Surgical Management of Jugular Foramen Schwannomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Outcome Parameters
2.2. Statistics
2.3. Surgical Approaches
2.3.1. Retrosigmoid Approach
2.3.2. Extreme Lateral Infrajugular Transcondylar (ELITE) Approach
3. Results
3.1. Patient Population
3.2. Postoperative Outcome
4. Discussion
4.1. Choosing a Suitable Approach
4.2. Extent of Resection and Functional Outcome
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, E.S.; Lee, E.J.; Park, J.B.; Cho, Y.H.; Hong, S.H.; Kim, J.H.; Kim, C.J. A Single-Institution Retrospective Study of Jugular Foramen Schwannoma Management: Radical Resection Versus Subtotal Intracranial Resection Through a Retrosigmoid Suboccipital Approach Followed by Radiosurgery. World Neurosurg. 2016, 88, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Babu, R.P.; Tatagiba, M.; Sepehrnia, A. Surgical treatment of jugular foramen schwannomas. J. Neurosurg. 1995, 82, 924–932. [Google Scholar] [CrossRef]
- Wang, X.; Long, W.; Liu, D.; Yuan, J.; Xiao, Q.; Liu, Q. Optimal surgical approaches and treatment outcomes in patients with jugular foramen schwannomas: A single institution series of 31 cases and a literature review. Neurosurg. Rev. 2020, 43, 1–12. [Google Scholar] [CrossRef]
- Bulsara, K.R.; Sameshima, T.; Friedman, A.H.; Fukushima, T. Microsurgical management of 53 jugular foramen schwannomas: Lessons learned incorporated into a modified grading system. J. Neurosurg. 2008, 109, 794–803. [Google Scholar] [CrossRef]
- Ryu, S.M.; Lee, J.-I.; Park, K.; Choi, J.W.; Kong, D.-S.; Nam, D.-H.; Jeong, H.-S.; Cho, Y.-S.; Seol, H.J. Optimal treatment of jugular foramen schwannomas: Long-term outcome of a multidisciplinary approach for a series of 29 cases in a single institute. Acta Neurochir. 2017, 159, 1517–1527. [Google Scholar] [CrossRef]
- Sanna, M.; Bacciu, A.; Falcioni, M.; Taibah, A. Surgical Management of Jugular Foramen Schwannomas with Hearing and Facial Nerve Function Preservation: A Series of 23 Cases and Review of the Literature. Laryngoscope 2006, 116, 2191–2204. [Google Scholar] [CrossRef] [PubMed]
