Extranodal Extension Predicts Poor Survival Outcomes among Patients with Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection from Taiwan Cancer Registry
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Demographics
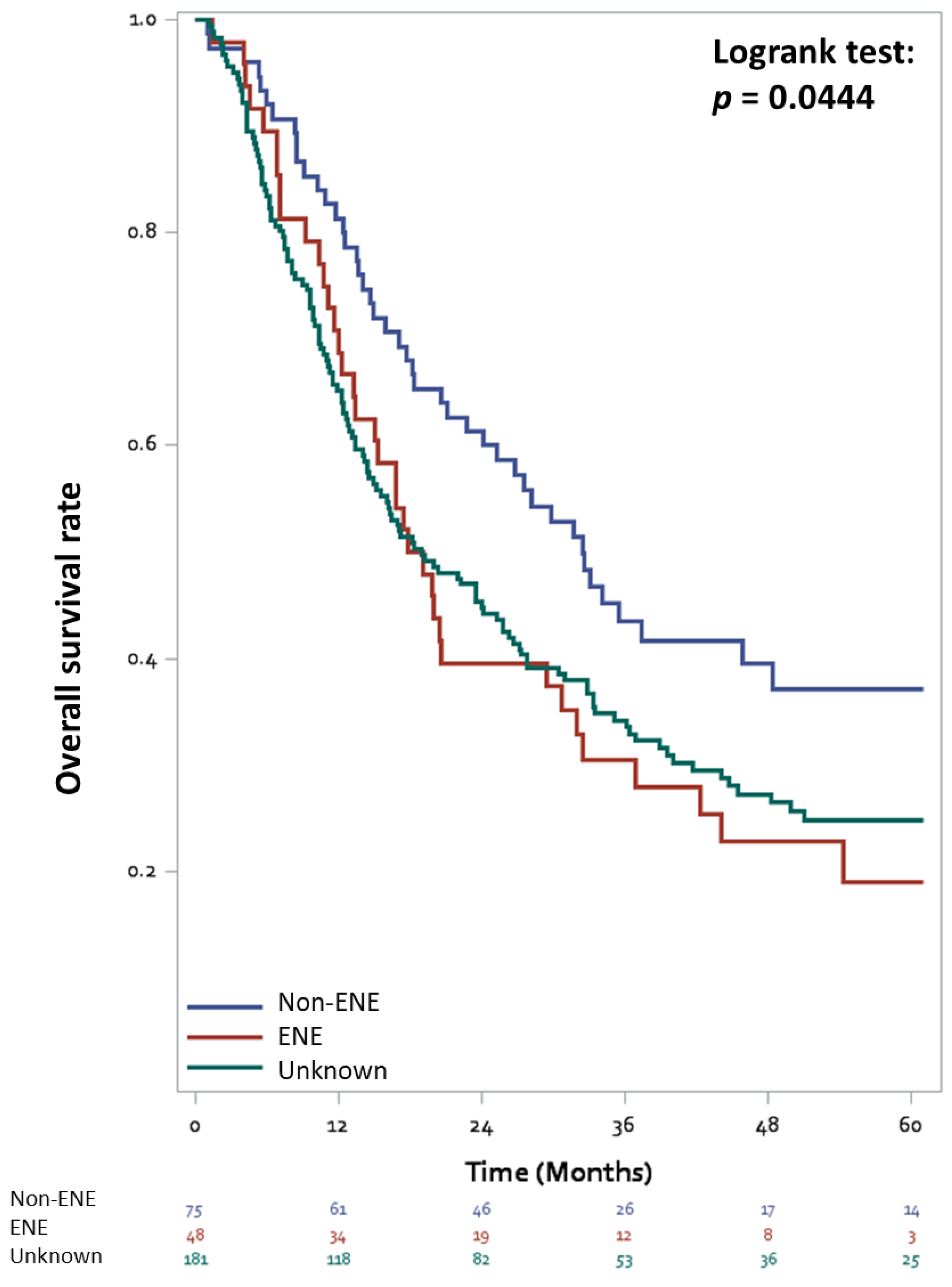
3.2. Overall Survival
3.3. Cancer-Specific Survival
3.4. Association between Perioperative Chemotherapy and ENE Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Quek, M.L.; Sanderson, K.M.; Daneshmand, S.; Stein, J.P. The importance of an extended lymphadenectomy in the management of high-grade invasive bladder cancer. Expert Rev. Anticancer Ther. 2004, 4, 1007–1016. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.-C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Cai, J.; Groshen, S.; Skinner, D.G. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: The concept of lymph node density. J. Urol. 2003, 170, 35–41. [Google Scholar] [CrossRef]
- Fleischmann, A.; Thalmann, G.N.; Markwalder, R.; Studer, U.E. Extracapsular extension of pelvic lymph node metastases from urothelial carcinoma of the bladder is an independent prognostic factor. J. Clin. Oncol. 2005, 23, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, C.; Salzer, A.; Thomas, C.; Gellermann-Schultes, C.; Gillitzer, R.; Hampel, C.; Thüroff, J.W. Cancer-specific survival after radical cystectomy and standardized extended lymphadenectomy for node-positive bladder cancer: Prediction by lymph node positivity and density. BJU Int. 2009, 104, 331–335. [Google Scholar] [CrossRef]
- Herr, H.W.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Reuter, V.E.; Bajorin, D.F. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J. Urol. 2002, 167, 1295–1298. [Google Scholar] [CrossRef]
- Fajkovic, H.; Cha, E.K.; Jeldres, C.; Robinson, B.D.; Rink, M.; Xylinas, E.; Chromecki, T.F.; Breinl, E.; Svatek, R.S.; Donner, G. Extranodal extension is a powerful prognostic factor in bladder cancer patients with lymph node metastasis. Eur. Urol. 2013, 64, 837–845. [Google Scholar] [CrossRef]
- Jeong, I.G.; Ro, J.Y.; Kim, S.C.; You, D.; Song, C.; Hong, J.H.; Ahn, H.; Kim, C.S. Extranodal extension in node-positive bladder cancer: The continuing controversy. BJU Int. 2011, 108, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; You, S.L.; Chen, C.J.; Yang, Y.W.; Lo, W.C.; Lai, M.S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015, 45, 291–296. [Google Scholar] [CrossRef]
