Usp22 Overexpression Leads to Aberrant Signal Transduction of Cancer-Related Pathways but Is Not Sufficient to Drive Tumor Formation in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
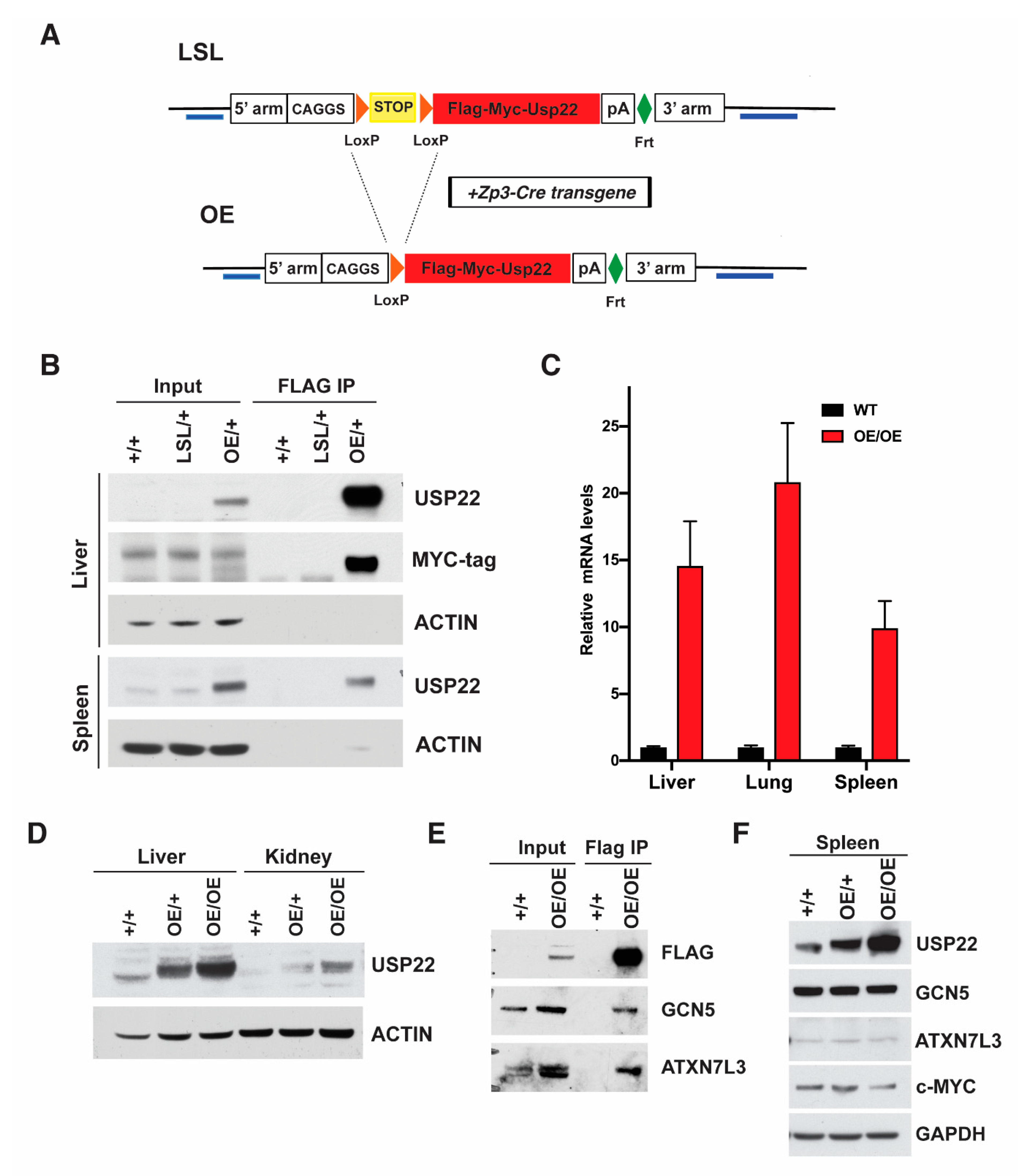
2.1. Generation of Overexpressing Usp22 Mice
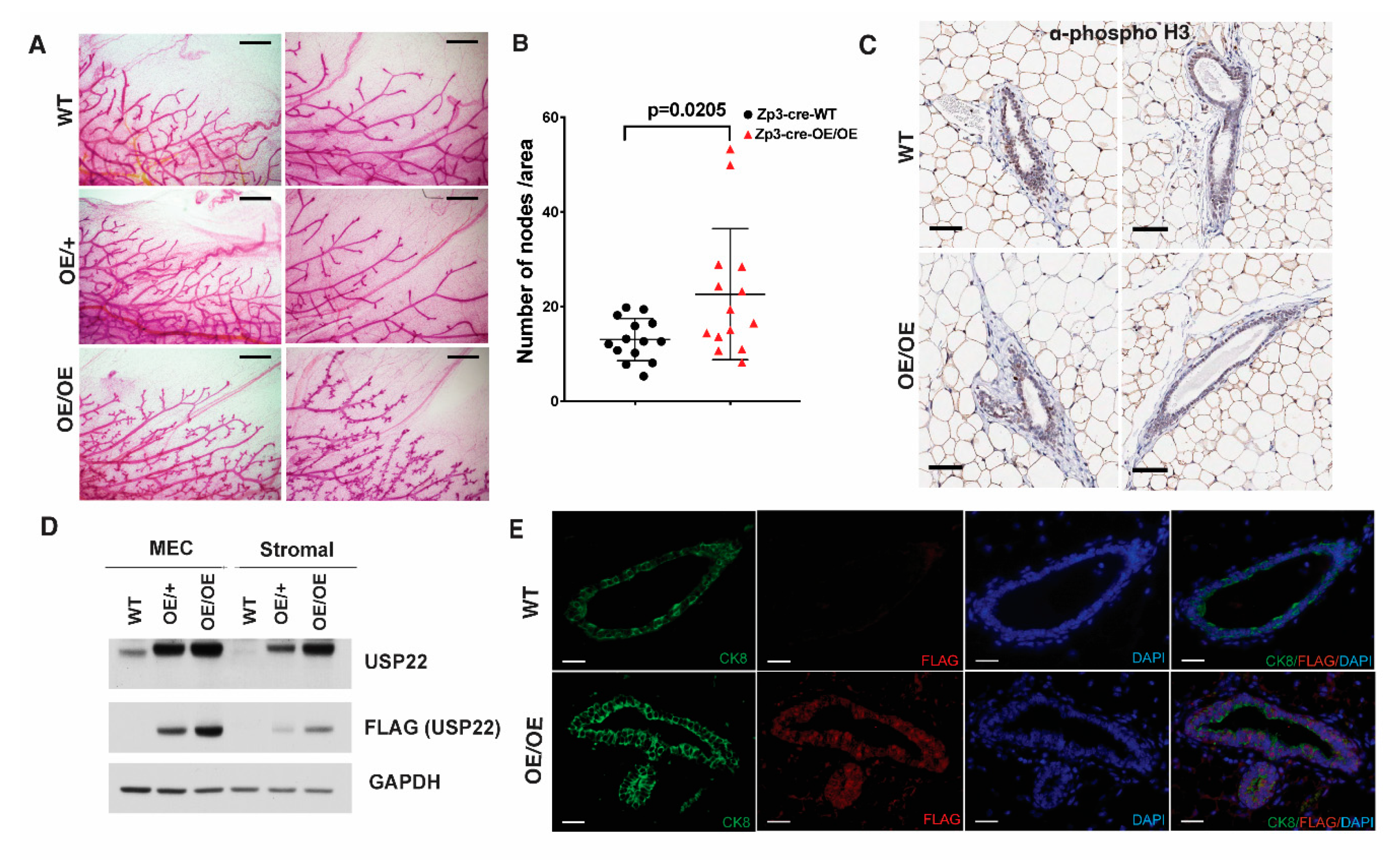
2.2. Mammary Gland Over-Branching in Usp22 Overexpressing Mice
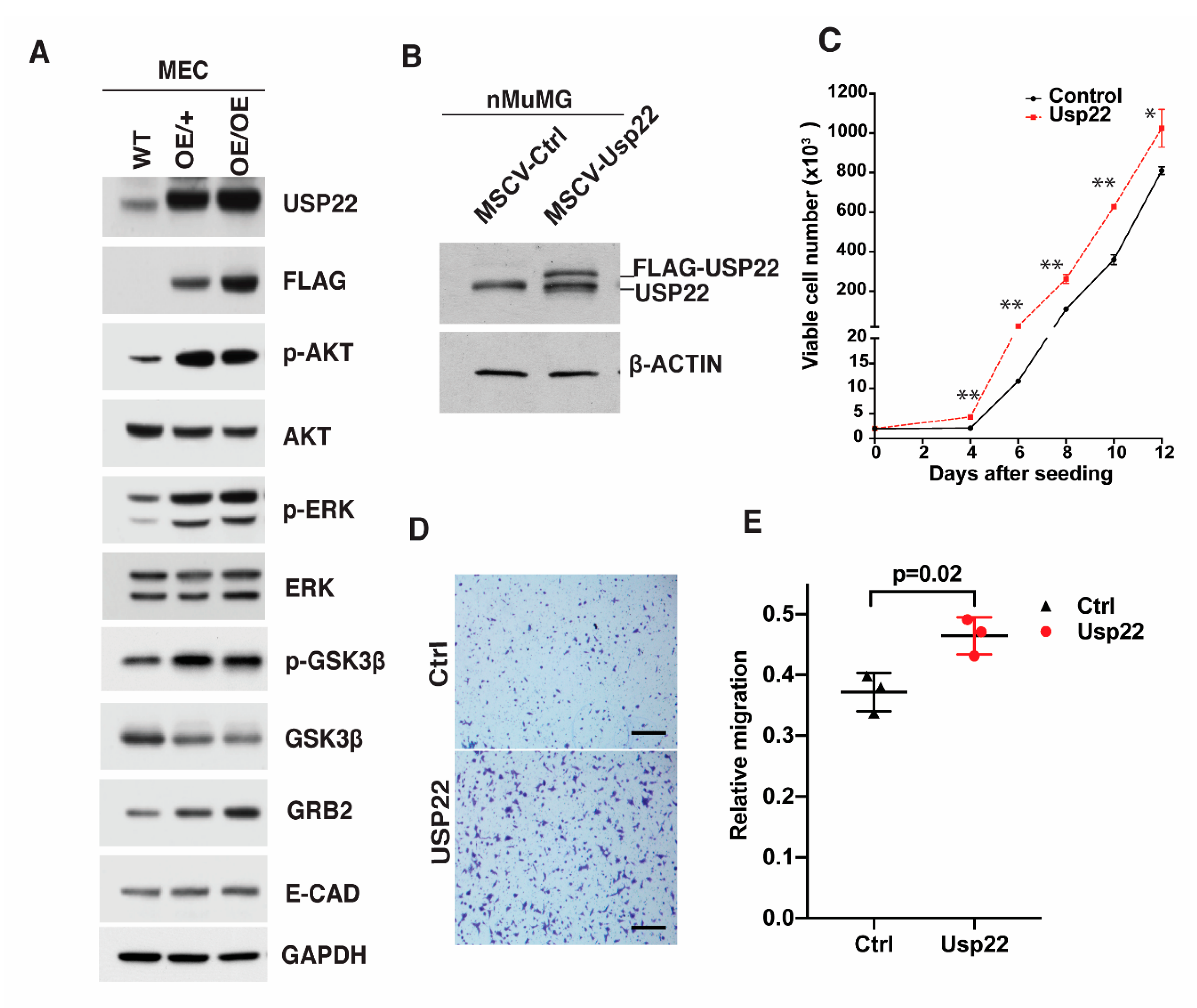
2.3. Altered Gene Expression Profiles in Usp22 OE Primary Mammary Epithelial Cells
2.4. Deregulation of Signaling Cascades in Primary Mammary Epithelial Cells Overexpressing Usp22
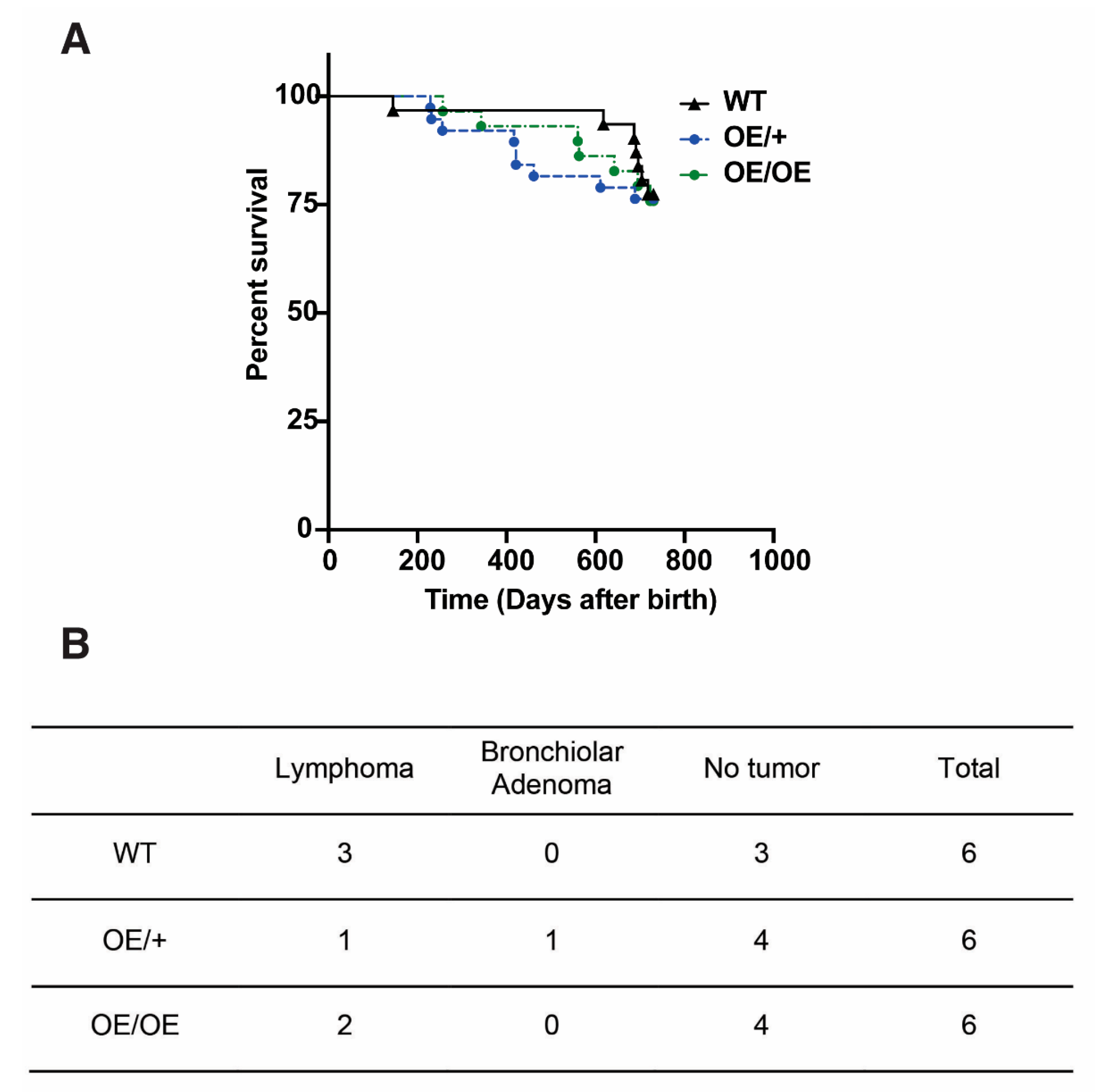
2.5. No Increase in Spontaneous Tumor Formation upon Ubiquitous Overexpression of Usp22 in Mice
3. Discussion
4. Methods
4.1. Generation of the Targeting Construct
4.2. Gene Targeting in ES Cells and Experimental Mice
4.3. Preparation of NMuMG Cells Stably Expressing Usp22 and Proliferation Assay
4.4. Isolation and Confirmation of Mouse Primary Mammary Gland Epithelial and Fibroblast Cells
4.5. Western Blotting
4.6. Immunoprecipitation
4.7. RNA Isolation and qRT-PCR
4.8. Mammary Gland Whole-Mount Staining
4.9. Mammary Gland Immunofluorescence Staining
4.10. Whole Transcriptome Sequencing (RNA-seq)
4.11. Transwell Migration Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, S.; Allis, C.D.; Wang, G.G. The language of chromatin modification in human cancers. Nat. Rev. Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef]
- Glinsky, G.V. Death-From-cancer signatures and stem cell contribution to metastatic cancer. Cell Cycle 2005, 4, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Glinsky, G.V.; Berezovska, O.; Glinskii, A.B. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J. Clin. Investig. 2005, 115, 1503–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Dent, S. Functions of SAGA in development and disease. Epigenomics 2014, 6, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-L.; Jiang, S.