Piperlongumine, a Potent Anticancer Phytotherapeutic, Induces Cell Cycle Arrest and Apoptosis In Vitro and In Vivo through the ROS/Akt Pathway in Human Thyroid Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
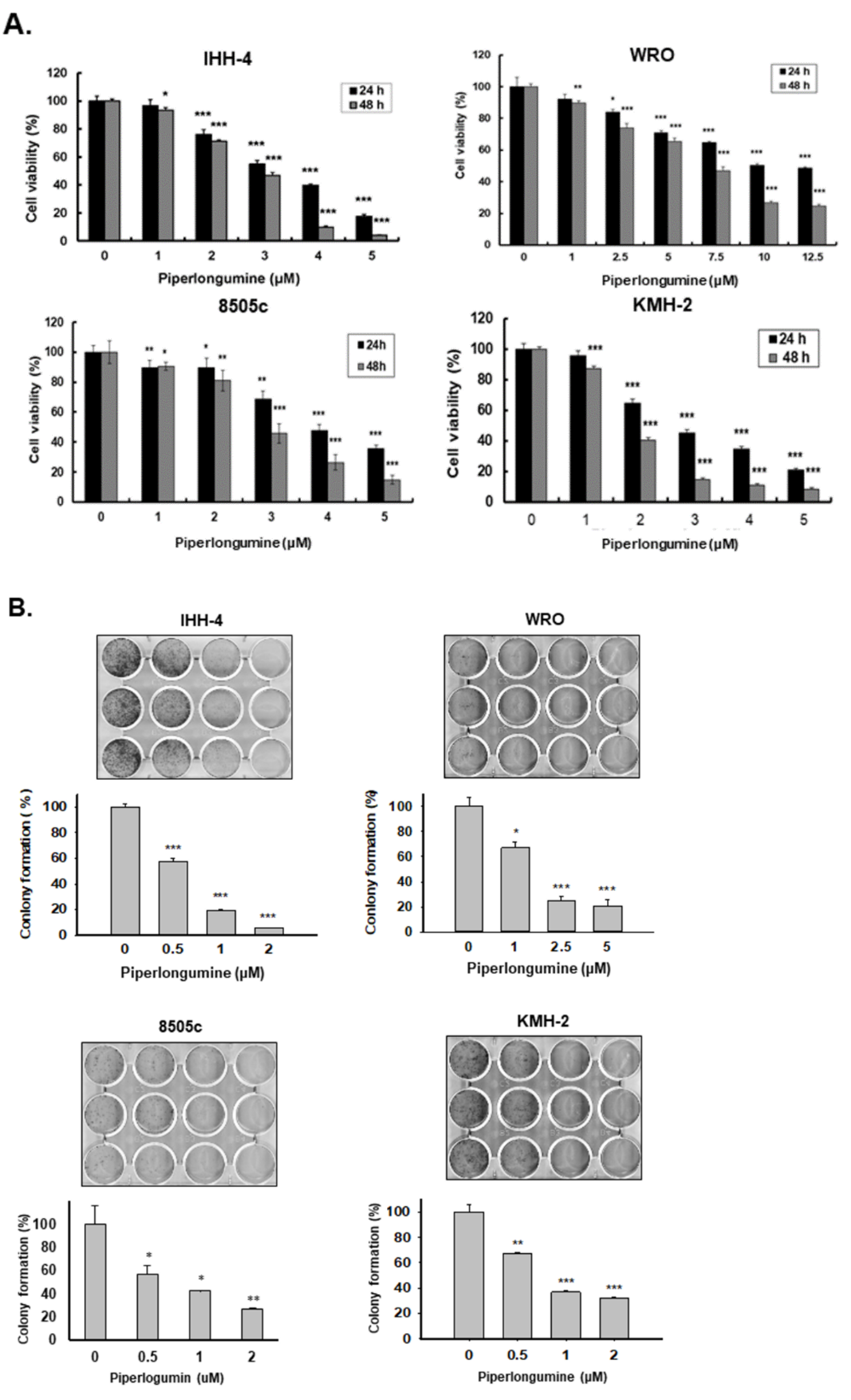
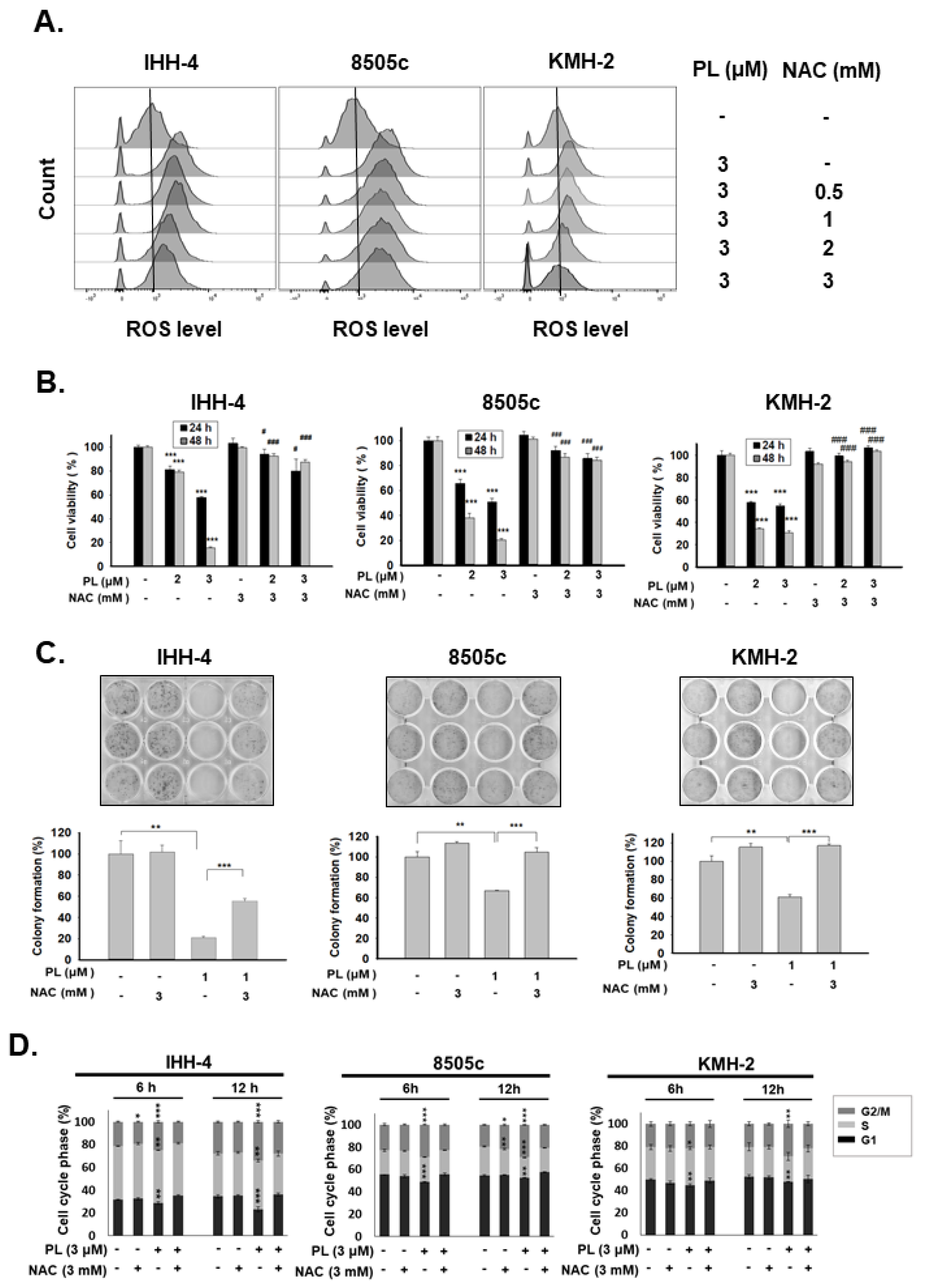
2.1. Piperlongumine Inhibits Cell Proliferation and Colony Formation of Human Thyroid Cancer Cells
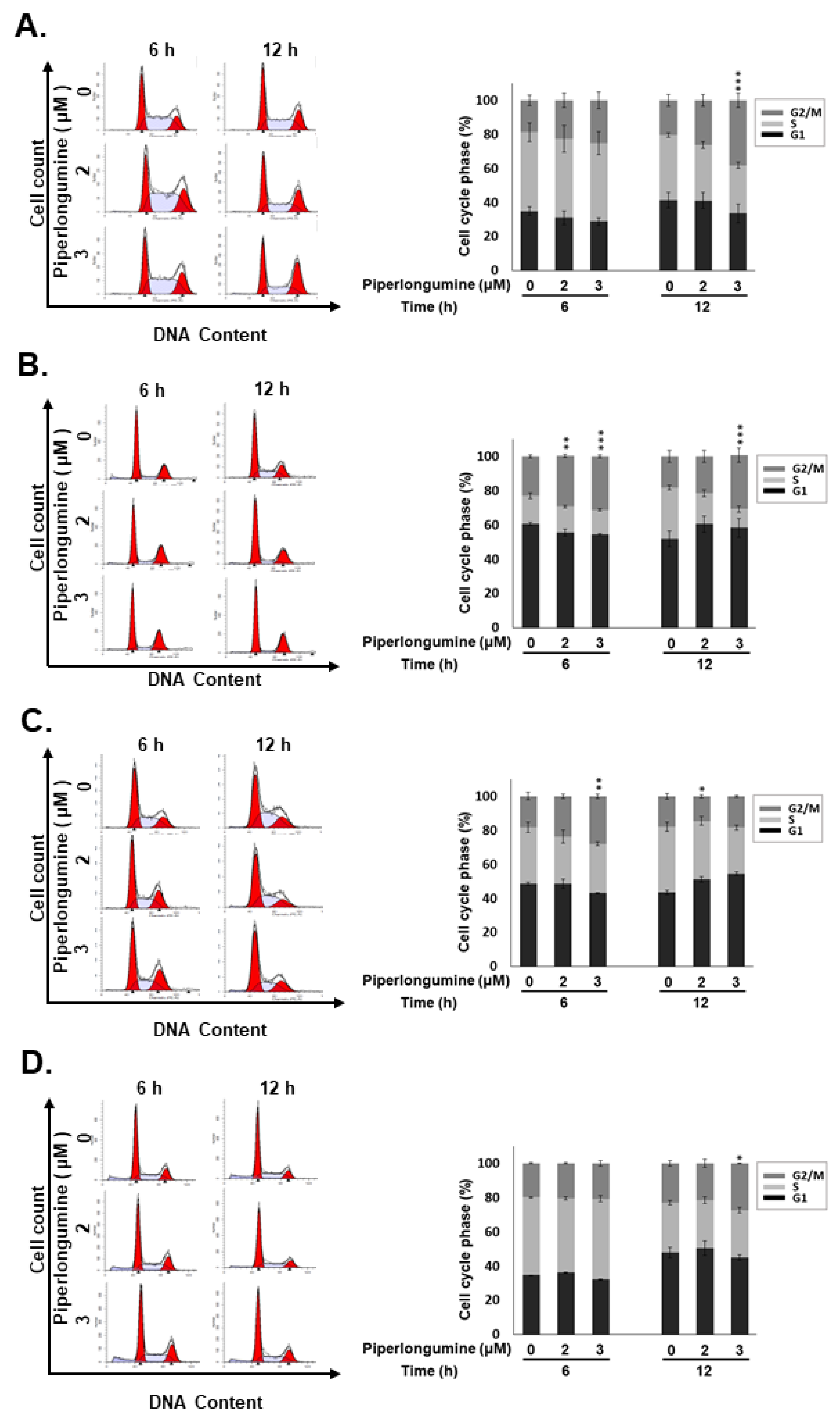
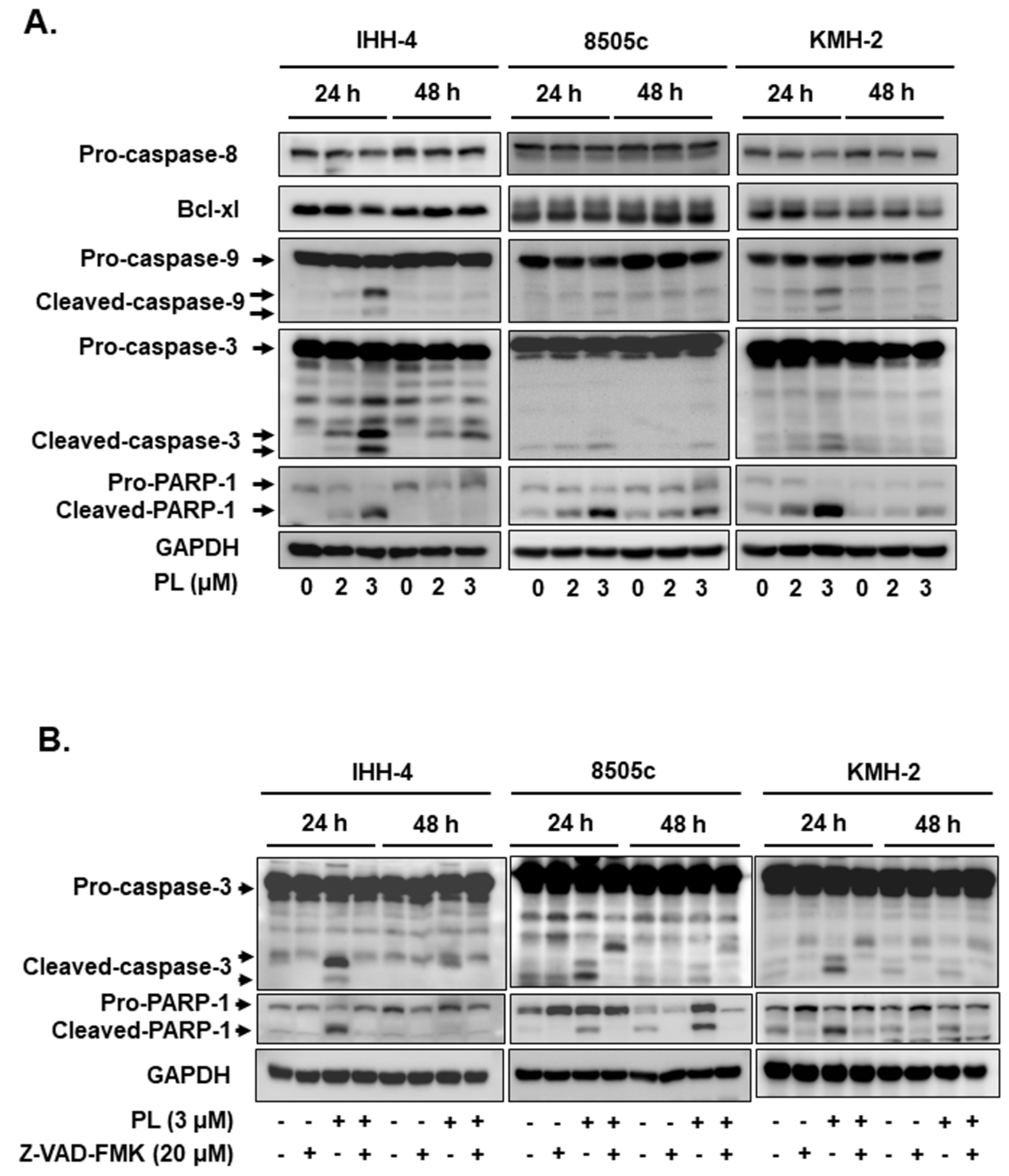
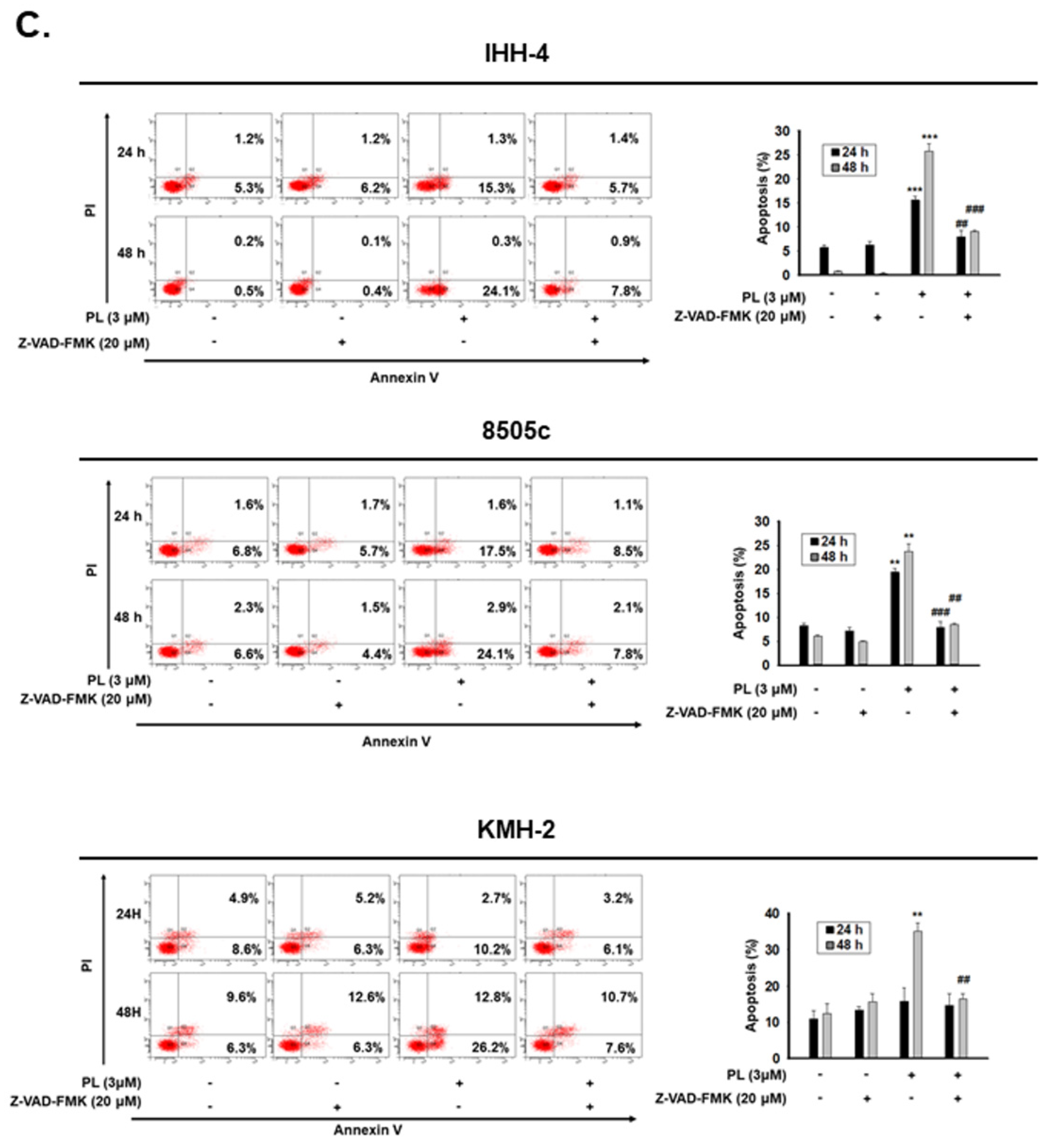
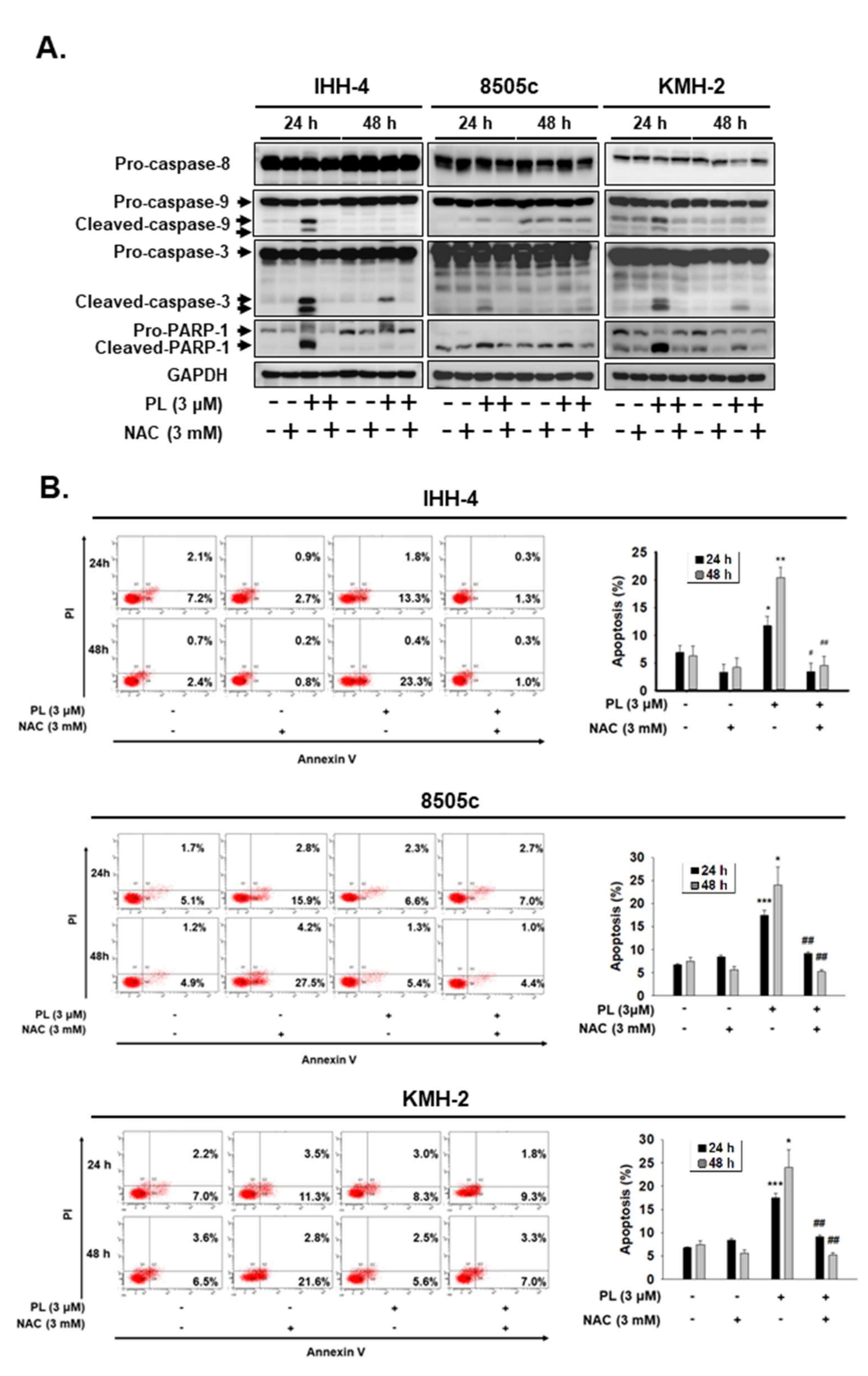
2.2. PL Induces G2/M Phase Cell Cycle Arrest and Cellular Apoptosis through the Intrinsic Caspase-Dependent Pathway in Human Thyroid Cancer Cells
2.3. ROS Induction Is Involved in PL-Mediated Antitumor Behavior in Human Thyroid Cancer Cells
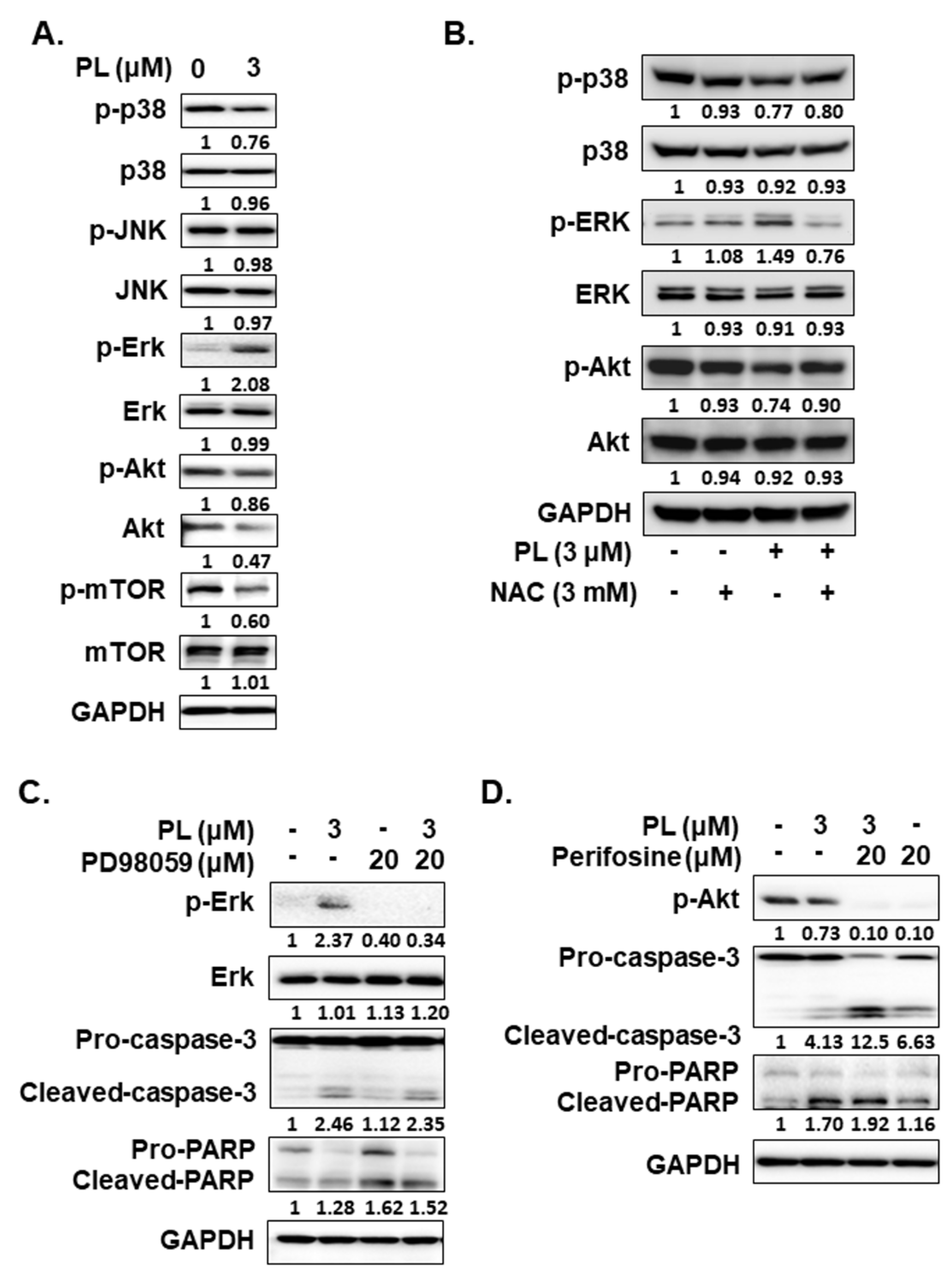
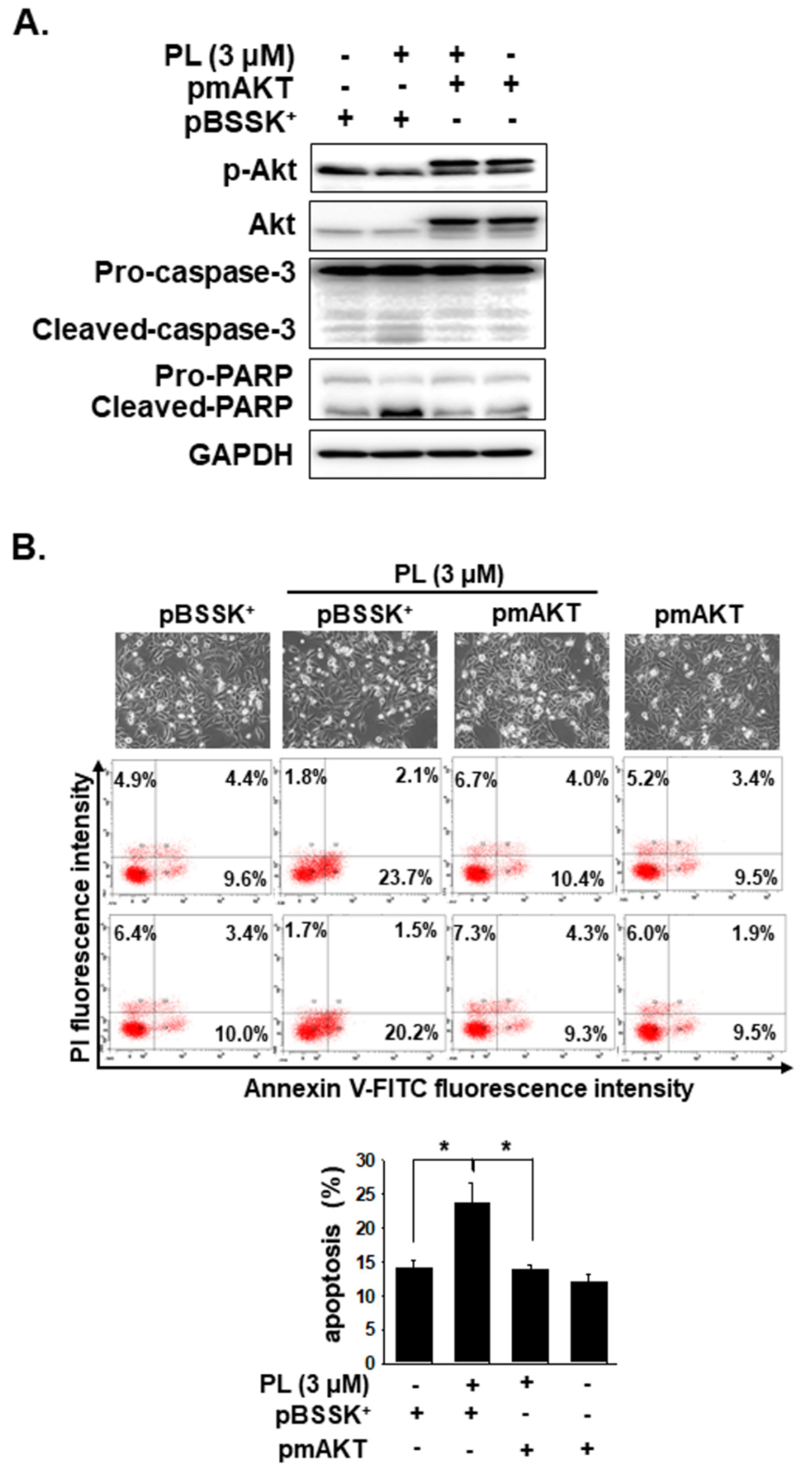
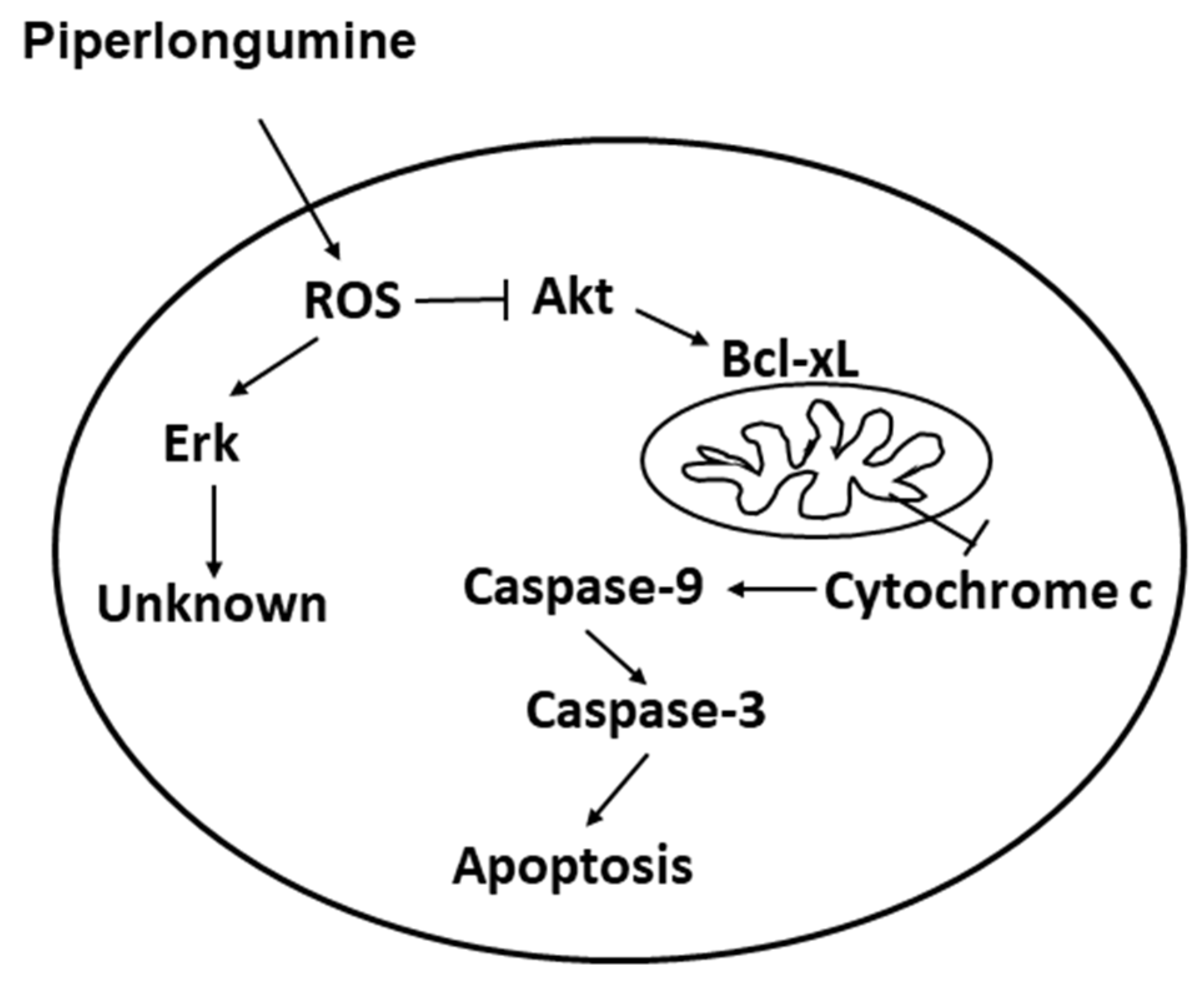
2.4. PL Induces Cellular Apoptosis through the ROS-Modulated Akt Pathway
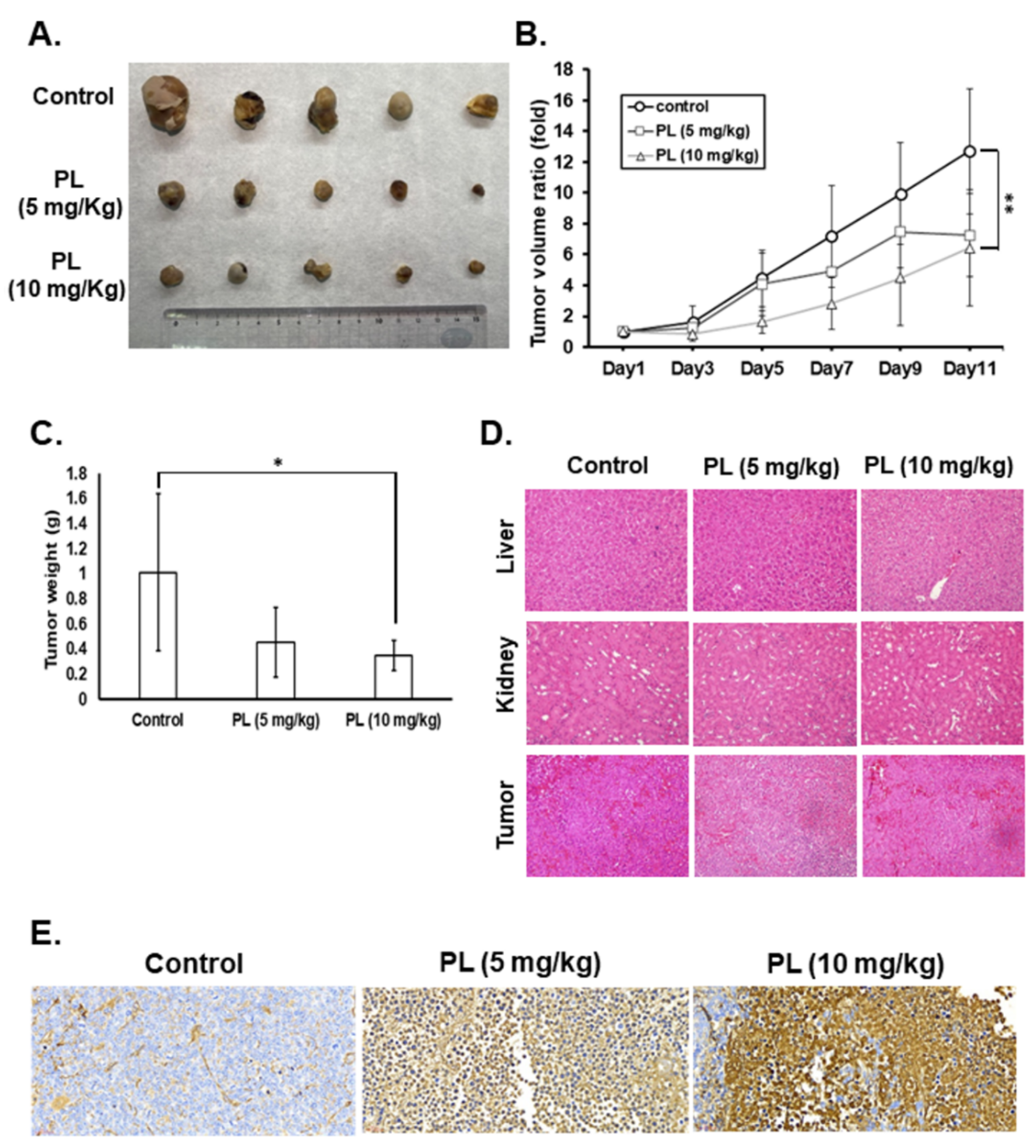
2.5. PL Reduces Tumor Growth and Induces Tumor Cell Apoptosis In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell lines and Cell Culture
4.2. Cell Viability Assay
4.3. Colony Formation Assay
4.4. Cell Cycle Analysis
4.5. Cell Death Analysis
4.6. Measurement of Cellular ROS
4.7. Western Blotting
4.8. Xenograft Study
4.9. Immunohistochemical Staining and TUNEL Assay of Tissue Sections
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgson, N.C.; Button, J.; Solorzano, C.C. Thyroid Cancer: Is the Incidence Still Increasing? Ann. Surg. Oncol. 2004, 11, 1093–1097. [Google Scholar] [CrossRef]
- Vanderpump, M.P. The Epidemiology of Thyroid Disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; McMillan, A. Anaplastic Thyroid Carcinoma. Treatment Outcome and Prognostic Factors. Cancer 2005, 103, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.C.; Chang, T.C.; Chen, H.R.; Lu, C.H.; Liu, Y.W.; Chen, S.H.; Yu, H.I.; Chang, Y.P.; Lee, Y.R. Reversine, a 2,6-Disubstituted Purine, as an Anti-cancer Agent in Differentiated and Undifferentiated Thyroid Cancer Cells. Pharm. Res. 2012, 29, 1990–2005. [Google Scholar] [CrossRef] [PubMed]