- Chiang, C.J.; Wang, Y.W.; Lee, W.C. Taiwan’s Nationwide Cancer Registry System of 40 years: Past, present, and future. J. Formos Med. Assoc. 2019, 118, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D. (Eds.) International Classification of Diseases for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Edge, S.; Byrd, D.; Compton, C.; Fritz, A.; Greene, F.; Trotti, A. AJCC cancer staging handbook. In AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Ilknur, G.B.; Hilmi, A.; Tülay, C.; Oguz, Ç.; Selma, S.; Serdar, S.; Uğur, Y.; Pınar, B.; Ömer, H.; Münir, K. The importance of extracapsular extension of axillary lymph node metastases in breast cancer. Tumori J. 2004, 90, 107–111. [Google Scholar] [CrossRef]
- Dong, R.-Z.; Guo, J.-M.; Zhang, Z.-W.; Zhou, Y.-M.; Su, Y. Prognostic impact and implications of extracapsular lymph node spread in Borrmann type IV gastric cancer. Oncotarget 2017, 8, 97593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vander, A.J.; Sherman, J.H.; Luciano, D.S. Human Physiology: The Mechanisms of Body Function; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic Vessel Network Structure and Physiology. Compr. Physiol. 2018, 9, 207–299. [Google Scholar] [CrossRef]
- Margaris, K.; Black, R.A. Modelling the lymphatic system: Challenges and opportunities. J. R. Soc. Interface 2012, 9, 601–612. [Google Scholar] [CrossRef]
- Bazigou, E.; Wilson, J.T.; Moore, J.E., Jr. Primary and secondary lymphatic valve development: Molecular, functional and mechanical insights. Microvasc. Res. 2014, 96, 38–45. [Google Scholar] [CrossRef]
- Nottegar, A.; Veronese, N.; Senthil, M.; Roumen, R.; Stubbs, B.; Choi, A.; Verheuvel, N.; Solmi, M.; Pea, A.; Capelli, P. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 919–925. [Google Scholar] [CrossRef]
- Yang, X.; Ma, X.; Yang, W.; Shui, R. Clinical significance of extranodal extension in sentinel lymph node positive breast cancer. Sci. Rep. 2020, 10, 14684. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Yajima, R.; Yamaguchi, S.; Yanagita, Y.; Fujisawa, T.; Hirakata, T.; Tsutsumi, S.; Asao, T.; Iijima, M.; Kuwano, H. Extracapsular invasion of sentinel lymph nodes is not associated with disease recurrence in breast cancer. Int. Surg. 2014, 99, 305–308. [Google Scholar] [CrossRef]
- Kassouf, W.; Leibovici, D.; Luongo, T.; Munsell, M.F.; Vakar, F.; Dinney, C.P.; Grossman, H.B.; Kamat, A.M. Relevance of extracapsular extension of pelvic lymph node metastasis in patients with bladder cancer treated in the contemporary era. Cancer 2006, 107, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Trinh, Q.-D.; Seisen, T.; Vetterlein, M.W.; Sammon, J.; Preston, M.A.; Lipsitz, S.R.; Bellmunt, J.; Menon, M.; Choueiri, T.K. Effectiveness of neoadjuvant chemotherapy for muscle-invasive bladder cancer in the current real world setting in the USA. Eur. Urol. Oncol. 2018, 1, 83–90. [Google Scholar] [CrossRef]
