Role of Chemotherapy in Vulvar Cancers: Time to Rethink Standard of Care?
Abstract
:Simple Summary
Abstract
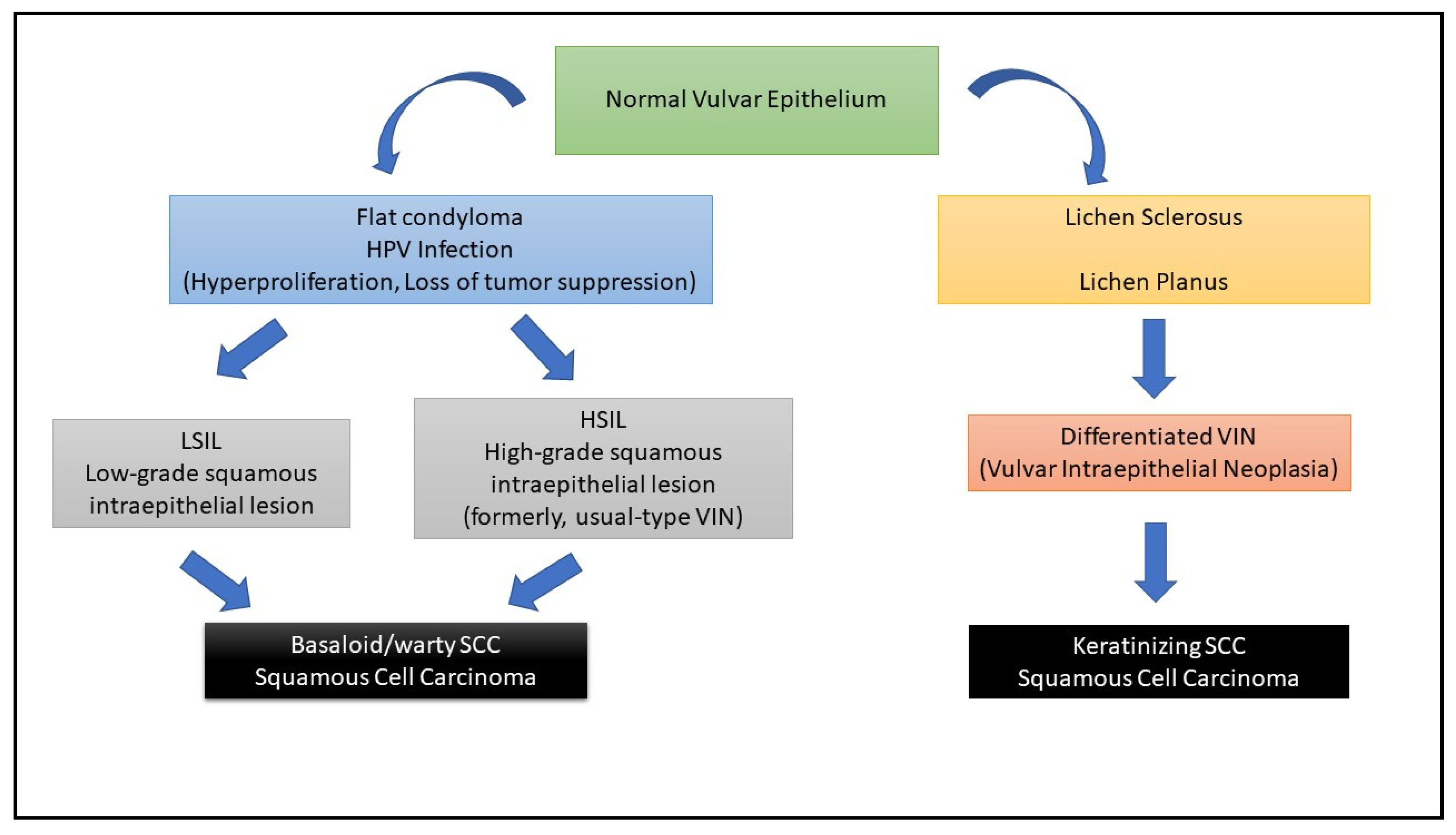
1. Introduction
2. Early Setting
2.1. Neoadjuvant Chemoradiation
2.2. Primary Chemoradiation
2.3. Neoadjuvant Chemotherapy
2.4. Adjuvant Chemotherapy
2.5. Adjuvant Chemoradiation
3. Recurrent Disease
4. Advanced Disease
4.1. Chemotherapy, Chemoradiation and Electrochemotherapy
4.2. Targeted Agents
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Cuello, M.A. Cancer of the vulva. Int. J. Gynaecol. Obs. 2018, 143 (Suppl. S2), 4–13. [Google Scholar] [CrossRef]
- Patel, H.; Wagner, M.; Singhal, P.; Kothari, S. Systematic review of the incidence and prevalence of genital warts. BMC Infect. Dis. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Hampl, M.; Sarajuuri, H.; Wentzensen, N.; Bender, H.G.; Kueppers, V. Effect of human papillomavirus vaccines on vulvar, vaginal, and anal intraepithelial lesions and vulvar cancer. Obs. Gynecol. 2006, 108, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.T.; Campbell, S.; Nygård, M. Long-term incidence trends of HPV-related cancers, and cases preventable by HPV vaccination: A registry-based study in Norway. BMJ Open 2018, 8, e019005. [Google Scholar] [CrossRef] [Green Version]
- Xing, D.; Fadare, O. Molecular events in the pathogenesis of vulvar squamous cell carcinoma. Semin. Diagn. Pathol. 2021, 38, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zięba, S.; Pouwer, A.W.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Bakuła-Zalewska, E.; Kopczyński, J.; Pijnenborg, J.; de Hullu, J.A.; Kowalewska, M. Somatic Mutation Profiling in Premalignant Lesions of Vulvar Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 4880. [Google Scholar] [CrossRef]
- Singh, N.; Gilks, C.B. Vulval squamous cell carcinoma and its precursors. Histopathology 2020, 76, 128–138. [Google Scholar] [CrossRef]
- Tomao, F.; Di Tucci, C.; Marchetti, C.; Perniola, G.; Bellati, F.; Panici, P.B. Role of chemotherapy in the management of vulvar carcinoma. Crit. Rev. Oncol. Hematol. 2012, 82, 25–39. [Google Scholar] [CrossRef]
- Reade, C.J.; Eiriksson, L.R.; Mackay, H. Systemic therapy in squamous cell carcinoma of the vulva: Current status and future directions. Gynecol. Oncol. 2014, 132, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.; Planchamp, F.; Baldwin, P.; Bidzinski, M.; Brännström, M.; Landoni, F.; Mahner, S.; Mahantshetty, U.; Mirza, M.; Petersen, C.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 832–837. [Google Scholar] [CrossRef]
- Gaffney, D.K.; King, B.; Viswanathan, A.N.; Barkati, M.; Beriwal, S.; Eifel, P.; Erickson, B.; Fyles, A.; Goulart, J.; Harkenrider, M.; et al. Consensus Recommendations for Radiation Therapy Contouring and Treatment of Vulvar Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1191–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montana, G.S. Carcinoma of the vulva: Combined modality treatment. Curr. Treat. Options Oncol. 2004, 5, 85–95. [Google Scholar] [CrossRef]
- Rotmensch, J.; Rubin, S.J.; Sutton, H.G.; Javaheri, G.; Halpern, H.J.; Schwartz, J.L.; Stewart, M.; Weichselbaum, R.R.; Herbst, A.L. Preoperative radiotherapy followed by radical vulvectomy with inguinal lymphadenectomy for advanced vulvar carcinomas. Gynecol. Oncol. 1990, 36, 181–184. [Google Scholar] [CrossRef]
