Efficacy of Immune Checkpoint Inhibitors in Upper Tract Urothelial Carcinomas: Current Knowledge and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
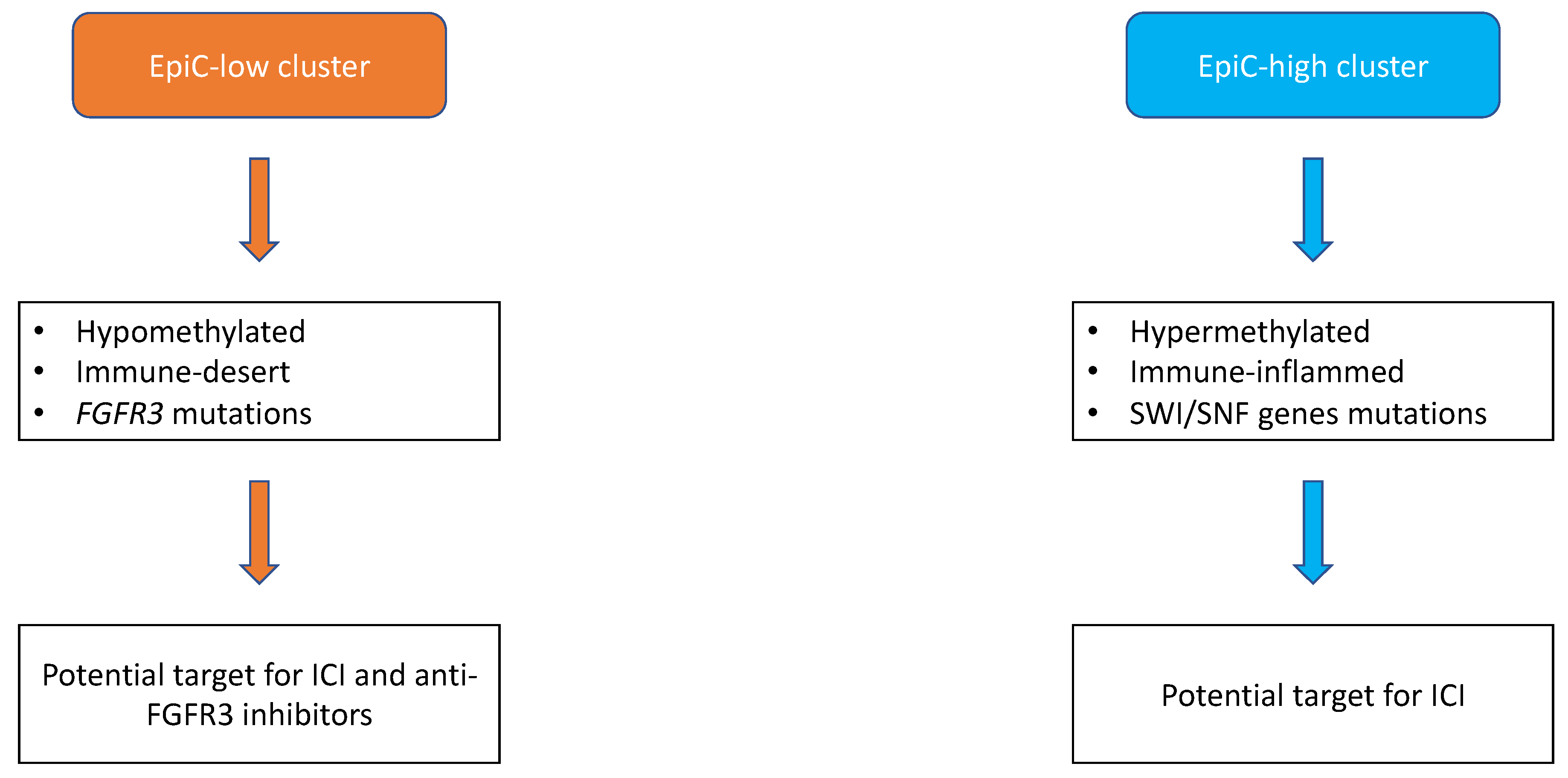
2. The Molecular Landscape of UTUC
3. The Immune Microenvironment of UTUC
4. Current Systemic Management of UTUC
4.1. Localized Disease
4.2. Metastatic Disease
5. Immune Checkpoint Inhibition in UTUC
5.1. Immune Checkpoint Inhibitors in the Perioperative Setting
5.2. Immune Checkpoint Inhibitors in the Metastatic Setting
6. Perspectives in UTUC Management
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H.; et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur. Urol. 2020, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European association of urology (EAU) prognostic factor risk groups for non–muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: An update from the EAU NMIBC guidelines panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Buti, S.; Giannatempo, P.; Raggi, D.; Necchi, A.; Leonetti, A.; Banna, G.L.; Petrelli, F. Outcome of patients with advanced upper tract urothelial carcinoma treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Crit. Rev. Oncol. 2021, 159, 103241. [Google Scholar] [CrossRef] [PubMed]
- Moss, T.J.; Qi, Y.; Xi, L.; Peng, B.; Kim, T.-B.; Ezzedine, N.E.; Mosqueda, M.E.; Guo, C.C.; Czerniak, B.A.; Ittmann, M.; et al. Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur. Urol. 2017, 72, 641–649. [Google Scholar] [CrossRef]
- Sfakianos, J.P.; Cha, E.K.; Iyer, G.; Scott, S.N.; Zabor, E.C.; Shah, R.; Ren, Q.; Bagrodia, A.; Kim, P.H.; Hakimi, A.A.; et al. Genomic characterization of upper tract urothelial carcinoma. Eur. Urol. 2015, 68, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, K.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.-Y.; Kwon, G.-Y.; et al. Molecular characterization of urothelial carcinoma of the bladder and upper urinary tract. Transl. Oncol. 2017, 11, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.; Wood, C.G.; et al. Outcomes of radical nephroureterectomy: A series from the upper tract urothelial carcinoma collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Chong, K.T.; Chang, S.L.; Bellmunt, J. Upper tract urothelial carcinoma: A different disease entity in terms of management. ESMO Open 2016, 1, e000126. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2020, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Rink, M.; Xylinas, E.; Matin, S.F.; Stenzl, A.; Roupret, M.; Karakiewicz, P.I.; Scherr, D.; Shariat, S.F. Urothelial carcinoma of the bladder and the upper tract: Disparate twins. J. Urol. 2012, 189, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.D.; Vlachostergios, P.; Bhinder, B.; Liu, W.; Li, K.; Moss, T.J.; Bareja, R.; Park, K.; Tavassoli, P.; Cyrta, J.; et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lu, X.; Bazai, S.K.; Compérat, E.; Mouawad, R.; Yao, H.; Rouprêt, M.; Spano, J.