- Fahmy, O.; Khairul-Asri, M.G.; Schubert, T.; Renninger, M.; Malek, R.; Kubler, H.; Stenzl, A.; Gakis, G. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol. Oncol. 2018, 36, 43–53. [Google Scholar] [CrossRef]
- Vetterlein, M.W.; Seisen, T.; May, M.; Nuhn, P.; Gierth, M.; Mayr, R.; Fritsche, H.-M.; Burger, M.; Novotny, V.; Froehner, M. Effectiveness of adjuvant chemotherapy after radical cystectomy for locally advanced and/or pelvic lymph node–positive muscle-invasive urothelial carcinoma of the bladder: A propensity score–weighted competing risks analysis. Eur. Urol. Focus 2018, 4, 252–259. [Google Scholar] [CrossRef]
- Seisen, T.; Jamzadeh, A.; Leow, J.J.; Roupret, M.; Cole, A.P.; Lipsitz, S.R.; Kibel, A.S.; Nguyen, P.L.; Sun, M.; Menon, M.; et al. Adjuvant Chemotherapy vs Observation for Patients with Adverse Pathologic Features at Radical Cystectomy Previously Treated with Neoadjuvant Chemotherapy. JAMA Oncol. 2018, 4, 225–229. [Google Scholar] [CrossRef]
- Leow, J.J.; Martin-Doyle, W.; Rajagopal, P.S.; Patel, C.G.; Anderson, E.M.; Rothman, A.T.; Cote, R.J.; Urun, Y.; Chang, S.L.; Choueiri, T.K. Adjuvant chemotherapy for invasive bladder cancer: A 2013 updated systematic review and meta-analysis of randomized trials. Eur. Urol. 2014, 66, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L. Adjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis of individual patient data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur. Urol. 2005, 48, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Yousem, D.M.; Gatewood, O.; Goldman, S.M.; Marshall, F.F. Synchronous and metachronous transitional cell carcinoma of the urinary tract: Prevalence, incidence, and radiographic detection. Radiology 1988, 167, 613–618. [Google Scholar] [CrossRef]
- Westhoff, E.; Witjes, J.A.; Fleshner, N.E.; Lerner, S.P.; Shariat, S.F.; Steineck, G.; Kampman, E.; Kiemeney, L.A.; Vrieling, A. Body mass index, diet-related factors, and bladder cancer prognosis: A systematic review and meta-analysis. Bladder Cancer 2018, 4, 91–112. [Google Scholar] [CrossRef] [PubMed]


| Variables | Case Number | Extranodal Extension | p Value * | ||
|---|---|---|---|---|---|
| Negative | Positive | Unknown | |||
| All | 304 | 75 (24.7%) | 48 (15.8%) | 181 (59.5%) | |
| Age (years) | 0.98 | ||||
| 25–64 | 136 (44.7%) | 35 (46.7%) | 22 (45.8%) | 79 (43.6%) | |
| 65–74 | 111 (36.5%) | 24 (32.0%) | 15 (31.3%) | 72 (39.8%) | |
| ≥75 | 57 (18.8%) | 16 (21.3%) | 11 (22.9%) | 30 (16.6%) | |
| Gender | 0.6 | ||||
| male | 214 (70.4%) | 53 (70.7%) | 36 (75%) | 125 (69.1%) | |
| female | 90 (29.6%) | 22 (29.3%) | 12 (25%) | 56 (30.9%) | |
| Tumor size (cm) | 0.44 | ||||