- NCCN Guidelines Vulvar Cancer Version 3.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 28 April 2021).
- Rao, Y.J.; Chin, R.I.; Hui, C.; Mutch, D.G.; Powell, M.A.; Schwarz, J.K.; Grigsby, P.W.; Markovina, S. Improved survival with definitive chemoradiation compared to definitive radiation alone in squamous cell carcinoma of the vulva: A review of the National Cancer Database. Gynecol. Oncol. 2017, 146, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Bernard, M.E.; Lin, J.F.; Balasubramani, G.K.; Rajagopalan, M.S.; Sukumvanich, P.; Krivak, T.C.; Olawaiye, A.B.; Kelley, J.L.; Beriwal, S. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: A National Cancer Data Base (NCDB) analysis. Gynecol. Oncol. 2015, 137, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Dembo, A.; DePetrillo, A.; Pringle, J.; Ackerman, I.; Bryson, P.; Balogh, J.; Osborne, R.; Rosen, B.; Fyles, A. Concurrent radiation and chemotherapy in vulvar carcinoma. Gynecol. Oncol. 1989, 34, 263–267. [Google Scholar] [CrossRef]
- Berek, J.S.; Heaps, J.M.; Fu, Y.S.; Juillard, G.J.; Hacker, N.F. Concurrent cisplatin and 5-fluorouracil chemotherapy and radiation therapy for advanced-stage squamous carcinoma of the vulva. Gynecol. Oncol. 1991, 42, 197–201. [Google Scholar] [CrossRef]
- Eifel, P.J.; Morris, M.; Burke, T.W.; Levenback, C.; Gershenson, D.M. Prolonged continuous infusion cisplatin and 5-fluorouracil with radiation for locally advanced carcinoma of the vulva. Gynecol. Oncol. 1995, 59, 51–56. [Google Scholar] [CrossRef]
- Scheiströen, M.; Tropé, C. Combined bleomycin and irradiation in preoperative treatment of advanced squamous cell carcinoma of the vulva. Acta Oncol. 1993, 32, 657–661. [Google Scholar] [CrossRef] [Green Version]
- Landoni, F.; Maneo, A.; Zanetta, G.; Colombo, A.; Nava, S.; Placa, F.; Tancini, G.; Mangioni, C. Concurrent preoperative chemotherapy with 5-fluorouracil and mitomycin C and radiotherapy (FUMIR) followed by limited surgery in locally advanced and recurrent vulvar carcinoma. Gynecol. Oncol. 1996, 61, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lupi, G.; Raspagliesi, F.; Zucali, R.; Fontanelli, R.; Paladini, D.; Kenda, R.; di Re, F. Combined preoperative chemoradiotherapy followed by radical surgery in locally advanced vulvar carcinoma: A pilot study. Cancer 1996, 77, 1472–1478. [Google Scholar] [CrossRef]
- Leiserowitz, G.S.; Russell, A.H.; Kinney, W.K.; Smith, L.H.; Taylor, M.H.; Scudder, S.A. Prophylactic chemoradiation of inguinofemoral lymph nodes in patients with locally extensive vulvar cancer. Gynecol. Oncol. 1997, 66, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.H.; Thomas, G.M.; Montana, G.S.; Saxer, A.; Gallup, D.G.; Olt, G. Preoperative chemoradiation for advanced vulvar cancer: A phase II study of the Gynecologic Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 79–85. [Google Scholar] [CrossRef]
- Montana, G.S.; Thomas, G.M.; Moore, D.H.; Saxer, A.; Mangan, C.E.; Lentz, S.S.; Averette, H.E. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: A gynecologic oncology group study. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1007–1013. [Google Scholar] [CrossRef]
- Han, S.C.; Kim, D.H.; Higgins, S.A.; Carcangiu, M.L.; Kacinski, B.M. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1235–1244. [Google Scholar] [CrossRef]
