The Association of Annexin A1 and Chemosensitivity to Osimertinib in Lung Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Small Interfering RNA (siRNA) Transfection
2.3. Cell Viability Assay
2.4. Colony Formation Assay
2.5. Three-Dimensional Culture
2.6. Wound Healing Assay
2.7. Invasion Assay
2.8. Western Bot Analysis
2.9. Apoptosis Detection
2.10. Mice Xenograft Model
2.11. Immuno-Histochemical (IHC) Staining
2.12. Transfection of Plasmids
2.13. Establishment of Lung Cancer Cells with C797S EGFR Mutation
2.14. Semi-Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR) for EGFR
3. Statistical Analysis
4. Results
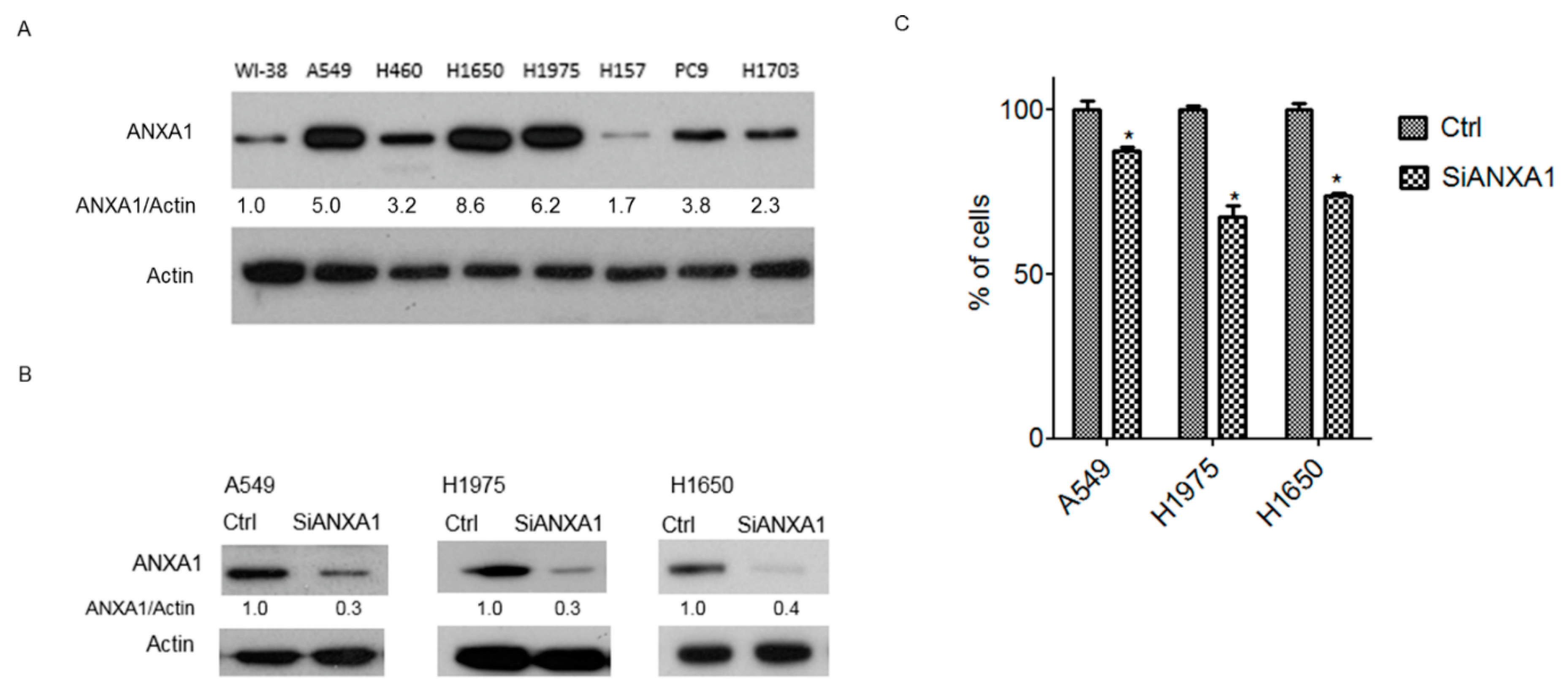
4.1. Overexpression of ANXA1 in Lung Cancer Cells
4.2. Knockdown of ANXA1 Inhibits the Growth of Lung Cancer Cells
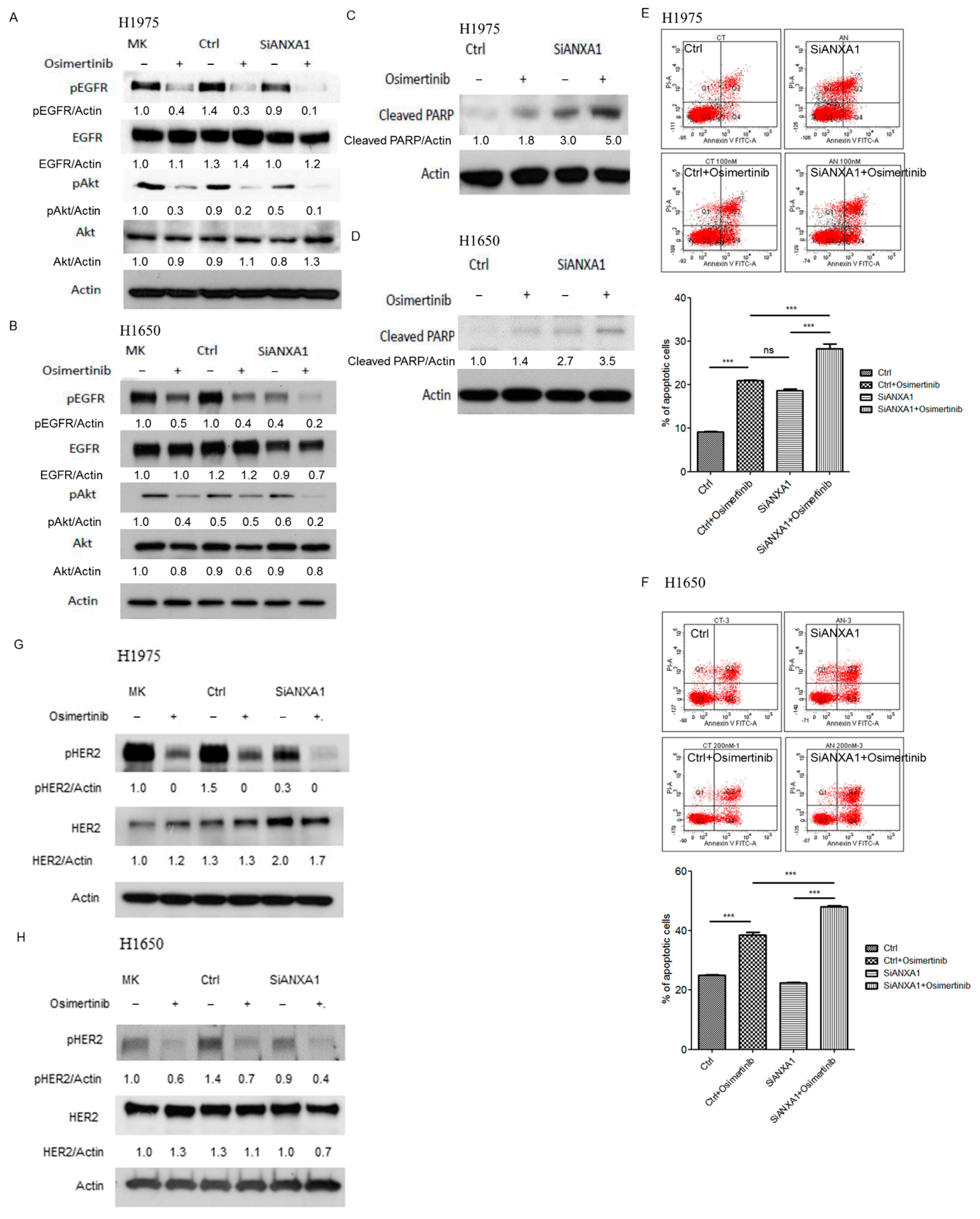
4.3. Knockdown of ANXA1 Enhanced Osimertinib Chemosensitivity and Inhibited Tumorigenesis in Lung Cancer Cells with EGFR Mutations
4.4. Knockdown of ANXA1 Inhibited Invasion and Migration in Lung Cancer Cells with EGFR Mutations
4.5. Knockdown of ANXA1 with Osimertinib Inhibited EGFR Down-Stream Pathways and Increased Apoptosis in Lung Cancer Cells with EGFR Mutations
4.6. Knockdown of ANXA1 with Osimertinib Inhibited Tumor Growth in the Mice Xenograft Model of Lung Cancer Cells with EGFR Mutations
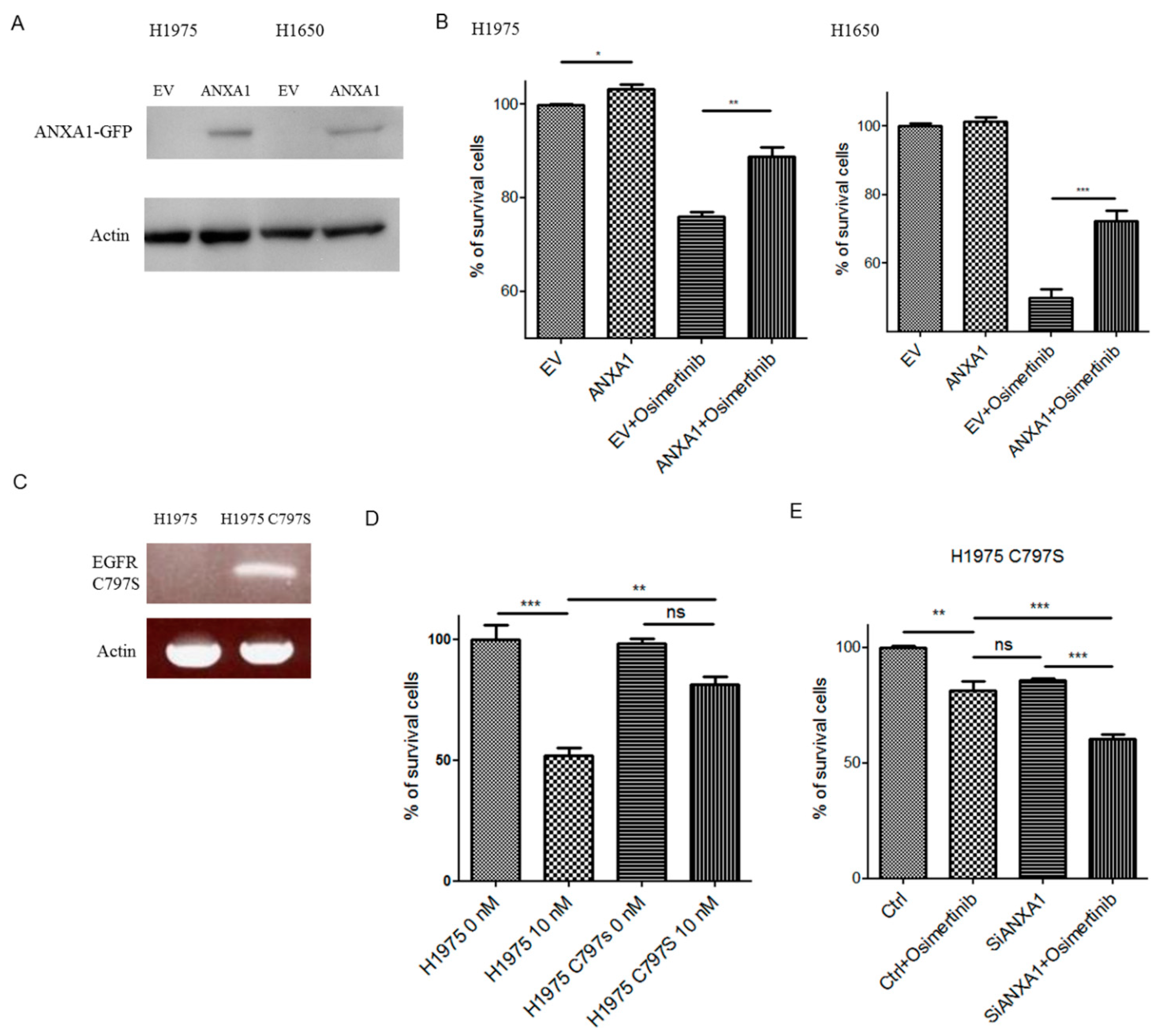
4.7. Overexpression of ANXA1 Decreased Chemosensitivity to Osimertinib
4.8. Knockdown of ANXA1 Enhanced Osimertinib Chemosensitivity in Osimertinib-Resistant C797S Lung Cancer Cells
