Useful MRI Findings for Minimally Invasive Surgery for Early Cervical Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. MRI Protocols
2.1. Patient Preparation and Technical Tips
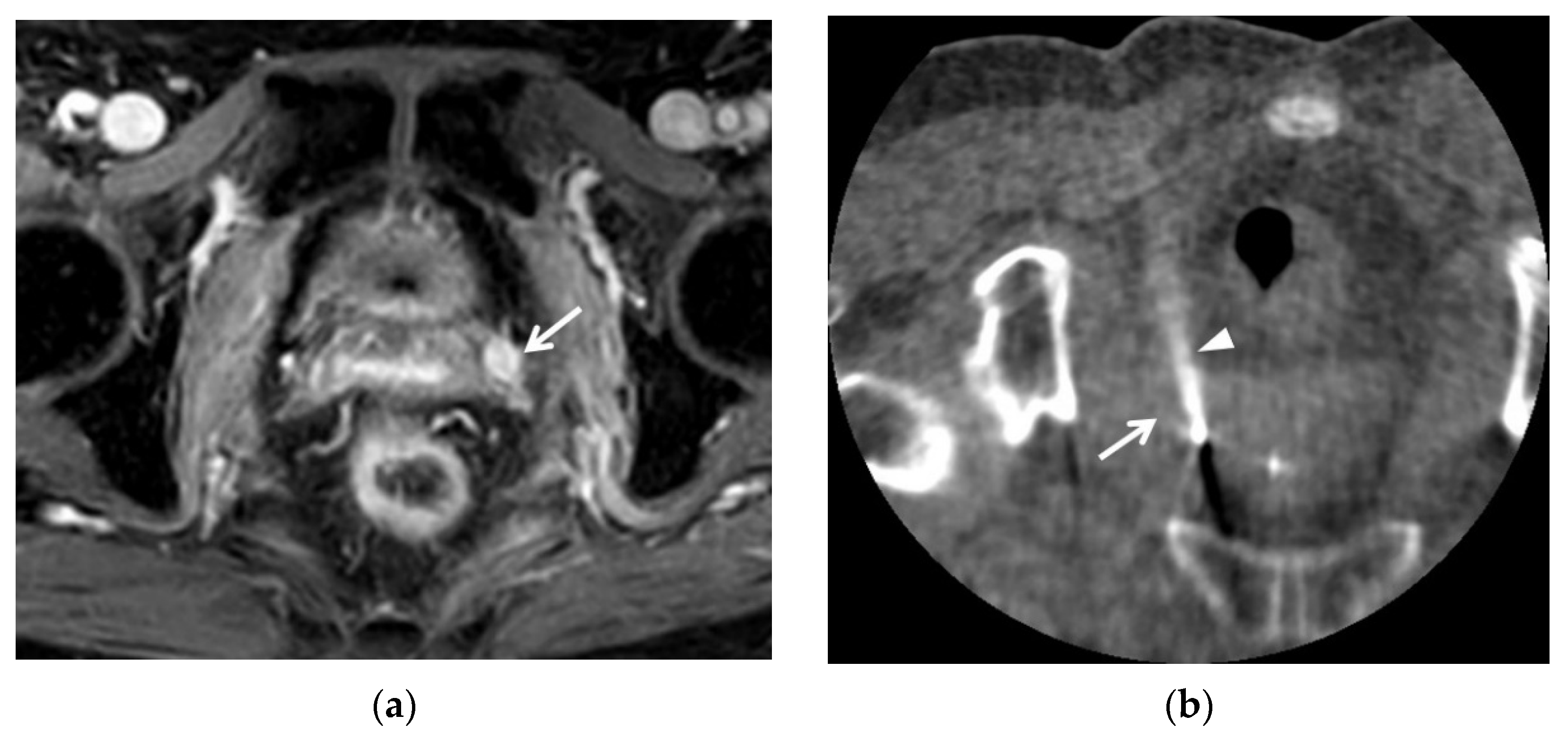
2.2. T2-Weighted Imaging (T2WI)
2.3. T1-Weighted Imaging (T1WI)
2.4. Diffusion-Weighted Imaging (DWI)
2.5. Contrast-Enhanced Imaging (CEI)
3. MRI Findings
3.1. T2WI Findings
3.2. T1WI Findings
3.3. DWI Findings
3.4. CEI Findings
3.5. MRI Findings Indicative of Prognosis
4. Post-Conization MRI
5. Post-Trachelectomy MRI
6. MRI for Endocervical Cancer
7. Early Detection of Recurrent Cancer
8. Lymph Node Metastasis
9. Invisible Cancer on 3T MRI as an Indicator
10. New MRI Techniques
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Piver, M.S.; Rutledge, F.; Smith, J.P. Five classes of extended hysterectomy for women with cervical cancer. Obstet. Gynecol. 1974, 44, 265–272. [Google Scholar] [CrossRef]
- Kristensen, G.B.; Abeler, V.M.; Risberg, B.; Tropé, C.; Bryne, M. Tumor Size, Depth of Invasion, and Grading of the Invasive Tumor Front Are the Main Prognostic Factors in Early Squamous Cell Cervical Carcinoma. Gynecol. Oncol. 1999, 74, 245–251. [Google Scholar] [CrossRef]
- Panici, P.B.; Angioli, R.; Palaia, I.; Muzii, L.; Zullo, M.A.; Manci, N.; Rabitti, C. Tailoring the parametrectomy in stages IA2–IB1 cervical carcinoma: Is it feasible and safe? Gynecol. Oncol. 2005, 96, 792–798. [Google Scholar] [CrossRef]
- Kamimori, T.; Sakamoto, K.; Fujiwara, K.; Umayahara, K.; Sugiyama, Y.; Utsugi, K.; Takeshima, N.; Tanaka, H.; Gomi, N.; Takizawa, K. Parametrial Involvement in FIGO Stage IB1 Cervical Carcinoma: Diagnostic Impact of Tumor Diameter in Preoperative Magnetic Resonance Imaging. Int. J. Gynecol. Cancer 2011, 21, 349–354. [Google Scholar] [CrossRef]
- Artman, L.E.; Hoskins, W.J.; Bibro, M.C.; Heller, P.B.; Weiser, E.B.; Barnhill, D.R.; Park, R.C. Radical hysterectomy and pelvic lymphadenectomy for Stage IB carcinoma of the cervix: 21 years experience. Gynecol. Oncol. 1987, 28, 8–13. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Cormio, G.; Perego, P.; Milani, R.; Caruso, O.; Mangioni, C. Class II versus Class III Radical Hysterectomy in Stage IB–IIA Cervical Cancer: A Prospective Randomized Study. Gynecol. Oncol. 2001, 80, 3–12. [Google Scholar] [CrossRef]
- Rob, L.; Halaska, M.; Robova, H. Nerve-sparing and individually tailored surgery for cervical cancer. Lancet Oncol. 2010, 11, 292–301. [Google Scholar] [CrossRef]