- Gerszten, K.; Selvaraj, R.N.; Kelley, J.; Faul, C. Preoperative chemoradiation for locally advanced carcinoma of the vulva. Gynecol. Oncol. 2005, 99, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Gaudineau, A.; Weitbruch, D.; Quetin, P.; Heymann, S.; Petit, T.; Volkmar, P.; Bodin, F.; Velten, M.; Rodier, J.F. Neoadjuvant chemoradiotherapy followed by surgery in locally advanced squamous cell carcinoma of the vulva. Oncol. Lett. 2012, 4, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.H.; Ali, S.; Koh, W.J.; Michael, H.; Barnes, M.N.; McCourt, C.K.; Homesley, H.D.; Walker, J.L. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: A gynecologic oncology group study. Gynecol. Oncol. 2012, 124, 529–533. [Google Scholar] [CrossRef]
- Beriwal, S.; Shukla, G.; Shinde, A.; Heron, D.E.; Kelley, J.L.; Edwards, R.P.; Sukumvanich, P.; Richards, S.; Olawaiye, A.B.; Krivak, T.C. Preoperative intensity modulated radiation therapy and chemotherapy for locally advanced vulvar carcinoma: Analysis of pattern of relapse. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, H.C.; Ansink, A.; Verhaar-Langereis, M.; Stalpers, L. Neoadjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst. Rev. 2006, 3, CD003752. [Google Scholar]
- Shylasree, T.S.; Bryant, A.; Howells, R.E. Chemoradiation for advanced primary vulval cancer. Cochrane Database Syst. Rev. 2011, 4, CD003752. [Google Scholar] [CrossRef]
- Maneo, A.; Landoni, F.; Colombo, A.; Villa, A.; Caspani, G. Randomised study between neoadjuvant chemoradiotherapy and primary surgery for the treatment of advanced vulval cancer. Int. J. Gynecol. Cancer 2003, 13 (Suppl. S1), 6. [Google Scholar] [CrossRef]
- Stuckey, A.; Schutzer, M.; Rizack, T.; Dizon, D. Locally advanced vulvar cancer in elderly women: Is chemoradiation beneficial? Am. J. Clin. Oncol. 2013, 36, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Aletti, G.D. Locally advanced squamous cell carcinoma of the vulva: A challenging question for gynecologic oncologists. Gynecol. Oncol. 2020, 158, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.H.; Mesic, J.B.; Scudder, S.A.; Rosenberg, P.J.; Smith, L.H.; Kinney, W.K.; Townsend, D.E.; Trelford, J.D.; Taylor, M.H.; Zukowski, C.L. Synchronous radiation and cytotoxic chemotherapy for locally advanced or recurrent squamous cancer of the vulva. Gynecol. Oncol. 1992, 47, 14–20. [Google Scholar] [CrossRef]
- Koh, W.J.; Wallace, H.J., 3rd; Greer, B.E.; Cain, J.; Stelzer, K.J.; Russell, K.J.; Tamimi, H.K.; Figge, D.C.; Russell, A.H.; Griffin, T.W. Combined radiotherapy and chemotherapy in the management of local-regionally advanced vulvarcancer. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 809–816. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.J.; McLean, C.; Arnott, S.J.; Blake, P.; Van Dam, P.; Hudson, C.N.; Shepherd, J.H. Treatment of advanced carcinoma of the vulva with chemoradiotherapy—Can exenterative surgery be avoided? Int. J. Gynecol. Cancer 1994, 4, 150–155. [Google Scholar] [CrossRef]
- Wahlen, S.A.; Slater, J.D.; Wagner, R.J.; Wang, W.A.; Keeney, E.D.; Hocko, J.M.; King, A.; Slater, J.M. Concurrent radiation therapy and chemotherapy in the treatment of primary squamous cell carcinoma of the vulva. Cancer 1995, 75, 2289–2294. [Google Scholar] [CrossRef]
- Cunningham, M.J.; Goyer, R.P.; Gibbons, S.K.; Kredentser, D.C.; Malfetano, J.H.; Keys, H. Primary radiation, cisplatin, and 5-fluorouracil for advanced squamous carcinoma of the vulva. Gynecol. Oncol. 1997, 66, 258–261. [Google Scholar] [CrossRef]