| <4.0 | 88 (28.9%) | 25 (33.3%) | 11 (22.9%) | 52 (28.7%) | |
| ≥4.0 | 155 (51%) | 39 (52%) | 30 (62.5%) | 86 (47.5%) | |
| Unknown | 61 (20.1%) | 11 (14.7%) | 7 (14.6%) | 43 (23.8%) | |
| Tumor grade | 0.21 | ||||
| Low | 3 (1%) | 0 | 1 (2.1%) | 2 (1.1%) | |
| High | 301 (99%) | 75 (100%) | 47 (97.9%) | 179 (98.9%) | |
| pT stage | 0.73 | ||||
| T0 | 9 (3.0%) | 1 (1.3%) | 1 (2.1%) | 7 (3.9%) | |
| Tis/a/1 | 6 (2.0%) | 1 (1.3%) | 0 | 5 (2.8%) | |
| T2 | 47 (15.5%) | 12 (16.0%) | 5 (10.4%) | 30 (16.6%) | |
| T3 | 156 (51.3%) | 40 (53.3%) | 24 (50.0%) | 92 (50.0%) | |
| T4 | 86 (28.3%) | 21 (28.0%) | 18 (37.5%) | 47 (26.0%) | |
| pN stage | < 0.01 | ||||
| N1 | 112 (36.8%) | 31 (41.3%) | 8 (16.7%) | 73 (40.3%) | |
| N2 | 156 (51.3%) | 38 (50.7%) | 29 (60.4%) | 89 (49.2%) | |
| N3 | 36 (11.8%) | 6 (8%) | 11 (22.9%) | 19 (10.5%) | |
| Specimen margin | 0.18 | ||||
| Free | 247 (81.3%) | 63 (84%) | 37 (77.1%) | 147 (81.2%) | |
| Not free | 50 (16.4%) | 12 (16%) | 9 (18.8%) | 29 (16%) | |
| Unknown | 7 (2.3%) | 0 | 2 (4.2%) | 5 (2.8%) | |
| Lymph node density | 0.043 | ||||
| Unknown | 14 (4.6%) | 2 (2.7%) | 1 (2.1%) | 11 (6.1%) | |
| <20% | 148 (48.7%) | 45 (60.0%) | 18 (37.5%) | 85 (47%) | |
| ≥20% | 142 (46.7%) | 28 (37.3%) | 29 (60.4%) | 85 (47%) | |
| Multiple primaries | < 0.01 | ||||
| Single | 248 (81.6%) | 54 (72%) | 45 (93.8%) | 149 (82.3%) | |
| Multiple | 56 (18.4%) | 21 (28%) | 3 (6.2%) | 32 (17.7%) | |
| Smoking history | 0.67 | ||||
| Never | 188 (61.8%) | 44 (58.7%) | 26 (54.2%) | 118 (65.2%) | |
| Current | 67 (22%) | 16 (21.3%) | 14 (29.1%) | 37 (20.4%) | |
| Ever | 46 (15.1%) | 14 (18.7%) | 8 (16.7%) | 24 (13.3%) | |
| Unknown | 3 (1%) | 1 (1.3%) | 0 | 2 (1.1%) | |
| Body mass index | 0.17 | ||||
| <18 | 54 (17.8%) | 20 (26.7%) | 5 (10.4%) | 29 (16%) | |
| 18–23.9 | 121 (39.8%) | 29 (38.7%) | 22 (45.8%) | 70 (38.7%) | |
| 24–26.9 | 77 (25.3%) | 18 (24%) | 13 (27.1%) | 46 (25.4%) | |
| ≥27 | 52 (17.1%) | 8 (10.7%) | 8 (16.7%) | 36 (19.9%) | |
| Peri-operativechemotherapy | 0.02 | ||||
| No | 101 (33.2%) | 32 (42.7%) | 9 (18.8%) | 60 (33.1%) | |
| NAC alone | 54 (17.8%) | 8 (10.7%) | 10 (20.8%) | 36 (19.9%) | |
| AC alone | 129 (42.4%) | 31 (41.3%) | 22 (45.8%) | 76 (42%) | |
| NAC plus AC | 20 (6.5%) | 4 (5.3%) | 7 (14.6%) | 9 (5.0%) | |
| Variables | Case Number | All Cause Death | Cancer Specific Death | Univariable Analysis | Multivariable Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Survival | Cancer Specific Survival | Overall Survival | Cancer Specific Survival | ||||||||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95%CI | p Value | ||||
| Age (years) | |||||||||||||||