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [Green Version]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [Green Version]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Passaro, A.; Gori, B.; de Marinis, F. Afatinib as first-line treatment for patients with advanced non-small-cell lung cancer harboring EGFR mutations: Focus on LUX-Lung 3 and LUX-Lung 6 phase III trials. J. Thorac. Dis. 2013, 5, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ercan, D.; Chen, L.; Yun, C.H.; Li, D.; Capelletti, M.; Cortot, A.B.; Chirieac, L.; Iacob, R.E.; Padera, R.; et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009, 462, 1070–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.H.; Pervaiz, S. Annexin 1: The new face of an old molecule. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 968–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bist, P.; Shu, S.; Lee, H.; Arora, S.; Nair, S.; Lim, J.Y.; Dayalan, J.; Gasser, S.; Biswas, S.K.; Fairhurst, A.M.; et al. Annexin-A1 regulates TLR-mediated IFN-beta production through an interaction with TANK-binding kinase 1. J. Immunol. 2013, 191, 4375–4382. [Google Scholar] [CrossRef]
- Fang, Y.; Guan, X.; Cai, T.; Long, J.; Wang, H.; Xie, X.; Zhang, Y. Knockdown of ANXA1 suppresses the biological behavior of human NSCLC cells in vitro. Mol. Med. Rep. 2016, 13, 3858–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Xiao, Q.; Li, Y.W.; Zhao, C.; Jia, N.; Li, R.L.; Cao, S.S.; Cui, J.; Wang, L.; Wu, Y.; et al. Regulatory mechanisms of annexin-induced chemotherapy resistance in cisplatin resistant lung adenocarcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 3191–3194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, B.; Zhao, C.; Liu, H.; Ming, Z.; Cai, X.; Gao, W.; Yang, S. Elevated serum annexin A1 as potential diagnostic marker for lung cancer: A retrospective case-control study. Am. J. Transl. Res. 2014, 6, 558–569. [Google Scholar]