- Shaha, A.R. Implications of Prognostic Factors and Risk Groups in the Management of Differentiated Thyroid Cancer. Laryngoscope 2004, 114, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Dutta, C.P. Alkaloids of Piper longum Linn. I. Structure and Synthesis of Piperlongumine and Piperlonguminine. Tetrahedron 1967, 23, 1769–1781. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the Therapeutic Potential of Piplartine (Piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Son, D.J.; Yun, H.; Kamberos, N.L.; Janz, S. Piperlongumine Inhibits Proliferation and Survival of Burkitt Lymphoma In Vitro. Leuk. Res. 2013, 37, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Gao, T.; Lei, Q.; Zhang, L.; Yao, Y.; Xiong, J. Piperlongumine Induces Apoptosis in Human Melanoma Cells Via Reactive Oxygen Species Mediated Mitochondria Disruption. Nutr. Cancer 2018, 70, 502–511. [Google Scholar] [CrossRef]
- Liu, J.M.; Pan, F.; Li, L.; Liu, Q.R.; Chen, Y.; Xiong, X.X.; Cheng, K.; Yu, S.B.; Shi, Z.; Yu, A.C.; et al. Piperlongumine Selectively Kills Glioblastoma Multiforme Cells via Reactive Oxygen Species Accumulation Dependent JNK and p38 Activation. Biochem. Biophys. Res. Commun. 2013, 437, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Liu, G.H.; Chao, W.Y.; Shi, C.S.; Lin, C.Y.; Lim, Y.P.; Lu, C.H.; Lai, P.Y.; Chen, H.R.; Lee, Y.R. Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence. Int. J. Mol. Sci. 2016, 17, 616. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.L.; Kim, E.H.; Park, J.Y.; Kim, J.W.; Kwon, M.; Lee, B.H. Piperlongumine Selectively Kills Cancer Cells and Increases Cisplatin Antitumor Activity in Head and Neck Cancer. Oncotarget 2014, 5, 9227–9238. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Son, D.J.; Gu, S.M.; Woo, J.R.; Ham, Y.W.; Lee, H.P.; Kim, W.J.; Jung, J.K.; Hong, J.T. Piperlongumine Inhibits Lung Tumor Growth via Inhibition of Nuclear Factor Kappa B signaling Pathway. Sci. Rep. 2016, 6, 26357. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Jin, H.O.; Park, J.A.; Kim, J.H.; Kim, J.Y.; Kim, B.; Kim, W.; Hong, S.E.; Lee, Y.H.; Chang, Y.H.; et al. Heme Oxygenase-1 Determines the Differential Response of Breast Cancer and Normal Cells to Piperlongumine. Mol. Cells 2015, 38, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, J.M.; Xiong, X.X.; Qiu, X.Y.; Pan, F.; Liu, D.; Lan, S.J.; Jin, S.; Yu, S.B.; Chen, X.Q. Piperlongumine Selectively Kills Hepatocellular Carcinoma Cells and Preferentially Inhibits their Invasion via ROS-ER-MAPKs-CHOP. Oncotarget 2015, 6, 6406–6421. [Google Scholar] [CrossRef] [Green Version]
- Thongsom, S.; Suginta, W.; Lee, K.J.; Choe, H.; Talabnin, C. Piperlongumine Induces G2/M Phase Arrest and Apoptosis in Cholangiocarcinoma Cells through the ROS-JNK-ERK Signaling Pathway. Apoptosis 2017, 22, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Golovine, K.; Makhov, P.; Naito, S.; Raiyani, H.; Tomaszewski, J.; Mehrazin, R.; Tulin, A.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine and its Analogs Down-regulate Expression of c-Met in Renal Cell Carcinoma. Cancer Biol. Ther. 2015, 16, 743–749. [Google Scholar] [CrossRef]
- Mohammad, J.; Singh, R.R.; Riggle, C.; Haugrud, B.; Abdalla, M.Y.; Reindl, K.M. JNK Inhibition Blocks Piperlongumine-Induced Cell Death and Transcriptional Activation of Heme Oxygenase-1 in Pancreatic Cancer Cells. Apoptosis 2019, 24, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Zhang, B.; Deng, C.; Cao, Y.; Zhou, F.; Wu, L.; Chen, M.; Shen, S.; Xu, G.; Zhang, S.; et al. Piperlongumine Induces Gastric Cancer Cell Apoptosis and G2/M cell Cycle Arrest both In Vitro and In Vivo. Tumour Biol. 2016, 37, 10793–10804. [Google Scholar] [CrossRef]