- Akl, A.; Akl, M.; Boike, G.; Hebert, J.; Graham, J. Preliminary results of chemoradiation as a primary treatment for vulvar carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 415–420. [Google Scholar] [CrossRef]
- Mulayim, N.; Foster Silver, D.; Schwartz, P.E.; Higgins, S. Chemoradiation with 5-fluorouracil and mitomycin C in the treatment of vulvar squamous cell carcinoma. Gynecol. Oncol. 2004, 93, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Landrum, L.M.; Skaggs, V.; Gould, N.; Walker, J.L.; McMeekin, D.S. Comparison of outcome measures in patients with advanced squamous cell carcinoma of the vulva treated with surgery or primary chemoradiation. Gynecol. Oncol. 2008, 108, 584–590. [Google Scholar] [CrossRef]
- Mak, R.H.; Halasz, L.M.; Tanaka, C.K.; Ancukiewicz, M.; Schultz, D.J.; Russell, A.H.; Viswanathan, A.N. Outcomes after radiation therapy with concurrent weekly platinum-based chemotherapy or every-3-4-week 5-fluorouracil-containing regimens for squamous cell carcinoma of the vulva. Gynecol. Oncol. 2011, 120, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tans, L.; Ansink, A.C.; van Rooij, P.H.; Kleijnen, C.; Mens, J.W. The role of chemo-radiotherapy in the management of locally advanced carcinoma of the vulva: Single institutional experience and review of literature. Am. J. Clin. Oncol. 2010, 34, 22–26. [Google Scholar] [CrossRef]
- Natesan, D.; Hong, J.C.; Foote, J.; Sosa, J.A.; Havrilesky, L.; Chino, J. Primary Versus Preoperative Radiation for Locally Advanced Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 794–804. [Google Scholar] [CrossRef]
- Clinical Trial: “Radiation Therapy, Gemcitabine Hydrochloride, and Cisplatin in Treating Patients with Locally Advanced Squamous Cell Cancer of the Vulva”. Available online: https://clinicaltrials.gov/ct2/show/NCT01595061 (accessed on 15 April 2021).
- Shimizu, Y.; Hasumi, K.; Masubuchi, K. Effective chemotherapy consisting of bleomycin, vincristine, mitomycin C, and cisplatin (BOMP) for a patient with inoperable vulvar cancer. Gynecol. Oncol. 1990, 36, 423–427. [Google Scholar] [CrossRef]
- Durrant, K.R.; Mangioni, C.; Lacave, A.J.; George, M.; van der Burg, M.E.; Guthrie, D.; Rotmenz, N.; Dalesio, O.; Vermorken, J.B. Bleomycin, methotrexate, and CCNU in advanced inoperable squamous cell carcinoma of the vulva: A phase II study of the EORTC Gynaecological Cancer Cooperative Group (GCCG). Gynecol. Oncol. 1990, 37, 359–362. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Greggi, S.; Scambia, G.; Salerno, G.; Mancuso, S. Cisplatin (P), bleomycin (B), and methotrexate (M) preoperative chemotherapy in locally advanced vulvar carcinoma. Gynecol. Oncol. 1993, 50, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, H.C.; Colombo, N.; Vergote, I.; Hoctin-Boes, G.; Zanetta, G.; Pecorelli, S.; Lacave, A.J.; van Hoesel, Q.; Cervantes, A.; Bolis, G.; et al. Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: An EORTC Gynaecological Cancer Cooperative Group Study. European Organization for Research and Treatment of Cancer. Gynecol. Oncol. 2001, 81, 348–354. [Google Scholar] [CrossRef]
- Geisler, J.P.; Manahan, K.J.; Buller, R.E. Neoadjuvant chemotherapy in vulvar cancer: Avoiding primary exenteration. Gynecol. Oncol. 2006, 100, 53–57. [Google Scholar] [CrossRef]
- Domingues, A.P.; Mota, F.; Durão, M.; Frutuoso, C.; Amaral, N.; de Oliveira, C.F. Neoadjuvant chemotherapy in advanced vulvar cancer. Int. J. Gynecol. Cancer 2010, 20, 294–298. [Google Scholar] [CrossRef]