-P.; Khayat, D.; Davidson, I.; et al. Comprehensive integrative profiling of upper tract urothelial carcinomas. Genome Biol. 2021, 22, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Isharwal, S.; Cha, E.K.; Donoghue, M.T.A.; Drill, E.N.; Ostrovnaya, I.; Pietzak, E.J.; Sfakianos, J.P.; Bagrodia, A.; Murugan, P.; et al. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin. Cancer Res. 2018, 25, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.; Akbani, R.; et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed]
- Atlas, N.C.G. The cancer genome atlas research network comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 2019, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Hassler, M.R.; Bray, F.; Catto, J.W.; Grollman, A.P.; Hartmann, A.; Margulis, V.; Matin, S.F.; Roupret, M.; Sfakianos, J.P.; Shariat, S.F.; et al. Molecular characterization of upper tract urothelial carcinoma in the era of next-generation sequencing: A systematic review of the current literature. Eur. Urol. 2020, 78, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Madison, R.; Pal, S.K.; Ross, J.S.; Agarwal, N.; Sonpavde, G.; Joshi, M.; Yin, M.; Miller, V.A.; Grivas, P.; et al. Comprehensive genomic profiling of upper-tract and bladder urothelial carcinoma. Eur. Urol. Focus 2020, in press. [Google Scholar] [CrossRef]
- Bagrodia, A.; Cha, E.K.; Sfakianos, J.P.; Zabor, E.C.; Bochner, B.; Al-Ahmadie, H.A.; Solit, D.B.; Coleman, J.; Iyer, G.; Scott, S.N.; et al. Genomic biomarkers for the prediction of stage and prognosis of upper tract urothelial carcinoma. J. Urol. 2016, 195, 1684–1689. [Google Scholar] [CrossRef][Green Version]
- Bagrodia, A.; Audenet, F.; Pietzak, E.J.; Kim, K.; Murray, K.S.; Cha, E.K.; Sfakianos, J.P.; Iyer, G.; Singla, N.; Arcila, M.; et al. Genomic profile of urothelial carcinoma of the upper tract from ureteroscopic biopsy: Feasibility and validation using matched radical nephroureterectomy specimens. Eur. Urol. Focus 2018, 5, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.F.; Bagrodia, A.; Audenet, F.; Donoghue, M.T.; Cha, E.K.; Sfakianos, J.P.; Sperling, D.; Al-Ahmadie, H.; Clendenning, M.; Rosty, C.; et al. Genomic characterization of upper-tract urothelial carcinoma in patients with lynch syndrome. JCO Precis. Oncol. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.H.; Umeton, R.; Kim, J.; Lundgren, K.; Harshman, L.; Van Allen, E.M.; Preston, M.; Dong, F.; Bellmunt, J.; Mouw, K.W.; et al. Mutational analysis of 472 urothelial carcinoma across grades and anatomic sites. Clin. Cancer Res. 2018, 25, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.L.; Chen, C.-H.; Sidorenko, V.S.; He, J.; Dickman, K.G.; Yun, B.H.; Moriya, M.; Niknafs, N.; Douville, C.; Karchin, R.; et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Sci. Transl. Med. 2013, 5, 197ra102. [Google Scholar] [CrossRef] [PubMed]