- Sedney, C.L.; Nonaka, Y.; Bulsara, K.R.; Fukushima, T. Microsurgical Management of Jugular Foramen Schwannomas. Neurosurgery 2012, 72, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Suri, A.; Bansal, S.; Singh, M.; Mahapatra, A.K.; Sharma, B.S. Jugular foramen schwannomas: A single institution patient series. J. Clin. Neurosci. 2014, 21, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Sutiono, A.B.; Kawase, T.; Tabuse, M.; Kitamura, Y.; Arifin, M.Z.; Horiguchi, T.; Yoshida, K. Importance of Preserved Periosteum Around Jugular Foramen Neurinomas for Functional Outcome of Lower Cranial Nerves: Anatomic and Clinical Studies. Oper. Neurosurg. 2011, 69, ons230–ons240. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.-J.; Li, D.; Hao, S.-Y.; Wang, L.; Tang, J.; Xiao, X.-R.; Meng, G.-L.; Jia, G.-J.; Zhang, L.-W.; Wu, Z.; et al. Long-Term Functional and Recurrence Outcomes of Surgically Treated Jugular Foramen Schwannomas: A 20-Year Experience. World Neurosurg. 2016, 86, 134–146. [Google Scholar] [CrossRef]
- Kaye, A.H.; Hahn, J.F.; Kinney, S.E.; Hardy, R.W., Jr.; Bay, J.W. Jugular foramen schwannomas. J. Neurosurg. 1984, 60, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Pellet, W.; Cannoni, M.; Pech, A. The widened transcochlear approach to jugular foramen tumors. J. Neurosurg. 1988, 69, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Darwish, H.; Adada, B. A Small Glossopharyngeal Schwannoma Presenting with Intractable Vomiting: Case Presentation and Literature Review. World Neurosurg. 2018, 115, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Aftahy, A.; Groll, M.; Barz, M.; Wagner, A.; Lange, N.; Butenschön, V.; Delbridge, C.; Bernhardt, D.; Meyer, B.; Negwer, C.; et al. Surgical Outcome of Trigeminal Schwannomas. Cancers 2021, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Tatagiba, M.; Acioly, M.A. Retrosigmoid Approach to the Posterior and Middle Fossae. In Samii’s Essentials in Neurosurgery; Springer: Berlin/Heidelberg, Germany, 2008; pp. 137–153. [Google Scholar] [CrossRef]