- Bonneau, C.; Cortez, A.; Lis, R.; Mirshahi, M.; Fauconnier, A.; Ballester, M.; Daraï, E.; Touboul, C. Lymphatic and nerve distribution throughout the parametrium. Gynecol. Oncol. 2013, 131, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Rebegea, L.F.; Stoleriu, G.; Manolache, N.; Serban, C.; Craescu, M.; Lupu, M.-N.; Voinescu, D.C.; Firescu, D.; Ciobotaru, O.R. Associated risk factors of lower limb lymphedema after treatment of cervical and endometrial cancer. Exp. Ther. Med. 2020, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Togami, S.; Kawamura, T.; Fukuda, M.; Yanazume, S.; Kamio, M.; Kobayashi, H. Risk factors for lymphatic complications following lymphadenectomy in patients with cervical cancer. Jpn. J. Clin. Oncol. 2018, 48, 1036–1040. [Google Scholar] [CrossRef] [Green Version]
- Togami, S.; Kubo, R.; Kawamura, T.; Yanazume, S.; Kamio, M.; Kobayashi, H. Comparison of lymphatic complications between sentinel node navigation surgery and pelvic lymphadenectomy in patients with cervical cancer. Jpn. J. Clin. Oncol. 2020, 50, 543–547. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, B.I.; Lee, H.P.; Kang, S.B.; Choi, Y.M.; Han, M.C.; Kim, C.W. Uterine cervical carcinoma: Comparison of CT and MR findings. Radiology 1990, 175, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, J.W.; Park, B.K.; Lee, Y.Y.; Choi, C.H.; Kim, T.J.; Bae, D.S.; Kim, B.G.; Park, J.J.; Park, S.Y.; et al. Postoperative outcomes of MR-invisible stage IB1 cervical cancer. Am. J. Obstet. Gynecol. 2014, 211, 168.e1–168.e7. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.G.; Snyder, B.; Coakley, F.; Reinhold, C.; Thomas, G.; Amendola, M.; Schwartz, L.H.; Woodward, P.; Pannu, H.; Hricak, H. Early invasive cervical cancer: Tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. J. Clin. Oncol. 2006, 24, 5687–5694. [Google Scholar] [CrossRef]
- Jung, D.C.; Ju, W.; Choi, H.J.; Kang, S.; Park, S.; Yoo, C.W.; Park, S.-Y. The validity of tumour diameter assessed by magnetic resonance imaging and gross specimen with regard to tumour volume in cervical cancer patients. Eur. J. Cancer 2008, 44, 1524–1528. [Google Scholar] [CrossRef]
- Hoffman, M.S.; Cardosi, R.J.; Roberts, W.S.; Fiorica, J.V.; Grendys, E.C., Jr.; Griffin, D. Accuracy of pelvic examination in the assessment of patients with operable cervical cancer. Am. J. Obstet. Gynecol. 2004, 190, 986–993. [Google Scholar] [CrossRef]
- Otero-García, M.M.; Mesa-Álvarez, A.; Nikolic, O.; Blanco-Lobato, P.; Basta-Nikolic, M.; de Llano-Ortega, R.M.; Paredes-Velázquez, L.; Nikolic, N.; Szewczyk-Bieda, M. Role of MRI in staging and follow-up of endometrial and cervical cancer: Pitfalls and mimickers. Insights Imaging 2019, 10, 19. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Brambs, C.E.; Opitz, S.; Ulrich, U.A.; Höhn, A.K. The 2019 FIGO classification for cervical carcinoma-what’s new? Pathologe 2019, 40, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Virarkar, M.; Javadi, S.; Elsherif, S.B.; de Castro Faria, S.; Bhosale, P. Cervical Cancer: 2018 Revised International Federation of Gynecology and Obstetrics Staging System and the Role of Imaging. AJR Am. J. Roentgenol. 