- Castells, X.; Karanović, S.; Ardin, M.; Tomić, K.; Xylinas, E.; Durand, G.; Villar, S.; Forey, N.; Le Calvez-Kelm, F.; Voegele, C.; et al. Low-coverage exome sequencing screen in formalin-fixed paraffin-embedded tumors reveals evidence of exposure to carcinogenic aristolochic acid. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, R.; Leng, N.; Zill, O.; Sokol, E.; Liu, G.; Pavlick, D.; Maund, S.; Liu, L.-F.; Kadel, E.; Baldwin, N.; et al. Molecular determinants of response to PD-L1 blockade across tumor types. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Loeffler, M.; Steinke, V.; Rahner, N.; Holinski-Feder, E.; Dietmaier, W.; Schackert, H.K.; Goergens, H.; Doeberitz, M.V.K.; Goecke, T.O.; et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J. Clin. Oncol. 2012, 30, 4409–4415. [Google Scholar] [CrossRef]
- Van Der Post, R.S.; Kiemeney, L.; Ligtenberg, M.J.L.; Witjes, J.A.; De Kaa, C.A.H.-V.; Bodmer, D.; Schaap, L.; Kets, C.M.; Van Krieken, J.H.J.M.; Hoogerbrugge, N. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J. Med. Genet. 2010, 47, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Vasen, H.; Mecklin, J.-P.; Bernstein, I.; Aarnio, M.; Järvinen, H.J.; Myrhøj, T.; Sunde, L.; Wijnen, J.T.; Lynch, H.T. The risk of extra-colonic, extra-endometrial cancer in the lynch syndrome. Int. J. Cancer 2008, 123, 444–449. [Google Scholar] [CrossRef]
- Joost, P.; Therkildsen, C.; Dominguez-Valentin, M.; Jönsson, M.; Nilbert, M. Urinary tract cancer in lynch syndrome; increased risk in carriers of MSH2 mutations. Urology 2015, 86, 1212–1217. [Google Scholar] [CrossRef]
- Andreev-Drakhlin, A.; Shah, A.Y.; Adriazola, A.C.; Shaw, L.; Lopez, L.; James, M.; Matin, S.F.; Alhalabi, O.; Gao, J.; Siefker-Radtke, A.O.; et al. Efficacy of immune checkpoint blockade in patients with advanced upper tract urothelial cancer and mismatch repair deficiency or microsatellite instability (MSI). J. Clin. Oncol. 2021, 39, 487. [Google Scholar] [CrossRef]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; McGrath, J.E.; Xiu, J.; de Souza, A.L.; Gulati, S.; Abuali, I.; Sagaram, S.; Nabhan, C.; Korn, W.M.; Ryan, C.J.; et al. Characterization of microsatellite instability (dMMR/MSI-H) and mutational landscape in a large contemporary cohort of upper tract urothelial cancer (UTUC) patients. J. Clin. Oncol. 2021, 39, 465. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of microsatellite instability across 39 cancer types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Fromont, G.; Azzouzi, A.-R.; Catto, J.W.; Vallancien, G.; Hamdy, F.C.; Cussenot, O. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology 2005, 65, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Sato, Y.; Suzuki, H.; Kakiuchi, N.; Yoshizato, T.; Lenis, A.T.; Maekawa, S.; Yokoyama, A.; Takeuchi, Y.; Inoue, Y.; et al. Molecular classification and diagnostics of upper urinary tract urothelial carcinoma. Cancer Cell 2021, 39, 793–809.e8. [Google Scholar] [CrossRef]