- Aragona, A.M.; Cuneo, N.; Soderini, A.H.; Alcoba, E.; Greco, A.; Reyes, C.; Lekmann, S. Tailoring the treatment of locally advanced squamous cell carcinoma of the vulva: Neoadjuvant chemotherapy followed by radical surgery: Results from a multicenter study. Int. J. Gynecol. Cancer 2012, 22, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Raspagliesi, F.; Zanaboni, F.; Martinelli, F.; Scasso, S.; Laufer, J.; Ditto, A. Role of paclitaxel and cisplatin as the neoadjuvant treatment for locally advanced squamous cell carcinoma of the vulva. J. Gynecol. Oncol. 2014, 25, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellati, F.; Angioli, R.; Manci, N.; Angelo Zullo, M.; Muzii, L.; Plotti, F.; Basile, S.; Panici, P.B. Single agent cisplatin chemotherapy in surgically resected vulvar cancer patients with multiple inguinal lymph node metastases. Gynecol. Oncol. 2005, 96, 227–231. [Google Scholar] [CrossRef]
- Morrison, J.; Baldwin, P.; Buckley, L.; Cogswell, L.; Edey, K.; Faruqi, A.; Ganesan, R.; Hall, M.; Hillaby, K.; Reed, N.; et al. British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: Recommendations for practice. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 252, 502–525. [Google Scholar] [CrossRef]
- Gray, H.J. Advances in vulvar and vaginal cancer treatment. Gynecol. Oncol. 2010, 118, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, P.O.; van der Velden, J.; Vergote, I.; Guerra, C.; Scarabeli, C.; Coens, C.; Demonty, G.; Reed, N. Phase II study on paclitaxel in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy: A study of the EORTC-GCG (European Organisation for Research and Treatment of Cancer—Gynaecological Cancer Group). Ann. Oncol. 2009, 20, 1511–1516. [Google Scholar] [CrossRef]
- Thigpen, J.T.; Blessing, J.A.; Homesley, H.D.; Lewis, G.C., Jr. Phase II trials of cisplatin and piperazinedione in advanced or recurrent squamous cell carcinoma of the vulva: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1986, 23, 358–363. [Google Scholar] [CrossRef]
- Cormio, G.; Loizzi, V.; Gissi, F.; Serrati, G.; Panzarino, M.; Carriero, C.; Selvaggi, L. Cisplatin and vinorelbine chemotherapy in recurrent vulvar carcinoma. Oncology 2009, 77, 281–284. [Google Scholar] [CrossRef]
- Han, S.N.; Vergote, I.; Amant, F. Weekly paclitaxel/carboplatin in the treatment of locally advanced, recurrent, or metastatic vulvar cancer. Int. J. Gynecol. Cancer 2012, 22, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Cutillo, G.; Fragomeni, S.M.; Bruno, V.; Tagliaferri, L.; Mancini, E.; Certelli, C.; Paris, I.; Vizza, E.; Scambia, G.; et al. Palliative electrochemotherapy in primary or recurrent vulvar cancer. Int. J. Gynecol. Cancer 2020, 30, 927–931. [Google Scholar] [CrossRef]
- Muss, H.B.; Bundy, B.N.; Christopherson, W.A. Mitoxantrone in the treatment of advanced vulvar and vaginal carcinoma. A gynecologic oncology group study. Am. J. Clin. Oncol. 1989, 12, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Clancy, A.A.; Spaans, J.N.; Weberpals, J.I. The forgotten woman’s cancer: Vulvar squamous cell carcinoma (VSCC) and a targeted approach to therapy. Ann. Oncol. 2016, 27, 1696–1705. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- Klavans, M.R.; Erickson, S.H.; Modesitt, S.C. Neoadjuvant chemotherapy with paclitaxel/carboplatin/bevacizumab in advanced vulvar cancer: Time to rethink standard of care? Gynecol. Oncol. Rep. 2020, 34, 100631. [Google Scholar] [CrossRef]