- Samii, M.; Migliori, M.M.; Tatagiba, M.; Babu, R. Surgical treatment of trigeminal schwannomas. J. Neurosurg. 1995, 82, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Ramina, R.; Mattei, T.A.; Sória, M.G.; Da Silva, E.B., Jr.; Leal, A.G.; Neto, M.C.; Fernandes, Y.B. Surgical management of trigeminal schwannomas. Neurosurg. Focus 2008, 25, E6, discussion E6. [Google Scholar] [CrossRef] [Green Version]
- Elsmore, A.J.; Mendoza, N.D. The operative learning curve for vestibular schwannoma excision via the retrosigmoid approach. Br. J. Neurosurg. 2002, 16, 448–455. [Google Scholar] [CrossRef]
- Lau, T.; Olivera, R.; Miller, J.T.; Downes, K.; Danner, C.; Van Loveren, H.R.; Agazzi, S. Paradoxical trends in the management of vestibular schwannoma in the United States. J. Neurosurg. 2012, 117, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Fisch, U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J. Laryngol. Otol. 1978, 92, 949–967. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, M.; Oishi, M.; Saito, A.; Fujii, Y. Long-Term Outcomes after Surgical Treatment of Jugular Foramen Schwannoma. Semin. Neurol. 2009, 19, 401–408. [Google Scholar] [CrossRef]
- Tucci, D.L.; Pilkington, T.M.; Fukushima, T. Chapter 59—Extreme Lateral Infrajugular Transcondylar Approach for Resection of Skull Base Tumors. In Otologic Surgery, 3rd ed.; Brackmann, D.E., Shelton, C., Arriaga, M.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2010; pp. 715–726. [Google Scholar] [CrossRef]
- Katsuta, T.; Rhoton, A.L., Jr.; Matsushima, T. The jugular foramen: Microsurgical anatomy and operative approaches. Neurosurgery 1997, 41, 149–201, discussion 201–142. [Google Scholar] [CrossRef]
- Samii, M.; Alimohamadi, M.; Gerganov, V. Surgical Treatment of Jugular Foramen Schwannoma: Surgical Treatment Based on a New Classification. Neurosurgery 2015, 77, 424–432. [Google Scholar] [CrossRef]