- Babbin, B.A.; Lee, W.Y.; Parkos, C.A.; Winfree, L.M.; Akyildiz, A.; Perretti, M.; Nusrat, A. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J. Biol. Chem. 2006, 281, 19588–19599. [Google Scholar] [CrossRef] [Green Version]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der Pathol. 1987, 8, 138–140. [Google Scholar]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef] [Green Version]
- de Graauw, M.; van Miltenburg, M.H.; Schmidt, M.K.; Pont, C.; Lalai, R.; Kartopawiro, J.; Pardali, E.; Le Devedec, S.E.; Smit, V.T.; van der Wal, A.; et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6340–6345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belvedere, R.; Bizzarro, V.; Forte, G.; Dal Piaz, F.; Parente, L.; Petrella, A. Annexin A1 contributes to pancreatic cancer cell phenotype, behaviour and metastatic potential independently of Formyl Peptide Receptor pathway. Sci. Rep. 2016, 6, 29660. [Google Scholar] [CrossRef] [Green Version]
- Belvedere, R.; Bizzarro, V.; Popolo, A.; Dal Piaz, F.; Vasaturo, M.; Picardi, P.; Parente, L.; Petrella, A. Role of intracellular and extracellular annexin A1 in migration and invasion of human pancreatic carcinoma cells. BMC Cancer 2014, 14, 961. [Google Scholar] [CrossRef] [Green Version]
- Boudhraa, Z.; Rondepierre, F.; Ouchchane, L.; Kintossou, R.; Trzeciakiewicz, A.; Franck, F.; Kanitakis, J.; Labeille, B.; Joubert-Zakeyh, J.; Bouchon, B.; et al. Annexin A1 in primary tumors promotes melanoma dissemination. Clin. Exp. Metastasis 2014, 31, 749–760. [Google Scholar] [CrossRef]
- Zhu, J.F.; Huang, W.; Yi, H.M.; Xiao, T.; Li, J.Y.; Feng, J.; Yi, H.; Lu, S.S.; Li, X.H.; Lu, R.H.; et al. Annexin A1-suppressed autophagy promotes nasopharyngeal carcinoma cell invasion and metastasis by PI3K/AKT signaling activation. Cell Death Dis. 2018, 9, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Lu, S.S.; Xiao, T.; Huang, W.; Yi, H.; Zhu, W.; Fan, S.; Feng, X.P.; Li, J.Y.; Yu, Z.Z.; et al. ANXA1 Binds and Stabilizes EphA2 to Promote Nasopharyngeal Carcinoma Growth and Metastasis. Cancer Res. 2020, 80, 4386–4398. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, W.; Li, X.; Li, H.; Li, J.; Li, H.; He, F. ANXA1 enhances tumor proliferation and migration by regulating epithelial-mesenchymal transition and IL-6/JAK2/STAT3 pathway in papillary thyroid carcinoma. J. Cancer 2021, 12, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Krasinskas, A.M. EGFR Signaling in Colorectal Carcinoma. Pathol. Res. Int. 2011, 2011, 932932. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Hai, J.; Wang, X.; Chen, T.; Quinn, M.M.; Gao, P.; Zhang, Y.; Ji, H.; Cross, D.A.E.; et al. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2594–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takezawa, K.; Pirazzoli, V.; Arcila, M.E.; Nebhan, C.A.; Song, X.; de Stanchina, E.; Ohashi, K.; Janjigian, Y.Y.; Spitzler, P.J.; Melnick, M.A.; et al. HER2 amplification: A potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012, 2, 922–933. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Liu, S.; Sun, M.Z. Potential role of Anxa1 in cancer. Future Oncol. 2013, 9, 1773–1793. [Google Scholar] [CrossRef]
- Wan, Y.M.; Tian, J.; Qi, L.; Liu, L.M.; Xu, N. ANXA1 affects cell proliferation, invasion and epithelial-mesenchymal transition of oral squamous cell carcinoma. Exp. Ther. Med. 2017, 14, 5214–5218. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Teijeiro, S.; Menendez, S.T.; Villaronga, M.A.; Pena-Alonso, E.; Rodrigo, J.P.; Morgan, R.O.; Granda-Diaz, R.; Salom, C.; Fernandez, M.P.; Garcia-Pedrero, J.M. Annexin A1 down-regulation in head and neck squamous cell carcinoma is mediated via transcriptional control with direct involvement of miR-196a/b. Sci. Rep. 2017, 7, 6790. [Google Scholar] [CrossRef] [Green Version]