- Mantovani, G.; Fragomeni, S.M.; Inzani, F.; Fagotti, A.; Della Corte, L.; Gentileschi, S.; Tagliaferri, L.; Zannoni, G.F.; Scambia, G.; Garganese, G. Molecular pathways in vulvar squamous cell carcinoma: Implications for target therapeutic strategies. J. Cancer Res. Clin. Oncol. 2020, 146, 1647–1658. [Google Scholar]
- Horowitz, N.S.; Olawaiye, A.B.; Borger, D.R.; Growdon, W.B.; Krasner, C.N.; Matulonis, U.A.; Liu, J.F.; Lee, J.; Brard, L.; Dizon, D.S. Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecol. Oncol. 2012, 127, 141–146. [Google Scholar] [CrossRef]
- Bartoletti, M.; Mazzeo, R.; De Scordilli, M.; Del Fabro, A.; Vitale, M.G.; Bortot, L.; Nicoloso, M.S.; Corsetti, S.; Bonotto, M.; Scalone, S.; et al. Human epidermal growth factor receptor-2 (HER2) is a potential therapeutic target in extramammary Paget’s disease of the vulva. Int. J. Gynecol. Cancer 2020, 30, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.H.; Song, Y.C.; Song, Y.S. Celecoxib potentiates the anticancer effect of cisplatin on vulvar cancer cells independently of cyclooxygenase. Ann. N. Y. Acad. Sci. 2009, 1171, 635–641. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, Y.C.; Kim, S.H.; Jo, H.; Song, Y.S. Effect of epidermal growth factor receptor inhibitor alone and in combination with cisplatin on growth of vulvar cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 642–648. [Google Scholar] [CrossRef]
- Woelber, L.; Mathey, S.; Prieske, K.; Kuerti, S.; Hillen, C.; Burandt, E.; Coym, A.; Mueller, V.; Schmalfeldt, B.; Jaeger, A. Targeted Therapeutic Approaches in Vulvar Squamous Cell Cancer (VSCC): Case Series and Review of the Literature. Oncol. Res. 2021, 28, 645–659. [Google Scholar] [CrossRef]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.P.; Delord, J.P.; Evans, T.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef]
- Yeku, O.; Russo, A.L.; Lee, H.; Spriggs, D. A phase 2 study of combined chemo-immunotherapy with cisplatin-pembrolizumab and radiation for unresectable vulvar squamous cell carcinoma. J. Transl. Med. 2020, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Smith, M.; Barlow, E.; Coffey, K.; Hacker, N.; Canfell, K. Vulvar cancer in high-income countries: Increasing burden of disease. Int. J. Cancer 2017, 141, 2174–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network 2021 Vulvar Cancer (Version 3.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 5 April 2021).
- Gadducci, A.; Cionini, L.; Romanini, A.; Fanucchi, A.; Genazzani, A.R. Old and new perspectives in the management of high-risk, locally advanced or recurrent, and metastatic vulvar cancer. Crit. Rev. Oncol. Hematol. 2006, 60, 227–241. [Google Scholar] [CrossRef]
- Beller, U.; Quinn, M.A.; Benedet, J.L.; Creasman, W.T.; Ngan, H.; Maisonneuve, P.; Pecorelli, S.; Odicino, F.; Heintz, A. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obs. 2006, 95, S7–S27. [Google Scholar] [CrossRef]
- How, J.A.; Jazaeri, A.A.; Soliman, P.T.; Fleming, N.D.; Gong, J.; Piha-Paul, S.A.; Janku, F.; Stephen, B.; Naing, A. Pembrolizumab in vaginal and vulvar squamous cell carcinoma: A case series from a phase II basket trial. Sci. Rep. 2021, 11, 3667. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Førsund, M.S.; Trope, C.G.; Nesland, J.M.; Holm, R.; Slipicevic, A. Evaluation of CHK1 activation in vulvar squamous cell carcinoma and its potential as a therapeutic target in vitro. Cancer Med. 2018, 7, 3955–3964. [Google Scholar] [CrossRef]