-X.; Yang, Y.-M.; Xu, H.; Liu, J.-L.; Wang, X.-S. USP22 Acts as an oncogene by the activation of BMI-1-mediated INK4A/ARF pathway and akt pathway. Cell Biophys. 2012, 62, 229–235. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Varthi, M.; Sykes, S.M.; Phillips, C.; Warzecha, C.; Zhu, W.; Wyce, A.; Thorne, A.W.; Berger, S.L.; McMahon, S.B. The Putative Cancer Stem Cell Marker USP22 Is a Subunit of the Human SAGA complex required for activated transcription and cell-cycle progression. Mol. Cell 2008, 29, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Yang, H.; Kong, Q.; Li, J.; Lee, S.-M.; Gao, B.; Dong, H.; Wei, J.; Song, J.; Zhang, D.D.; et al. USP22 Antagonizes p53 Transcriptional Activation by Deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol. Cell 2012, 46, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Gennaro, V.J.; Stanek, T.J.; Peck, A.R.; Sun, Y.; Wang, F.; Qie, S.; Knudsen, K.; Rui, H.; Butt, T.; Diehl, J.A.; et al. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, E9298–E9307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, A.; Lin, K.; Zhang, S.; Chen, Y.; Zhang, N.; Xue, J.; Wang, Z.; Aldape, K.D.; Xie, K.; Woodgett, J.R.; et al. Nuclear GSK3β promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat. Cell Biol. 2016, 18, 954–966. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Bao, C.; Tian, Y.; Xiao, H.; Wang, M.; Xie, X.; Hu, F.; Mei, J. USP22 Promotes NSCLC Tumorigenesis via MDMX Up-Regulation and Subsequent p53 Inhibition. Int. J. Mol. Sci. 2014, 16, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.; Xiao, X.-Y.; Gu, Z.-H.; Zeng, F.-Q.; Huang, L.-Q.; Jiang, G.-S. Silencing USP22 by asymmetric structure of interfering RNA inhibits proliferation and induces cell cycle arrest in bladder cancer cells. Mol. Cell. Biochem. 2011, 346, 11–21. [Google Scholar] [CrossRef]
