The Dimeric Form of HPV16 E6 Is Crucial to Drive YAP/TAZ Upregulation through the Targeting of hScrib
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Plasmid Construction and Mutagenesis
2.3. Transfection Techniques and the Generation of Stable Cell Lines
2.4. Bioluminescence Resonance Energy Transfer (BRET) Data Acquisition and Analysis
2.5. Proximity Ligation Assays (PLA)
2.6. Immunofluorescence and Live-Imaging Analyses
2.7. Western Blotting
2.8. Coimmunoprecipitation (CoIP) Assays
2.9. Quantitative Real-Time PCR (qPCR)
2.10. Three-Dimensional Spheroid Formation Assays
2.11. Differential Gene Expression Analysis
2.12. Quantification and Statistical Analysis
3. Results
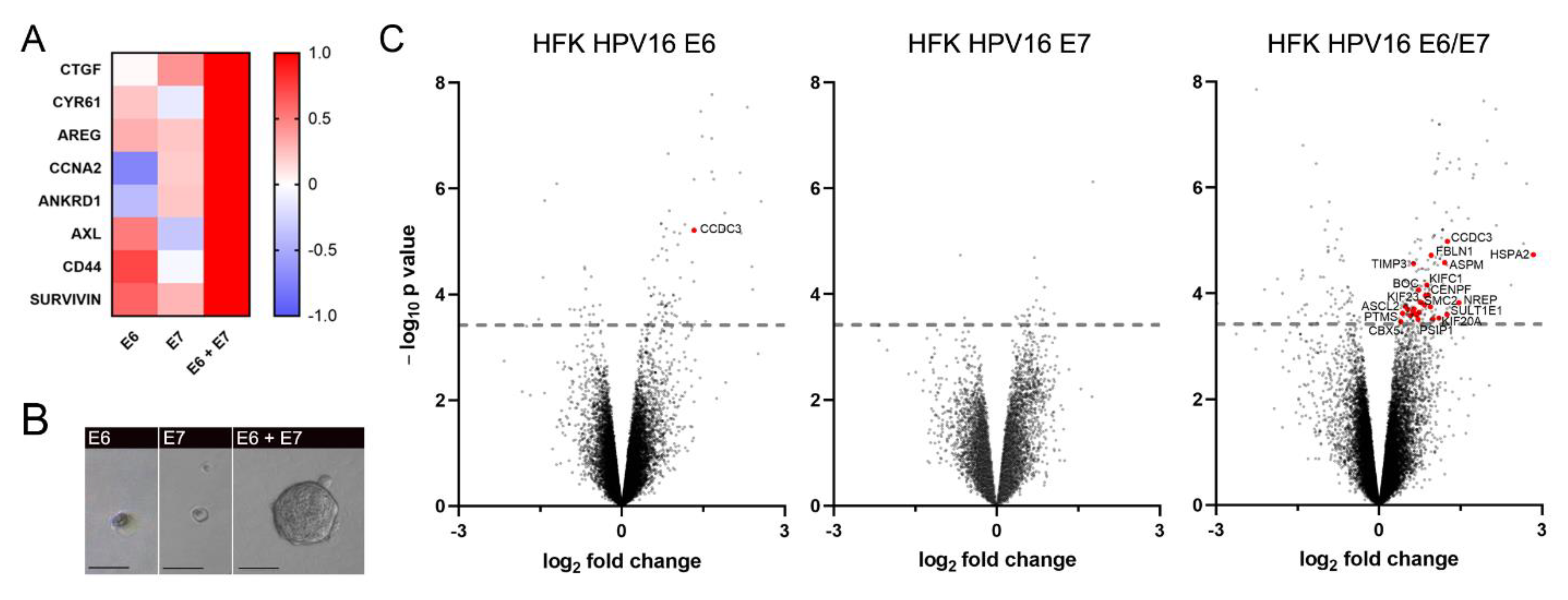
3.1. HPV16 E6 and E7 Are Concomitantly Required to Promote YAP/TAZ Signaling and Stemness Properties
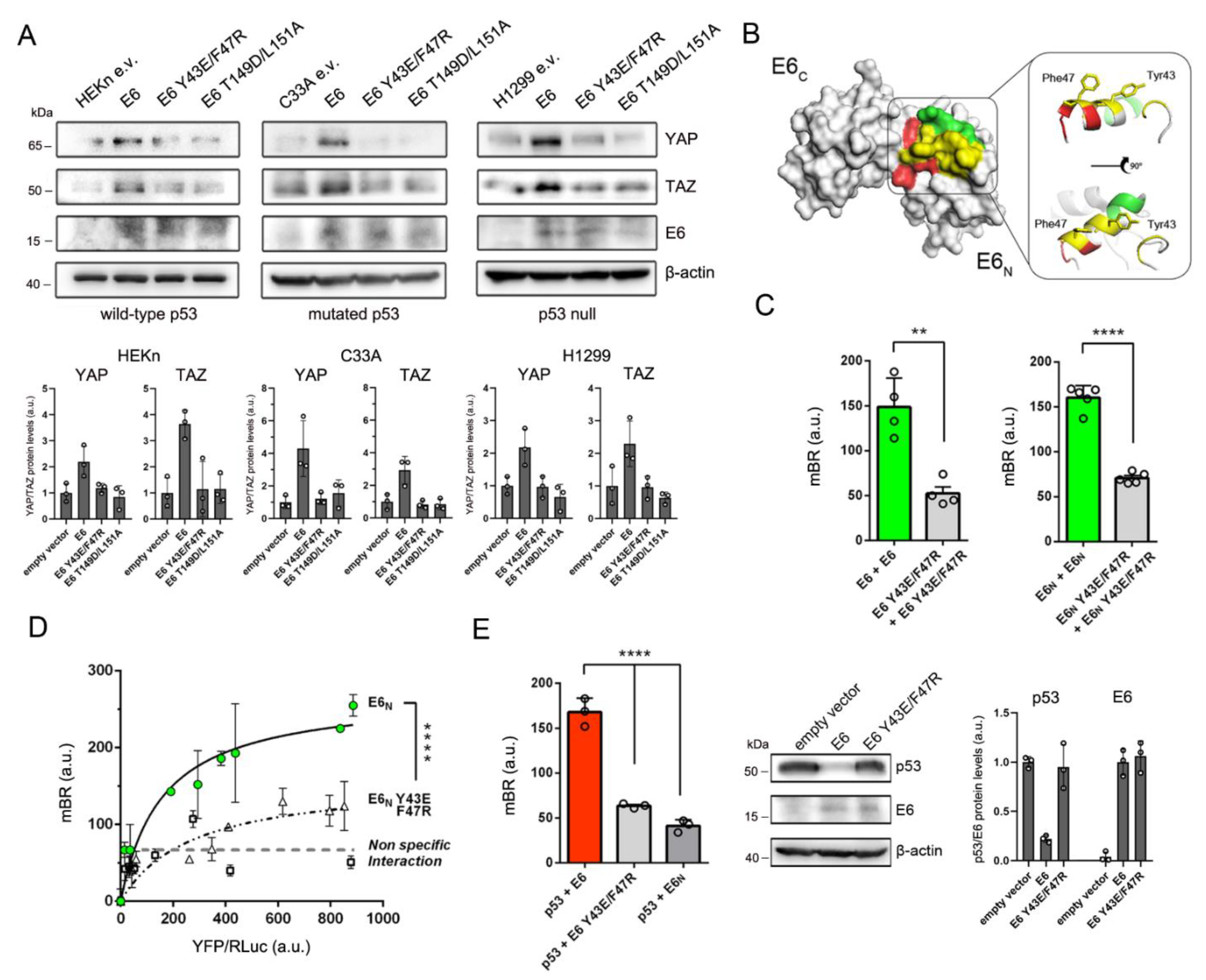
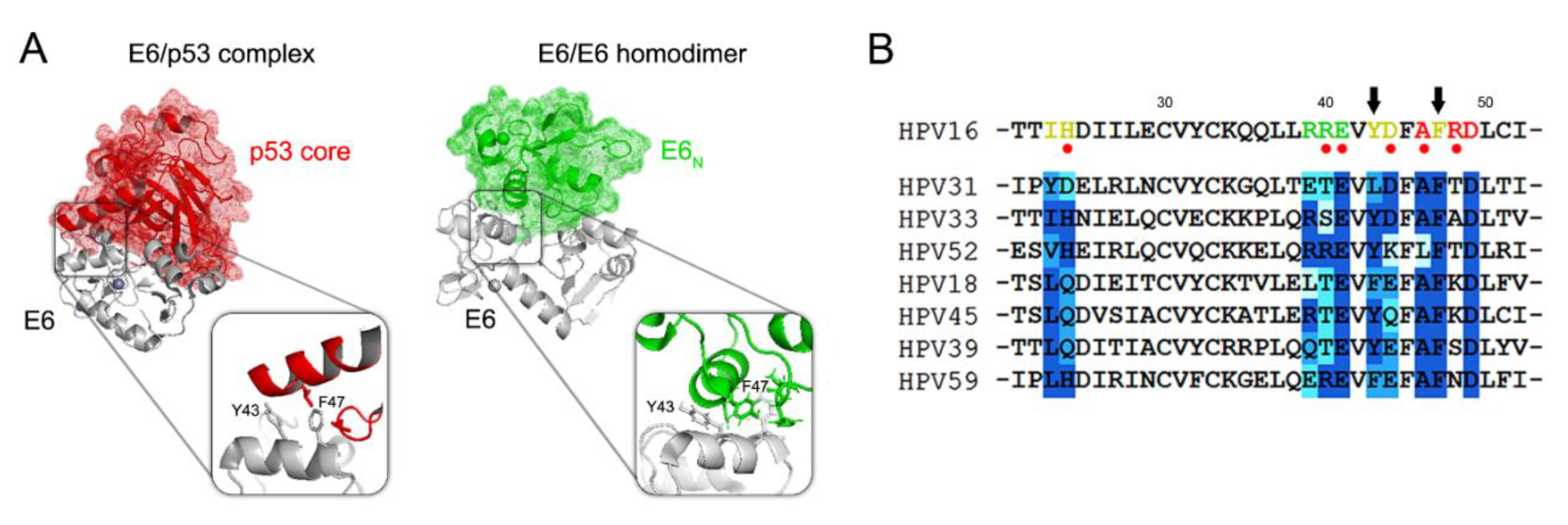
3.2. HPV16 E6 Forms Dimers in the Cell and Its Homodimerization Is Linked to YAP/TAZ Upregulation
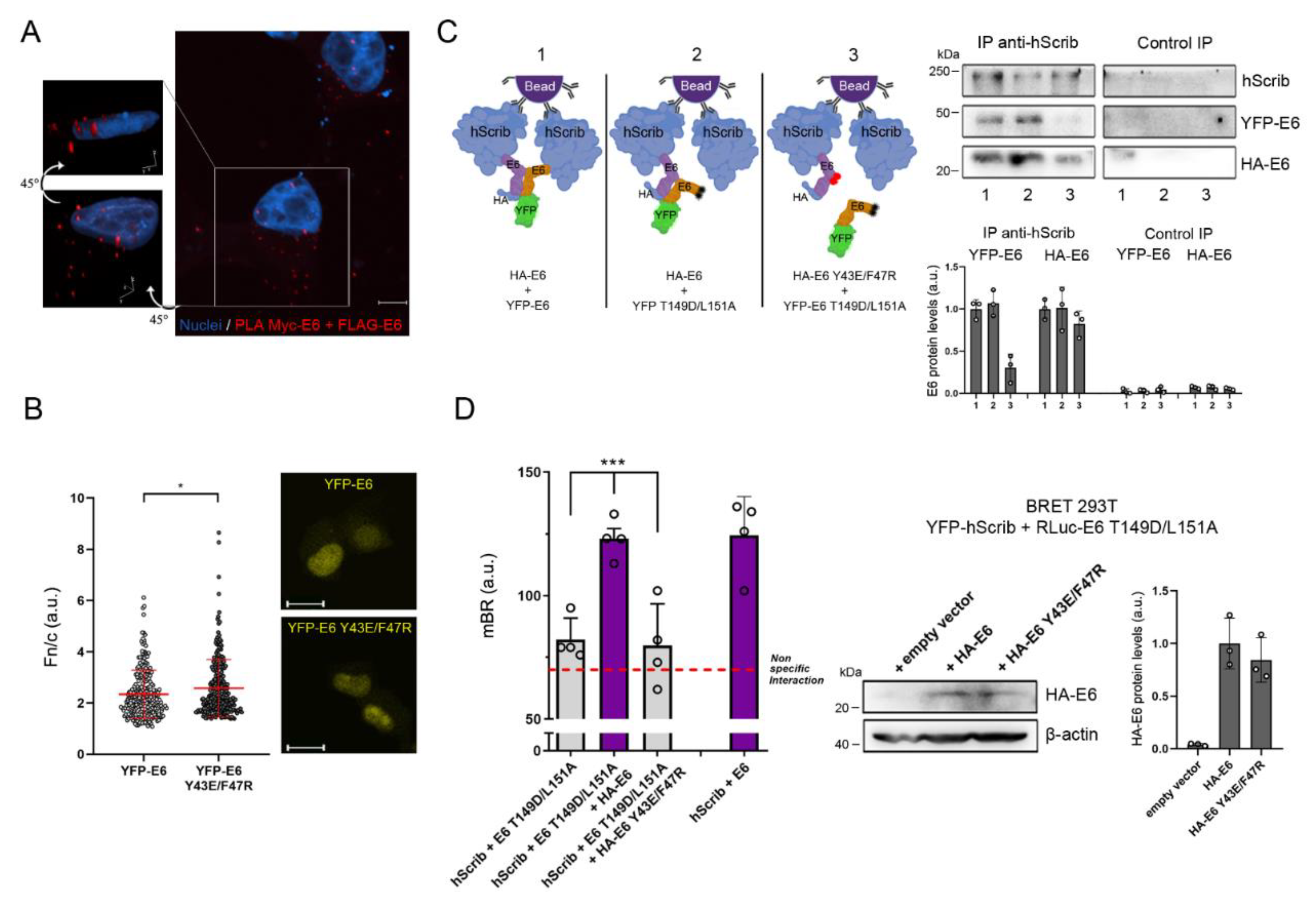
3.3. The Dimeric Form of E6 Localizes in the Cytosol and Takes Part in the E6–hScrib Complex Formation
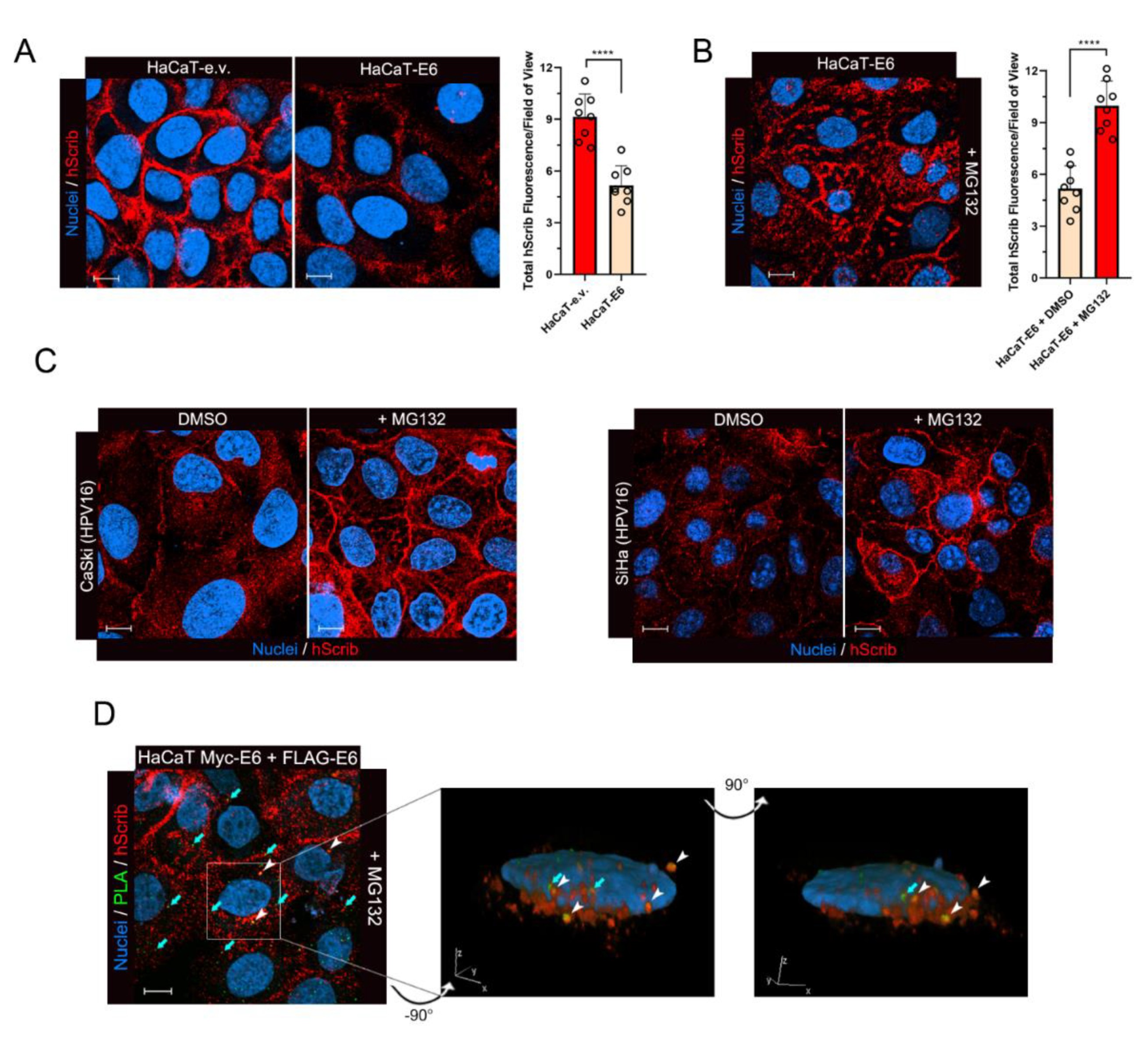
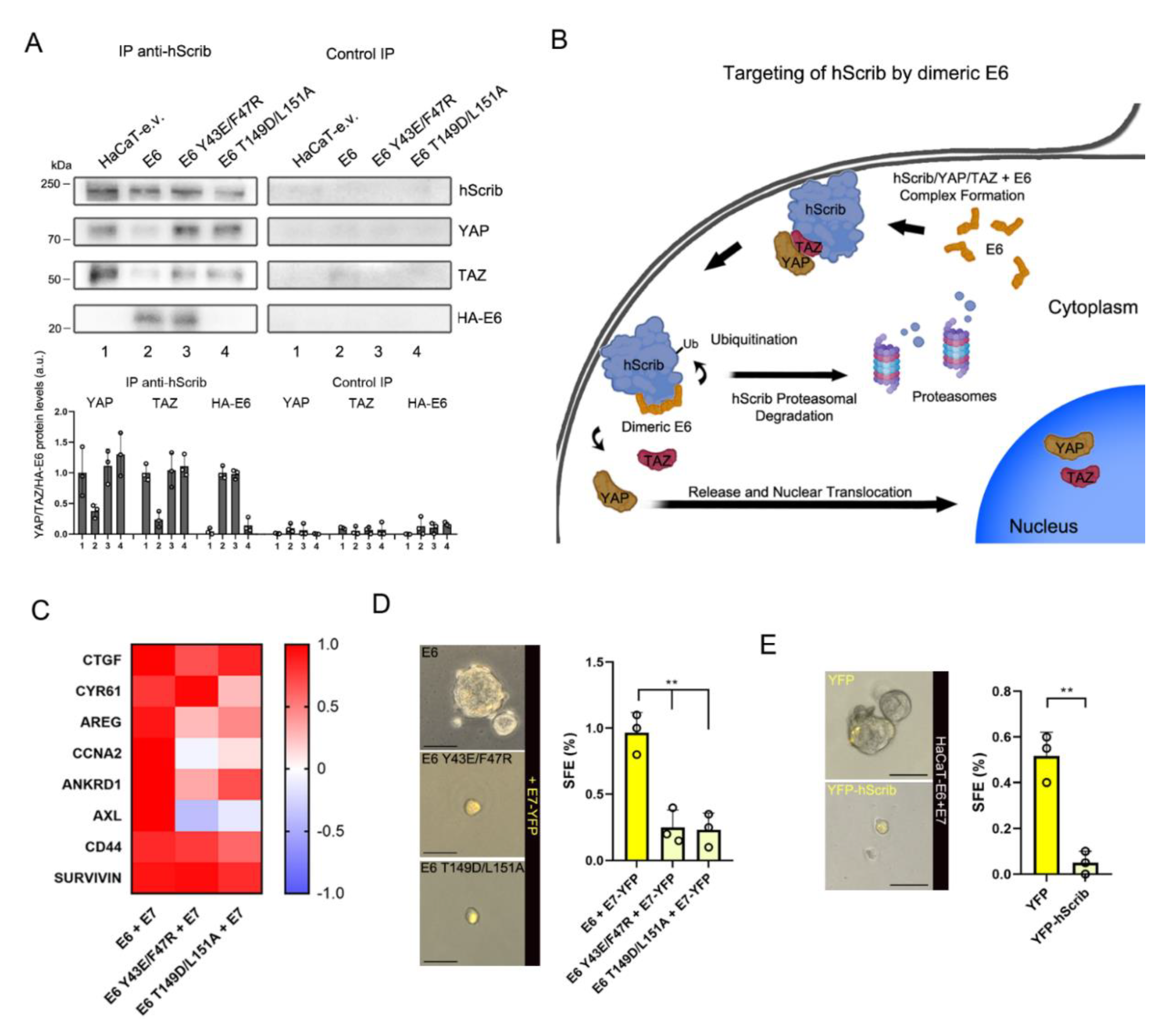
3.4. The Dimeric Form of E6 Is Involved in hScrib Proteasomal Degradation
3.5. HPV16 E6 Sustains YAP/TAZ Induction through Its Dimerization-Dependent Targeting of hScrib
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [Green Version]
- Bzhalava, D.; Guan, P.; Franceschi, S.; Dillner, J.; Clifford, G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013, 445, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Bhargava, R.; Badin, S.; Cullen, K.J. The role of human papillomavirus in nongenital cancers. CA Cancer J. Clin. 2012, 63, 57–81. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef]