2020, 214, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Devine, C.; Viswanathan, C.; Faria, S.; Marcal, L.; Sagebiel, T.L. Imaging and Staging of Cervical Cancer. Semin. Ultrasound CT MR 2019, 40, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Merz, J.; Bossart, M.; Bamberg, F.; Eisenblaetter, M. Revised FIGO Staging for Cervical Cancer—A New Role for MRI. Rofo 2020, 192, 937–944. [Google Scholar]
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynaecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef]
- Pecorelli, S.; Zigliani, L.; Odicino, F. Revised FIGO staging for carcinoma of the cervix. Int. J. Gynaecol. Obstet. 2009, 105, 107–108. [Google Scholar] [CrossRef]
- Luo, L.; Luo, Q.; Tang, L. Diagnostic value and clinical significance of MRI and CT in detecting lymph node metastasis of early cervical cancer. Oncol. Lett. 2020, 19, 700–706. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, B.I.; Han, J.K.; Kim, H.D.; Lee, H.P.; Kang, S.B.; Lee, J.Y.; Han, M.C. Preoperative staging of uterine cervical carcinoma: Comparison of CT and MRI in 99 patients. J. Comput. Assist. Tomogr. 1993, 17, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Haldorsen, I.S.; Lura, N.; Blaakær, J.; Fischerova, D.; Werner, H.M.J. What Is the Role of Imaging at Primary Diagnostic Work-Up in Uterine Cervical Cancer? Curr. Oncol. Rep. 2019, 21, 77. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.; Kim, H.S.; Chung, H.H.; Kim, S.Y.; Kim, S.H.; Cho, J.Y. Early stage cervical cancer: Role of magnetic resonance imaging after conization in determining residual tumor. Acta Radiol. 2016, 57, 1268–1276. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Bradley, W.G., Jr. Pros and cons of 3 tesla MRI. J. Am. Coll. Radiol. 2008, 5, 871–878. [Google Scholar] [CrossRef]
- Kataoka, M.; Kido, A.; Koyama, T.; Isoda, H.; Umeoka, S.; Tamai, K.; Nakamoto, Y.; Maetani, Y.; Morisawa, N.; Saga, T.; et al. MRI of the female pelvis at 3T compared to 1.5T: Evaluation on high-resolution T2-weighted and HASTE images. J. Magn. Reson. Imaging 2007, 25, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.J.; Kim, K.B.; Lee, J.H.; Kim, H.J.; Kwon, Y.-S.; Lee, S.H. Early Cervical Cancer: Predictive Relevance of Preoperative 3-Tesla Multiparametric Magnetic Resonance Imaging. Int. J. Surg. Oncol. 2018, 2018, 9120753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutzeit, A.; Binkert, C.; Koh, D.-M.; Hergan, K.; Weymarn, C.; Graf, N.; Patak, M.; Horstmann, M.; Kos, S.; Hungerbühler, S.; et al. Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: Comparison of intravenous and intramuscular drug administration. Eur. Radiol. 2012, 22, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Martí-Bonmatí, L.; Graells, M.; Ronchera-Oms, C.L. Reduction of peristaltic artifacts on magnetic resonance imaging of the abdomen: A comparative evaluation of three drugs. Abdom. Imaging 1996, 21, 309–313. [Google Scholar] [CrossRef]