- Schrecengost, R.S.; Dean, J.L.; Goodwin, J.F.; Schiewer, M.J.; Urban, M.W.; Stanek, T.J.; Sussman, R.; Hicks, J.L.; Birbe, R.; Draganova-Tacheva, R.A.; et al. USP22 regulates oncogenic signaling pathways to drive lethal cancer progression. Cancer Res. 2014, 74, 272–286. [Google Scholar] [CrossRef] [Green Version]
- McCann, J.J.; Vasilevskaya, I.A.; Neupane, N.P.; Shafi, A.A.; McNair, C.; Dylgjeri, E.; Mandigo, A.C.; Schiewer, M.J.; Schrecengost, R.S.; Gallagher, P.; et al. USP22 Functions as an oncogenic driver in prostate cancer by regulating cell proliferation and DNA Repair. Cancer Res. 2020, 80, 430–443. [Google Scholar] [CrossRef]
- Kosinsky, R.L.; Zerche, M.; Saul, D.; Wang, X.; Wohn, L.; Wegwitz, F.; Begus-Nahrmann, Y.; Johnsen, S.A. USP22 exerts tumor-suppressive functions in colorectal cancer by decreasing mTOR activity. Cell Death Differ. 2019, 27, 1328–1340. [Google Scholar] [CrossRef]
- Koutelou, E.; Wang, L.; Schibler, A.; Chao, H.-P.; Kuang, X.; Lin, K.; Lu, Y.; Shen, J.; Jeter, C.R.; Salinger, A.; et al. Usp22 controls multiple signaling pathways that are essential for vasculature formation in the mouse placenta. Development 2019, 146, dev.174037. [Google Scholar] [CrossRef] [Green Version]
- Lewandoski, M.; Wassarman, K.M.; Martin, G.R. Zp3–cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 1997, 7, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Bach, K.; Pensa, S.; Grzelak, M.; Hadfield, J.; Adams, D.J.; Marioni, J.C.; Khaled, W.T. Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurlin, J.W.; Nelson, C.M. Building branched tissue structures: From single cell guidance to coordinated construction. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20150527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, C.J.; Khaled, W. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development 2008, 135, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocchinfuso, W.P.; Lindzey, J.K.; Hewitt, S.C.; Clark, J.A.; Myers, P.H.; Cooper, R.; Korach, K.S. Induction of mammary gland development in estrogen receptor-α knockout mice. Endocrinology 2000, 141, 2982–2994. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Manka, D.; Wagner, K.-U.; Khan, S.A. Estrogen receptor- expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 14718–14723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hens, J.R.; Dann, P.; Zhang, J.-P.; Harris, S.; Robinson, G.W.; Wysolmerski, J. BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development 2007, 134, 1221–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hens, J.R.; Wysolmerski, J.J. Key stages of mammary gland development: Molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005, 7, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Mallepell, S.; Krust, A.; Chambon, P.; Brisken, C. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc. Natl. Acad. Sci. USA 2006, 103, 2196–2201. [Google Scholar] [CrossRef] [Green Version]
- Moraes, R.; Zhang, X.; Harrington, N.; Fung, J.Y.; Wu, M.-F.; Hilsenbeck, S.G.; Allred, D.C.; Lewis, M.T. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development 2007, 134, 1231–1242. [Google Scholar] [CrossRef] [Green Version]
- Bean, J.; Mohan, R.D. Hepatocellular carcinoma reduces ATXN7L3 to evade estrogen-dependent growth suppression. EBioMedicine 2021, 63, 103179. [Google Scholar] [CrossRef]
- Sun, N.; Zhong, X.; Wang, S.; Zeng, K.; Sun, H.; Sun, G.; Zou, R.; Liu, W.; Liu, W.; Lin, L.; et al. ATXN7L3 positively regulates SMAD7 transcription in hepatocellular carcinoma with growth inhibitory function. EBioMedicine 2020, 62, 103108. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, X.; Wang, C.; Luo, H.; Lin, L.; Sun, H.; Sun, G.; Zeng, K.; Zou, R.; Liu, W.; et al. USP22 positively modulates ERα action via its deubiquitinase activity in breast cancer. Cell Death Differ. 2020, 27, 3131–3145. [Google Scholar] [CrossRef]