- Poirson, J.; Biquand, E.; Straub, M.-L.; Cassonnet, P.; Nominé, Y.; Jones, L.; van der Werf, S.; Travé, G.; Zanier, K.; Jacob, Y.; et al. Mapping the interactome of HPV E6 and E7 oncoproteins with the ubiquitin-proteasome system. FEBS J. 2017, 284, 3171–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, L.; Ramirez, N.; Kim, J. Context-dependent roles of YAP/TAZ in stem cell fates and cancer. Cell. Mol. Life Sci. 2021, 78, 4201–4219. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Fujimura, A.; Di Biagio, D.; Frasson, C.; Bresolin, S.; Soligo, S.; Basso, G.; Bicciato, S.; Rosato, A.; et al. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 2016, 19, 725–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Shin, J.E.; Park, H.W. The role of hippo pathway in cancer stem cell biology. Mol. Cells 2018, 41, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Nieva, L.; Muñoz-Bello, J.O.; Manzo-Merino, J.; Lizano, M. New insights in hippo signalling alteration in human papillomavirus-related cancers. Cell. Signal. 2020, 76, 109815. [Google Scholar] [CrossRef]
- Hatterschide, J.; Bohidar, A.E.; Grace, M.; Nulton, T.J.; Kim, H.W.; Windle, B.; Morgan, I.M.; Munger, K.; White, E.A. PTPN14 degradation by high-risk human papillomavirus E7 limits keratinocyte differentiation and contributes to HPV-mediated oncogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 7033–7042. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.-Y.; Kim, M.W.; Lee, H.S.; Kim, W.; Shin, J.H.; Kim, H.; Shin, H.-C.; Park, H.; Oh, B.-H.; Kim, W.K.; et al. Structural basis for recognition of the tumor suppressor protein PTPN14 by the oncoprotein E7 of human papillomavirus. PLoS Biol. 2019, 17, e3000367. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.E.; Li, Y.-W.; Yang, N.; Shen, H.; Orillion, A.R.; Zhang, J. PTPN14 Forms a complex with kibra and lats1 proteins and negatively regulates the YAP oncogenic function. J. Biol. Chem. 2014, 289, 23693–23700. [Google Scholar] [CrossRef] [Green Version]