- Ogura, A.; Maeda, F.; Miyai, A.; Kikumoto, R. Effects of slice thickness and matrix size on MRI for signal detection. Nihon Hoshasen Gijutsu Gakkai Zasshi 2005, 61, 1140–1143. [Google Scholar] [CrossRef] [Green Version]
- Lin, I.T.; Yang, H.C.; Chen, J.H. Enlargement of the field of view and maintenance of a high signal-to-noise ratio using a two-element high-Tc superconducting array in a 3T MRI. PLoS ONE 2012, 7, e42509. [Google Scholar]
- Balleyguier, C.; Sala, E.; Da Cunha, T.; Bergman, A.; Brkljacic, B.; Danza, F.; Forstner, R.; Hamm, B.; Kubik-Huch, R.; Lopez, C.; et al. Staging of uterine cervical cancer with MRI: Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2011, 21, 1102–1110. [Google Scholar] [CrossRef]
- Noël, P.; Dubé, M.; Plante, M.; St-Laurent, G. Early cervical carcinoma and fertility-sparing treatment options: MR imaging as a tool in patient selection and a follow-up modality. Radiographics 2014, 34, 1099–1119. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.C.; Choi, B.I.; Han, M.C. Uterine cervical carcinoma: Evaluation of pelvic lymph node metastasis with MR imaging. Radiology 1994, 190, 807–811. [Google Scholar] [CrossRef]
- Roh, H.J.; Go, E.B.; Kim, K.B.; Lee, J.H.; Lee, S.H. The Diagnostic Accuracy and Postoperative Outcomes of Cervical Cancer Patients for MR-invisible or MR-visible Diagnosis of Combined T2- and Diffusion-weighted 3T MRI Using the External Phased-array Receiver. Anticancer Res. 2019, 39, 6945–6956. [Google Scholar] [CrossRef]
- McVeigh, P.Z.; Syed, A.M.; Milosevic, M.; Fyles, A.; Haider, M.A. Diffusion-weighted MRI in cervical cancer. Eur. Radiol. 2008, 18, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Liang, B.; Yang, Z. The utility of diffusion-weighted MR imaging in cervical cancer. Eur. J. Radiol. 2010, 74, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, R.; Sun, H.; Liu, H.; Wang, D. Diffusion-Weighted Magnetic Resonance Imaging of Uterine Cervical Cancer. J. Comput. Assist. Tomogr. 2009, 33, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Thoeny, H.C.; Forstner, R.; De Keyzer, F. Genitourinary applications of diffusion-weighted MR imaging in the pelvis. Radiology 2012, 263, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.Z.; Zhong, Y.; Zhou, D.Y.; Wu, W.Q.; Wu, G.Y.; Quan, H. Application of brain multi-b-value diffusion-weighted imaging (DWI) in adolescent orphans from AIDS families. Br. J. Radiol. 2016, 89, 20150732. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Shi, F.; Zhang, J.; Ling, C.; Dong, F.; Jiang, B. A Modified Tri-Exponential Model for Multi-b-value Diffusion-Weighted Imaging: A Method to Detect the Strictly Diffusion-Limited Compartment in Brain. Front. Neurosci. 2018, 12, 102. [Google Scholar] [CrossRef]