- Wellenstein, M.D.; De Visser, K.E. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity 2018, 48, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Y.; Saci, A.; Szabo, P.M.; Martini, A.; Necchi, A.; Siefker-Radtke, A.; Pal, S.; Plimack, E.R.; Sfakianos, J.P.; et al. Fibroblast growth factor receptor 3 alterations and response to PD-1/PD-L1 blockade in patients with metastatic urothelial cancer. Eur. Urol. 2019, 76, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.L.; Weir, W.H.; Mayhew, G.M.; Shibata, Y.; Eulitt, P.; Uronis, J.M.; Zhou, M.; Nielsen, M.; Smith, A.B.; Woods, M.; et al. Fibroblast growth factor receptor 3 alterations and response to immune checkpoint inhibition in metastatic urothelial cancer: A real world experience. Br. J. Cancer 2021, 125, 1–10. [Google Scholar] [CrossRef]
- Chen, C.-H.; Tsai, M.-Y.; Chiang, P.-C.; Sung, M.-T.; Luo, H.-L.; Suen, J.-L.; Tsai, E.-M.; Chiang, P.-H. Prognostic value of PD-L1 combined positive score in patients with upper tract urothelial carcinoma. Cancer Immunol. Immunother. 2021, 70, 1–10. [Google Scholar] [CrossRef]
- Lu, Y.; Kang, J.; Luo, Z.; Song, Y.; Tian, J.; Li, Z.; Wang, X.; Liu, L.; Yang, Y.; Liu, X. The prevalence and prognostic role of PD-L1 in upper tract urothelial carcinoma patients underwent radical nephroureterectomy: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 1400. [Google Scholar] [CrossRef] [PubMed]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef]
- Birtle, A.J.; Chester, J.D.; Jones, R.J.; Jenkins, B.; Johnson, M.; Catto, J.W.; Powles, T.; Bryan, R.T.; Blacker, A.; Chakraborti, P.R.; et al. Updated outcomes of POUT: A phase III randomized trial of peri-operative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J. Clin. Oncol. 2021, 39, 455. [Google Scholar] [CrossRef]
- Lane, B.R.; Smith, A.K.; Larson, B.T.; Gong, M.C.; Campbell, S.C.; Raghavan, D.; Dreicer, R.; Hansel, D.E.; Stephenson, A.J. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 2010, 116, 2967–2973. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Zennami, K.; Sumitomo, M.; Takahara, K.; Nukaya, T.; Takenaka, M.; Fukaya, K.; Ichino, M.; Fukami, N.; Sasaki, H.; Kusaka, M.; et al. Two cycles of neoadjuvant chemotherapy improves survival in patients with high-risk upper tract urothelial carcinoma. BJU Int. 2020, 127, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Chong, Y.L.; Chang, S.L.; Valderrama, B.P.; Powles, T.; Bellmunt, J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: A 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur. Urol. 2021, 79, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, M.; Nguyen, D.-D.; Modonutti, D.; Haeuser, L.; Lipsitz, S.; Mossanen, M.; Kibel, A.S.; Lughezzani, G.; Trinh, Q.-D.; Cole, A.P. Impact of high-intensity local treatment on overall survival in stage IV upper tract urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 436.e1–436.e10. [Google Scholar] [CrossRef]