- Furth, N.; Aylon, Y.; Oren, M. p53 shades of hippo. Cell Death Differ. 2018, 25, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef]
- Mohseni, M.; Sun, J.; Lau, A.; Curtis, S.; Goldsmith, J.; Fox, V.L.; Wei, C.; Frazier, M.; Samson, O.; Wong, K.K.; et al. A genetic screen identifies an LKB1/PAR1 signaling axis controlling the Hippo/YAP pathway. Nat. Cell Biol. 2014, 16, 108–117. [Google Scholar] [CrossRef]
- Yang, C.-C.; Graves, H.K.; Moya, I.M.; Tao, C.; Hamaratoglu, F.; Gladden, A.B.; Halder, G. Differential regulation of the hippo pathway by adherens junctions and apical–basal cell polarity modules. Proc. Natl. Acad. Sci. USA 2015, 112, 1785–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb Strickland, S.; Brimer, N.; Lyons, C.; Vande Pol, S.B. Human papillomavirus E6 interaction with cellular PDZ domain proteins modulates YAP nuclear localization. Virology 2018, 516, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Messa, L.; Celegato, M.; Bertagnin, C.; Mercorelli, B.; Nannetti, G.; Palù, G.; Loregian, A. A quantitative LumiFluo assay to test inhibitory compounds blocking p53 degradation induced by human papillomavirus oncoprotein E6 in living cells. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaunsinger, B.A.; Lee, S.S.; Thomas, M.; Banks, L.; Javier, R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 2000, 19, 5270–5280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvisi, G.; Roth, D.M.; Camozzi, D.; Pari, G.S.; Loregian, A.; Ripalti, A.; Jans, D. The Flexible loop of the human cytomegalovirus DNA polymerase processivity factor ppUL44 is required for efficient DNA binding and replication in cells. J. Virol. 2009, 83, 9567–9576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsis, N.; Lannigan, D.A.; Macara, I.G. The function of the p190 Rho GTPase-activating protein is controlled by its n-terminal GTP binding domain. J. Biol. Chem. 1998, 273, 34631–34638. [Google Scholar] [CrossRef] [Green Version]