| 25–64 | 136 | 79 | 69 | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| 65–74 | 111 | 84 | 68 | 1.55 | 1.14–2.11 | 0.005 | 1.43 | 1.02–2.0 | 0.04 | 1.47 | 1.08–2.02 | 0.02 | 1.36 | 0.96–1.92 | 0.08 |
| ≥75 | 57 | 49 | 38 | 2.4 | 1.66–3.42 | <0.001 | 2.11 | 1.4–3.13 | <0.001 | 2.19 | 1.48–3.21 | <0.001 | 1.87 | 1.21–2.85 | 0.004 |
| Gender | |||||||||||||||
| Male | 214 | 150 | 120 | 1 | (ref) | 1 | (ref) | - | - | - | - | - | - | ||
| Female | 90 | 62 | 55 | 0.98 | 0.72–1.31 | 0.89 | 1.08 | 0.78–1.48 | 0.62 | - | - | - | - | - | - |
| Tumor size (cm) | |||||||||||||||
| <4 | 88 | 54 | 42 | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| ≥4 | 155 | 115 | 100 | 1.55 | 1.13–2.16 | 0.008 | 1.73 | 1.22–2.51 | 0.003 | 1.57 | 1.13–2.20 | 0.01 | 1.73 | 1.20–2.53 | 0.004 |
| Unknown | 61 | 43 | 33 | 1.3 | 0.86–1.93 | 0.21 | 1.28 | 0.81–2.01 | 0.29 | 1.26 | 0.80–1.98 | 0.32 | 1.09 | 0.65–1.82 | 0.74 |
| Tumor grade | |||||||||||||||
| Low | 3 | 2 | 2 | 1.13 | 0.19–3.53 | 0.87 | 1.34 | 0.22–4.21 | 0.68 | - | - | - | - | - | - |
| High | 301 | 210 | 173 | 1 | (ref) | 1 | (ref) | - | - | - | - | - | - | ||
| Pathological T stage | |||||||||||||||
| T0 | 9 | 4 | 4 | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| Tis/a/1 | 6 | 4 | 3 | 1.55 | 0.37–6.57 | 0.53 | 1.17 | 0.23–5.32 | 0.83 | 2.68 | 0.56–12.8 | 0.20 | 2.08 | 0.36–10.8 | 0.38 |
| T2 | 47 | 24 | 19 | 1.13 | 0.44–3.84 | 0.82 | 0.90 | 0.34–3.09 | 0.84 | 1.58 | 0.53–5.90 | 0.45 | 1.44 | 0.46–5.54 | 0.56 |
| T3 | 156 | 109 | 94 | 1.96 | 0.82–6.38 | 0.19 | 1.69 | 0.71–5.52 | 0.30 | 2.80 | 0.98–10.2 | 0.08 | 2.65 | 0.90–9.85 | 0.10 |
| T4 | 86 | 71 | 55 | 2.71 | 1.12–8.89 | 0.05 | 2.10 | 0.86–6.93 | 0.15 | 3.69 | 1.28–13.6 | 0.03 | 3.12 | 1.04–11.7 | 0.05 |
| Surgical margin | |||||||||||||||
| Free | 247 | 164 | 136 | 1 | (ref) | 1 | (ref) | - | - | - | |||||
| Not free | 50 | 42 | 34 | 1.66 | 1.16–2.30 | 0.004 | 1.61 | 1.09–2.32 | 0.01 | - | - | - | |||
| Unknown | 7 | 6 | 5 | 2.03 | 0.8–4.19 | 0.09 | 2.05 | 0.72–4.5 | 0.12 | - | - | - | |||
| Lymph node density | |||||||||||||||
| <20% | 148 | 88 | 67 | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| ≥20% | 142 | 114 | 98 | 1.83 | 1.38–2.43 | < 0.001 | 2.06 | 1.51–2.82 | < 0.001 | 1.47 | 1.10–1.97 | 0.01 | 1.69 | 1.22–2.35 | 0.002 |
| Unknown | 14 | 10 | 10 | 1.63 | 0.79–2.98 | 0.14 | 2.14 | 1.04–3.97 | 0.02 | 2.16 | 0.97–4.31 | 0.05 | 2.91 | 1.28–5.94 | 0.01 |
| Extranodal extension | |||||||||||||||
| No | 75 | 44 | 36 | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| Yes | 48 | 37 | 31 | 1.59 | 1.02–2.46 | 0.038 | 1.62 | 1.0–2.63 | 0.048 | 1.74 | 1.09–2.78 | 0.02 | 1.69 | 1.01–2.83 | 0.045 |