- Jiang, D.-Z.; Zhou, D.-Y.; Wu, W.-Q.; Wu, G.-Y.; Quan, H. Value of multi-b value DWI in the assessment of early cerebral changes in asymptomatic HIV-positive adolescents. Acta Radiol. 2016, 58, 867–875. [Google Scholar] [CrossRef]
- Abou khadrah, R.S.; Imam, H.H. Multiple b values of diffusion-weighted magnetic resonance imaging in evaluation of solid head and neck masses. Egypt. J. Radiol. Nucl. Med. 2019, 50, 54. [Google Scholar] [CrossRef]
- Yamashita, Y.; Baba, T.; Baba, Y.; Nishimura, R.; Ikeda, S.; Takahashi, M.; Ohtake, H.; Okamura, H. Dynamic Contrast-enhanced MR Imaging of Uterine Cervical Cancer: Pharmacokinetic Analysis with Histopathologic Correlation and Its Importance in Predicting the Outcome of Radiation Therapy. Radiology 2000, 216, 803–809. [Google Scholar] [CrossRef]
- Halle, C.; Andersen, E.; Lando, M.; Aarnes, E.; Hasvold, G.; Holden, M.; Syljuåsen, R.; Sundfør, K.; Kristensen, G.; Holm, R.; et al. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res. 2012, 72, 5285–5295. [Google Scholar] [CrossRef] [Green Version]
- Andersen, E.K.; Hole, K.H.; Lund, K.V.; Sundfør, K.; Kristensen, G.B.; Lyng, H.; Malinen, E. Dynamic contrast-enhanced MRI of cervical cancers: Temporal percentile screening of contrast enhancement identifies parameters for prediction of chemoradioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e485–e492. [Google Scholar] [CrossRef]
- Delgado, G.; Bundy, B.; Zaino, R.; Sevin, B.-U.; Creasman, W.T.; Major, F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol. Oncol. 1990, 38, 352–357. [Google Scholar] [CrossRef]
- Kamura, T.; Tsukamoto, N.; Tsuruchi, N.; Saito, T.; Matsuyama, T.; Akazawa, K.; Nakano, H. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer 1992, 69, 181–186. [Google Scholar] [CrossRef]
- Van de Putte, G.; Lie, A.K.; Vach, W.; Baekelandt, M.; Kristensen, G.B. Risk grouping in stage IB squamous cell cervical carcinoma. Gynecol. Oncol. 2005, 99, 106–112. [Google Scholar] [CrossRef]
- Halle, M.K.; Ojesina, A.I.; Engerud, H.; Woie, K.; Tangen, I.L.; Holst, F.; Høivik, E.; Kusonmano, K.; Haldorsen, I.S.; Vintermyr, O.K.; et al. Clinicopathologic and molecular markers in cervical carcinoma: A prospective cohort study. Am. J. Obstet. Gynecol. 2017, 217, 432.e1–432.e17. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Song, J.C.; Chen, T.; Yang, M.; Ma, Z.L. Making the invisible visible: Improving detectability of MRI-invisible residual cervical cancer after conisation by DCE-MRI. Clin. Radiol. 2019, 74, 166.e15–166.e21. [Google Scholar] [CrossRef]