- Vos, R.M.; Altreuter, J.; White, E.A.; Howley, P.M. The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. J. Virol. 2009, 83, 8885–8892. [Google Scholar] [CrossRef] [Green Version]
- Boon, S.S.; Banks, L. High-risk human papillomavirus E6 oncoproteins interact with 14-3-3ζ in a PDZ binding motif-dependent manner. J. Virol. 2013, 87, 1586–1595. [Google Scholar] [CrossRef] [Green Version]
- Szalmás, A.; Tomaić, V.; Basukala, O.; Massimi, P.; Mittal, S.; Kónya, J.; Banks, L. The PTPN14 tumor suppressor is a degradation target of human papillomavirus E7. J. Virol. 2017, 91, e00057-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisan, M.; Di Antonio, V.; Radeghieri, A.; Palù, G.; Ghildyal, R.; Alvisi, G. Molecular requirements for self-interaction of the respiratory syncytial virus matrix protein in living mammalian cells. Viruses 2018, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Bodaghi, S.; Jia, R.; Zheng, Z.-M. Human papillomavirus type 16 E2 and E6 are RNA-binding proteins and inhibit in vitro splicing of pre-mRNAs with suboptimal splice sites. Virology 2009, 386, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Di Antonio, V.; Bellucci, L.; Thomas, D.; Caporuscio, F.; Ciccarese, F.; Ghassabian, H.; Wagstaff, K.; Forwood, J.; Jans, D.; et al. Contribution of the residue at position 4 within classical nuclear localization signals to modulating interaction with importins and nuclear targeting. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- Celegato, M.; Messa, L.; Goracci, L.; Mercorelli, B.; Bertagnin, C.; Spyrakis, F.; Suarez, I.; Cousido-Siah, A.; Travé, G.; Banks, L.; et al. A novel small-molecule inhibitor of the human papillomavirus E6-p53 interaction that reactivates p53 function and blocks cancer cells growth. Cancer Lett. 2020, 470, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, E.; Szalmás, A.; Ferenczi, A.; Póliska, S.; Kónya, J.; Veress, G. Transcriptional regulation of genes involved in keratinocyte differentiation by human papillomavirus 16 oncoproteins. Arch. Virol. 2014, 160, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Nallet-Staub, F.; Yin, X.; Gilbert, C.; Marsaud, V.; Ben Mimoun, S.; Javelaud, D.; Leof, E.B.; Mauviel, A. Cell density sensing alters TGF-β signaling in a cell-type-specific manner, independent from hippo pathway activation. Dev. Cell 2015, 32, 640–651. [Google Scholar] [CrossRef] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nat. Cell Biol. 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.; Rundsten, C.F.; et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 2018, 22, 35–49.e7. [Google Scholar] [CrossRef] [Green Version]
- Pastrana, E.; Silva-Vargas, V.; Doetsch, F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011, 8, 486–498. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Drummond, M.L.; Guerrero-Juarez, C.F.; Tarapore, E.; MacLean, A.L.; Stabell, A.R.; Wu, S.C.; Gutierrez, G.; That, B.T.; Benavente, C.A.; et al. Single cell transcriptomics of human epidermis identifies basal stem cell transition states. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Yang, N.; Figel, A.S.; Wilson, E.K.; Morrison, C.D.; Gelman, I.H.; Zhang, J. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene 2012, 32, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Zanier, K.; ould M’hamed ould Sidi, A.; Boulade-Ladame, C.; Rybin, V.; Chappelle, A.; Atkinson, A.; Kieffer, B.; Travé, G. Solution structure analysis of the HPV16 E6 oncoprotein reveals a self-association mechanism required for E6-mediated degradation