| Unknown | 181 | 131 | 108 | 1.5 | 1.07–2.13 | 0.02 | 1.51 | 1.05–2.23 | 0.03 | 1.76 ** | 1.24–2.54 | 0.001 | 1.76 ** | 1.20–2.64 | 0.01 |
| Multiple primaries | |||||||||||||||
| No | 248 | 178 | 151 | 1 | (ref) | 1 | (ref) | - | - | - | - | - | - | ||
| Yes | 56 | 34 | 24 | 0.76 | 0.52–1.08 | 0.14 | 0.63 | 0.4–0.95 | 0.04 | - | - | - | - | - | - |
| Smoking history | |||||||||||||||
| Never | 188 | 130 | 109 | 1 | (ref) | 1 | (ref) | - | - | - | - | - | - | ||
| Current | 67 | 45 | 39 | 0.97 | 0.68–1.34 | 0.84 | 1 | 0.68–1.43 | 1 | - | - | - | - | - | - |
| Ever | 46 | 36 | 26 | 1.47 | 1–2.10 | 0.04 | 1.26 | 0.81–1.91 | 0.28 | - | - | - | - | - | - |
| Unknown | 3 | 1 | 1 | 0.34 | 0.02–1.52 | 0.28 | 0.41 | 0.02–1.84 | 0.38 | - | - | - | - | - | - |
| Body mass index | |||||||||||||||
| <18 | 54 | 34 | 26 | 0.72 | 0.48–1.06 | 0.11 | 0.68 | 0.43–1.05 | 0.09 | - | - | - | - | - | - |
| 18–23.9 | 121 | 91 | 74 | 1 | (ref) | 1 | (ref) | - | - | - | - | - | - | ||
| 24–26.9 | 77 | 54 | 47 | 0.87 | 0.62–1.22 | 0.43 | 0.93 | 0.64–1.34 | 0.71 | - | - | - | - | - | - |
| ≥27 | 52 | 33 | 28 | 0.71 | 0.47–1.04 | 0.09 | 0.74 | 0.47–1.13 | 0.17 | - | - | - | - | - | - |
| Neoadjuvant chemotherapy * | |||||||||||||||
| No | 230 | 160 | 128 | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| Yes | 74 | 52 | 47 | 0.91 | 0.66–1.23 | 0.54 | 1.02 | 0.73–1.42 | 0.89 | 0.72 | 0.49–1.03 | 0.08 | 0.86 | 0.57–1.27 | 0.44 |
| Adjuvant chemotherapy * | |||||||||||||||
| No | 155 | 118 | 99 | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| Yes | 149 | 94 | 76 | 0.62 | 0.47–0.81 | <0.001 | 0.60 | 0.44–0.80 | <0.001 | 0.58 | 0.43–0.79 | <0.001 | 0.57 | 0.40–0.80 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.-A.; Chiang, C.-J.; Lee, W.-C.; Zhuang, B.-Z.; Chen, C.-H.; Pu, Y.-S. Extranodal Extension Predicts Poor Survival Outcomes among Patients with Bladder Cancer. Cancers 2021, 13, 4108. https://doi.org/10.3390/cancers13164108
Liao Y-A, Chiang C-J, Lee W-C, Zhuang B-Z, Chen C-H, Pu Y-S. Extranodal Extension Predicts Poor Survival Outcomes among Patients with Bladder Cancer. Cancers. 2021; 13(16):4108. https://doi.org/10.3390/cancers13164108
Chicago/Turabian StyleLiao, Yi-An, Chun-Ju Chiang, Wen-Chung Lee, Bo-Zhi Zhuang, Chung-Hsin Chen, and Yeong-Shiau Pu. 2021. "Extranodal Extension Predicts Poor Survival Outcomes among Patients with Bladder Cancer" Cancers 13, no. 16: 4108. https://doi.org/10.3390/cancers13164108
APA StyleLiao, Y.-A., Chiang, C.-J., Lee, W.-C., Zhuang, B.-Z., Chen, C.-H., & Pu, Y.-S. (2021). Extranodal Extension Predicts Poor Survival Outcomes among Patients with Bladder Cancer. Cancers, 13(16), 4108. https://doi.org/10.3390/cancers13164108