- Kumar, S.; Agnihotri, N. Piperlongumine, a Piper Alkaloid Targets Ras/PI3K/Akt/mTOR Signaling Axis to Inhibit Tumor Cell Growth and Proliferation in DMH/DSS Induced Experimental Colon Cancer. Biomed. Pharmacother. 2019, 109, 1462–1477. [Google Scholar] [CrossRef]
- Liu, D.; Qiu, X.Y.; Wu, X.; Hu, D.X.; Li, C.Y.; Yu, S.B.; Pan, F.; Chen, X.Q. Piperlongumine Suppresses Bladder Cancer Invasion via Inhibiting Epithelial Mesenchymal Transition and F-actin Reorganization. Biochem. Biophys. Res. Commun. 2017, 494, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Oblad, R.; Doughty, H.; Lawson, J.; Christensen, M.; Kenealey, J. Application of Mixture Design Response Surface Methodology for Combination Chemotherapy in PC-3 Human Prostate Cancer Cells. Mol. Pharmacol. 2018, 94, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, S.K.; Biswal, B.K. Piperlongumine, a Potent Anticancer Phytotherapeutic: Perspectives on Contemporary Status and Future Possibilities as an Anticancer Agent. Pharmacol. Res. 2020, 156, 104772. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wen, C.; Bai, H.; Wang, X.; Zhang, X.; Huang, L.; Yang, X.; Iwamoto, A.; Liu, H. JNK Signaling Pathway is Involved in Piperlongumine-mediated Apoptosis in Human Colorectal Cancer HCT116 Cells. Onco.l Lett. 2015, 10, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; Costa-Lotufo, L.V. In Vivo Growth-inhibition of Sarcoma 180 by Piplartine and Piperine, Two Alkaloid Amides from Piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef]
- Bezerra, D.P.; de Castro, F.O.; Alves, A.P.; Pessoa, C.; de Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; de Alencar, N.M.; Mesquita, R.O.; et al. In Vitro and In Vivo Antitumor Effect of 5-FU Combined with Piplartine and Piperine. J. Appl Toxicol. 2008, 28, 156–163. [Google Scholar] [CrossRef]