- Gjorevski, N.; Nelson, C.M. Integrated morphodynamic signalling of the mammary gland. Nat. Rev. Mol. Cell Biol. 2011, 12, 581–593. [Google Scholar] [CrossRef]
- Atanassov, B.; Dent, S. USP22 regulates cell proliferation by deubiquitinating the transcriptional regulator FBP1. EMBO Rep. 2011, 12, 924–930. [Google Scholar] [CrossRef]
- Atanassov, B.; Evrard, Y.A.; Multani, A.S.; Zhang, Z.; Tora, L.; Devys, D.; Chang, S.; Dent, S.Y. Gcn5 and SAGA regulate shelterin protein turnover and telomere maintenance. Mol. Cell 2009, 35, 352–364. [Google Scholar] [CrossRef] [Green Version]
- Atanassov, B.S.; Mohan, R.D.; Lan, X.; Kuang, X.; Lu, Y.; Lin, K.; McIvor, E.; Li, W.; Zhang, Y.; Florens, L.; et al. ATXN7L3 and ENY2 Coordinate Activity of Multiple H2B Deubiquitinases Important for Cellular Proliferation and Tumor Growth. Mol. Cell 2016, 62, 558–571. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Hong, A.; Park, H.I.; Shin, W.H.; Yoo, L.; Jeon, S.J.; Chung, K.C. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J. Cell. Physiol. 2017, 232, 3664–3676. [Google Scholar] [CrossRef] [PubMed]
- Prokakis, E.; Dyas, A.; Grun, R.; Fritzsche, S.; Bedi, U.; Kazerouni, Z.B.; Kosinsky, R.L.; Johnsen, S.A.; Wegwitz, F. USP22 promotes HER2-driven mammary carcinoma aggressiveness by suppressing the unfolded protein response. Oncogene 2021, 43, 2017–2027. [Google Scholar] [CrossRef]
- Wang, Z.; Hausmann, S.; Lyu, R.; Li, T.-M.; Lofgren, S.M.; Flores, N.M.; Fuentes, M.E.; Caporicci, M.; Yang, Z.; Meiners, M.J.; et al. SETD5-coordinated chromatin reprogramming regulates adaptive resistance to targeted pancreatic cancer therapy. Cancer Cell 2020, 37, 834–849.e13. [Google Scholar] [CrossRef]