| Study (First Author) | Number pts | Year | Chemotherapy Regimen | Radiotherapy Total Dose | Outcomes |
|---|---|---|---|---|---|
| Thomas et al. [18] | 9 * | 1989 | 5-FU 1000 mg/m2/d d1–4 with or without MMC 6 mg/m2 | 45–51 GY | cCR: 66.6% |
| Berek et al. [19] | 12 | 1991 | 5-FU 1000 mg/m2 d1–4 + CisP 100 mg/m2 d1 for 2 cycles | 40–52 Gy | cCR: 67% |
| Eifel et al. [20] | 12 | 1995 | CisP 4 mg/m2/d d1–4 + 5-FU 250 mg/m2/d d1–4 for 4 weeks | 40 Gy | cPR: 100% pCR: 50% |
| Scheistroen et al. [21] | 20 | 1993 | Bleo 30 mg IV d1, 3, 5 for 2 courses | 30–45 Gy | cCR: 25% cPR: 50% |
| Landoni et al. [22] | 41 | 1996 | 5-FU 750 mg/m2 d1–5 + MMC 15 mg/m2 IV d1 for 2 courses | 54 Gy in 2 courses | pCR: 24.4% |
| Lupi et al. [23] | 24 | 1996 | 5-FU 750 mg/m2 d1–5 + MMC 15 mg/m2 IV d1 for 2 courses | 54 Gy in 2 courses | cPR: 91.6% |
| Leiserowitz et al. [24] | 23 | 1997 | 5-FU 1000 mg/m2 d1–4 + CisP 100 mg/m2 IV d2 for 2–3 cycles | 54 Gy | cCR: 60.9% |
| Moore et al. [25] | 71 | 1998 | 5-FU 1000 mg/m2 d1–4 + CisP 50 mg/m2 IV d1 for 2 courses | 2 courses of 23.8 Gy | cCR: 48% pCR: 70% |
| Montana et al. [26] | 46 | 2000 | 5-FU 1000 mg/m2 d1–4 + CisP 50 mg/m2 IV d1 for 2 courses | 2 courses of 23.8 Gy | pCR (nodes): 40% pCR (vulva): 52% |
| Han et al. [27] | 12 | 2000 | 5-FU 1000 mg/m2 d1–4 + MMC 10 mg/m2 IV d1 for 2 cycles | 45 Gy | cCR: 71.4% cPR: 28.6% |
| Gerszten et al. [28] | 18 | 2005 | 5-FU 1000 mg/m2 d1–4 + CisP 50 mg/m2 IV d1 for 2 courses | 44.6 Gy | cCR: 72.2% |
| Gaudineau et al. [29] | 22 | 2012 | Carbo AUC 2 weekly | 50 Gy | pCR: 27% pPR: 95% |
| Moore et al. [30] | 58 | 2012 | Weekly CisP 40 mg/m2 IV | 57.6 Gy | cCR: 64% pCR: 50% |
| Beriwal et al. [31] | 42 | 2013 | CisP 40 mg/m2 d1 or 5-FU 1000 mg/m2 d1–5 for 2 courses | IMRT 46 Gy | cCR: 51.2% pCR: 48.5% |
| Study (First Author) | Number pts | Year | Chemotherapy Regimen | Radiotherapy Total Dose | Outcomes |
|---|---|---|---|---|---|
| Russel et al. [37] | 18 | 1992 | 5-FU 750–1000 mg/m2 d1–4 + CisP 100 mg/m2 IV d1 for 2–3 cycles | 54 Gy | CR: 50% PR: 6% |
| Koh et al. [38] | 14 | 1993 | 5-FU 750–1000 mg/m2 IV d1–4 weekly | 54 Gy | CR: 57% PR: 36% |
| Sebag-Montefiore et al. [39] | 16 | 1994 | 5-FU 750 mg/m2 d1–5 + MMC 10 mg/m2 IV d1 for 2 cycles | 45 Gy | CR: 44% PR: 37% |
| Wahlen et al. [40] | 19 | 1995 | 5-FU 1000 mg/m2 d1–4 for 2 cycles with/without MMC 10 mg/m2 IV d1 | 45–50 Gy | CR: 52% PR: 36% |
| Cunningham et al. [41] | 14 | 1997 | 5-FU 1000 mg/m2 d1–4 + CisP 50 mg/m2 d1 for 2 cycles | 45–50 Gy | CR: 64% PR: 29% |
| Akl et al. [42] | 12 | 2000 | 5-FU 1000 mg/m2/24h d1–4 and d29–32 + MMC 15 mg/m2 IV d1 | 30–36 Gy | CR: 100% |
| Mulayim et al. [43] | 17 | 2004 | 5-FU 1000 mg/m2 d1–4 with/without MMC 10 mg/m2 IV d1 | 45–60 Gy | CR: 86% |
| Landrum et al. [44] | 33 | 2008 | Weekly CisP 40 mg/m2 or 2 cycles of CisP 50 mg/m2 IV d1 + 5-FU 1000 mg/m2 IV d1–4 | 47.6 Gy | CR: 87% |
| Mak et al. [45] | 24 | 2011 | weekly CisP or 5-FU based | 50 Gy | CR: 58% |
| Tans et al. [46] | 20 | 2011 | 5-FU 1000 mg/m2 infusion d1–4 + MMC 10 mg/m2 IV d1 for 2 courses | 40 + 20 Gy | CR: 70% |
| Study (First Author) | Number pts | Year | Chemotherapy Regimen | Outcomes |
|---|---|---|---|---|