- Mohler, H.; Pfirrmann, R.W.; Frei, K. Redox-directed Cancer Therapeutics: Taurolidine and Piperlongumine as Broadly Effective Antineoplastic Agents (Review). Int. J. Oncol. 2014, 45, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS Signaling under Metabolic Stress: Cross-Talk between AMPK and AKT Pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McArthur, K.; Kile, B.T. Apoptotic Caspases: Multiple or Mistaken Identities? Trends Cell Biol. 2018, 28, 475–493. [Google Scholar] [CrossRef]
- Stassi, G.; Todaro, M.; Zerilli, M.; Ricci-Vitiani, L.; Di Liberto, D.; Patti, M.; Florena, A.; Di Gaudio, F.; Di Gesu, G.; De Maria, R. Thyroid Cancer Resistance to Chemotherapeutic Drugs Via Autocrine Production of Interleukin-4 and Interleukin-10. Cancer Res. 2003, 63, 6784–6790. [Google Scholar] [PubMed]
- Bezerra, D.P.; Pessoa, C.; Moraes, M.O.; Alencar, N.M.; Mesquita, R.O.; Lima, M.W.; Alves, A.P.; Pessoa, O.D.; Chaves, J.H.; Silveira, E.R.; et al. In Vivo Growth Inhibition of Sarcoma 180 by Piperlonguminine, an Alkaloid Amide from the Piper Species. J. Appl. Toxicol. 2008, 28, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.Y.; Wang, Y.P.; Liu, Y.M.; Liu, B.; Liu, M.M.; Liu, B.M. Spectroscopic Investigation of the Anticancer Alkaloid Piperlongumine Binding to Human Serum Albumin from the Viewpoint of Drug Delivery. Luminescence 2018, 33, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Mazzi, V.; Vita, R.; Ferrari, S.M.; Materazzi, G.; Galleri, D.; Benvenga, S.; Miccoli, P.; Antonelli, A. New Therapies for Dedifferentiated Papillary Thyroid Cancer. Int. J. Mol. Sci. 2015, 16, 6153–6182. [Google Scholar] [CrossRef] [Green Version]
- Pistollato, F.; Masias, M.; Agudo, P.; Giampieri, F.; Battino, M. Effects of Phytochemicals on Thyroid Function and Their Possible Role in Thyroid Disease. Ann. N. Y. Acad. Sci. 2019, 1443, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Park, Y.; Hollenbeck, A.R.; Kitahara, C.M. Dietary Flavonoid Intake and Thyroid Cancer Risk in the NIH-AARP Diet and Health Study. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Mao, Y.; You, Q.; Hua, D.; Cai, D. Piperlongumine Induces Apoptosis and Autophagy in Human Lung Cancer Cells through Inhibition of PI3K/Akt/mTOR Pathway. Int. J. Immunopathol. Pharmacol. 2015, 28, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Piska, K.; Gunia-Krzyzak, A.; Koczurkiewicz, P.; Wojcik-Pszczola, K.; Pekala, E. Piperlongumine (Piplartine) as a Lead Compound for Anticancer Agents-Synthesis and Properties of Analogues: A Mini-review. Eur. J. Med. Chem. 2018, 156, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, Y.; Yang, C.; Sang, Z.; Yang, T.; Ang, W.; Ye, W.; Wei, Y.; Gong, C.; Luo, Y. Biodegradable Nanoassemblies of Piperlongumine Display Enhanced Anti-angiogenesis and Anti-tumor Activities. Nanoscale 2014, 6, 4325–4337. [Google Scholar] [CrossRef]
- Piska, K.; Koczurkiewicz, P.; Wnuk, D.; Karnas, E.; Bucki, A.; Wojcik-Pszczola, K.; Jamrozik, M.; Michalik, M.; Kolaczkowski, M.; Pekala, E. Synergistic anticancer activity of doxorubicin and piperlongumine on DU-145 Prostate Cancer Cells-The Involvement of Carbonyl Reductase 1 Inhibition. Chem. Biol. Interact. 2019, 300, 40–48. [Google Scholar] [CrossRef]
- Yao, J.X.; Yao, Z.F.; Li, Z.F.; Liu, Y.B. Radio-sensitization by Piper Longumine of Human Breast Adenoma MDA-MB-231 Cells In Vitro. Asian Pac. J. Cancer Prev. 2014, 15, 3211–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; Xia, Y.; He, W.; Zhang, T.; Hong, L.; Zheng, P.; Shen, X.; Liang, G.; Cui, R.; Zou, P. Enhancement of Oxaliplatin-induced Colon Cancer Cell Apoptosis by Alantolactone, a Natural Product Inducer of ROS. Int. J. Biol. Sci. 2019, 15, 1676–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Circu, M.L.; Aw, T.Y. Reactive Oxygen Species, Cellular Redox Systems, and Apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.X.; Liu, J.M.; Qiu, X.Y.; Pan, F.; Yu, S.B.; Chen, X.Q. Piperlongumine Induces Apoptotic and Autophagic Death of the Primary Myeloid Leukemia Cells from Patients via Activation of ROS-p38/JNK Pathways. Acta. Pharmacol. Sin. 2015, 36, 362–374. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Z. Increased Oxidative Stress as a Selective Anticancer Therapy. Oxid. Med. Cell Longev. 2015, 2015, 294303. [Google Scholar] [CrossRef] [Green Version]
- van Staveren, W.C.; Solis, D.Y.; Hebrant, A.; Detours, V.; Dumont, J.E.; Maenhaut, C. Human Cancer Cell Lines: Experimental Models for Cancer Cells in Situ? For Cancer Stem Cells? Biochim. Biophys. Acta. 2009, 1795, 92–103. [Google Scholar] [CrossRef]
- Chintakuntlawar, A.V.; Foote, R.L.; Kasperbauer, J.L.; Bible, K.C. Diagnosis and Management of Anaplastic Thyroid Cancer. Endocrinol. Metab. Clin. North. Am. 2019, 48, 269–284. [Google Scholar] [CrossRef]
- Lu, C.H.; Liu, Y.W.; Hua, S.C.; Yu, H.I.; Chang, Y.P.; Lee, Y.R. Autophagy Induction of Reversine on Human Follicular Thyroid Cancer Cells. Biomed. Pharmacother. 2012, 66, 642–647. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, S.H.; Lin, C.Y.; Chao, W.Y.; Lim, Y.P.; Yu, H.I.; Lu, C.H. In Vitro Antitumor Activity of Aloperine on Human Thyroid Cancer Cells through Caspase-Dependent Apoptosis. Int. J. Mol. Sci. 2018, 19, 312. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.H.; Chen, S.H.; Chang, Y.S.; Liu, Y.W.; Wu, J.Y.; Lim, Y.P.; Yu, H.I.; Lee, Y.R. Honokiol, a Potential Therapeutic Agent, Induces Cell Cycle Arrest and Program Cell Death In Vitro and In Vivo in Human Thyroid Cancer Cells. Pharmacol. Res. 2017, 115, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Kam, K.H.; Chao, W.Y.; Zhao, P.W.; Chen, S.H.; Chung, H.C.; Li, Y.Z.; Wu, J.Y.; Lee, Y.R. Berberine Derivatives Suppress Cellular Proliferation and Tumorigenesis In Vitro in Human Non-Small-Cell Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4218. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Kuo, C.H.; Chen, S.H.; Lin, C.Y.; Lee, Y.R. Honokiol Inhibits In Vitro and In Vivo Growth of Oral Squamous Cell Carcinoma through Induction of Apoptosis, Cell Cycle Arrest and Autophagy. J. Cell Mol. Med. 2018, 22, 1894–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Time (h) | Cell Line | ||||