- Wu, S.-P.; Lee, D.-K.; DeMayo, F.J.; Tsai, S.Y.; Tsai, M.-J. Generation of ES cells for conditional expression of nuclear receptors and coregulatorsin vivo. Mol. Endocrinol. 2010, 24, 1297–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, K.; Guermah, M.; Yuan, C.-X.; Ito, M.; Wallberg, A.E.; Spiegelman, B.M.; Roeder, R.G. Transcription coactivator TRAP220 is required for PPARγ2-stimulated adipogenesis. Nat. Cell Biol. 2002, 417, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Bray, K.; Gillette, M.; Young, J.; Loughran, E.; Hwang, M.; Sears, J.C.; Vargo-Gogola, T. Cdc42 overexpression induces hyperbranching in the developing mammary gland by enhancing cell migration. Breast Cancer Res. 2013, 15, R91. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lang, G.; Ito, S.; Bonnet, J.; Metzger, E.; Sawatsubashi, S.; Suzuki, E.; LE Guezennec, X.; Stunnenberg, H.G.; Krasnov, A.; et al. A TFTC/STAGA Module Mediates Histone H2A and H2B Deubiquitination, Coactivates Nuclear Receptors, and Counteracts Heterochromatin Silencing. Mol. Cell 2008, 29, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [Green Version]
- Frankish, A.; Diekhans, M.; Ferreira, A.-M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Nat. Prec. 2010. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 1–5. [Google Scholar] [CrossRef]

| +/+ | OE/+ | OE/OE | Total | |
|---|---|---|---|---|
| Observed number | 45 (25%) | 81 (46%) | 52 (29%) | 178 (100%) |
| Expected number | 44 (25%) | 89 (50%) | 45 (25%) | 178 (100%) |
| Chi square | 0.23 | 0.72 | 1.09 | |
| p value | >0.75 | >0.25 | >0.25 |
| Total Number: 34 | R26OE-Usp22: OE/+ Total Number: 108 | R26OE-Usp22: OE/OE Total Number: 56 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Usp22 +/+ | Usp22 +/- | Usp22 -/- | Usp22 +/+ | Usp22 +/- | Usp22 -/- | Usp22 +/+ | Usp22 +/- | Usp22 -/- | |