- Kadri, P.A.S.; Al-Mefty, O. Surgical treatment of dumbbell-shaped jugular foramen schwannomas. Neurosurg. Focus 2004, 17, 56–62. [Google Scholar] [CrossRef]
- Komune, N.; Matsushima, K.; Matsushima, T.; Komune, S.; Rhoton, A.L. Surgical approaches to jugular foramen schwannomas: An anatomic study. Head Neck 2015, 38, E1041–E1053. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Alimohamadi, M.; Gerganov, V.M. Endoscope-assisted retrosigmoid infralabyrinthine approach to jugular foramen tumors. J. Neurosurg. 2016, 124, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- Bertalanffy, H.; Seeger, W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery 1991, 29, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.A.; Tatagiba, M.; Samii, M. Cystic Schwannomas of the Jugular Foramen: Clinical and Surgical Remarks. Neurosurgery 2000, 46, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Bakar, B. The Jugular Foramen Schwannomas: Review of the Large Surgical Series. J. Korean Neurosurg. Soc. 2008, 44, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Champ, C.E.; Mishra, M.V.; Shi, W.; Siglin, J.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J. Stereotactic Radiotherapy for Trigeminal Schwannomas. Neurosurgery 2012, 71, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Combs, S.E.; Engelhard, C.; Kopp, C.; Wiedenmann, N.; Schramm, O.; Prokic, V.; Debus, J.; Molls, M.; Grosu, A.-L. Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas—Pooled results from 3 large German centers. Radiother. Oncol. 2015, 114, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.; Xu, Z.; Schlesinger, D.; Sheehan, J.P. Gamma Knife surgery for nonvestibular schwannomas: Radiological and clinical outcomes. J. Neurosurg. 2012, 116, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.H.; Shepard, M.J.; Chen, C.-J.; Sheehan, J.P. Stereotactic Radiosurgery for Trigeminal Schwannomas: A 28-Year Single-Center Experience and Review of the Literature. World Neurosurg. 2018, 119, e874–e881. [Google Scholar] [CrossRef]