|---|---|---|---|---|---|
| IHH-4 | WRO | 8505c | KMH-2 | ||
| Piperlongumine (μM) | 24 | 3.2 | 12.52 | 3.3 | 2.4 |
| 48 | 2.8 | 5.58 | 2.8 | 1.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kung, F.-P.; Lim, Y.-P.; Chao, W.-Y.; Zhang, Y.-S.; Yu, H.-I.; Tai, T.-S.; Lu, C.-H.; Chen, S.-H.; Li, Y.-Z.; Zhao, P.-W.; et al. Piperlongumine, a Potent Anticancer Phytotherapeutic, Induces Cell Cycle Arrest and Apoptosis In Vitro and In Vivo through the ROS/Akt Pathway in Human Thyroid Cancer Cells. Cancers 2021, 13, 4266. https://doi.org/10.3390/cancers13174266
Kung F-P, Lim Y-P, Chao W-Y, Zhang Y-S, Yu H-I, Tai T-S, Lu C-H, Chen S-H, Li Y-Z, Zhao P-W, et al. Piperlongumine, a Potent Anticancer Phytotherapeutic, Induces Cell Cycle Arrest and Apoptosis In Vitro and In Vivo through the ROS/Akt Pathway in Human Thyroid Cancer Cells. Cancers. 2021; 13(17):4266. https://doi.org/10.3390/cancers13174266
Chicago/Turabian StyleKung, Fang-Ping, Yun-Ping Lim, Wen-Ying Chao, Yi-Sheng Zhang, Hui-I Yu, Tsai-Sung Tai, Chieh-Hsiang Lu, Shu-Hsin Chen, Yi-Zhen Li, Pei-Wen Zhao, and et al. 2021. "Piperlongumine, a Potent Anticancer Phytotherapeutic, Induces Cell Cycle Arrest and Apoptosis In Vitro and In Vivo through the ROS/Akt Pathway in Human Thyroid Cancer Cells" Cancers 13, no. 17: 4266. https://doi.org/10.3390/cancers13174266
APA StyleKung, F.-P., Lim, Y.-P., Chao, W.-Y., Zhang, Y.-S., Yu, H.-I., Tai, T.-S., Lu, C.-H., Chen, S.-H., Li, Y.-Z., Zhao, P.-W., Yen, Y.-P., & Lee, Y.-R. (2021). Piperlongumine, a Potent Anticancer Phytotherapeutic, Induces Cell Cycle Arrest and Apoptosis In Vitro and In Vivo through the ROS/Akt Pathway in Human Thyroid Cancer Cells. Cancers, 13(17), 4266. https://doi.org/10.3390/cancers13174266