- Bourgioti, C.; Koutoulidis, V.; Chatoupis, K.; Rodolakis, A.; Koureas, A.; Thomakos, N.; Moulopoulos, L.A. MRI findings before and after abdominal radical trachelectomy (ART) for cervical cancer: A prospective study and review of the literature. Clin. Radiol. 2014, 69, 678–686. [Google Scholar] [CrossRef]
- Downey, K.; Shepherd, J.H.; Attygalle, A.D.; Hazell, S.; Morgan, V.A.; Giles, S.L.; Ind, T.E.J.; deSouza, N.M. Preoperative imaging in patients undergoing trachelectomy for cervical cancer: Validation of a combined T2- and diffusion-weighted endovaginal MRI technique at 3.0T. Gynecol. Oncol. 2014, 133, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, R.M.; Biliatis, I.; Rockall, A.; Papadakou, E.; Sohaib, S.A.; deSouza, N.M.; Butler, J.; Nobbenhuis, M.; Barton, D.; Shepherd, J.H.; et al. MRI measurement of residual cervical length after radical trachelectomy for cervical cancer and the risk of adverse pregnancy outcomes: A blinded imaging analysis. BJOG 2018, 125, 1726–1733. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.Y.; Kang, H.K.; Chung, T.W.; Seo, J.J.; Park, J.G. Uterine Cervical Carcinoma after Therapy: CT and MR Imaging Findings. RadioGraphics 2003, 23, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Valduvieco, I.; Biete, A.; Rios, I.; Llorente, R.; Rovirosa, A.; Pahisa, J.; Vidal, L.; Farrús, B.; Samper, P. Correlation between clinical findings and magnetic resonance imaging for the assessment of local response after standard treatment in cervical cancer. Rep. Pract. Oncol. Radiother. 2013, 18, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeles, M.A.; Baissas, P.; Leblanc, E.; Lusque, A.; Ferron, G.; Ducassou, A.; Martínez-Gómez, C.; Querleu, D.; Martinez, A. Magnetic resonance imaging after external beam radiotherapy and concurrent chemotherapy for locally advanced cervical cancer helps to identify patients at risk of recurrence. Int. J. Gynecol. Cancer 2019, 29, 480–486. [Google Scholar] [CrossRef]
- Sala, E.; Rockall, A.; Rangarajan, D.; Kubik-Huch, R.A. The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur. J. Radiol. 2010, 76, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, M.J.; Ehritt-Braun, C.; Vogelgesang, D.; Ihling, C.; Högerle, S.; Mix, M.; Moser, E.; Krause, T.M. Metastatic Lymph Nodes in Patients with Cervical Cancer: Detection with MR Imaging and FDG PET. Radiology 2001, 218, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Bipat, S.; Glas, A.S.; Velden, J.v.d.; Zwinderman, A.H.; Bossuyt, P.M.M.; Stoker, J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: A systematic review. Gynecol. Oncol. 2003, 91, 59–66. [Google Scholar] [CrossRef]
- Choi, H.J.; Roh, J.W.; Seo, S.-S.; Lee, S.; Kim, J.-Y.; Kim, S.-K.; Kang, K.W.; Lee, J.S.; Jeong, J.Y.; Park, S.-Y. Comparison of the accuracy of magnetic resonance imaging and positron emission tomography/computed tomography in the presurgical detection of lymph node metastases in patients with uterine cervical carcinoma. Cancer 2006, 106, 914–922. [Google Scholar] [CrossRef]