- Combs, S.E.; Welzel, T.; Kessel, K.; Habermehl, D.; Rieken, S.; Schramm, O.; Debus, J. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcome. Radiother. Oncol. 2013, 106, 175–180. [Google Scholar] [CrossRef]
- Chan, A.W.; Black, P.M.; Ojemann, R.G.; Barker, F.G.; Kooy, H.M.; Lopes, V.V.; McKenna, M.J.; Shrieve, D.C.; Martuza, R.L.; Loeffler, J.S. Stereotactic Radiotherapy for Vestibular Schwannomas: Favorable Outcome with Minimal Toxicity. Neurosurgery 2005, 57, 60–70, discussion 60–70. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhang, H.; Han, Y.C.; Fan, Z.; Li, J.F.; Fan, Z.M. Surgical management of jugular foramen tumors. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2008, 43, 570–576. [Google Scholar]
- Combs, S.E.; Thilmann, C.; Debus, J.; Schulz-Ertner, D. Long-term outcome of stereotactic radiosurgery (SRS) in patients with acoustic neuromas. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.J.; Kondziolka, D.; Flickinger, J.C.; Mathieu, D.; Niranjan, A.; Lunsford, L.D. Cranial Nerve Preservation and Outcomes after Stereotactic Radiosurgery for Jugular Foramen Schwannomas. Neurosurgery 2007, 61, 76–81. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Yu, X.; Qi, S.; Du, Y.; Ni, W.; Hu, Y.; Tian, Z. Stereotactic Radiosurgery for Trigeminal Schwannoma: A Clinical Retrospective Study in 52 Cases. Ster. Funct. Neurosurg. 2013, 91, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kato, T.; Iizuka, H.; Kida, Y. Long-Term Results for Trigeminal Schwannomas Treated With Gamma Knife Surgery. Int. J. Radiat. Oncol. 2013, 87, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Golfinos, J.G.; Hill, T.C.; Rokosh, R.; Choudhry, O.; Shinseki, M.; Mansouri, A.; Friedmann, D.; Roland, J.T.; Kondziolka, D. A matched cohort comparison of clinical outcomes following microsurgical resection or stereotactic radiosurgery for patients with small- and medium-sized vestibular schwannomas. J. Neurosurg. 2016, 125, 1472–1482. [Google Scholar] [CrossRef] [Green Version]