of p53. Structure 2012, 20, 604–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illiano, E.; Demurtas, O.C.; Massa, S.; Di Bonito, P.; Consalvi, V.; Chiaraluce, R.; Zanotto, C.; Morghen, C.D.G.; Radaelli, A.; Venuti, A.; et al. Production of functional, stable, unmutated recombinant human papillomavirus E6 oncoprotein: Implications for HPV-tumor diagnosis and therapy. J. Transl. Med. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Massimi, P.; Navarro, C.; Borg, J.-P.; Banks, L. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene 2005, 24, 6222–6230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.; Myers, M.P.; Massimi, P.; Guarnaccia, C.; Banks, L. Analysis of multiple HPV E6 PDZ interactions defines type-specific PDZ fingerprints that predict oncogenic potential. PLoS Pathog. 2016, 12, e1005766. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Huibregtse, J.M. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 2000, 20, 8244–8253. [Google Scholar] [CrossRef]
- Humbert, P.O.; Grzeschik, N.A.; Brumby, A.M.; Galea, R.; Elsum, I.; Richardson, H. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 2008, 27, 6888–6907. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, X.; Li, X.; Qiu, K.; Jiao, F.; Liu, Y.; Kong, Q.; Liu, Y.; Wu, Y. Proteasome inhibition activates autophagy-lysosome pathway associated with TFEB dephosphorylation and nuclear translocation. Front. Cell Dev. Biol. 2019, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Dunner, K.; McConkey, D.J. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene 2009, 29, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.A.; Ward, C.L.; Kopito, R.R. Aggresomes: A cellular response to misfolded proteins. J. Cell. Biol. 1998, 143, 1883–1898. [Google Scholar] [CrossRef] [Green Version]