- He, X.Q.; Wei, L.N. Diagnostic value of lymph node metastasis by diffusion-weighted magnetic resonance imaging in cervical cancer. J. Cancer Res. Ther. 2016, 12, 77–83. [Google Scholar]
- Liu, Y.; Liu, H.; Bai, X.; Ye, Z.; Sun, H.; Bai, R.; Wang, D. Differentiation of metastatic from non-metastatic lymph nodes in patients with uterine cervical cancer using diffusion-weighted imaging. Gynecol. Oncol. 2011, 122, 19–24. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, K.A.; Park, B.-W.; Kim, N.; Cho, K.-S. Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: Early experience. J. Magn. Reson. Imaging 2008, 28, 714–719. [Google Scholar] [CrossRef]
- Martí-Bonmatí, L. Lymph node assessment by diffusion weighted imaging in cervical cancer. Eur. Radiol. 2011, 21, 474–477. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Zhou, H.; Jia, Z.; Deng, H. Diagnostic performance of diffusion-weighted MRI for detection of pelvic metastatic lymph nodes in patients with cervical cancer: A systematic review and meta-analysis. Br. J. Radiol. 2015, 88, 20150063. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.; Zhang, Y.; Ren, H. Meta-analysis of PET/CT Detect Lymph Nodes Metastases of Cervical Cancer. Open Med. (Wars) 2018, 13, 436–442. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.; Park, B.K.; Kim, T.J.; Kim, C.K.; Park, J.J.; Choi, C.H.; Lee, Y.Y.; Lee, J.W.; Bae, D.S.; et al. Long-term outcomes of magnetic resonance imaging-invisible endometrial cancer. J. Gynecol. Oncol. 2016, 27, e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, B.R.; Friedrich, F.; Fischer, C.; Paech, D.; Ladd, M.E. Beyond T2 and 3T: New MRI techniques for clinicians. Clin. Transl. Radiat. Oncol. 2019, 18, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, K.T.; Park, S.H.; Moon, C.H.; Kim, J.H.; Kaya, D.; Zhao, T. Dual-echo arteriovenography imaging with 7T MRI. J. Magn. Reson. Imaging 2010, 31, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangerter, N.K.; Taylor, M.D.; Tarbox, G.J.; Palmer, A.J.; Park, D.J. Quantitative techniques for musculoskeletal MRI at 7 Tesla. Quant. Imaging Med. Surg. 2016, 6, 715–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, U.; Mallia, A.; Stirling, J.; Joemon, J.; MacKewn, J.; Charles-Edwards, G.; Goh, V.; Cook, G.J. PET/MRI in Oncological Imaging: State of the Art. Diagnostics 2015, 5, 333–357. [Google Scholar] [CrossRef] [Green Version]
- Czernin, J.; Ta, L.; Herrmann, K. Does PET/MR Imaging Improve Cancer Assessments? Literature Evidence from More Than 900 Patients. J. Nucl. Med. 2014, 55, 59s–62s. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Choi, H.J.; Park, S.Y.; Lee, H.Y.; Seo, S.S.; Yoo, C.W.; Jung, D.C.; Kang, S.; Cho, K.S. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur. J. Cancer 2009, 45, 2103–2109. [Google Scholar] [CrossRef]