- Wolbers, J.G.; Dallenga, A.H.; Mendez Romero, A.; van Linge, A. What intervention is best practice for vestibular schwannomas? A systematic review of controlled studies. BMJ Open 2013, 3, e001345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; AlAhmari, M.; Temel, Y.; Hovinga, K. Therapy of Sporadic and NF2-Related Vestibular Schwannoma. Cancers 2020, 12, 835. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, D.; Kondziolka, D.; Flickinger, J.C.; Niranjan, A.; Williamson, R.; Martin, J.J.; Lunsford, L.D. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: An analysis of tumor control, complications, and hearing preservation rates. Neurosurgery 2007, 60, 460–468, discussion 468–470. [Google Scholar] [CrossRef] [PubMed]
- Phi, J.H.; Kim, D.G.; Chung, H.-T.; Lee, J.; Paek, S.H.; Jung, H.-W. Radiosurgical treatment of vestibular schwannomas in patients with neurofibromatosis type 2. Cancer 2009, 115, 390–398. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
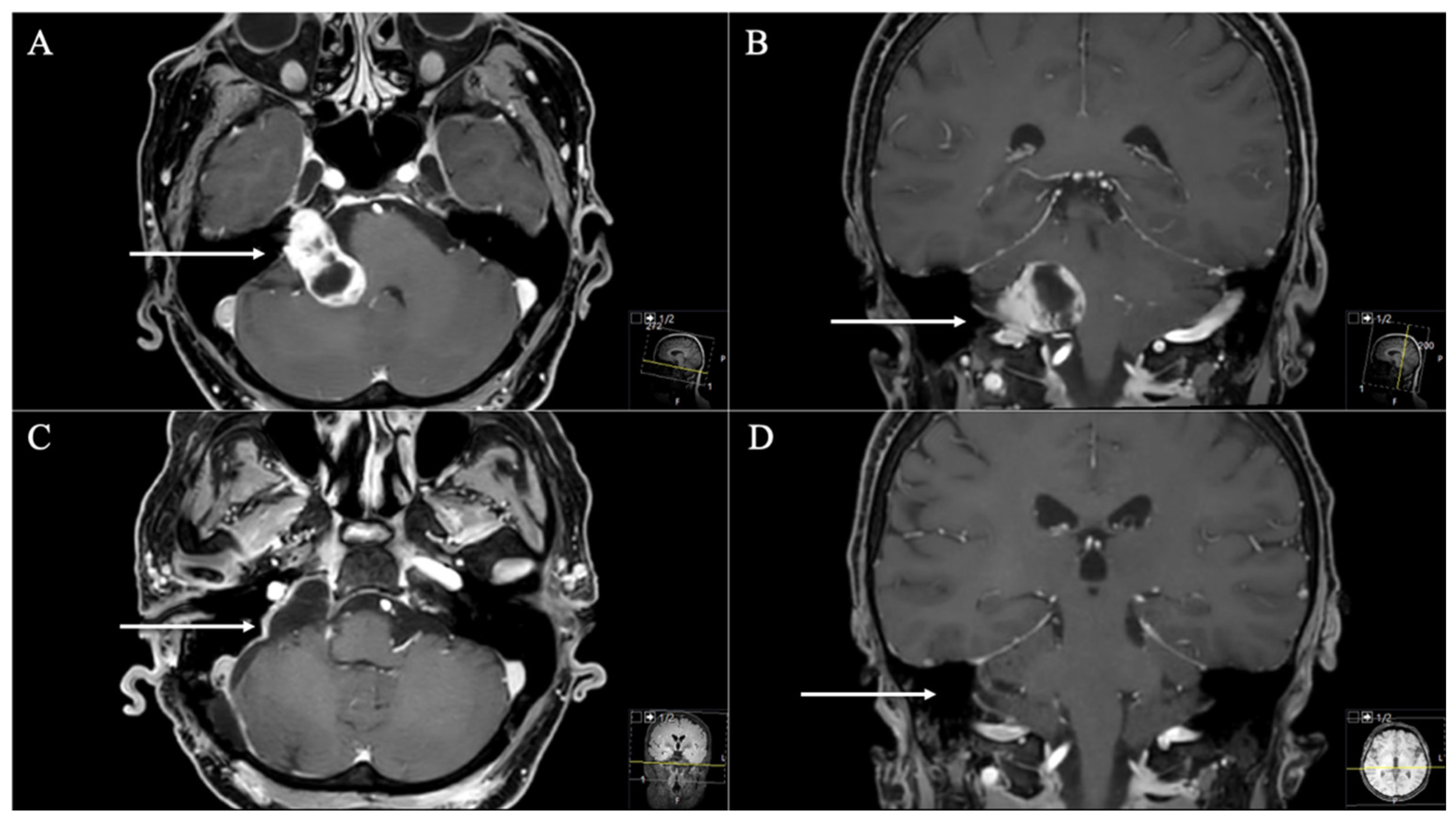
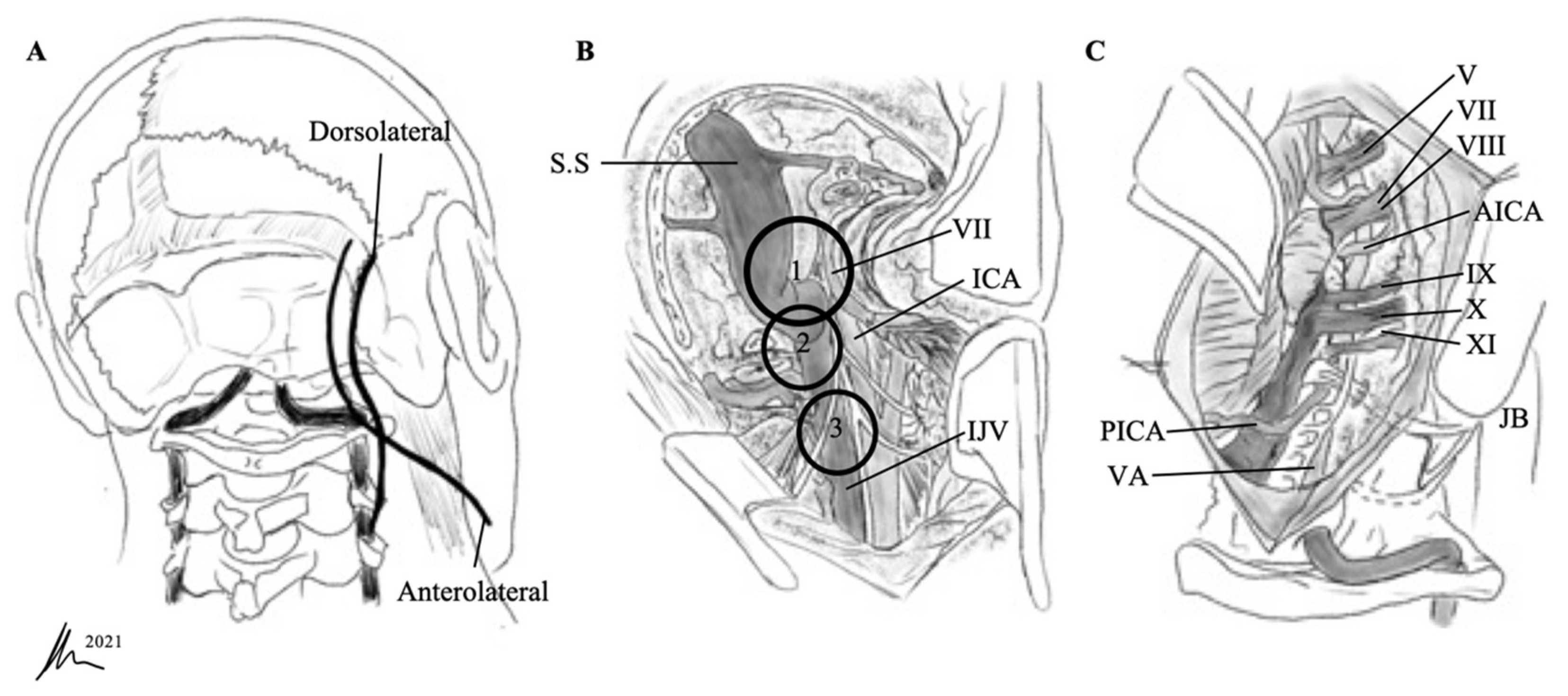

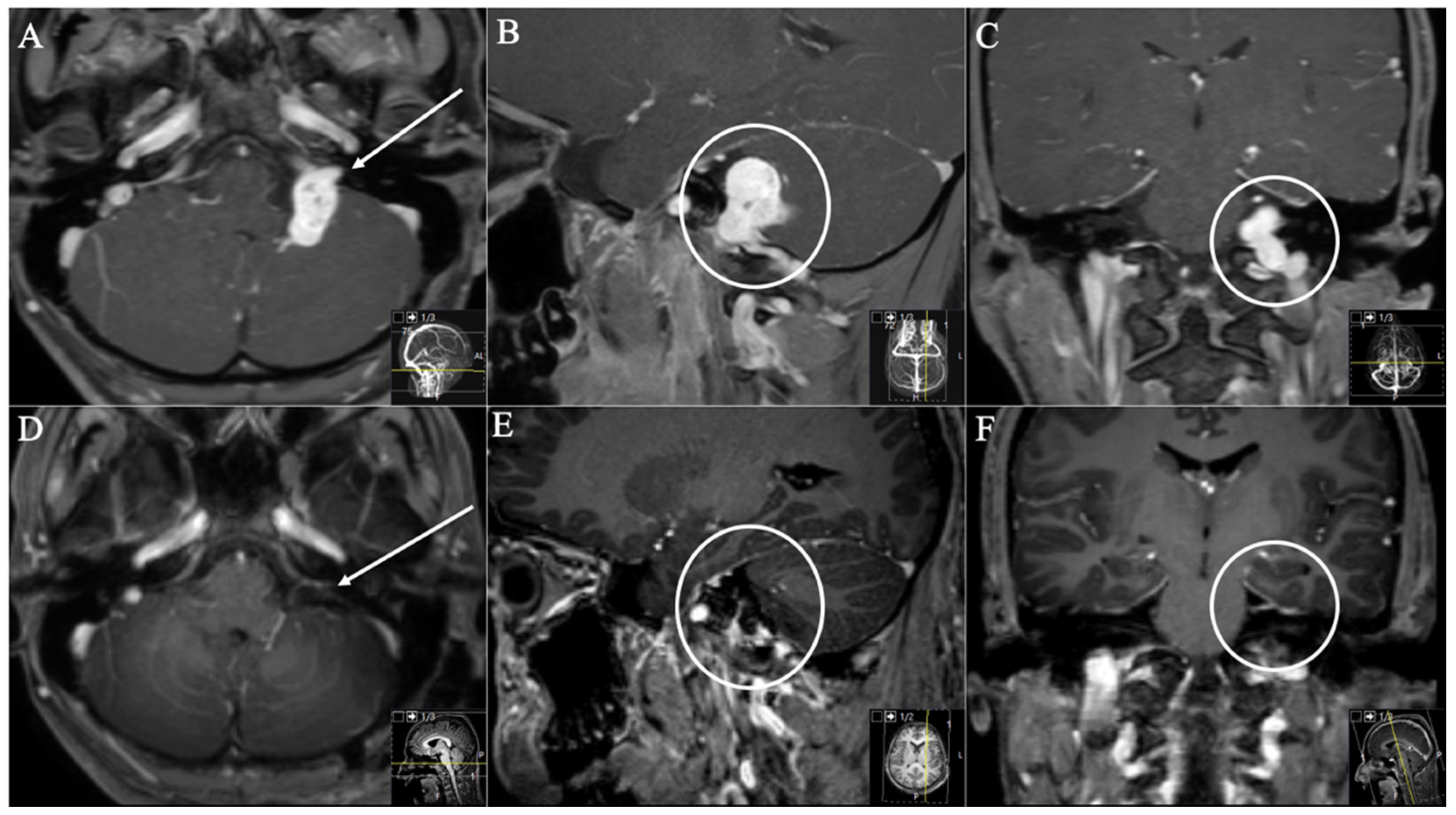

| Samii et al. [2] | Kaye et al. [11] and Pellet et al. [12] | |
|---|---|---|
| A | JFS in the CPA with minimum enlargement of JF | JFS in the CPA with minimum enlargement of JF and with a small extension into the bone |
| B | Main portion at JF with intracranial extension | JFS invading the bone with or without intradural parts |
| C | Extracranial JFS with extension into JF | Extracranial JFS with minor extension to the bone |
| D | Dumbbell-shaped JFS with both intra- and extracranial parts | Saddle-bag-shaped tumor with intra- and extracranial parts |
| Mdn. Age (Years). | 43 | (20–71) | |
| Sex | Female | 7 | (77.8%) |
| Male | 2 | (22.2%) | |
| Preoperative deficits | |||
| Hoarseness | 3 | (33.3%) | |
| Dysphagia | 4 | (44.4%) | |