- Oshi, M.; Tokumaru, Y.; Mukhopadhyay, S.; Yan, L.; Matsuyama, R.; Endo, I.; Takabe, K. Annexin A1 Expression Is Associated with Epithelial-Mesenchymal Transition (EMT), Cell Proliferation, Prognosis, and Drug Response in Pancreatic Cancer. Cells 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Ganesan, N.; Tachibana, K.; Rajapakshe, K.; Albarracin, C.T.; Gunaratne, P.H.; Coarfa, C.; Bedrosian, I. Annexin A1 Preferentially Predicts Poor Prognosis of Basal-Like Breast Cancer Patients by Activating mTOR-S6 Signaling. PLoS ONE 2015, 10, e0127678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Lin, G.; Fang, W.; Zhu, H.; Chu, K. Increased expression of annexin A1 predicts poor prognosis in human hepatocellular carcinoma and enhances cell malignant phenotype. Med. Oncol. 2014, 31, 327. [Google Scholar] [CrossRef]
- Biaoxue, R.; Xiling, J.; Shuanying, Y.; Wei, Z.; Xiguang, C.; Jinsui, W.; Min, Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J. Exp. Clin. Cancer Res. 2012, 31, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Tian, Y.; Duan, B.; Sheng, H.; Gao, H.; Huang, J. Association of nuclear annexin A1 with prognosis of patients with esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 751–759. [Google Scholar]
- Dong, S.X.M.; Caballero, R.; Ali, H.; Roy, D.L.F.; Cassol, E.; Kumar, A. Transfection of hard-to-transfect primary human macrophages with Bax siRNA to reverse Resveratrol-induced apoptosis. RNA Biol. 2020, 17, 755–764. [Google Scholar] [CrossRef]
- Hazzan, T.; Guhl, S.; Artuc, M.; Franke, K.; Worm, M.; Zuberbier, T.; Babina, M. An efficient method for gene knock-down by RNA interference in human skin mast cells. Exp. Dermatol. 2017, 26, 1136–1139. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Kubo, T.; Semi, Y.; Itakura, M.; Kuwamura, M.; Izawa, T.; Azuma, Y.T.; Takeuchi, T. A rapid, targeted, neuron-selective, in vivo knockdown following a single intracerebroventricular injection of a novel chemically modified siRNA in the adult rat brain. J. Biotechnol. 2012, 157, 326–333. [Google Scholar] [CrossRef]
- Ruigrok, M.J.R.; Maggan, N.; Willaert, D.; Frijlink, H.W.; Melgert, B.N.; Olinga, P.; Hinrichs, W.L.J. siRNA-Mediated RNA Interference in Precision-Cut Tissue Slices Prepared from Mouse Lung and Kidney. AAPS J. 2017, 19, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Devi, G.R. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006, 13, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Ledford, H. Gene-silencing technology gets first drug approval after 20-year wait. Nature 2018, 560, 291–292. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, M.-C.; Lung, J.-H.; Chen, Y.-C.; Lin, Y.-C.; Li, Y.-C.; Hung, M.-S. The Association of Annexin A1 and Chemosensitivity to Osimertinib in Lung Cancer Cells. Cancers 2021, 13, 4106. https://doi.org/10.3390/cancers13164106
Chuang M-C, Lung J-H, Chen Y-C, Lin Y-C, Li Y-C, Hung M-S. The Association of Annexin A1 and Chemosensitivity to Osimertinib in Lung Cancer Cells. Cancers. 2021; 13(16):4106. https://doi.org/10.3390/cancers13164106
Chicago/Turabian StyleChuang, Min-Chun, Jr-Hau Lung, Yi-Chuan Chen, Yu-Ching Lin, Ya-Chin Li, and Ming-Szu Hung. 2021. "The Association of Annexin A1 and Chemosensitivity to Osimertinib in Lung Cancer Cells" Cancers 13, no. 16: 4106. https://doi.org/10.3390/cancers13164106
APA StyleChuang, M.-C., Lung, J.-H., Chen, Y.-C., Lin, Y.-C., Li, Y.-C., & Hung, M.-S. (2021). The Association of Annexin A1 and Chemosensitivity to Osimertinib in Lung Cancer Cells. Cancers, 13(16), 4106. https://doi.org/10.3390/cancers13164106