| Observed number | 12 | 22 | 0 | 32 | 56 | 20 | 18 | 22 | 16 |
| Expected number | 11 | 22 | 11 | 27 | 56 | 27 | 14 | 28 | 14 |
| Chi square | 0.09 | 0 | 11 | 0.93 | 0 | 1.81 | 1.14 | 1.29 | 0.29 |
| p value | >0.75 | 0.99 | <0.001 | >0.25 | 0.99 | >0.10 | >0.25 | >0.25 | >0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, X.; McAndrew, M.J.; Mustachio, L.M.; Chen, Y.-J.C.; Atanassov, B.S.; Lin, K.; Lu, Y.; Shen, J.; Salinger, A.; Macatee, T.; et al. Usp22 Overexpression Leads to Aberrant Signal Transduction of Cancer-Related Pathways but Is Not Sufficient to Drive Tumor Formation in Mice. Cancers 2021, 13, 4276. https://doi.org/10.3390/cancers13174276
Kuang X, McAndrew MJ, Mustachio LM, Chen Y-JC, Atanassov BS, Lin K, Lu Y, Shen J, Salinger A, Macatee T, et al. Usp22 Overexpression Leads to Aberrant Signal Transduction of Cancer-Related Pathways but Is Not Sufficient to Drive Tumor Formation in Mice. Cancers. 2021; 13(17):4276. https://doi.org/10.3390/cancers13174276
Chicago/Turabian StyleKuang, Xianghong, Michael J. McAndrew, Lisa Maria Mustachio, Ying-Jiun C. Chen, Boyko S. Atanassov, Kevin Lin, Yue Lu, Jianjun Shen, Andrew Salinger, Timothy Macatee, and et al. 2021. "Usp22 Overexpression Leads to Aberrant Signal Transduction of Cancer-Related Pathways but Is Not Sufficient to Drive Tumor Formation in Mice" Cancers 13, no. 17: 4276. https://doi.org/10.3390/cancers13174276
APA StyleKuang, X., McAndrew, M. J., Mustachio, L. M., Chen, Y.-J. C., Atanassov, B. S., Lin, K., Lu, Y., Shen, J., Salinger, A., Macatee, T., Dent, S. Y. R., & Koutelou, E. (2021). Usp22 Overexpression Leads to Aberrant Signal Transduction of Cancer-Related Pathways but Is Not Sufficient to Drive Tumor Formation in Mice. Cancers, 13(17), 4276. https://doi.org/10.3390/cancers13174276