| Durrant et al. [50] | 18 | 1990 | Bleo 5 mg IM d1–5 + MTX 15 mg PO d1 and 4 + CCNU 40 mg PO d5–7 week 1, then Bleo 5 mg IM d1 and 4 + MTX 15 mg PO d1 and 4 | ORR: 64% |
| Benedetti-Panici et al. [51] | 21 | 1993 | CisP 100 mg/m2 d1 + Bleo 15 mg d1 and 8 + MTX 300 mg/m2 d8 | ORR (vulva): 90.5% ORR (nodes): 67% |
| Wagenaar et al. [52] | 12 | 2001 | Week 1: Bleo 5 mg IM d1–5 + CCNU 40 mg PO d5–7 + MTX 10 mg PO d1 + 4 Weeks 2–6: Bleo 5 mg IM d1 + 4 + MTX 15 mg PO d1. | ORR: 58% |
| Geisler et al. [53] | 13 | 2006 | 5-FU 1000 mg/m2/24 h d1–5 + CisP 50 mg/m2 IV d1 (n = 10) | pPR: 60%, pCR: 40% |
| CisP 50 mg/m2 IV d1 (n = 3) | ORR: 0% | |||
| Domingues et al. [54] | 25 | 2010 | Bleo 20 mg/m2 IV d1–10 (n = 10) | ORR: 60% |
| Tax 100 mg/m2 IV weekly (n = 5) | ORR: 40% | |||
| 5-FU 750 mg/m2 d1–4 + CisP 60–80 mg/m2 IV d1, weekly (n = 10) | ORR: 20% | |||
| Aragona et al. [55] | 35 | 2012 | Cis + 5-FU (n = 12) CisP + Tax (n = 6) CisP +5-FU + Tax (n = 6) VinC + Bleo + CisP (n = 6) Bleo alone (n = 5) | cPR: 85.7% |
| Raspagliesi et al. [56] | 10 | 2014 | Tax 175 mg/m2 d1 + ifosfamide 5 g/m2 in 24 h in d2 + CisP 50 mg/m2 Tax 175 mg/m2 d1 + CisP 70 mg/m2 | pCR: 10% |
| Study (First Author) | Number pts | Year | Chemotherapy Regimen | Outcomes |
|---|---|---|---|---|
| Thigpen et al. [61] | 22 | 1986 | CisP 50 mg/m2 IV q21 or Piperazinedione 9 mg/m2 IV q21 | ORR: 0% mPFS: NA mOS: NA |
| Muss et al. [65] | 11 | 1989 | Mitoxantrone 12 mg/m2 IV q21 | ORR: 0% mPFS: 1.3 m mOS: 3.2 m |
| Durrant et al. [50] | 11 | 1990 | Bleo 5 mg IM d1–5 + MTX 15 mg PO d1,4 + CCNU 40 mg PO d5–7 week 1 then Bleo 5 mg IM d1,4 + MTX 15 mg PO d1,4 weeks 2–5 | ORR: 60% mPFS: NA mOS: NA |
| Wagenaar et al. [52] | 13 | 2001 | Week 1: Bleo 5 mg IM d1–5 + CCNU 40 mg PO d5–7 + MTX 10 mg PO d1,4 Weeks 2–6: Bleo 5 mg IM d1,4 + MTX 15 mg PO d1 | ORR: 54% mPFS: 4.8 m mOS: 7.8 m |
| Cormio et al. [62] | 15 | 2009 | CisP 80 mg/m2 IV d1 + Vinorelbine 25 mg/m2 IV d1 and d8, q21 | ORR: 40% mPFS: 10 m mOS: 19 m |
| Witteveen et al. [60] | 29 | 2009 | Tax 175 mg/m2 IV q3 weeks | ORR: 13.8% mPFS: 2.6 m mOS: 6.8 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzotta, M.; Pizzuti, L.; Krasniqi, E.; Di Lisa, F.S.; Cappuzzo, F.; Landi, L.; Sergi, D.; Pelle, F.; Cappelli, S.; Botti, C.; et al. Role of Chemotherapy in Vulvar Cancers: Time to Rethink Standard of Care? Cancers 2021, 13, 4061. https://doi.org/10.3390/cancers13164061
Mazzotta M, Pizzuti L, Krasniqi E, Di Lisa FS, Cappuzzo F, Landi L, Sergi D, Pelle F, Cappelli S, Botti C, et al. Role of Chemotherapy in Vulvar Cancers: Time to Rethink Standard of Care? Cancers. 2021; 13(16):4061. https://doi.org/10.3390/cancers13164061
Chicago/Turabian StyleMazzotta, Marco, Laura Pizzuti, Eriseld Krasniqi, Francesca Sofia Di Lisa, Federico Cappuzzo, Lorenza Landi, Domenico Sergi, Fabio Pelle, Sonia Cappelli, Claudio Botti, and et al. 2021. "Role of Chemotherapy in Vulvar Cancers: Time to Rethink Standard of Care?" Cancers 13, no. 16: 4061. https://doi.org/10.3390/cancers13164061
APA StyleMazzotta, M., Pizzuti, L., Krasniqi, E., Di Lisa, F. S., Cappuzzo, F., Landi, L., Sergi, D., Pelle, F., Cappelli, S., Botti, C., Vizza, E., Tomao, S., Marchetti, L., Sanguineti, G., Botticelli, A., Marchetti, P., Magri, V., Pisegna, S., Venuti, A., ... Vici, P. (2021). Role of Chemotherapy in Vulvar Cancers: Time to Rethink Standard of Care? Cancers, 13(16), 4061. https://doi.org/10.3390/cancers13164061