- Dong, F.; Fu, H.; Shi, X.; Shen, Y.; Xu, T.; Gao, F.; Wang, X.; Zhong, S.; Ding, Q.; Shen, Z.; et al. How do organ-specific metastases affect prognosis and surgical treatment for patients with metastatic upper tract urothelial carcinoma: First evidence from population based data. Clin. Exp. Metastasis 2017, 34, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Seisen, T.; Jindal, T.; Karabon, P.; Sood, A.; Bellmunt, J.; Rouprêt, M.; Leow, J.J.; Vetterlein, M.; Sun, M.; Alanee, S.; et al. Efficacy of Systemic Chemotherapy Plus Radical Nephroureterectomy for Metastatic Upper Tract Urothelial Carcinoma. Eur. Urol. 2016, 71, 714–718. [Google Scholar] [CrossRef]
- Moschini, M.; Xylinas, E.; Zamboni, S.; Mattei, A.; Niegisch, G.; Yu, E.Y.; Bamias, A.; Agarwal, N.; Sridhar, S.S.; Sternberg, C.N.; et al. Efficacy of surgery in the primary tumor site for metastatic urothelial cancer: Analysis of an international, multicenter, multidisciplinary database. Eur. Urol. Oncol. 2019, 3, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nazzani, S.; Preisser, F.; Mazzone, E.; Marchioni, M.; Bandini, M.; Tian, Z.; Mistretta, F.; Shariat, S.; Soulières, D.; Saad, F.; et al. Survival effect of nephroureterectomy in metastatic upper urinary tract urothelial carcinoma. Clin. Genitourin. Cancer 2019, 17, e602–e611. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.; Liu, Z.; Tan, T.W.; Lee, Y.M.; Yeo, E.K.; Chong, Y.-L. Optimal management of upper tract urothelial carcinoma: Current perspectives. OncoTargets Ther. 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.; Lee, J.; Fong, L.; Vogelzang, N.; Climent, M.; Petrylak, D.; Choueiri, T.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Raggi, D.; Miceli, R.; Sonpavde, G.; Giannatempo, P.; Mariani, L.; Galsky, M.D.; Bellmunt, J.; Necchi, A. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 49–61. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): An open-label, single-arm, phase II study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef]
- Necchi, A.; Raggi, D.; Gallina, A.; Madison, R.; Colecchia, M.; Lucianò, R.; Montironi, R.; Giannatempo, P.; Farè, E.; Pederzoli, F.; et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur. Urol. 2020, 77, 439–446. [Google Scholar] [CrossRef]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.L.; Harrison, M.R.; Deal, A.M.; Osterman, C.K.; Ramalingam, S.; Whang, Y.E.; Brower, B.Y.; Bjurlin, M.; Smith, A.B.; Nielsen, M.E.; et al. Phase II study of gemcitabine and split-dose cisplatin plus pembrolizumab as neoadjuvant therapy prior to radical cystectomy (RC) in patients with muscle-invasive bladder cancer (MIBC). J. Clin. Oncol. 