- Johnston, H.E.; Samant, R.S. Alternative systems for misfolded protein clearance: Life beyond the proteasome. FEBS J. 2020, 288, 4464–4487. [Google Scholar] [CrossRef] [PubMed]
- Howie, H.L.; Katzenellenbogen, R.; Galloway, D.A. Papillomavirus E6 proteins. Virology 2009, 384, 324–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Mao, D.; Hua, G.; Lv, X.; Chen, X.; Angeletti, P.C.; Dong, J.; Remmenga, S.W.; Rodabaugh, K.J.; Zhou, J.; et al. The Hippo YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol. Med. 2015, 7, 1426–1449. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, Z.; Zhou, T.; Aiman, S.; Gong, W.; Li, C. Inferring virus-host relationship between HPV and its host homo sapiens using protein interaction network. Sci. Rep. 2020, 10, 8719. [Google Scholar]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Simonson, S.J.; Difilippantonio, M.J.; Lambert, P.F. Two distinct activities contribute to human papillomavirus 16 E6′s oncogenic potential. Cancer Res. 2005, 65, 8266–8273. [Google Scholar] [CrossRef] [Green Version]
- White, E.A.; Kramer, R.E.; Tan, M.J.A.; Hayes, S.D.; Harper, J.; Howley, P.M. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J. Virol. 2012, 86, 13174–13186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, S.; Yano, T.; Takizawa, S.; Suzuki, Y.; Yasugi, T.; Huibregtse, J.M.; Taketani, Y.; Nakagawa, K. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br. J. Cancer 2004, 90, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Yoshimatsu, Y.; Nakahara, T.; Tanaka, K.; Inagawa, Y.; Narisawa-Saito, M.; Yugawa, T.; Ohno, S.; Fujita, M.; Nakagama, H.; Kiyono, T. Roles of the PDZ-binding motif of HPV 16 E6 protein in oncogenic transformation of human cervical keratinocytes. Cancer Sci. 2017, 108, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Vande Pol, S. Papillomavirus E6 oncoproteins take common structural approaches to solve different biological problems. PLoS Pathog. 2015, 11, e1005138. [Google Scholar] [CrossRef]