| Cerebellar dysfunction | 4 | (44.4%) | |
| Extinguished gag reflex | 2 | (22.2%) | |
| Headache | 4 | (44.4%) | |
| Hearing loss | 6 | (66.6%) | |
| Facial nerve palsy | 3 | (33.3%) | |
| Hypoglossal nerve palsy | 2 | (22.2%) | |
| Tumor type and approach | Retrosigmoid | ELITE | |
| A | 3 (33.3%) | 3 | |
| B | 1 (0.0%) | 1 | |
| C | 1 (11.1%) | 1 | |
| D | 4 (44.4%) | 3 | 1 |
| Tumor origin | |||
| CN IX | 5 | (55.6%) | |
| CN X | 2 | (22.2%) | |
| CN XI | 0 | 0% | |
| Mixed involvement (particularly CN X and XI) | 2 | (22.2%) | |
| Mdn. Tumor Volume (cm3) | 7.08 | [3.27–50.1] | |
| Mdn. max. Tumor Diameter (cm) | 3.10 | [2.4–5.7] | |
| Mean max. Tumor Diameter (cm) | 3.45 | ||
| Extent of Resection | ||
|---|---|---|
| GTR | 9 | (100%) |
| Postoperative outcome (permanent) | ||
| Follow-up (months) | 16.5 | (3–84) |
| Surgery-related mortality | 0 | (0.0%) |
| New hoarseness | 1 | (11.1%) |
| New hearing loss | 1 | (11.1%) |
| New facial nerve palsy | 1 | (11.1%) |
| Shunt-dependent hydrocephalus | 2 | (22.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aftahy, A.K.; Groll, M.; Barz, M.; Bernhardt, D.; Combs, S.E.; Meyer, B.; Negwer, C.; Gempt, J. Surgical Management of Jugular Foramen Schwannomas. Cancers 2021, 13, 4218. https://doi.org/10.3390/cancers13164218
Aftahy AK, Groll M, Barz M, Bernhardt D, Combs SE, Meyer B, Negwer C, Gempt J. Surgical Management of Jugular Foramen Schwannomas. Cancers. 2021; 13(16):4218. https://doi.org/10.3390/cancers13164218
Chicago/Turabian StyleAftahy, Amir Kaywan, Maximilian Groll, Melanie Barz, Denise Bernhardt, Stephanie E. Combs, Bernhard Meyer, Chiara Negwer, and Jens Gempt. 2021. "Surgical Management of Jugular Foramen Schwannomas" Cancers 13, no. 16: 4218. https://doi.org/10.3390/cancers13164218
APA StyleAftahy, A. K., Groll, M., Barz, M., Bernhardt, D., Combs, S. E., Meyer, B., Negwer, C., & Gempt, J. (2021). Surgical Management of Jugular Foramen Schwannomas. Cancers, 13(16), 4218. https://doi.org/10.3390/cancers13164218