2021, 39, 396. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers 2021, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Califano, G.; Ouzaid, I.; Verze, P.; Hermieu, J.-F.; Mirone, V.; Xylinas, E. Immune checkpoint inhibition in upper tract urothelial carcinoma. World J. Urol. 2020, 39, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2016, 389, 67–76. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Ding, X.; Zong, J.; Li, X.; Bai, X.; Tan, B.; Sun, W.; Wang, R.; Ding, Y. Dramatic responses of recurrent upper urinary tract urothelial carcinoma harboring FGFR3 and TP53 activating mutations to pembrolizumab in combination with erdafitinib: A case report. OncoTargets Ther. 2021, 14, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
| Trial | Drug | Study Design | Line | Overall pts n, UTUC pts n. (%) | Outcomes (Primary Endpoint) |
|---|---|---|---|---|---|
| IMvigor 010 [61] | Atezolizumab | Phase 3 RCT | Adjuvant | 809; 54 (6.7%) | Median disease-free survival, 19.4 months (95% CI 15.9–24.8) |
| Checkmate 274 [62] | Nivolumab | Phase 3 RCT | Adjuvant | 709; 149 (21%) | Median disease-free survival 20.8 months (95% confidence interval [CI], 16.5 to 27.6) |
| NCT02690558 [63] | Cisplatin, gemcitabine, pembrolizumab | Phase 2 | Neoadjuvant | 39; na | pCR:36% |
| POUT [43] | Cisplatin or carboplatin + gemcitabine | Phase 3 RCT | Adjuvant | 261 | Disease-free survival (hazard ratio 0.45, 95% CI 0.30–0.68; p = 0.0001) |
| Trial | Drug/Control Arm | Study Design | Line | Overall pts n, UTUC pts n. (%) | Outcomes (Primary Endpoint) |
|---|---|---|---|---|---|
| JAVELIN-100 [55] | Avelumab/BSC | Phase 3 RCT | 1L | 700, 187 (27%) | median OS: 21.4 months vs. 14.3 months; hazard ratio for death, 0.69; 95% confidence interval [CI], 0.56 to 0.86; p = 0.001 |
| KEYNOTE 052 [67] | Pembrolizumab | Phase 2 | 1L | 370, 69 (19%) | ORR: 24%, 95% CI 20–29) |
| IMvigor 130 [68] | Atezolizumab + platinum-based chemotherapy (A)/Atezolizumab (B)/Platinum-based chemotherapy | Phase 3 RCT | 1L | 1213, 312 (26%) | median PFS: 8.2 months (95% CI 6.5–8.3) in group A and 6.3 months (6.2–7.0) in group C (stratified hazard ratio [HR] 0.82, 95% CI 0.70–0.96; one-sided p = 0.007). median OS: 16.0 months (13.9–18.9) in group A and 13.4 months (12.0–15.2) in group C (0.83, 0.69–1.00; one-sided p = 0.027). Median overall survival was 15.7 months (13.1–17.8) for group B and 13.1 months (11.7–15.1) for group C (1.02, 0.83–1.24) |
| KEYNOTE 361 [69] | Cisplatin or Carboplatin + Gemcitabine + Pembrolizumab/Pembrolizumab/Cisplatin or Carboplatin + Gemcitabine | Phase 3 RCT | 1L | 1010, 211 (21%) | median OS: 17·0 months (14.5–19.5) in the pembrolizumab plus chemotherapy group versus 14.3 months (12.3–16.7) in the chemotherapy group (0.86, 0.72–1.02; p = 0.0407) median PFS: 8.3 months (95% CI 7.5–8.5) in the pembrolizumab plus chemotherapy group versus 7.1 months (6.4–7.9) in the chemotherapy group (hazard ratio [HR] 0.78, 95% CI 0.65–0.93; p = 0.0033) |