- Jethanandani, A.; Lin, T.A.; Volpe, S.; Elhalawani, H.; Mohamed, A.S.R.; Yang, P.; Fuller, C.D. Exploring Applications of Radiomics in Magnetic Resonance Imaging of Head and Neck Cancer: A Systematic Review. Front. Oncol. 2018, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwier, M.; van Griethuysen, J.; Vangel, M.G.; Pieper, S.; Peled, S.; Tempany, C.; Aerts, H.J.W.L.; Kikinis, R.; Fennessy, F.M.; Fedorov, A. Repeatability of Multiparametric Prostate MRI Radiomics Features. Sci. Rep. 2019, 9, 9441. [Google Scholar] [CrossRef] [PubMed]
- Wormald, B.W.; Doran, S.J.; Ind, T.E.; D’Arcy, J.; Petts, J.; deSouza, N.M. Radiomic features of cervical cancer on T2-and diffusion-weighted MRI: Prognostic value in low-volume tumors suitable for trachelectomy. Gynecol. Oncol. 2020, 156, 107–114. [Google Scholar] [CrossRef] [PubMed]
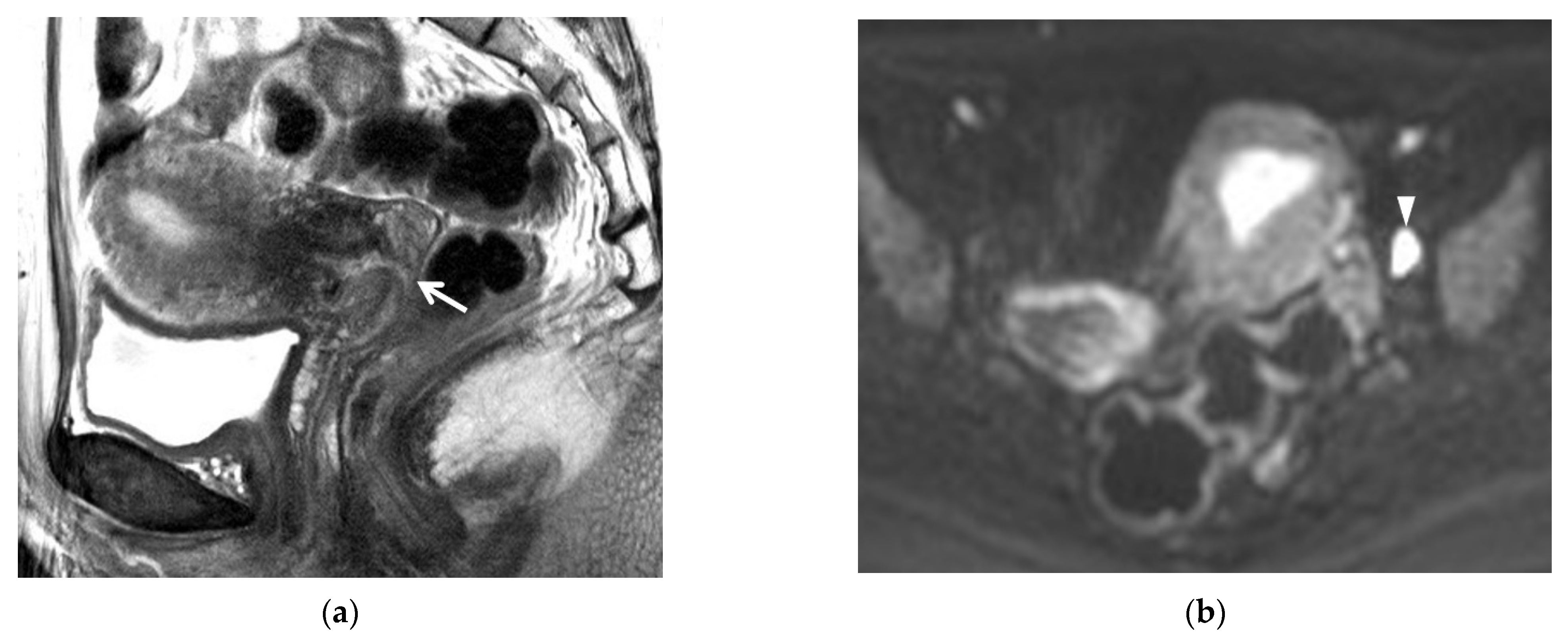
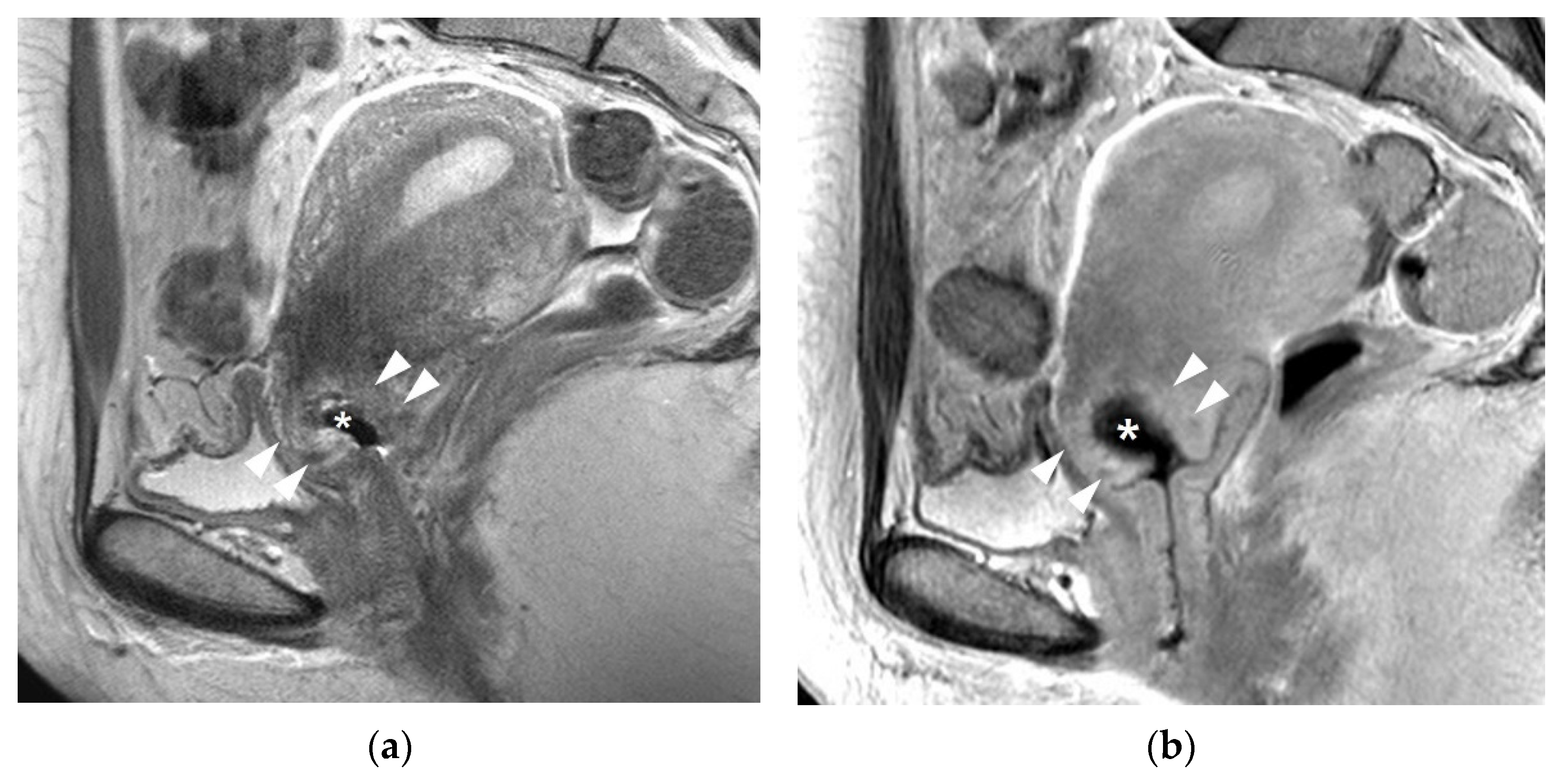
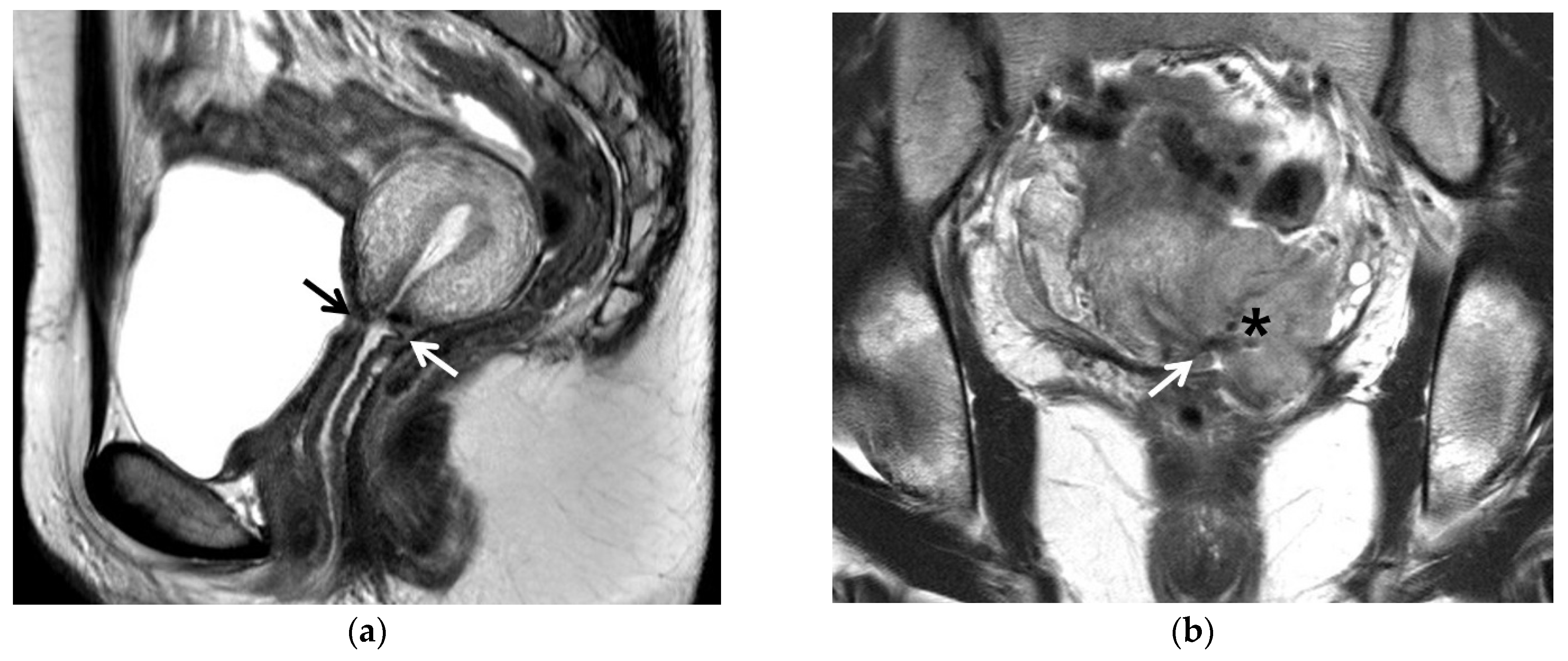
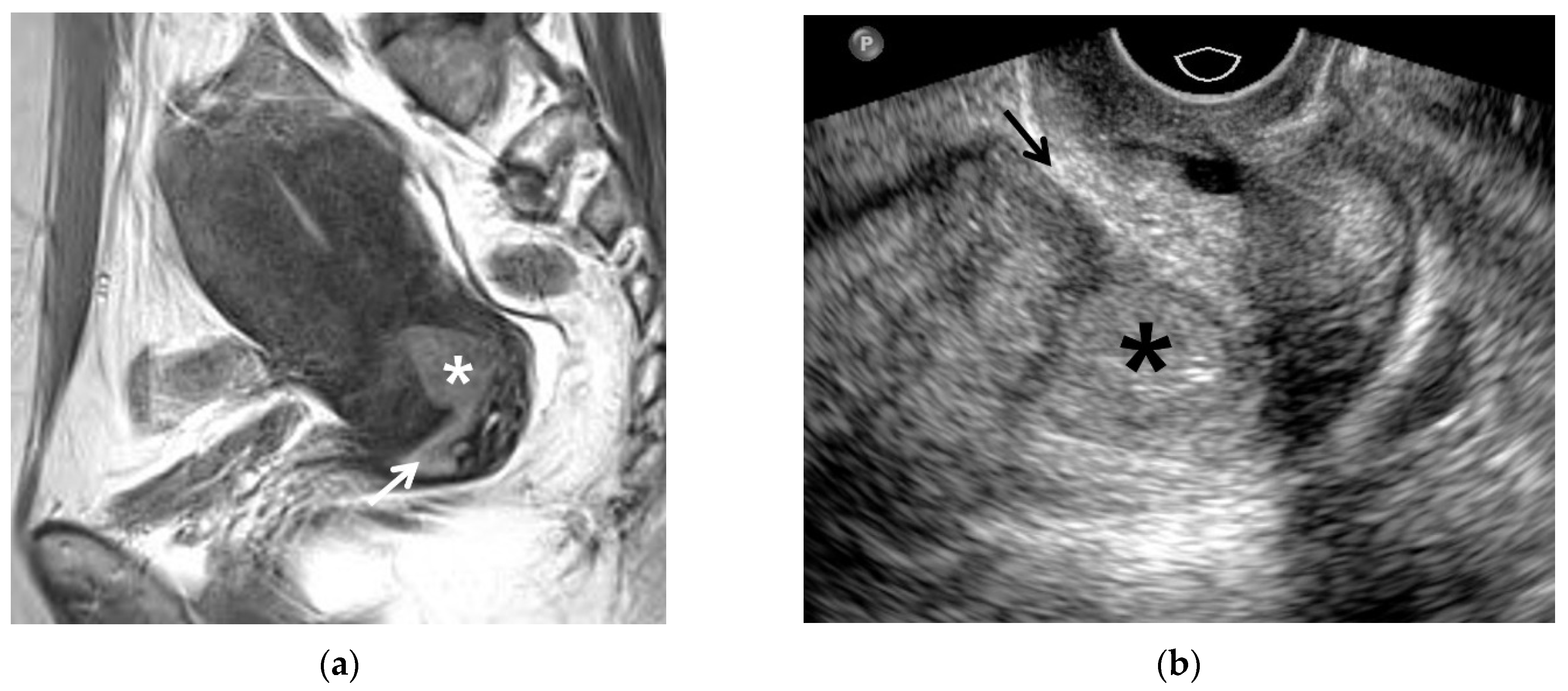
| MRI Sequences | T2WI | T1WI | DWI | Dynamic CEI | Delayed CEI |
|---|---|---|---|---|---|
| Scan techniques | Fast spin echo | Fast spin echo | Echo planar imaging | 3D T1 mDIXON | Fast spin echo |
| Imaging planes | Axial/sagittal/coronal | Axial | Axial | Axial | Sagittal |
| Repetition time (ms) | 3000–4044 | 500 | 6900 | 3.5 | 596 |
| Echo time (ms) | 100 | 10 | 64 | 0 | 10 |
| Matrix size | 640 × 640 | 384 × 381 | 144 × 206 | 208 × 207 | 384 × 380 |
| Slice thickness (mm) | 4 | 5 | 4 | 4 | 4 |
| Slice spacing (mm) | 4.4 | 7 | 4.4 | 2 | 4.4 |
| Field of view (cm) | 28 × 28 or 30 × 30 | 28 × 28 | 28 × 28 | 28 × 24 | 30 × 30 |
| Echo train length | 16 | 4 | 83 | 2 | 5 |
| Number of excitations | 1 | 1 | 3 | 1 | 1 |
| B value (s/mm2) | NA | NA | 0, 100, 1000 | NA | NA |
| Flip angle (°) | 90 | 90 | 90 | 10 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.K.; Kim, T.-J. Useful MRI Findings for Minimally Invasive Surgery for Early Cervical Cancer. Cancers 2021, 13, 4078. https://doi.org/10.3390/cancers13164078
Park BK, Kim T-J. Useful MRI Findings for Minimally Invasive Surgery for Early Cervical Cancer. Cancers. 2021; 13(16):4078. https://doi.org/10.3390/cancers13164078
Chicago/Turabian StylePark, Byung Kwan, and Tae-Joong Kim. 2021. "Useful MRI Findings for Minimally Invasive Surgery for Early Cervical Cancer" Cancers 13, no. 16: 4078. https://doi.org/10.3390/cancers13164078
APA StylePark, B. K., & Kim, T.-J. (2021). Useful MRI Findings for Minimally Invasive Surgery for Early Cervical Cancer. Cancers, 13(16), 4078. https://doi.org/10.3390/cancers13164078