- Mirabello, L.; Yeager, M.; Yu, K.; Clifford, G.M.; Xiao, Y.; Zhu, B.; Cullen, M.; Boland, J.F.; Wentzensen, N.; Nelson, C.; et al. HPV16 E7 genetic conservation is critical to carcinogenesis. Cell 2017, 170, 1164–1174.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loregian, A.; Marsden, H.S.; Palù, G. Protein-protein interactions as targets for antiviral chemotherapy. Rev. Med. Virol. 2002, 12, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Loregian, A.; Palù, G. How academic labs can approach the drug discovery process as a way to synergize with big pharma. Trends Microbiol. 2013, 21, 261–264. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messa, L.; Celegato, M.; Bertagnin, C.; Mercorelli, B.; Alvisi, G.; Banks, L.; Palù, G.; Loregian, A. The Dimeric Form of HPV16 E6 Is Crucial to Drive YAP/TAZ Upregulation through the Targeting of hScrib. Cancers 2021, 13, 4083. https://doi.org/10.3390/cancers13164083
Messa L, Celegato M, Bertagnin C, Mercorelli B, Alvisi G, Banks L, Palù G, Loregian A. The Dimeric Form of HPV16 E6 Is Crucial to Drive YAP/TAZ Upregulation through the Targeting of hScrib. Cancers. 2021; 13(16):4083. https://doi.org/10.3390/cancers13164083
Chicago/Turabian StyleMessa, Lorenzo, Marta Celegato, Chiara Bertagnin, Beatrice Mercorelli, Gualtiero Alvisi, Lawrence Banks, Giorgio Palù, and Arianna Loregian. 2021. "The Dimeric Form of HPV16 E6 Is Crucial to Drive YAP/TAZ Upregulation through the Targeting of hScrib" Cancers 13, no. 16: 4083. https://doi.org/10.3390/cancers13164083
APA StyleMessa, L., Celegato, M., Bertagnin, C., Mercorelli, B., Alvisi, G., Banks, L., Palù, G., & Loregian, A. (2021). The Dimeric Form of HPV16 E6 Is Crucial to Drive YAP/TAZ Upregulation through the Targeting of hScrib. Cancers, 13(16), 4083. https://doi.org/10.3390/cancers13164083