| KEYNOTE-045 [56] | Pembrolizumab/Paclitaxel or Docetaxel or Vinflunine | Phase 3 RCT | 2L | 748, 75 (10%) | median OS: 10.3 months (95% CI 8.0 to 11.8) vs. 7.4 months (95% CI, 6.1 to 8.3) (hazard ratio for death, 0.73; 95% CI, 0.59 to 0.91; p = 0.002) median PFS: 2.1 months (95% CI, 2.0 to 2.2) vs. 3.3 months (95% CI, 2.3 to 3.5) (HR 0.98; 95% CI, 0.81 to 1.19; p = 0.42) |
| IMvigor 211 [70] | Atezolizumab/Paclitaxel or Docetaxel or Vinflunine | Phase 3 RCT | 2L | 931, 236 (25%) | median OS: 11.1 (95% CI 8.6–15.5) vs. 10.6 months (95% CI 8.4–12.2) p = 0.41 |
| IMvigor 210 [66] | Atezolizumab | Phase 2 | 2L | 119, 33 (28%) | ORR: 23% (95% CI 16–31) |
| Trial Identification | Drugs | Comparative Arm | Administration | Study Design | Line | Primary Endpoint |
|---|---|---|---|---|---|---|
| NCT03513952 | Atezolizumab/CYT107 | Atezolizumab | IV | Phase 2 | ≥2 | ORR |
| NCT03237780 | Atezolizumab/eribulin | Eribulin | IV | Phase 2 | >2 | ORR |
| NCT02496208 | Cabozantinib/Nivolumab ± Ipilimumab | NA | PO/IV | Phase 1 | >1 | RP2D/safety |
| NCT04940299 | Tocilizumab/Ipilimumab/Nivolumab | NA | IV | Phase 2 | 1 | Safety/DLT |
| NCT03606174 | Sitravatinib/Nivolumab and Sitravatinib/Pembrolizumab/Enfortumab vedotin | NA | PO/IV and PO/IV/IV | Phase 2 | 1, ≥2 | ORR |
| NCT04602078 | Atezolizumab/Gemcitabine/Cisplatin | NA | IV | Phase 2 | 1 | ORR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thouvenin, J.; Martínez Chanzá, N.; Alhalabi, O.; Lang, H.; Tannir, N.M.; Barthélémy, P.; Malouf, G.G. Efficacy of Immune Checkpoint Inhibitors in Upper Tract Urothelial Carcinomas: Current Knowledge and Future Directions. Cancers 2021, 13, 4341. https://doi.org/10.3390/cancers13174341
Thouvenin J, Martínez Chanzá N, Alhalabi O, Lang H, Tannir NM, Barthélémy P, Malouf GG. Efficacy of Immune Checkpoint Inhibitors in Upper Tract Urothelial Carcinomas: Current Knowledge and Future Directions. Cancers. 2021; 13(17):4341. https://doi.org/10.3390/cancers13174341
Chicago/Turabian StyleThouvenin, Jonathan, Nieves Martínez Chanzá, Omar Alhalabi, Hervé Lang, Nizar M. Tannir, Philippe Barthélémy, and Gabriel G. Malouf. 2021. "Efficacy of Immune Checkpoint Inhibitors in Upper Tract Urothelial Carcinomas: Current Knowledge and Future Directions" Cancers 13, no. 17: 4341. https://doi.org/10.3390/cancers13174341
APA StyleThouvenin, J., Martínez Chanzá, N., Alhalabi, O., Lang, H., Tannir, N. M., Barthélémy, P., & Malouf, G. G. (2021). Efficacy of Immune Checkpoint Inhibitors in Upper Tract Urothelial Carcinomas: Current Knowledge and Future Directions. Cancers, 13(17), 4341. https://doi.org/10.3390/cancers13174341






