned to High-Risk Solely by MYCN Amplification. Cancers 2021, 13, 4360. https://doi.org/10.3390/cancers13174360
Berthold F, Ernst A, Ackermann S, Bartenhagen C, Christiansen H, Hero B, Rosswog C, von Schweinitz D, Klingebiel T, Schmid I, et al. Genetic Alterations and Resectability Predict Outcome in Patients with Neuroblastoma Assigned to High-Risk Solely by MYCN Amplification. Cancers. 2021; 13(17):4360. https://doi.org/10.3390/cancers13174360
Chicago/Turabian StyleBerthold, Frank, Angela Ernst, Sandra Ackermann, Christoph Bartenhagen, Holger Christiansen, Barbara Hero, Carolina Rosswog, Dietrich von Schweinitz, Thomas Klingebiel, Irene Schmid, and et al. 2021. "Genetic Alterations and Resectability Predict Outcome in Patients with Neuroblastoma Assigned to High-Risk Solely by MYCN Amplification" Cancers 13, no. 17: 4360. https://doi.org/10.3390/cancers13174360
APA StyleBerthold, F., Ernst, A., Ackermann, S., Bartenhagen, C., Christiansen, H., Hero, B., Rosswog, C., von Schweinitz, D., Klingebiel, T., Schmid, I., Simon, T., & Fischer, M. (2021). Genetic Alterations and Resectability Predict Outcome in Patients with Neuroblastoma Assigned to High-Risk Solely by MYCN Amplification. Cancers, 13(17), 4360. https://doi.org/10.3390/cancers13174360







